Abstract
Objective
Calcification of the aortic valve and adjacent structures involves inflammatory, lipid, and mineral metabolism pathways. We hypothesized that circulating biomarkers reflecting these pathways are associated with cardiac calcification in older adults.
Methods
We investigated the associations of various biomarkers with valvular and annular calcification in the Cardiovascular Health Study. Of 5888 participants, up to 3585 were eligible after exclusions for missing biomarker, covariate, or echocardiographic data. We evaluated analytes reflecting lipid (lipoprotein [Lp] (a), lipoprotein-associated phospholipase A2 [LpPLA2] mass and activity), inflammatory (interleukin-6, soluble [s] CD14), and mineral metabolism (fetuin-A, fibroblast growth factor [FGF]-23) that were measured within 5 years of echocardiography. The relationships of plasma biomarkers with aortic valve calcium (AVC), aortic annular calcium (AAC) and mitral annular calcium (MAC) were assessed with relative risk (RR) regression.
Results
Calcification was prevalent: AVC 59%, AAC 45% and MAC 41%. After adjustment, Lp(a), LpPLA2 mass and activity, and sCD14 were positively associated with AVC. RRs for AVC per SD (95% CI) were: Lp(a), 1.051 (1.022–1.081); LpPLA2 mass, 1.036 (1.006–1.066), and activity, 1.037 (1.004–1.071); sCD14, 1.039 (1.005–1.073). FGF-23 was positively associated with MAC, 1.040 (1.004–1.078), and fetuin-A was negatively associated, 0.949 (0.911–0.989). No biomarkers were significantly associated with AAC.
Conclusions
This study shows novel associations of circulating FGF-23 and fetuin-A with MAC, and LpPLA2 and sCD14 with AVC, confirming that previously reported for Lp(a). Further investigation of lipoprotein and inflammatory pathways may provide added insight into the etiology AVC, while study of phosphate regulation may illuminate the pathogenesis of MAC.
Keywords: valvular heart disease, inflammation, lipids, mineral metabolism
INTRODUCTION
Calcification of the aortic valve (AVC) and adjacent structures (aortic annulus [AAC], mitral annulus [MAC]) compromises valvular function, leading to morbidity and mortality in older individuals [1,2]. Surgical or transcatheter interventions for valvular disease are costly and risky. Accordingly, there is interest in developing medical approaches to prevent or reverse valvular calcification. Similarities between AVC and atherosclerosis have prompted testing of statin therapies in several randomized trials, but these failed to demonstrate small-to-moderate effects on progression of AVC [3]. Genetic analyses of population-based cohorts, however, have since implicated LDL cholesterol and lipoprotein (Lp) (a) in AVC in a causal manner, suggesting that the null findings with statins may relate to insufficient cholesterol lowering or their use after the disease process was already established [4,5]. Beyond lipids, animal models and histopathologic studies in humans point to inflammatory and mineral metabolism pathways in the pathogenesis of AVC and MAC [6,7]. Important gaps remain in understanding the proteins involved and their relationship with calcification of the fibrous skeleton of the heart in clinical cohorts. We tested the hypothesis that inflammatory, lipid, and mineral metabolism markers are associated with echocardiographic AVC, AAC and MAC in a large older cohort, to inform efforts in preventing cardiac calcification and its complications.
METHODS
Study population and procedures
The Cardiovascular Health Study (CHS) is a population-based study of risk factors for cardiovascular disease (CVD) in adults 65 and older [8]. CHS prospectively enrolled an original cohort of 5201 individuals in 1989–90 from 4 U.S. communities, followed by a supplemental cohort of 687 African-American individuals in 1992–93. Participants underwent standardized evaluations, including history, physical examination, phlebotomy, and diagnostic testing. All centers received institutional review board approval for the study and subjects gave informed consent.
Biomarkers were previously measured to investigate their associations with CVD. Lipid measures included Lp(a), a pro-atherogenic molecule previously associated with AVC in the first (1989–90) echocardiographic exam, but not examined in relation to MAC or AAC, which were only assessed in the second (1994–95) echocardiographic exam; and lipoprotein-associated phospholipase A2 (LpPLA2, mass and activity), an enzyme that circulates in association with lipid particles, generating pro-atherogenic lysophosphatidylcholine (lysoPC) and non-esterified fatty acids [9,10]. Inflammatory measures comprised the cytokine interleukin-6 (IL-6) and soluble cluster of differentiation (sCD) 14, a cell-surface protein released by macrophages and hepatocytes that binds bacterial lipopolysaccharide [11]. Measures of mineral metabolism included fetuin-A, a liver-derived protein that solubilizes calcium and phosphate [12], and fibroblast growth factor (FGF)-23, an osteocyte-secreted hormone that acts to lower phosphate levels [13]. The primary outcome measures were AVC, AAC and MAC, diagnosed by echocardiography in 1994–95.
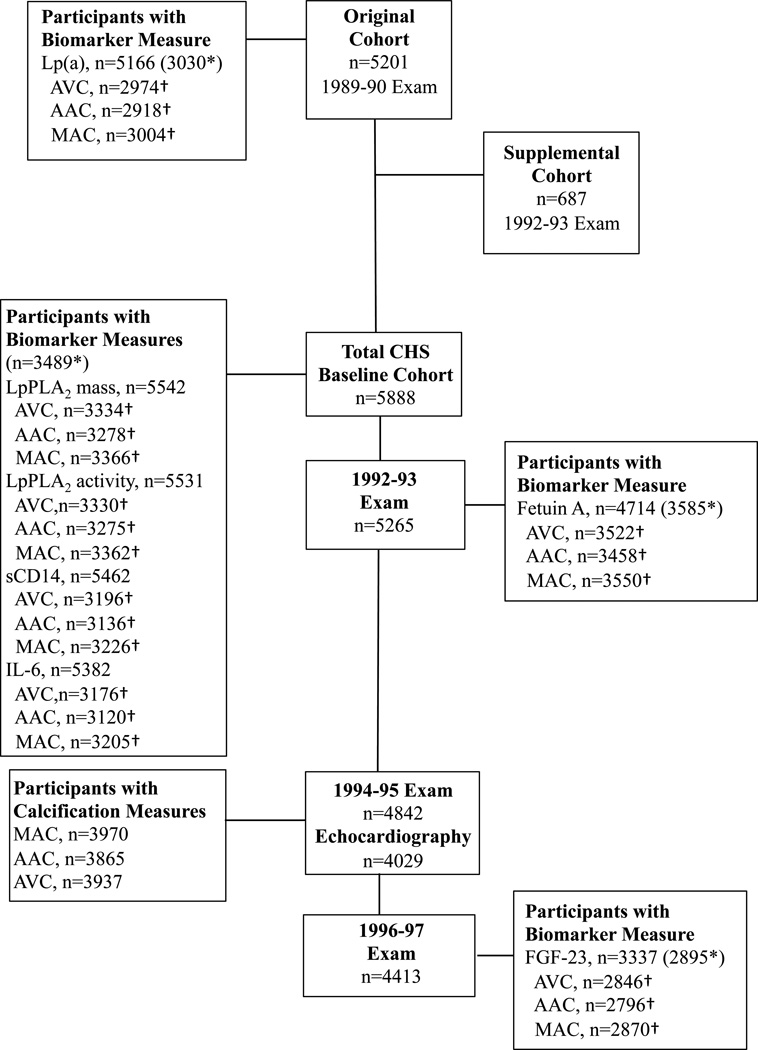
The numbers of participants attending each CHS exam, along with the timing and proportion with specimens available for specific biomarker measurements in relation to the echocardiographic assessment, are shown in Figure 1. Of 5888 participants at baseline (original and supplemental cohort), 1881 were missing all calcification measures, 31 were additionally missing all biomarkers, and 487 were additionally missing baseline covariates, leaving 3489 with at least one calcification measure available for analysis. The corresponding number for the 5201 participants from the original cohort was 3030. For the 5265 total participants attending the 1992–93 exam, 3585 with at lest one calcification measure were available for fetuin-A analysis, while of 4413 participants completing the 1996–97 exam, 2895 were available for FGF-23 analysis.
Figure 1.
Timing of Examinations, Biomarker Measurement, and Echocardiographic Outcome Assessment, Cardiovascular Health Study (1989–1997). The boxes provide the number of participants attending each visit; the number having measures for each biomarker at the corresponding visit; and the number with available cardiac calcification measures. The boxes also provide the number of participants who had at least one calcification measure after excluding missing biomarker and covariate measures (*); and, for each biomarker, the subset of participants (†) included in analyses for individual cardiac calcification measures after excluding missing covariates.
Laboratory methods
Fasting blood samples were collected, frozen at −70° C, and sent to the CHS Core Laboratory. Lp(a) was analyzed by a monoclonal antibody-based enzyme-linked immunosorbent assay [(ELISA), Genentech, San Francisco, CA] with a coefficient of variation (CV) of 7.5% [14]. Lp-PLA2 mass was measured using the PLAC™ Test (Diadexus, San Francisco, CA) with a CV of 6.3%. Lp-PLA2 activity was measured at GlaxoSmithKline (Research Triangle Park, NC) using tritium-labeled platelet activating factor (H3-PAF) as a substrate with a CV of 7.5% [15]. IL-6 and sCD14 were measured by ELISA (R&D Systems, Minneapolis, MN) [16,17]. The inter-assay CV for sCD14 of 5.3% to 12.4% [17]. Fetuin-A was determined by ELISA (Epitope Diagnostics, San Diego, CA) with CV of 3.3 to 9.1% [18]. FGF-23 was analyzed by ELISA (Immutopics, San Clemente, CA) with intra- and interassay CV of 7.4% to 10.6% [19].
Echocardiography
Standardized echocardiograms were performed and interpreted by blinded readers at a core laboratory. MAC was defined as an echodense structure at the junction of the atrioventricular groove and posterior mitral leaflet. MAC was graded as “mild”, a focal, limited increased echodensity of the mitral annulus; “moderate”, a marked echodensity involving >⅓ but <. of the ring circumference; and “severe”, a marked echodensity extending ≥. of the ring circumference or intruding into the left ventricular inflow tract. AAC was defined as increased echocardiographic density of the aortic root at the insertion of the aortic leaflets. AVC was defined as increased leaflet thickness without restriction of leaflet motion. AS was defined as thickened leaflets with reduced systolic opening on 2D imaging and/or an increased anterograde velocity (>2.0 m/s by continuous-wave Doppler) across the valve [20]. In a random subset of 116 participants, intra-observer κ scores for AAC, MAC and AVC were 0.60, 0.70, and 0.82, respectively, consistent with moderate or substantial agreement, respectively.
Covariates
Diabetes was defined by fasting glucose ≥126 mg/dl, non-fasting glucose ≥200 mg/dL, or anti-glycemic therapy. Estimated glomerular filtration rate (eGFR) was calculated using plasma cystatin C. Serum calcium, phosphate, and 25-hydroxyvitamin D were available from a subset in 1992–93. Atherosclerotic CVD, including coronary heart disease (CHD), stroke, transient ischemic attack (TIA), and peripheral arterial disease (PAD), was ascertained at the baseline examination and semi-annual contact thereafter, and adjudicated by specialized committees.
Statistical analysis
Levels of baseline covariates and candidate biomarkers were described by presence or absence of MAC, AAC, and AVC. Pairwise correlations of lipid, inflammatory, and mineral-metabolism biomarkers were assessed with Spearman coefficients. Multivariable relative risk (RR) regression was used to determine adjusted associations of biomarkers with AVC, MAC, and AAC. Linearity of these associations was assessed with generalized additive models. We adjusted sequentially for potential confounders. Model 1 was adjusted for age, sex and race-ethnicity. Model 2 additionally adjusted for education, body mass index, systolic blood pressure, antihypertensive medication, diabetes, smoking, prevalent CHD, prevalent stroke/TIA, prevalent PAD, LDL, HDL, log-transformed triglycerides, log-transformed C-reactive protein (CRP) and eGFR. Model 3 was adjusted for Model 2 variables plus Lp(a) for LpPLA2 mass and activity; LpPLA2 for Lp(a); sCD14 for IL-6; and IL-6 for sCD14. Analyses of IL-6 did not include adjustment for CRP, which is downstream in the causal pathway. FGF-23 was adjusted for fetuin-A and fetuin-A for FGF-23. Fetuin-A was further adjusted for calcium and phosphate as potential mediators. FGF-23 was additionally adjusted for calcium, phosphate, and 25-hydroxyvitamin D, which may be both potential confounders and mediators. First-order interactions were evaluated with age, sex, race-ethnicity, eGFR, prevalent CVD, and, in the case of fetuin-A, diabetes, and for biomarkers with each another. We used a two-tailed significance level of p<0.05 for main effects; for the interaction analysis, we adopted a Bonferroni-corrected p<(0.05/6)=0.008. As a sensitivity analysis we examined significant biomarker associations from the primary analysis with graded levels of AVC (AVC without AS vs. none and AVC with AS vs. none) and MAC (mild vs. none and moderate/severe vs. none). All analyses were performed with STATA v. 12.1 (College Station, TX).
RESULTS
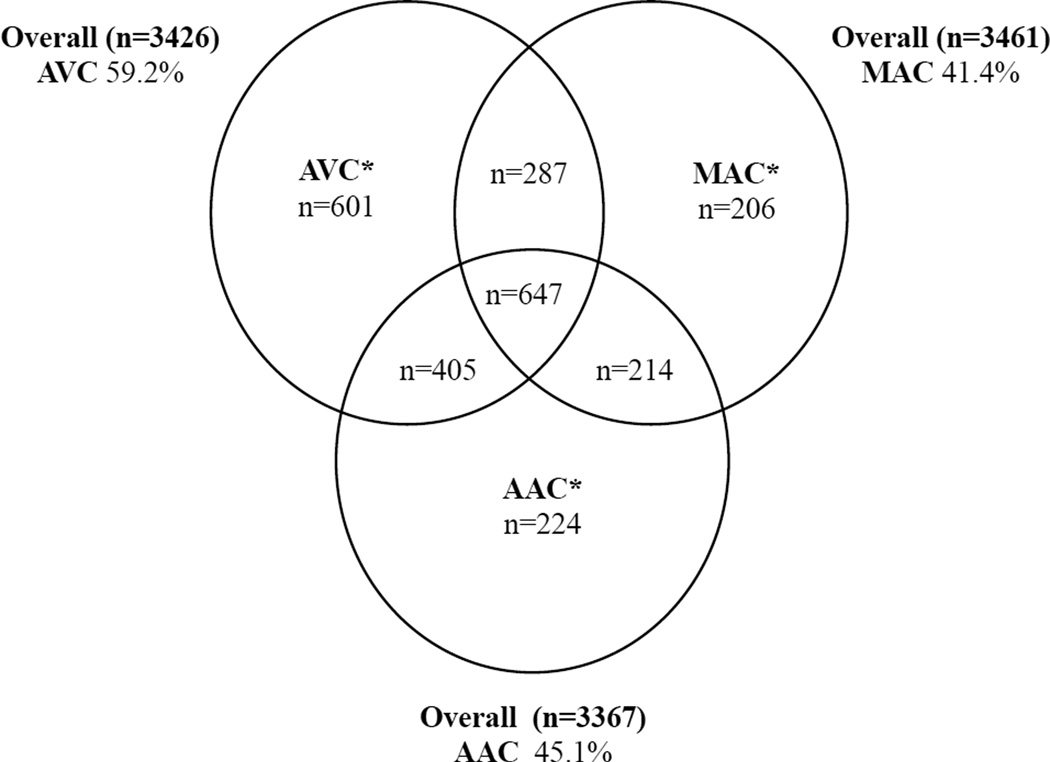
The mean age of the study sample at baseline was 72±5 years, and 63% were women. As shown in Figure 2, valvular and annular calcification was prevalent, and frequently occurred in combination. Individuals with calcification tended to be older; more frequently had hypertension, dyslipidemia, and prevalent CVD; and had lower eGFR and higher phosphate, as compared to those without calcification (Table 1). Participants with AVC were less commonly women, diabetic or ever smokers, while those with MAC exhibited higher CRP, as compared to those without calcification. In similar comparisons, LpPLA2 mass and activity were higher in those with than without calcification for AVC, AAC and MAC, while Lp(a) was higher for AVC, sCD14 for MAC, and FGF-23 for both AVC and MAC.
Figure 2.
Venn diagram of participants with AVC, MAC, and AAC alone or in combination. The total number of participants in the baseline analysis (original and supplemental cohorts) with each calcification measure after exclusion of those with missing covariate or biomarker data is provided outside the Venn diagram, along with the proportion having positive calcification. Values inside the Venn diagram (*) are for the subset of these participants with information on all three cardiac calcification measures, and give the number of participants having positive calcification for each of these measures, alone and in combination.
Table 1.
Associations of Covariates and Candidate Biomarkers with Annular and Valvular Calcification
| Mitral Annular Calcification | Aortic Annular Calcification | Aortic Valve Calcification | ||||
|---|---|---|---|---|---|---|
| Yes (n=1432) | No (n=2029) | Yes (n=1517) | No (n=1850) | Yes (n=2027) | No (n=1399) | |
| Covariates* | ||||||
| Age, years | 72±5 | 71±4 | 72±5 | 71±4 | 72±5 | 71±4 |
| Women, n (%) | 915 (63.9) | 1257 (62.0) | 931 (61.4) | 1185 (64.1) | 1198 (59.1) | 947(67.7) |
| Black race, n (%) | 205 (14.3) | 371 (18.3) | 229 (15.1) | 335 (18.1) | 343 (16.9) | 226 (16.2) |
| Education <high school, n (%) | 375 (26.2) | 495 (24.4) | 398 (26.2) | 444 (24.0) | 527 (26.0) | 341 (24.4) |
| Body mass index, kg/m2 | 27.0±4.6 | 26.6±4.4 | 26.7±4.5 | 26.8±4.5 | 26.7±4.5 | 26.8±4.5 |
| Systolic blood pressure, mm Hg | 137±22 | 133±21 | 136±21 | 134±21 | 136±21 | 134±21 |
| Diastolic blood pressure, mm Hg | 70±11 | 71±11 | 70±11 | 71±11 | 71±11 | 70±11 |
| Anti-hypertensive therapy, n (%) | 715 (49.9) | 849 (41.8) | 714 (47.1) | 803 (43.4) | 980 (48.3) | 575 (41.1) |
| Diabetes, n (%) | 191 (13.3) | 242 (11.9) | 196 (12.9) | 225 (12.2) | 273 (13.5) | 156(11.2) |
| Smoking status, n (%) | ||||||
| Never | 685 (47.8) | 971 (47.9) | 740 (48.8) | 876 (47.4) | 943 (46.5) | 696 (49.7) |
| Former | 593 (41.4) | 839 (41.4) | 633 (41.7) | 751 (40.6) | 874 (43.1) | 543 (38.8) |
| Current | 154 (10.8) | 219 (10.8) | 144 (9.5) | 223 (12.1) | 210 (10.4) | 160 (11.4) |
| Alcohol ≥14 (men) or ≥7 drinks/week (women), n (%) |
157 (11.0) |
232 (11.5) |
165 (10.9) |
217 (11.8) |
220 (10.9) |
165 (11.8) |
| LDL, mg/dl | 134±35 | 129±35 | 132±35 | 130±35 | 133±35 | 129±36 |
| HDL, mg/dl | 55±16 | 56±16 | 55±16 | 56±16 | 55±16 | 57±16 |
| TG, mg/dl | 136±60 | 132±59 | 135±61 | 132±57 | 135±61 | 132±56 |
| Lipid-lowering therapy, n (%) | 93 (6.5) | 111 (5.5) | 101 (6.7) | 98 (5.3) | 120 (5.9) | 80 (5.7) |
| CRP, mg/l | 4.7±7.8 | 4.1±7.0 | 4.4±7.5 | 4.3±7.4 | 4.3±7.1 | 4.4±7.90 |
| eGFR, ml/min/1.73 m2 | 79±19 | 82±18 | 80±19 | 82±18 | 80±19 | 82±18 |
| Prevalent CHD, n (%) | 274 (19.1) | 308 (15.2) | 269 (17.7) | 292 (15.8) | 388 (19.1) | 192 (13.7) |
| Prevalent stroke/TIA, n (%) | 79 (5.5) | 76 (3.7) | 66 (4.4) | 86 (4.6) | 98 (4.8) | 55 (3.9) |
| Prevalent PAD, n (%) | 25 (1.7) | 34 (1.7) | 27 (1.8) | 29 (1.6) | 40 (2.0) | 18 (1.3) |
| Serum calcium, mg/dl† | 9.5±0.4 | 9.5±0.4 | 9.5±0.4 | 9.5±0.4 | 9.5±0.4 | 9.5±0.3 |
| Serum phosphate, mg/dl† | 3.6±0.5 | 3.6±0.5 | 3.6±0.5 | 3.6±0.5 | 3.6±0.5 | 3.6±0.5 |
| 25-OH vit. D, ng/ml† | 25.6±13.5 | 26.1±10.3 | 25.4±12.8 | 26.4±10.8 | 25.9±11.2 | 26.1±12.5 |
| Biomarkers‡ | ||||||
| Lp(a), ug/ml | 54.2±52.4 | 51.5±48.1 | 51.8±50.5 | 53.0±48.7 | 55.5±52.1 | 48.9±46.4 |
| LpPLA2 mass, ng/ml | 342±113 | 333.3±116 | 341±118 | 332±111 | 343±116 | 328±112 |
| LpPLA2 activity, nmol/min.ml | 39.5±13.1 | 38.5±12.7 | 39.4±12.7 | 38.4±12.8 | 39.8±12.9 | 37.7±12.8 |
| IL-6, pg/ml | 2.1±1.7 | 1.9±1.8 | 2.0±1.5 | 2.0±2.0 | 2.0±1.8 | 1.9±1.6 |
| sCD14, ng/ml | 1635±351 | 1594±335 | 1620±348 | 1600±335 | 1619±349 | 1598±331 |
| Fetuin-A, g/l | 0.47±0.1 | 0.48±0.1 | 0.47±0.09 | 0.48±0.10 | 0.47±0.10 | 0.48±0.10 |
| FGF-23, RU/ml | 110.0±173.4 | 92.8±147.2 | 100.4±154.0 | 97.2±155.2 | 100.6±155.1 | 97.8±155.3 |
Values are given as mean±standard deviation or median(interquartile range).
CHD=Coronary heart disease. eGFR= Estimated glomerular filtration rate. CRP=C-reactive protein. OH=Hydroxy. PAD=Peripheral arterial disease. TG=Triglycerides. TIA=Transient ischemic attack. Vit.=Vitamin. Lp=lipoprotein. PLA2= phospholipase A2. sCD= soluble cluster of differentiation. IL= interleukin. FGF=fibroblast growth factor. RU=relative units.
Data from the 1989–90 exam for the original cohort, and the 1992–93 exam for the supplemental cohort, based on participants having each cardiac calcification measure at the baseline examination
Available in subset of n=1848, 1848, 1835 of 3584 participants available for the 1992–93 analysis.
See Figure 1 for timing of measurements.
Spearman correlations between biomarkers are shown in Table 2. LpPLA2 mass and activity were strongly correlated and both were modestly correlated with lipids. IL-6 and CRP were strongly correlated, and each was modestly correlated with sCD14. Fetuin-A was modestly correlated with triglycerides, while FGF-23 was weakly correlated with triglycerides and inflammatory markers.
Table 2.
Spearman Correlations between Lipid, Inflammatory, and Mineral Metabolism Biomarkers*
| Lp(a)† | LpPLA2 mass† |
LpPLA2 activity† |
LDL† | HDL† | TG† | CRP† | IL-6† | sCD14† | Fetuin-A‡ | FGF-23§ | Calcium‡ | Phosphate‡ | Vit. D‡ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lp(a) | 1.000 | |||||||||||||
| LpPLA2 mass | −0.014 | 1.000 | ||||||||||||
| LpPLA2 activity | −0.036 | 0.534 | 1.000 | |||||||||||
| LDL | 0.115 | 0.230 | 0.367 | 1.000 | ||||||||||
| HDL | 0.054 | −0.106 | −0.320 | −0.007 | 1.000 | |||||||||
| TG | −0.105 | 0.037 | 0.275 | 0.178 | −0.489 | 1.000 | ||||||||
| CRP | 0.065 | 0.088 | −0.010 | 0.002 | −0.182 | 0.149 | 1.000 | |||||||
| IL-6 | 0.055 | 0.096 | 0.040 | −0.087 | −0.233 | 0.092 | 0.520 | 1.000 | ||||||
| sCD14 | 0.068 | 0.117 | 0.077 | 0.062 | −0.005 | 0.044 | 0.258 | 0.193 | 1.000 | |||||
| Fetuin-A | −0.035 | 0.053 | 0.115 | 0.111 | −0.096 | 0.337 | −0.054 | −0.016 | 0.064 | 1.000 | ||||
| FGF-23 | 0.075 | −0.089 | −0.101 | −0.066 | −0.064 | 0.115 | 0.170 | 0.170 | 0.279 | 0.124 | 1.000 | |||
| Calcium | −0.013 | 0.015 | −0.002 | 0.182 | 0.137 | 0.104 | −0.057 | −0.019 | −0.034 | 0.049 | 0.098 | 1.000 | ||
| Phosphate | 0.035 | −0.052 | −0.070 | 0.059 | 0.138 | 0.016 | 0.006 | −0.058 | 0.206 | 0.082 | 0.089 | 0.099 | 1.000 | |
| Vit. D | 0.002 | −0.047 | 0.067 | −0.108 | 0.031 | 0.001 | −0.081 | −0.139 | −0.028 | 0.006 | −0.058 | 0.002 | 0.031 | 1.000 |
In subset of participants with at least one cardiac calcification measure.
1989 to 1990 for the original cohort and, 1992 to 1993 for the supplemental cohort.
1992 to 1993 for both cohorts, using baseline variables when variables from 1992 to 1993 were not available.
1996 to 1997 for both cohorts, using 1992 to 1993 and baseline variables when variables from 1996 to 1997 covariates were not available.
Abbreviations are as in Table 1. Vit. D = 25-hydroxyvitamin D
Generalized additive models showed no meaningful departures from linearity (Supplementary Figures 1–3). The adjusted associations between continuous levels of biomarkers and cardiac calcification measures are presented in Table 3. After full adjustment for potential confounders (Model 2), all lipid markers and sCD14 were associated with AVC, but not with MAC or AAC, at the nominal significance level. Associations were modest, ranging from 3.6 to 5.1% increased risk per SD for AVC, with 95% confidence intervals (CI’s) encompassing effect estimates as low as 0.4% and as high as 8.1%. After mutual adjustment, risk estimates for lipid markers with AVC were similar, as was that for sCD14. Fully adjusted models revealed modest associations of nominal significance between both mineral markers and MAC, but not with AVC or AAC. The relationship was inverse for fetuin-A, characterized by a 5.1% decreased risk per SD, ranging from as low as 1.1% to as high as 8.9%; it was instead positive for FGF-23, with a 4.0% increased risk per SD that ranged from 0.4% to 7.8%. These associations were not meaningfully changed after mutual adjustment. After additional adjustment for putative mediators of fetuin-A and FGF-23’s associations with MAC (calcium, phosphate and, for FGF-23, 25-hydroxyvitamin D) among participants with available measures (fetuin-A, n=1835; FGF-23, n=1534), there was no appreciable effect on the risk estimates.
Table 3.
Association between Biomarkers and Annular or Valvular Calcification.
| Biomarker | Mitral Annular Calcification | Aortic Annular Calcification | Aortic Valve Calcification |
|---|---|---|---|
| RR per SD (95% CI), P value | RR per SD (95% CI), P value | RR per SD (95% CI), P value | |
| Lp (a) | |||
| Model 1 | 1.030 (0.988,1.073) 0.166 |
0.991 (0.947,1.036) 0.687 |
1.057 (1.028,1.087) <0.001 |
| Model 2 | 1.015 (0.973,1.060) 0.489 |
0.990 (0.945,1.037) 0.676 |
1.051 (1.022,1.081) 0.001 |
| Model 3 | 1.016 (0.969,1.066) 0.511 |
0.983 (0.936,1.032) 0.494 |
1.055 (1.022,1.090) 0.001 |
| LpPLA2 mass | |||
| Model 1 | 1.026 (0.985,1.068) 0.219 |
1.022 (0.983,1.062) 0.273 |
1.048 (1.019,1.078) 0.001 |
| Model 2 | 1.001 (0.959,1.045) 0.959 |
1.022 (0.983,1.062) 0.402 |
1.036 (1.006,1.066) 0.019 |
| Model 3 | 1.002 (0.959,1.048) 0.922 |
1.014 (0.972,1.057) 0.528 |
1.040 (1.009,1.072) 0.012 |
| LpPLA2 activity | |||
| Model 1 | 1.041 (1.000,1.084) 0.048 |
1.029 (0.990,1.070) 0.140 |
1.058 (1.029,1.088) <0.001 |
| Model 2 | 0.994 (0.947,1.043) 0.802 |
1.019 (0.975,1.066) 0.403 |
1.037 (1.004,1.071) 0.027 |
| Model 3 | 0.988 (0.940,1.040) 0.649 |
1.017 (0.970,1.065) 0.489 |
1.033 (0.999,1.068) 0.054 |
| Interleukin-6 | |||
| Model 1 | 1.035 (0.992,1.081) 0.111 |
0.993 (0.954,1.033) 0.727 |
1.010 (0.984,1.037) 0.444 |
| Model 2 | 1.020 (0.976,1.065) 0.380 |
0.990 (0.950,1.032) 0.638 |
1.009 (0.981,1.038) 0.519 |
| Model 3 | 1.018 (0.974,1.064) 0.435 |
0.982 (0.941,1.026) 0.415 |
1.005 (0.976,1.035) 0.732 |
| sCD14 | |||
| Model 1 | 1.052 (1.008,1.097) 0.020 |
1.025 (0.983,1.068) 0.243 |
1.037 (1.007,1.068) 0.016 |
| Model 2 | 1.046 (0.997,1.098) 0.064 |
1.024 (0.978,1.073) 0.309 |
1.039 (1.005,1.073) 0.022 |
| Model 3 | 1.029 (0.977,1.084) 0.275 |
1.028 (0.979,1.080) 0.265 |
1.045 (1.010,1.081) 0.012 |
| Fetuin-A | |||
| Model 1 | 0.968 (0.930,1.001) 0.110 |
0.974 (0.937,1.012) 0.171 |
0.992 (0.964,1.020) 0.561 |
| Model 2 | 0.949 (0.911,0.989) 0.013 |
0.965 (0.927,1.004) 0.077 |
0.983 (0.955,1.013) 0.262 |
| Model 3 | 0.952 (0.908,0.998) 0.043 |
0.963 (0.919,1.009) 0.113 |
0.997 (0.963,1.032) 0.863 |
| FGF-23 | |||
| Model 1 | 1.043 (1.007,1.080) 0.019 |
1.003 (0.958,1.050) 0.889 |
1.004 (0.971,1.038) 0.823 |
| Model 2 | 1.040 (1.004,1.078) 0.030 |
1.007 (0.961,1.054) 0.781 |
1.004 (0.970,1.040) 0.804 |
| Model 3 | 1.038 (1.002,1.077) 0.041 |
1.005 (0.960,1.053) 0.818 |
1.005 (0.971,1.040) 0.794 |
Model 1. Adjusted for age, sex, race.
Model 2. Adjusted for covariates from Model 1, plus education, BMI, systolic blood pressure, antihypertensive treatment, diabetes, smoking, eGFR, prevalent coronary heart disease, prevalent stroke/transient ischemic attack, prevalent peripheral arterial disease, LDL, HDL, log (triglycerides) and (with the exception of IL-6) log (CRP).
Model 3. Adjusted for covariates in Model 2 plus, Lp(a) (for LpPLA2 mass and LpPLA2 activity), LpPLA2 (for Lp[a]), sCD14 (for IL-6), IL-6 (for sCD14), FGF-23 (for fetuin-A), and fetuin-A (for FGF-23).
Lp (a), SD=55.8 mg/dl; LpPLA2 mass SD=118.9 ng/ml; LpPLA2 activity, SD=13.0 nmol/min.ml; IL-6, SD =1.9 pg/ml; sCD14, SD=362 ng/ml, Fetuin-A, SD= 0.097 g/l, FGF-23 SD=174.4 RU/ml.
CI=Confidence Interval. RR=Relative Risk. RU=relative units.
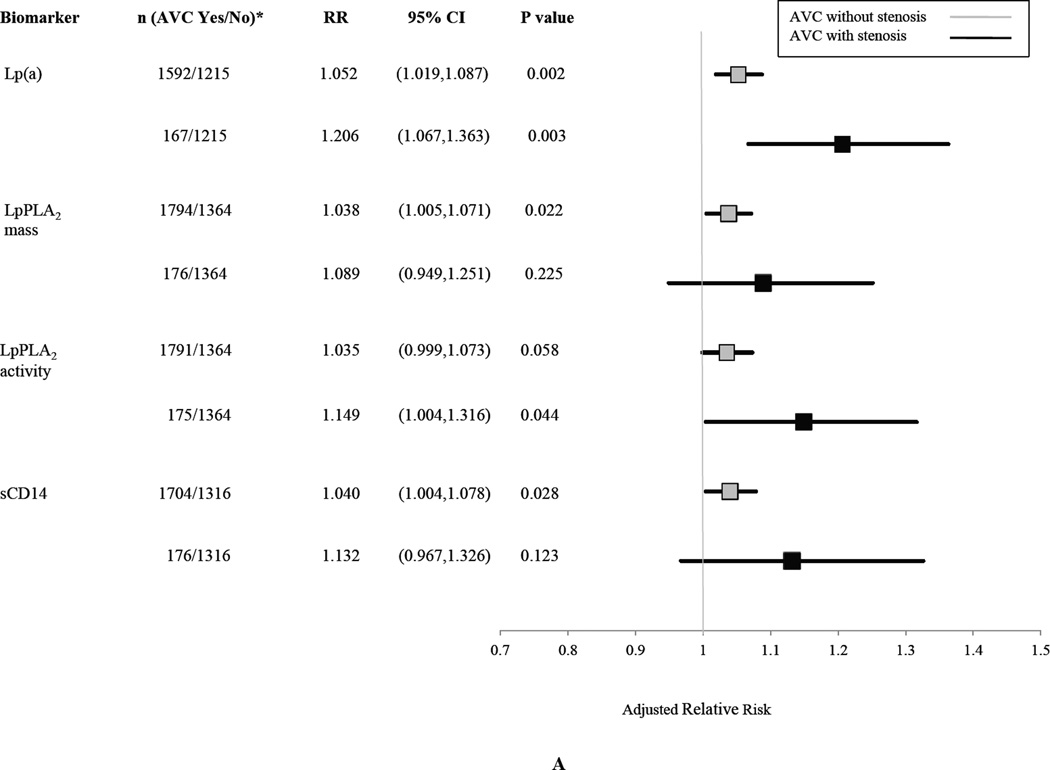
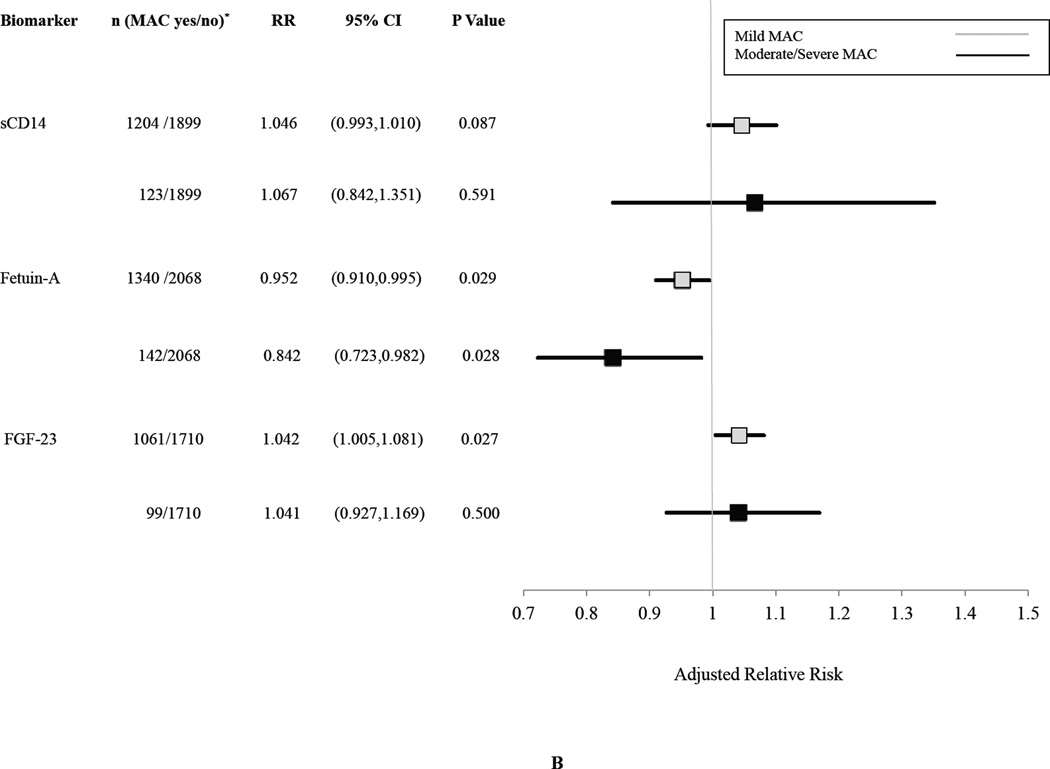
The associations of biomarkers with graded levels of AVC and MAC are shown in Figures 3A and 3B, respectively. There appeared to be strengthening of the association with greater severity of AVC, particularly for Lp(a) and LpPLA2 activity, although the number of participants having AVC with AS was modest, and there was overlap of the 95% CI’s for the risk estimates. In the case of MAC, there was an apparently graded inverse relationship for fetuin-A, but not for sCD14 and FGF-23, although again there were few participants in the higher MAC category.
Figure 3.
Association of Selected Biomarkers with Graded Levels of AVC (Panel A) or MAC (Panel B).
Relative risks are per SD increment in each biomarker comparing each level of calcification indicated versus the no calcification category. Risk estimates are adjusted for age, sex, race, education, BMI, systolic blood pressure, antihypertensive treatment, diabetes, smoking, eGFR, prevalent coronary heart disease, prevalent stroke/transient ischemic attack, prevalent peripheral arterial disease, LDL, HDL, log(triglycerides) and log(CRP). AVC yes/no indicates those having calcification with stenosis or without stenosis (yes) versus no calcification (no). MAC yes/no indicates those having calcification of mild or moderate/severe degree (yes) versus those without calcification (no).
There was evidence of effect modification of the association of LpPLA2 with binary AVC and AAC by age, whereby older participants tended to have a less pronounced association (p=0.006; RR per SD 0.992 [95% CI 0.987, 0.998] per year greater of age and p=0.005; RR per SD 0.990 [95% CI 0.983, 0.997] per year greater of age, respectively). In addition, there was evidence of interaction between FGF-23 and eGFR in relation to binary AVC (p=0.003; RR per SD 0.998 [95% CI 0.996, 0.999] per unit greater eGFR).
DISCUSSION
Main findings
In this large cohort of older adults, circulating measures of lipid metabolism or inflammation, namely, Lp(a), LpPLA2 mass and activity, and sCD14, were positively associated with AVC, at the nominal level of significance after adjustment for various traditional cardiovascular risk factors. Significant associations were not observed with respect to AAC or MAC, yet the upper 95% confidence bounds do not exclude similar associations of modest size. By contrast, the inflammatory marker IL-6 did not exhibit significant associations with any calcification, and in the case of AVC, the upper limit of the 95% CI excludes an association of the magnitude observed for sCD14. In turn, two mineral metabolism markers, fetuin-A and FGF-23, were associated with MAC after such adjustment, the first marker inversely and the second positively. Neither marker showed a significant association with AAC or AVC, and, in the case of AVC, the 95% confidence bounds excluded or all but excluded a modest association of the magnitude observed for MAC. The associations for Lp(a) and LpPLA2 activity persisted when graded levels of AVC were considered, as did that for fetuin-A with graded MAC, but these dose-response analyses were limited by small numbers in higher-severity categories.
Several of the relationships identified herein have not been previously reported, including that between the macrophage activation marker sCD14 and AVC, and the bone-derived hormone FGF-23 and MAC. The association of LpPLA2 mass or activity with AVC extends previous findings from pathological and small clinical studies to a broader population.
For LpPLA2 activity, there was a weakening of its relationship with AVC and AAC with older age, while for FGF-23, there was evidence of an association with AVC at lower eGFR. Effect modification suggests that the association for LpPLA2 may be overshadowed by other age-related risk factors, and that for FGF-23, there may also be a relationship to AVC that is especially relevant in the setting of kidney disease. These findings require replication given their post-hoc nature and multiple testing.
Context and implications of associations with AVC
Animal models and human histopathologic studies provide insights into the pathogenesis of cardiac calcification. The current conceptual model for AVC begins with an initiation phase characterized by deposition of oxidized LDL and subsequent macrophage infiltration of the leaflets [6]. Release of pro-inflammatory cytokines transdifferentiates valvular interstitial cells to osteoblast-like cells. Osteoprogenitors promote tissue mineralization, which restricts leaflet opening. Trauma from increased shear stress propagates a cycle of inflammation, oxidative stress, and mineralization that results in progressive AS.
Epidemiological studies accord with these preclinical and pathological observations, showing associations between AVC and various traditional atherosclerosis risk factors [8,20]. Previous analyses in CHS and elsewhere have also linked circulating Lp(a) levels with AVC [5,9], which we extend by demonstrating that the relationship remains significant after more extensive adjustment than undertaken previously, and by showing that associations of the same magnitude with other forms of calcification –AAC and MAC– are unlikely. Our findings advance knowledge of lipid determinants of AVC, demonstrating a relationship between circulating LpPLA2 mass or activity with AVC that was independent of lipid fractions and, for LpPLA2 activity, even of Lp(a). The action of LpPLA2 on lipoproteins generates lysoPC, which has been shown to activate valvular mineralization. LpPLA2 is expressed in stenotic aortic valve tissue, with higher circulating levels documented in a small AS study [21,22]. Furthermore, recent data from another clinical study suggest that increased LpPLA2 activity may stimulate faster progression of mild AS [23]. The present findings further implicate LpPLA2 in AVC, and strengthen the rationale for testing inhibition of LpPLA2 as a preventive strategy. This could complement drastic lowering of LDL and Lp(a) levels with another lipid-targeted approach using anti-proprotein convertase subtilisin/kexin 9 antibodies.
Various markers of inflammation, including CRP, have not been consistently linked to AVC in prior studies, and testing of IL-6 in our study similarly revealed no association [24]. The positive association documented between sCD14 and AVC is therefore notable. sCD14 is an acute phase protein secreted by the liver or released from macrophages. This protein stimulates innate immunity in sepsis, triggering release of cell-adhesion molecules and cytokines. sCD14 is elevated in atherosclerosis, and high levels have previously been related to CVD and mortality, independent of CRP, IL-6 and traditional risk factors in CHS [17]. That sCD14 was modestly associated with AVC in this study, independently of CRP and IL-6, points to the need for further study of macrophage-related pathways as potential therapeutic targets.
Context and implications of associations with MAC
MAC and AVC have histopathologic similarities [1,25]. Conditions associated with increased afterload impose high shear stress upon the ventricular aspect of the mitral leaflets, and annular calcification may be a response to the resulting injury. A foremost risk factor for MAC is chronic kidney disease (CKD), which is associated with derangements of mineral metabolism. Previous work from CHS has linked increased serum phosphate with AVC, AAC and MAC [26]. Development of MAC, however, may be even more sensitive to altered calcium and phosphorus metabolism than AVC or AAC, as supported by the strong association reported between CKD and MAC, but not AVC or AAC, in the Framingham Offspring Study [24].
Our findings also suggest a potentially stronger role of altered mineral metabolism as a determinant of MAC than of AVC. Both fetuin-A and FGF-23 are important regulators of mineral metabolism that forestall precipitation of calcium in soft tissues. Fetuin-A binds calcium and phosphate to inhibit their crystallization, but also engages the insulin receptor tyrosine kinase, inducing insulin resistance [27]. Fetuin-A was inversely associated with AS in a small, unmatched case-control study [28]. By contrast, in a cohort with prevalent CHD, fetuin-A was inversely associated with MAC, but there was effect modification of its relationship with AS, wherein a significant inverse association was only present in participants without diabetes [29]. Our findings extend fetuin-A’s inverse association with MAC to a general population, but do not confirm the presence of effect modification by diabetes in fetuin-A’s association with AVC. Raising fetuin-A levels to decrease MAC is intriguing, but may be marred by it adverse effects on insulin sensitivity.
In turn, FGF-23 controls serum phosphate by inhibiting renal tubular transport and 1,25-dihydroxyvitamin D (calcitriol) synthesis, increasing renal phosphate excretion and reducing its intestinal absorption. Higher FGF-23 levels occur in response to dietary phosphate, calcitriol, parathyroid hormone and calcium [30]. The positive association between FGF-23 and MAC could reflect more marked disturbance of kidney function and dysregulation of calcium and phosphate metabolism, perhaps signaling a higher level of resistance to FGF-23’s phosphate-lowering actions, with higher levels of the hormone necessary to handle a given phosphate load. Evidence of a stronger positive association at lower eGFR did not emerge for MAC, but it did for AVC, consistent with this premise in the latter instance. The significant association between FGF-23 and MAC persisted after adjustment for calcium, phosphate and 25-hydroxyvitamin D, as well as eGFR, suggesting an independent effect. Higher levels of FGF-23 may lower its co-receptor, Klotho, which has salutary actions, or stimulate the renin-angiotensin system. Notably, calcium mimetics have been shown to reduce events in direct proportion to lowering of FGF-23 levels, suggesting a potential intervention for testing [30].
LIMITATIONS
This study’s cross-sectional design limits inferences regarding causality. There was an offset of 2 to 5 years between biomarker measurement and echocardiography, but this would tend to bias associations toward the null. As noted, analyses of dose-response were limited by fewer participants with higher grades of calcification. Echocardiographic assessment of calcification is less accurate than computed tomography, but misclassification would diminish the strength of associations. Further misclassification is introduced by the one-time measurement of biomarkers, which would underestimate their cumulative impact over time. We did not correct for multiple testing, which can increase potential for chance findings. Hence, our findings will require independent replication.
CONCLUSIONS
This study shows novel associations of biomarkers of mineral metabolism, FGF-23 and fetuin-A, with MAC, and biomarkers of lipid metabolism and inflammation, Lp(a), LpPLA2 and sCD14, with AVC, suggesting some possible differences in the etiology of these disorders. Pending replication, these associations point to potentially new approaches for medical prevention of these prevalent conditions in older adults, whose implications for the health of our aging population could be profound.
Supplementary Material
KEY MESSAGES.
What is already known about this subject?
Histopathologic studies implicate lipid, inflammation and mineral metabolism pathways in calcification of the cardiac valves and annuli, and population-based genetic studies support a causal role for LDL cholesterol and lipoprotein (a) in aortic valve calcification (AVC). Smaller clinical studies have shown a positive association between plasma lipoprotein phospholipase A2 (LpPLA2) and AVC, and a negative association between plasma fetuin-A and mitral annular calcification (MAC).
What does this study add?
This investigation evaluates biomarkers in relation to echocardiographic cardiac calcification in a population-based study of older adults. The study shows significant associations of fibroblast growth factor-23 (positive) and fetuin-A (inverse) with MAC, and of Lp(a), LpPLA2 and soluble cluster of differentiation (sCD) 14 with AVC, that are independent of potential confounders and putative causal intermediates.
How might this impact on clinical practice?
MAC and AVC are important causes of cardiac dysfunction in older adults. The identification of high Lp(a), LpPLA2 and sCD14 as risk factors for AVC, and of elevated FGF-23 and low fetuin-A as risk factors for MAC, suggest new potential strategies for preventing cardiac calcification that warrant further study.
Acknowledgments
FUNDING
This work was supported by R01 HL-094555, as well as by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant U01HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). AEB is supported by a fellowship from the Empire Clinical Research Investigator Program. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
Mary Cushman and Jorge Kizer have received research funding from diaDexus (manufacturer of the LpPLA2 test). Dr. Kizer reports ownership of stock in Pfizer, Inc., and Gilead Sciences, Inc.
Footnotes
This work was presented, in part, at the American College of Cardiology Scientific Sessions, San Diego, California, 2015.
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive license (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in HEART editions and any other BMJPGL products to exploit all subsidiary rights
REFERENCES
- 1.Barasch E, Gottdiener JS, Marino Larsen EK, et al. Cardiovascular morbidity and mortality in community-dwelling elderly individuals with calcification of the fibrous skeleton of the base of the heart and aortosclerosis (The Cardiovascular Health Study) Am J Cardiol. 2006;97:1281–1286. doi: 10.1016/j.amjcard.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 2.Fox CS, Vasan RS, Parise H, et al. Mitral annular calcification predicts cardiovascular morbidity and mortality: the Framingham Heart Study. Circulation. 2003;107:1492–1496. doi: 10.1161/01.cir.0000058168.26163.bc. [DOI] [PubMed] [Google Scholar]
- 3.Teo KK, Corsi DJ, Tam JW, et al. Lipid lowering on progression of mild to moderate aortic stenosis: meta-analysis of the randomized placebo-controlled clinical trials on 2344 patients. Can J Cardiol. 2011;27:800–808. doi: 10.1016/j.cjca.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Smith JG, Luk K, Schulz C-A, et al. Association of low-density lipoprotein cholesterol-related genetic variants with aortic valve calcium and incident aortic stenosis. JAMA. 2014;312:1764–1771. doi: 10.1001/jama.2014.13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thanassoulis G, Campbell CY, Owens DS, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–512. doi: 10.1056/NEJMoa1109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pawade TA, Newby DE, Dweck MR. Calcification in Aortic Stenosis: The Skeleton Key. J Am Coll Cardiol. 2015;66:561–577. doi: 10.1016/j.jacc.2015.05.066. [DOI] [PubMed] [Google Scholar]
- 7.Arounlangsy P, Sawabe M, Izumiyama N, et al. Histopathogenesis of early-stage mitral annular calcification. J Med Dent Sci. 2004;51:35–44. [PubMed] [Google Scholar]
- 8.Novaro GM, Katz R, Aviles RJ, et al. Clinical factors, but not C-reactive protein, predict progression of calcific aortic-valve disease: the Cardiovascular Health Study. J Am Coll Cardiol. 2007;50:1992–1998. doi: 10.1016/j.jacc.2007.07.064. [DOI] [PubMed] [Google Scholar]
- 9.Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 10.Garg PK, McClelland RL, Jenny NS, et al. Lipoprotein-associated phospholipase A2 and risk of incident cardiovascular disease in a multi-ethnic cohort: The multi ethnic study of atherosclerosis. Atherosclerosis. 2015;241:176–182. doi: 10.1016/j.atherosclerosis.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaptoge S, Seshasai SRK, Gao P, et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J. 2014;35:578–589. doi: 10.1093/eurheartj/eht367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goustin A-S, Abou-Samra AB. The “thrifty” gene encoding Ahsg/Fetuin-A meets the insulin receptor: Insights into the mechanism of insulin resistance. Cell Signal. 2011;23:980–990. doi: 10.1016/j.cellsig.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 14.Wong WL, Eaton DL, Berloui A, et al. A monoclonal-antibody-based enzyme-linked immunosorbent assay of lipoprotein(a) Clin Chem. 1990;36:192–197. [PubMed] [Google Scholar]
- 15.Jenny NS, Solomon C, Cushman M, et al. Lipoprotein-associated phospholipase A(2) (Lp-PLA(2)) and risk of cardiovascular disease in older adults: results from the Cardiovascular Health Study. Atherosclerosis. 2010;209:528–532. doi: 10.1016/j.atherosclerosis.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tehrani DM, Gardin JM, Yanez D, et al. Impact of inflammatory biomarkers on relation of high density lipoprotein-cholesterol with incident coronary heart disease: cardiovascular Health Study. Atherosclerosis. 2013;231:246–251. doi: 10.1016/j.atherosclerosis.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reiner AP, Lange EM, Jenny NS, et al. Soluble CD14: genomewide association analysis and relationship to cardiovascular risk and mortality in older adults. Arterioscler Thromb Vasc Biol. 2013;33:158–164. doi: 10.1161/ATVBAHA.112.300421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen MK, Bartz TM, Djoussé L, et al. Genetically elevated fetuin-A levels, fasting glucose levels, and risk of type 2 diabetes: the cardiovascular health study. Diabetes Care. 2013;36:3121–3127. doi: 10.2337/dc12-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deo R, Katz R, de Boer IH, et al. Fibroblast Growth Factor 23 and Sudden Versus Non-sudden Cardiac Death: The Cardiovascular Health Study. Am J Kidney Dis Off J Natl Kidney Found. 2015;66:40–46. doi: 10.1053/j.ajkd.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otto CM, Lind BK, Kitzman DW, et al. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 21.Kolasa-Trela R, Fil K, Bazanek M, et al. Lipoprotein-associated phospholipase A2 is elevated in patients with severe aortic valve stenosis without clinically overt atherosclerosis. Clin Chem Lab Med CCLM FESCC. 2012;50:1825–1831. doi: 10.1515/cclm-2012-0015. [DOI] [PubMed] [Google Scholar]
- 22.Mahmut A, Boulanger M-C, Husseini DEl, et al. Elevated expression of lipoprotein-associated phospholipase A2 in calcific aortic valve disease: implications for valve mineralization. J Am Coll Cardiol. 2014;63:460–469. doi: 10.1016/j.jacc.2013.05.105. [DOI] [PubMed] [Google Scholar]
- 23.Capoulade R, Mahmut A, Tastet L, et al. Impact of plasma Lp-PLA2 activity on the progression of aortic stenosis: the PROGRESSA study. JACC Cardiovasc Imaging. 2015;8:26–33. doi: 10.1016/j.jcmg.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Fox CS, Guo C-Y, Larson MG, et al. Relations of inflammation and novel risk factors to valvular calcification. Am J Cardiol. 2006;97:1502–1505. doi: 10.1016/j.amjcard.2005.11.086. [DOI] [PubMed] [Google Scholar]
- 25.Kizer JR, Wiebers DO, Whisnant JP, et al. Mitral annular calcification, aortic valve sclerosis, and incident stroke in adults free of clinical cardiovascular disease: the Strong Heart Study. Stroke J Cereb Circ. 2005;36:2533–2537. doi: 10.1161/01.STR.0000190005.09442.ad. [DOI] [PubMed] [Google Scholar]
- 26.Linefsky JP, O’Brien KD, Katz R, et al. Association of serum phosphate levels with aortic valve sclerosis and annular calcification: the cardiovascular health study. J Am Coll Cardiol. 2011;58:291–297. doi: 10.1016/j.jacc.2010.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pal D, Dasgupta S, Kundu R, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18:1279–1285. doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari G, Sainger R, Beckmann E, et al. Validation of plasma biomarkers in degenerative calcific aortic stenosis. J Surg Res. 2010;163:12–17. doi: 10.1016/j.jss.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ix JH, Shlipak MG, Katz R, et al. Kidney function and aortic valve and mitral annular calcification in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis Off J Natl Kidney Found. 2007;50:412–420. doi: 10.1053/j.ajkd.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 30.Moe SM, Chertow GM, Parfrey PS, et al. Cinacalcet, Fibroblast Growth Factor-23, and Cardiovascular Disease in Hemodialysis: The Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) Trial. Circulation. 2015;132:27–39. doi: 10.1161/CIRCULATIONAHA.114.013876. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.