Abstract
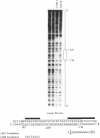
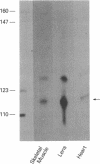
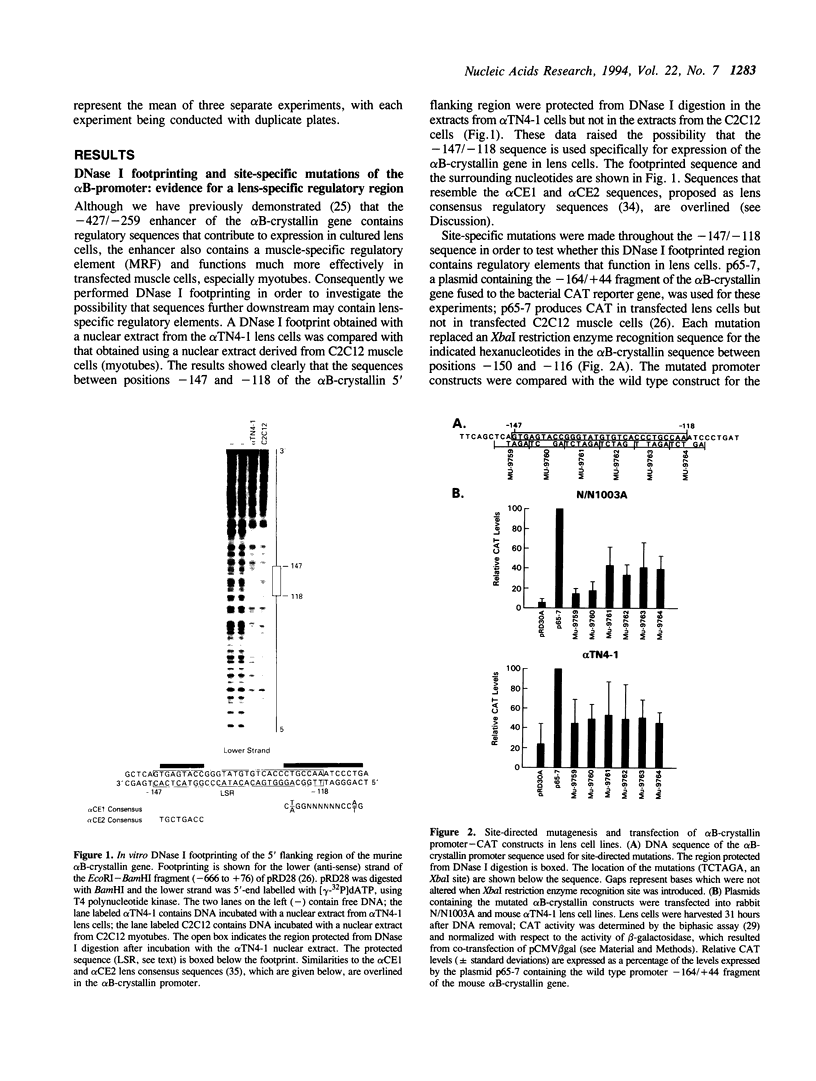
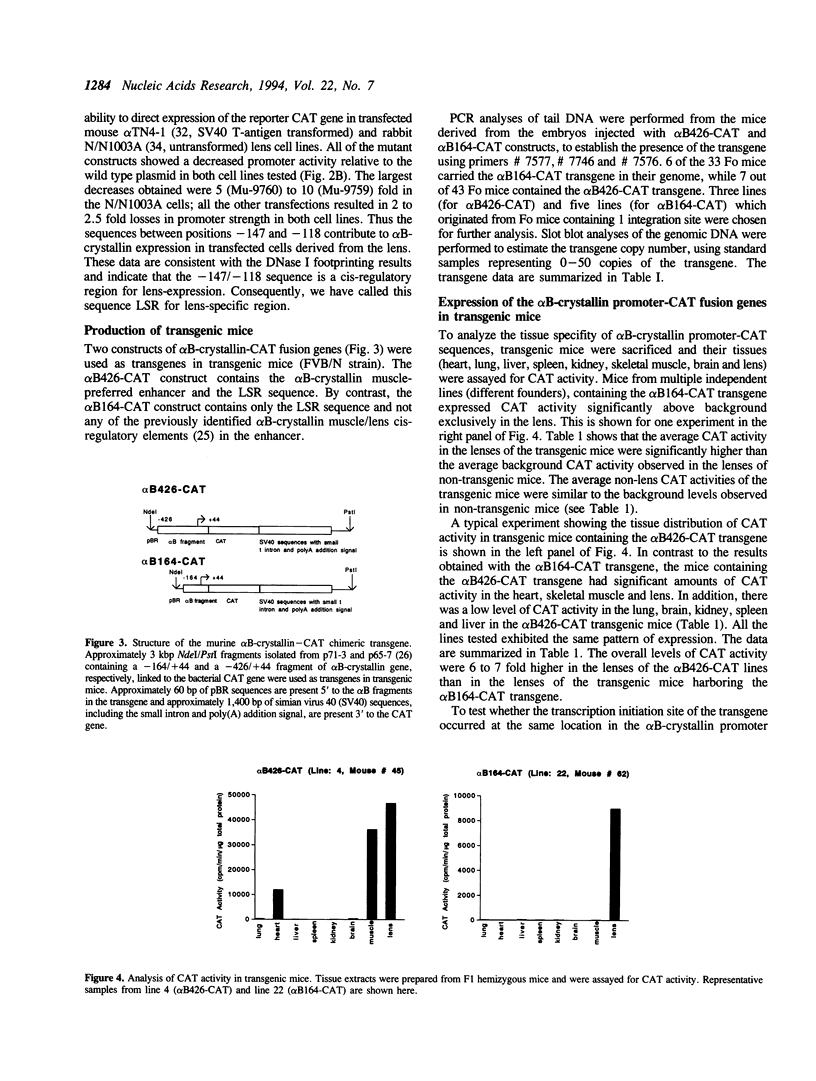
Previous studies have shown that the -661/+44 sequence of the murine alpha B-crystallin gene contains a muscle-preferred enhancer (-426/-257) and can drive the bacterial chloramphenicol acetyltransferase (CAT) gene in the lens, skeletal muscle and heart of transgenic mice. Here we show that transgenic mice carrying a truncated -164/+44 fragment of the alpha B-crystallin gene fused to the CAT gene expressed exclusively in the lens; by contrast mice carrying a -426/+44 fragment of the alpha B gene fused to CAT expressed highly in the lens, skeletal muscle and heart, and slightly in the lung, brain, kidney, spleen and liver. DNase I protection experiments indicated that the -147/-118 sequence is protected by nuclear proteins from alpha TN4-1 lens cell line, but not by nuclear proteins from myotubes of the C2C12 cell line. Site directed mutagenesis of this sequence decreased promoter activity in transiently-transfected lens cells, consistent with this sequence being a lens-specific regulatory region (LSR). We conclude that the -426/-257 enhancer is required for expression in skeletal muscle, heart and possibly other tissues, and that the -164/+44 sequence of the alpha B-crystallin gene is sufficient for expression in the lens of transgenic mice.
Full text
PDF

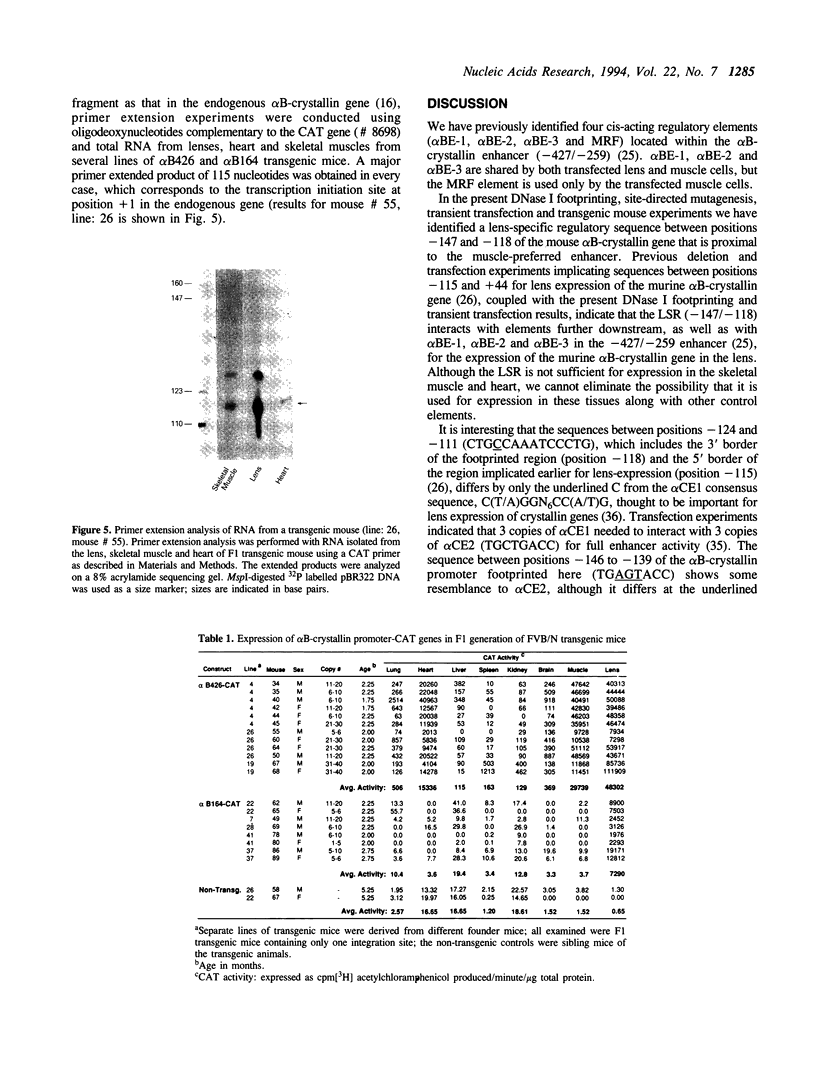

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama A., Fröhli E., Schäfer R., Klemenz R. Alpha B-crystallin expression in mouse NIH 3T3 fibroblasts: glucocorticoid responsiveness and involvement in thermal protection. Mol Cell Biol. 1993 Mar;13(3):1824–1835. doi: 10.1128/mcb.13.3.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat S. P., Nagineni C. N. alpha B subunit of lens-specific protein alpha-crystallin is present in other ocular and non-ocular tissues. Biochem Biophys Res Commun. 1989 Jan 16;158(1):319–325. doi: 10.1016/s0006-291x(89)80215-3. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Chiu C. P., Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell. 1983 Apr;32(4):1171–1180. doi: 10.1016/0092-8674(83)90300-8. [DOI] [PubMed] [Google Scholar]
- Bloemendal H. Lens proteins. CRC Crit Rev Biochem. 1982;12(1):1–38. doi: 10.3109/10409238209105849. [DOI] [PubMed] [Google Scholar]
- Bloemendal H., de Jong W. W. Lens proteins and their genes. Prog Nucleic Acid Res Mol Biol. 1991;41:259–281. doi: 10.1016/s0079-6603(08)60012-4. [DOI] [PubMed] [Google Scholar]
- Brakenhoff R. H., Guerts van Kessel A. H., Oldenburg M., Wijnen J. T., Bloemendal H., Meera Khan P., Schoenmakers J. G. Human alpha B-crystallin (CRYA2) gene mapped to chromosome 11q12-q23. Hum Genet. 1990 Jul;85(2):237–240. doi: 10.1007/BF00193203. [DOI] [PubMed] [Google Scholar]
- Dasgupta S., Hohman T. C., Carper D. Hypertonic stress induces alpha B-crystallin expression. Exp Eye Res. 1992 Mar;54(3):461–470. doi: 10.1016/0014-4835(92)90058-z. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin R. A., Gopal-Srivastava R., Wawrousek E. F., Piatigorsky J. Expression of the murine alpha B-crystallin gene in lens and skeletal muscle: identification of a muscle-preferred enhancer. Mol Cell Biol. 1991 Sep;11(9):4340–4349. doi: 10.1128/mcb.11.9.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin R. A., Wawrousek E. F., Piatigorsky J. Expression of the murine alpha B-crystallin gene is not restricted to the lens. Mol Cell Biol. 1989 Mar;9(3):1083–1091. doi: 10.1128/mcb.9.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal-Srivastava R., Piatigorsky J. The murine alpha B-crystallin/small heat shock protein enhancer: identification of alpha BE-1, alpha BE-2, alpha BE-3, and MRF control elements. Mol Cell Biol. 1993 Nov;13(11):7144–7152. doi: 10.1128/mcb.13.11.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. W., Scangos G. A., Plotkin D. J., Barbosa J. A., Ruddle F. H. Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7380–7384. doi: 10.1073/pnas.77.12.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins J. W., Van Keuren M. L., Piatigorsky J., Law M. L., Patterson D., Kao F. T. Confirmation of assignment of the human alpha 1-crystallin gene (CRYA1) to chromosome 21 with regional localization to q22.3. Hum Genet. 1987 Aug;76(4):375–380. doi: 10.1007/BF00272448. [DOI] [PubMed] [Google Scholar]
- Iwaki T., Kume-Iwaki A., Liem R. K., Goldman J. E. Alpha B-crystallin is expressed in non-lenticular tissues and accumulates in Alexander's disease brain. Cell. 1989 Apr 7;57(1):71–78. doi: 10.1016/0092-8674(89)90173-6. [DOI] [PubMed] [Google Scholar]
- Kato K., Shinohara H., Kurobe N., Goto S., Inaguma Y., Ohshima K. Immunoreactive alpha A crystallin in rat non-lenticular tissues detected with a sensitive immunoassay method. Biochim Biophys Acta. 1991 Oct 25;1080(2):173–180. doi: 10.1016/0167-4838(91)90146-q. [DOI] [PubMed] [Google Scholar]
- Klemenz R., Fröhli E., Aoyama A., Hoffmann S., Simpson R. J., Moritz R. L., Schäfer R. Alpha B crystallin accumulation is a specific response to Ha-ras and v-mos oncogene expression in mouse NIH 3T3 fibroblasts. Mol Cell Biol. 1991 Feb;11(2):803–812. doi: 10.1128/mcb.11.2.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R., Fröhli E., Steiger R. H., Schäfer R., Aoyama A. Alpha B-crystallin is a small heat shock protein. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3652–3656. doi: 10.1073/pnas.88.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R., Hoffmann S., Jaggi R., Werenskiold A. K. The v-mos and c-Ha-ras oncoproteins exert similar effects on the pattern of protein synthesis. Oncogene. 1989 Jun;4(6):799–803. [PubMed] [Google Scholar]
- Lowe J., McDermott H., Pike I., Spendlove I., Landon M., Mayer R. J. alpha B crystallin expression in non-lenticular tissues and selective presence in ubiquitinated inclusion bodies in human disease. J Pathol. 1992 Jan;166(1):61–68. doi: 10.1002/path.1711660110. [DOI] [PubMed] [Google Scholar]
- Matsuo I., Takeuchi M., Yasuda K. Identification of the contact sites of a factor that interacts with motif I (alpha CE1) of the chicken alpha A-crystallin lens-specific enhancer. Biochem Biophys Res Commun. 1992 Apr 15;184(1):24–30. doi: 10.1016/0006-291x(92)91152-g. [DOI] [PubMed] [Google Scholar]
- Matsuo I., Yasuda K. The cooperative interaction between two motifs of an enhancer element of the chicken alpha A-crystallin gene, alpha CE1 and alpha CE2, confers lens-specific expression. Nucleic Acids Res. 1992 Jul 25;20(14):3701–3712. doi: 10.1093/nar/20.14.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo J. T., Klisak I., Dubin R. A., Piatigorsky J., Mohandas T., Sparkes R. S., Bateman J. B. Assignment of the alpha B-crystallin gene to human chromosome 11. Genomics. 1989 Nov;5(4):665–669. doi: 10.1016/0888-7543(89)90106-7. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens crystallins. Innovation associated with changes in gene regulation. J Biol Chem. 1992 Mar 5;267(7):4277–4280. [PubMed] [Google Scholar]
- Piatigorsky J., Wistow G. J. Enzyme/crystallins: gene sharing as an evolutionary strategy. Cell. 1989 Apr 21;57(2):197–199. doi: 10.1016/0092-8674(89)90956-2. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., Wistow G. The recruitment of crystallins: new functions precede gene duplication. Science. 1991 May 24;252(5009):1078–1079. doi: 10.1126/science.252.5009.1078. [DOI] [PubMed] [Google Scholar]
- Reddan J. R., Chepelinsky A. B., Dziedzic D. C., Piatigorsky J., Goldenberg E. M. Retention of lens specificity in long-term cultures of diploid rabbit lens epithelial cells. Differentiation. 1986;33(2):168–174. doi: 10.1111/j.1432-0436.1986.tb00422.x. [DOI] [PubMed] [Google Scholar]
- Srinivasan A. N., Nagineni C. N., Bhat S. P. alpha A-crystallin is expressed in non-ocular tissues. J Biol Chem. 1992 Nov 15;267(32):23337–23341. [PubMed] [Google Scholar]
- Wistow G. J., Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- Yamada T., Nakamura T., Westphal H., Russell P. Synthesis of alpha-crystallin by a cell line derived from the lens of a transgenic animal. Curr Eye Res. 1990 Jan;9(1):31–37. doi: 10.3109/02713689009000052. [DOI] [PubMed] [Google Scholar]
- de Jong W. W., Hendriks W., Mulders J. W., Bloemendal H. Evolution of eye lens crystallins: the stress connection. Trends Biochem Sci. 1989 Sep;14(9):365–368. doi: 10.1016/0968-0004(89)90009-1. [DOI] [PubMed] [Google Scholar]
- de Jong W. W., Leunissen J. A., Voorter C. E. Evolution of the alpha-crystallin/small heat-shock protein family. Mol Biol Evol. 1993 Jan;10(1):103–126. doi: 10.1093/oxfordjournals.molbev.a039992. [DOI] [PubMed] [Google Scholar]