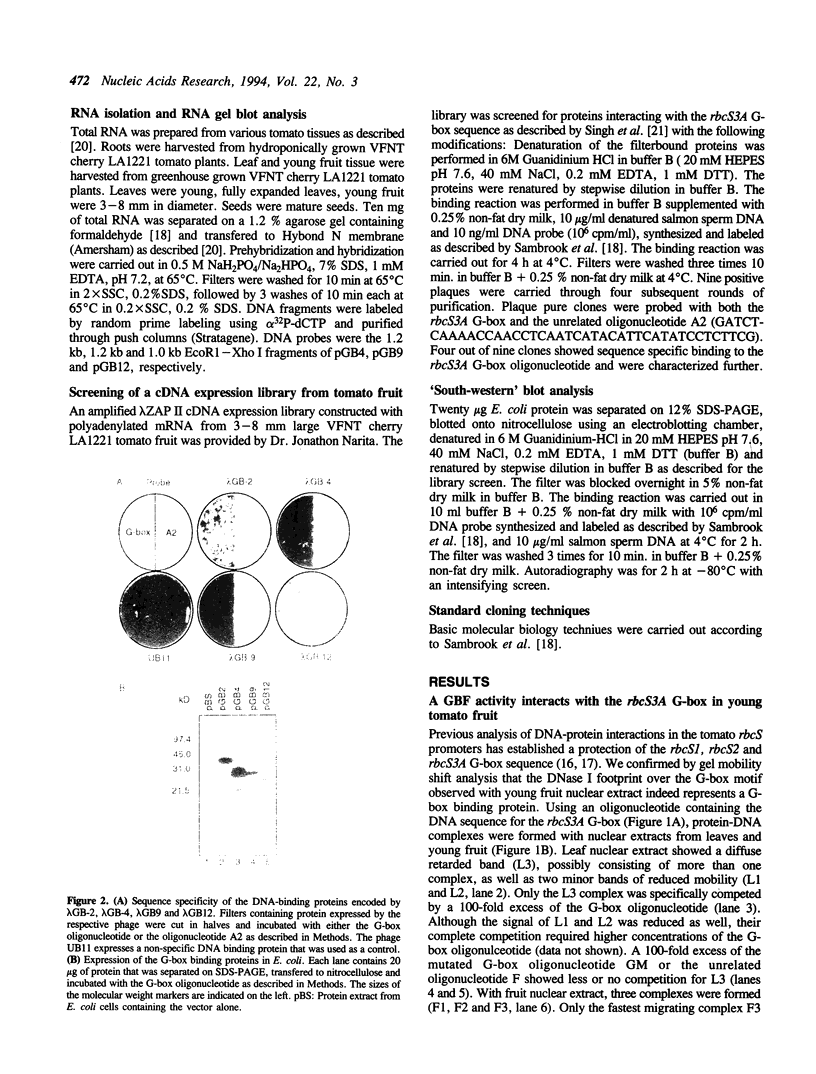
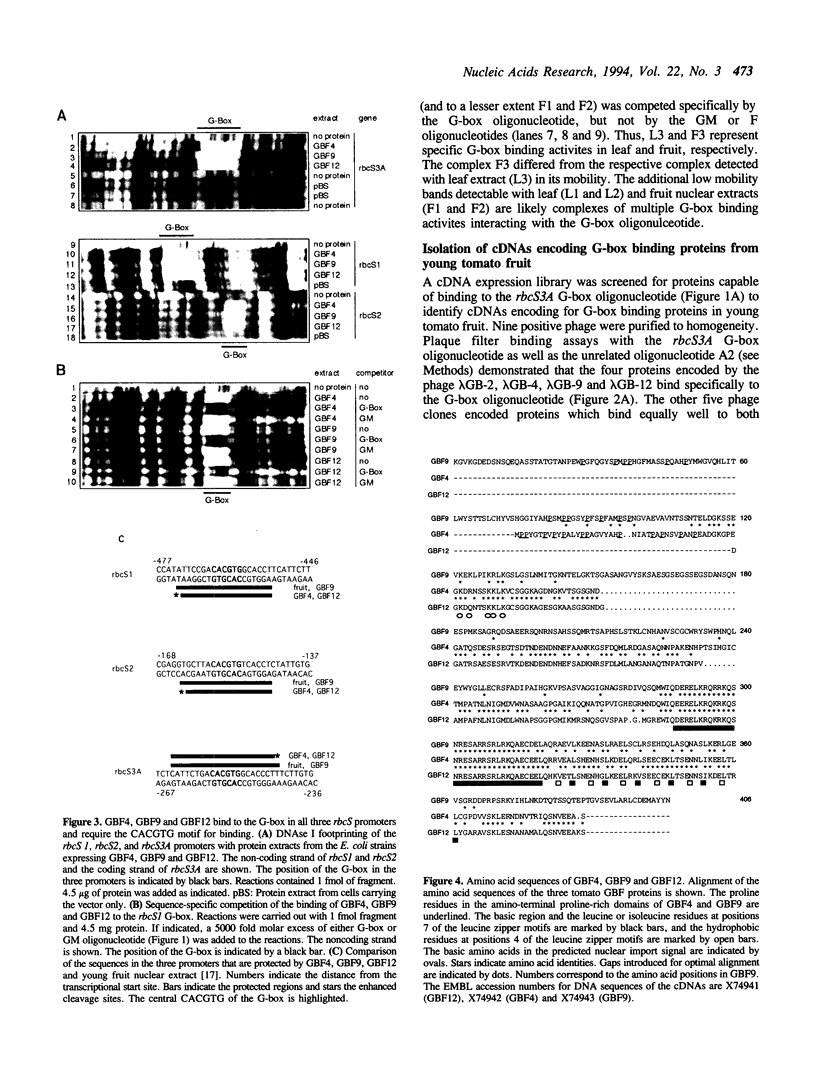
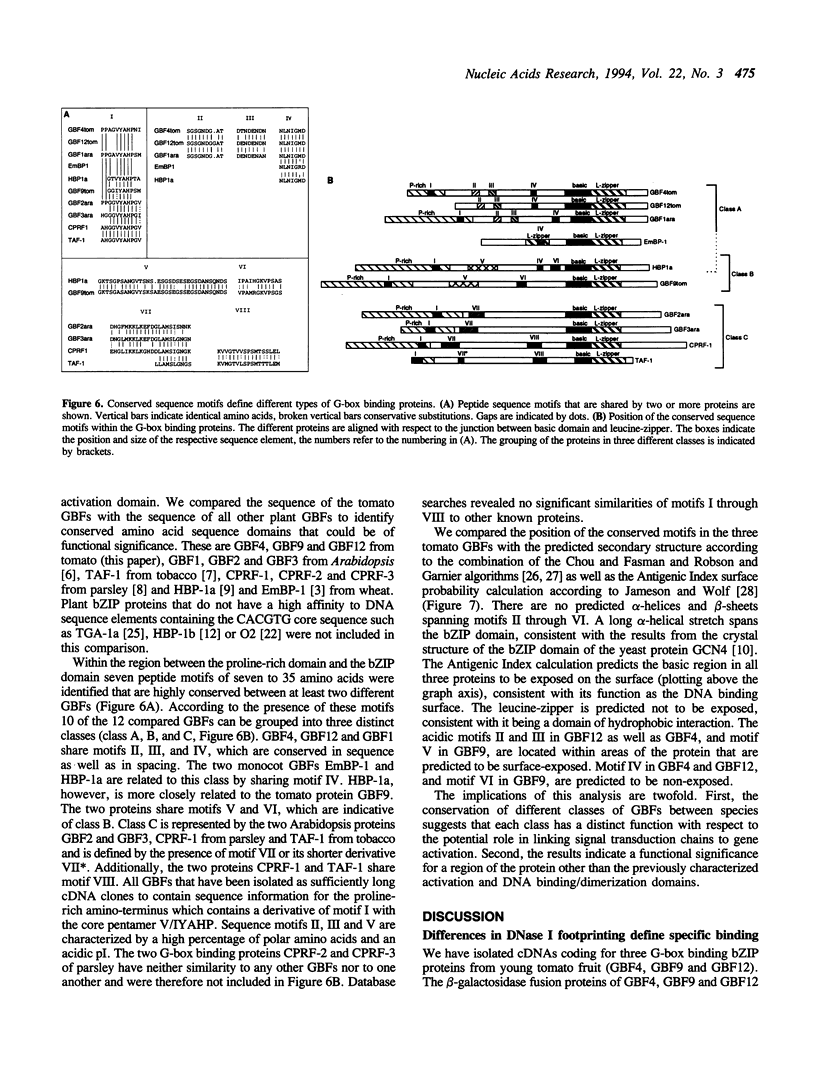
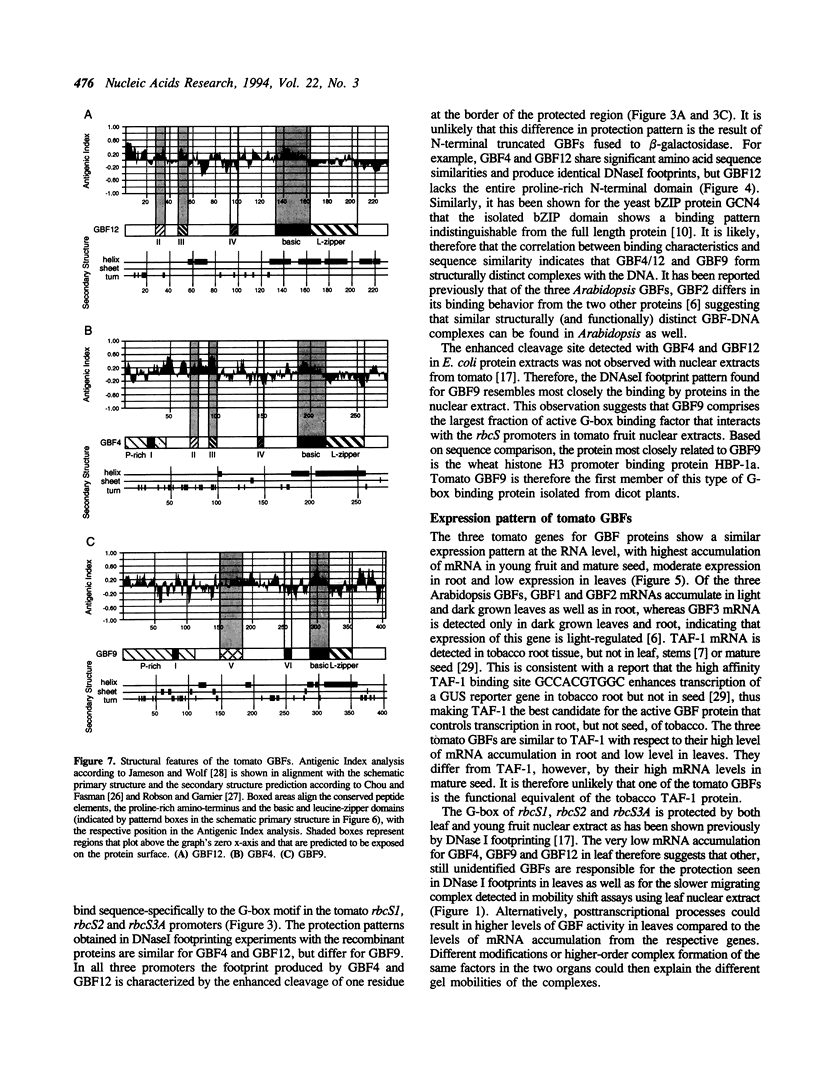
Abstract
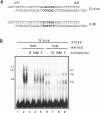
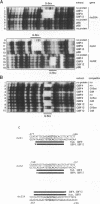
The G-box is a cis-acting DNA sequence present in several plant promoters that are regulated by diverse signals such as UV irradiation, anaerobiosis, abscissic acid and light. Several basic/leucine zipper (bZIP) proteins from different plant species have been identified as high affinity G-box binding proteins. Although their capability to enhance transcription has been demonstrated, their precise function in transcriptional activation is still unknown. We have isolated three cDNAs from young tomato fruit that encode bZIP G-box binding proteins (GBF4, GBF9 and GBF12). They bind to the G-box sequence in the tomato rbcS1, rbcS2 and rbcS3A promoters. GBF9 binding resulted in a DNase I footprint identical to that obtained with tomato nuclear extract and different from the DNase I protection obtained with GBF4 and GBF12. The mRNAs of all three GBFs were most abundant in tomato fruit and seeds, moderately abundant in root and least abundant in leaves. Protein sequences outside of the bZIP domains were compared with the known GBFs from other plants and seven conserved motifs of seven to 35 amino acids length have been identified. Based on the presence of these motifs, three classes of GBFs can be defined that are conserved among plant species. GBF9, the predominantly expressed tomato GBF, is the first member of its class isolated from dicot plants. Three conserved motifs from two of the classes are highly hydrophilic and are predicted to be exposed on the surface of the proteins. These motifs likely define novel interactive domains in the different classes of GBFs that could provide a new tool to determine how distinct regulatory signals are transmitted through GBFs to activate transcription.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- DeLisle A. J., Ferl R. J. Characterization of the Arabidopsis Adh G-box binding factor. Plant Cell. 1990 Jun;2(6):547–557. doi: 10.1105/tpc.2.6.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Nuclear targeting sequences--a consensus? Trends Biochem Sci. 1991 Dec;16(12):478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- Donald R. G., Cashmore A. R. Mutation of either G box or I box sequences profoundly affects expression from the Arabidopsis rbcS-1A promoter. EMBO J. 1990 Jun;9(6):1717–1726. doi: 10.1002/j.1460-2075.1990.tb08295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger T. E., Brandl C. J., Struhl K., Harrison S. C. The GCN4 basic region leucine zipper binds DNA as a dimer of uninterrupted alpha helices: crystal structure of the protein-DNA complex. Cell. 1992 Dec 24;71(7):1223–1237. doi: 10.1016/s0092-8674(05)80070-4. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Giuliano G., Pichersky E., Malik V. S., Timko M. P., Scolnik P. A., Cashmore A. R. An evolutionarily conserved protein binding sequence upstream of a plant light-regulated gene. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7089–7093. doi: 10.1073/pnas.85.19.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiltinan M. J., Marcotte W. R., Jr, Quatrano R. S. A plant leucine zipper protein that recognizes an abscisic acid response element. Science. 1990 Oct 12;250(4978):267–271. doi: 10.1126/science.2145628. [DOI] [PubMed] [Google Scholar]
- Hartings H., Maddaloni M., Lazzaroni N., Di Fonzo N., Motto M., Salamini F., Thompson R. The O2 gene which regulates zein deposition in maize endosperm encodes a protein with structural homologies to transcriptional activators. EMBO J. 1989 Oct;8(10):2795–2801. doi: 10.1002/j.1460-2075.1989.tb08425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson B. A., Wolf H. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput Appl Biosci. 1988 Mar;4(1):181–186. doi: 10.1093/bioinformatics/4.1.181. [DOI] [PubMed] [Google Scholar]
- Jorgensen W. L. Supramolecular chemistry. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1635–1636. doi: 10.1073/pnas.90.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri F., Lam E., Chua N. H. Two tobacco DNA-binding proteins with homology to the nuclear factor CREB. Nature. 1989 Aug 31;340(6236):727–730. doi: 10.1038/340727a0. [DOI] [PubMed] [Google Scholar]
- Lu G., DeLisle A. J., de Vetten N. C., Ferl R. J. Brain proteins in plants: an Arabidopsis homolog to neurotransmitter pathway activators is part of a DNA binding complex. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11490–11494. doi: 10.1073/pnas.89.23.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzara T., Carrasco P., Gruissem W. Developmental and organ-specific changes in DNA-protein interactions in the tomato rbcS1, rbcS2 and rbcS3A promoter regions. Plant Mol Biol. 1993 Jan;21(1):69–88. doi: 10.1007/BF00039619. [DOI] [PubMed] [Google Scholar]
- Manzara T., Carrasco P., Gruissem W. Developmental and organ-specific changes in promoter DNA-protein interactions in the tomato rbcS gene family. Plant Cell. 1991 Dec;3(12):1305–1316. doi: 10.1105/tpc.3.12.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKendree W. L., Jr, Ferl R. J. Functional elements of the Arabidopsis Adh promoter include the G-box. Plant Mol Biol. 1992 Aug;19(5):859–862. doi: 10.1007/BF00027081. [DOI] [PubMed] [Google Scholar]
- McKendree W. L., Paul A. L., DeLisle A. J., Ferl R. J. In vivo and in vitro characterization of protein interactions with the dyad G-box of the Arabidopsis Adh gene. Plant Cell. 1990 Mar;2(3):207–214. doi: 10.1105/tpc.2.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Oeda K., Salinas J., Chua N. H. A tobacco bZip transcription activator (TAF-1) binds to a G-box-like motif conserved in plant genes. EMBO J. 1991 Jul;10(7):1793–1802. doi: 10.1002/j.1460-2075.1991.tb07704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pysh L. D., Aukerman M. J., Schmidt R. J. OHP1: a maize basic domain/leucine zipper protein that interacts with opaque2. Plant Cell. 1993 Feb;5(2):227–236. doi: 10.1105/tpc.5.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas J., Oeda K., Chua N. H. Two G-box-related sequences confer different expression patterns in transgenic tobacco. Plant Cell. 1992 Dec;4(12):1485–1493. doi: 10.1105/tpc.4.12.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler U., Menkens A. E., Beckmann H., Ecker J. R., Cashmore A. R. Heterodimerization between light-regulated and ubiquitously expressed Arabidopsis GBF bZIP proteins. EMBO J. 1992 Apr;11(4):1261–1273. doi: 10.1002/j.1460-2075.1992.tb05170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler U., Terzaghi W., Beckmann H., Kadesch T., Cashmore A. R. DNA binding site preferences and transcriptional activation properties of the Arabidopsis transcription factor GBF1. EMBO J. 1992 Apr;11(4):1275–1289. doi: 10.1002/j.1460-2075.1992.tb05171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Lefert P., Dangl J. L., Becker-André M., Hahlbrock K., Schulz W. Inducible in vivo DNA footprints define sequences necessary for UV light activation of the parsley chalcone synthase gene. EMBO J. 1989 Mar;8(3):651–656. doi: 10.1002/j.1460-2075.1989.tb03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Clerc R. G., LeBowitz J. H. Molecular cloning of sequence-specific DNA binding proteins using recognition site probes. Biotechniques. 1989 Mar;7(3):252–261. [PubMed] [Google Scholar]
- Tabata T., Nakayama T., Mikami K., Iwabuchi M. HBP-1a and HBP-1b: leucine zipper-type transcription factors of wheat. EMBO J. 1991 Jun;10(6):1459–1467. doi: 10.1002/j.1460-2075.1991.tb07666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T., Takase H., Takayama S., Mikami K., Nakatsuka A., Kawata T., Nakayama T., Iwabuchi M. A protein that binds to a cis-acting element of wheat histone genes has a leucine zipper motif. Science. 1989 Sep 1;245(4921):965–967. doi: 10.1126/science.2772648. [DOI] [PubMed] [Google Scholar]
- Vinson C. R., Sigler P. B., McKnight S. L. Scissors-grip model for DNA recognition by a family of leucine zipper proteins. Science. 1989 Nov 17;246(4932):911–916. doi: 10.1126/science.2683088. [DOI] [PubMed] [Google Scholar]
- Wanner L. A., Gruissem W. Expression dynamics of the tomato rbcS gene family during development. Plant Cell. 1991 Dec;3(12):1289–1303. doi: 10.1105/tpc.3.12.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisshaar B., Armstrong G. A., Block A., da Costa e Silva O., Hahlbrock K. Light-inducible and constitutively expressed DNA-binding proteins recognizing a plant promoter element with functional relevance in light responsiveness. EMBO J. 1991 Jul;10(7):1777–1786. doi: 10.1002/j.1460-2075.1991.tb07702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]