Abstract
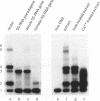
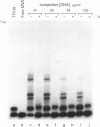
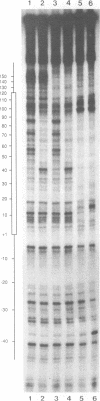
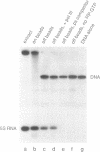
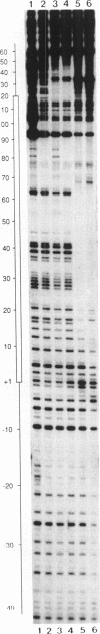
Specific protein-DNA complexes formed on a Xenopus 5S RNA gene were isolated and characterized using a novel technique. A DNA template reversibly immobilized on paramagnetic beads was used to capture, affinity purify, and concentrate protein--DNA complexes formed in a whole cell extract. The complexes were then released from the beads in a soluble and transcriptionally active form via restriction enzyme digestion of the DNA. A band-shift gel was used to separate and obtain the DNase I footprints of five individual complexes. Three of the complexes resulted from the independent binding of two proteins, TFIIIA and an unidentified protein binding to a large region just downstream of the 3' end of the gene. Two more slowly migrating complexes contained an additional large central protected region covering most of the gene. The most slowly migrating complex displayed protein interactions over the 5' flanking sequences. The formation of two of these complexes was shown to be dependent on TFIIIC activity. The correlation between transcriptional activity and the formation of these complexes suggests that the observed protein--DNA interactions are important for transcription of 5S RNA genes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun B. R., Riggs D. L., Kassavetis G. A., Geiduschek E. P. Multiple states of protein-DNA interaction in the assembly of transcription complexes on Saccharomyces cerevisiae 5S ribosomal RNA genes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2530–2534. doi: 10.1073/pnas.86.8.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey M. F., Gerrard S. P., Cozzarelli N. R. Analysis of RNA polymerase III transcription complexes by gel filtration. J Biol Chem. 1986 Mar 25;261(9):4309–4317. [PubMed] [Google Scholar]
- Engelke D. R., Gottesfeld J. M. Chromosomal footprinting of transcriptionally active and inactive oocyte-type 5S RNA genes of Xenopus laevis. Nucleic Acids Res. 1990 Oct 25;18(20):6031–6037. doi: 10.1093/nar/18.20.6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradkin L. G., Yoshinaga S. K., Berk A. J., Dasgupta A. Human transcription factor TFIIIC2 specifically interacts with a unique sequence in the Xenopus laevis 5S rRNA gene. Mol Cell Biol. 1989 Nov;9(11):4941–4950. doi: 10.1128/mcb.9.11.4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielsen O. S., Sentenac A. RNA polymerase III (C) and its transcription factors. Trends Biochem Sci. 1991 Nov;16(11):412–416. doi: 10.1016/0968-0004(91)90166-s. [DOI] [PubMed] [Google Scholar]
- Geiduschek E. P., Tocchini-Valentini G. P. Transcription by RNA polymerase III. Annu Rev Biochem. 1988;57:873–914. doi: 10.1146/annurev.bi.57.070188.004301. [DOI] [PubMed] [Google Scholar]
- Glikin G. C., Ruberti I., Worcel A. Chromatin assembly in Xenopus oocytes: in vitro studies. Cell. 1984 May;37(1):33–41. doi: 10.1016/0092-8674(84)90298-8. [DOI] [PubMed] [Google Scholar]
- Hayes J., Tullius T. D., Wolffe A. P. A protein-protein interaction is essential for stable complex formation on a 5 S RNA gene. J Biol Chem. 1989 Apr 15;264(11):6009–6012. [PubMed] [Google Scholar]
- Kassavetis G. A., Braun B. R., Nguyen L. H., Geiduschek E. P. S. cerevisiae TFIIIB is the transcription initiation factor proper of RNA polymerase III, while TFIIIA and TFIIIC are assembly factors. Cell. 1990 Jan 26;60(2):235–245. doi: 10.1016/0092-8674(90)90739-2. [DOI] [PubMed] [Google Scholar]
- Keller H. J., Romaniuk P. J., Gottesfeld J. M. Interaction of Xenopus TFIIIC with the TFIIIA.5 S RNA gene complex. J Biol Chem. 1992 Sep 5;267(25):18190–18198. [PubMed] [Google Scholar]
- Keller H. J., You Q. M., Romaniuk P. J., Gottesfeld J. M. Additional intragenic promoter elements of the Xenopus 5S RNA genes upstream from the TFIIIA-binding site. Mol Cell Biol. 1990 Oct;10(10):5166–5176. doi: 10.1128/mcb.10.10.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovelman R., Roeder R. G. Purification and characterization of two forms of human transcription factor IIIC. J Biol Chem. 1992 Dec 5;267(34):24446–24456. [PubMed] [Google Scholar]
- Lassar A. B., Martin P. L., Roeder R. G. Transcription of class III genes: formation of preinitiation complexes. Science. 1983 Nov 18;222(4625):740–748. doi: 10.1126/science.6356356. [DOI] [PubMed] [Google Scholar]
- Peck L. J., Millstein L., Eversole-Cire P., Gottesfeld J. M., Varshavsky A. Transcriptionally inactive oocyte-type 5S RNA genes of Xenopus laevis are complexed with TFIIIA in vitro. Mol Cell Biol. 1987 Oct;7(10):3503–3510. doi: 10.1128/mcb.7.10.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H. R., Waldschmidt R., Jahn D., Seifart K. H. Purification of human transcription factor IIIC and its binding to the gene for ribosomal 5S RNA. Nucleic Acids Res. 1989 Jul 11;17(13):5003–5016. doi: 10.1093/nar/17.13.5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel C. W., Peck L. J. Kinetic control of 5 S RNA gene transcription. J Mol Biol. 1992 Oct 20;227(4):1009–1018. doi: 10.1016/0022-2836(92)90517-n. [DOI] [PubMed] [Google Scholar]
- Setzer D. R., Brown D. D. Formation and stability of the 5 S RNA transcription complex. J Biol Chem. 1985 Feb 25;260(4):2483–2492. [PubMed] [Google Scholar]
- Taylor M. J., Segall J. Characterization of factors and DNA sequences required for accurate transcription of the Saccharomyces cerevisiae 5 S RNA gene. J Biol Chem. 1985 Apr 10;260(7):4531–4540. [PubMed] [Google Scholar]
- Wolffe A. P., Brown D. D. Developmental regulation of two 5S ribosomal RNA genes. Science. 1988 Sep 23;241(4873):1626–1632. doi: 10.1126/science.241.4873.1626. [DOI] [PubMed] [Google Scholar]
- Wolffe A. P., Jordan E., Brown D. D. A bacteriophage RNA polymerase transcribes through a Xenopus 5S RNA gene transcription complex without disrupting it. Cell. 1986 Feb 14;44(3):381–389. doi: 10.1016/0092-8674(86)90459-9. [DOI] [PubMed] [Google Scholar]
- Wolffe A. P., Morse R. H. The transcription complex of the Xenopus somatic 5 S RNA gene. A functional analysis of protein-DNA interactions outside of the internal control region. J Biol Chem. 1990 Mar 15;265(8):4592–4599. [PubMed] [Google Scholar]
- Wolffe A. P. Transcription fraction TFIIIC can regulate differential Xenopus 5S RNA gene transcription in vitro. EMBO J. 1988 Apr;7(4):1071–1079. doi: 10.1002/j.1460-2075.1988.tb02915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Rivier D. H., Sprague K. U. Sequences far downstream from the classical tRNA promoter elements bind RNA polymerase III transcription factors. Mol Cell Biol. 1991 Mar;11(3):1382–1392. doi: 10.1128/mcb.11.3.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]