Abstract
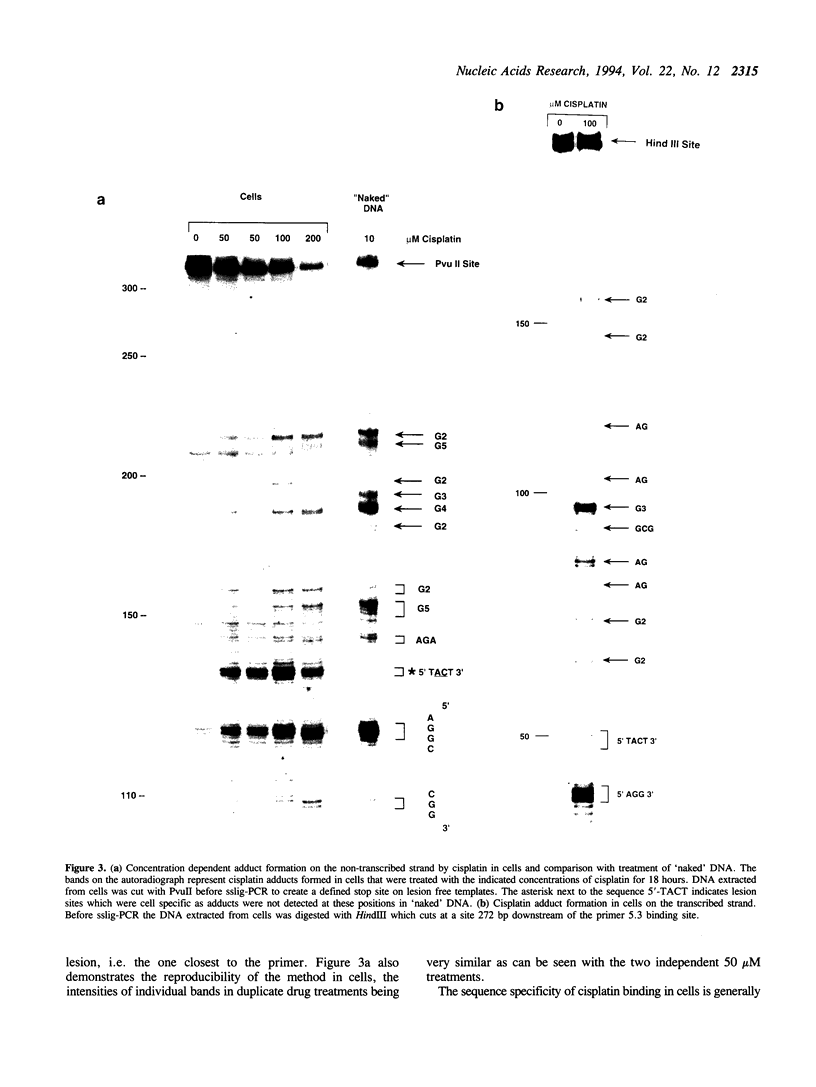
A new PCR based technique has been developed to investigate the sequence selectivity of adduct formation by DNA damaging agents in a single copy gene in isolated genomic DNA or in drug treated cells. Single-strand ligation PCR (sslig-PCR) demonstrated that cisplatin and nitrogen mustards reacted with guanine in an N-ras fragment with varying sequence specificity similar to that observed previously in plasmid DNA. In cisplatin-treated cells sslig-PCR demonstrated all the adducts found in isolated DNA and with the same sequence selectivity showing a preference for GG and AG sites. However, in cells an additional site of DNA binding of cisplatin was observed at the two occurrences of the sequence 5'-TACT-3' on the transcribed and non-transcribed strands. This sequence is not a recognised target for cisplatin and represents a novel adduct formed in cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernges F., Holler E. The reaction of platinum(II) complexes with DNA. Kinetics of intrastrand crosslink formation in vitro. Nucleic Acids Res. 1991 Apr 11;19(7):1483–1489. doi: 10.1093/nar/19.7.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A. Gene specific DNA repair. Carcinogenesis. 1991 Nov;12(11):1983–1992. doi: 10.1093/carcin/12.11.1983. [DOI] [PubMed] [Google Scholar]
- Bohr V. A., Okumoto D. S., Hanawalt P. C. Survival of UV-irradiated mammalian cells correlates with efficient DNA repair in an essential gene. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3830–3833. doi: 10.1073/pnas.83.11.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Chin A. J., Sanders S. P., Sherman F., Lang P., Norwood W. I., Castaneda A. R. Accuracy of subcostal two-dimensional echocardiography in prospective diagnosis of total anomalous pulmonary venous connection. Am Heart J. 1987 May;113(5):1153–1159. doi: 10.1016/0002-8703(87)90928-8. [DOI] [PubMed] [Google Scholar]
- Comess K. M., Burstyn J. N., Essigmann J. M., Lippard S. J. Replication inhibition and translesion synthesis on templates containing site-specifically placed cis-diamminedichloroplatinum(II) DNA adducts. Biochemistry. 1992 Apr 28;31(16):3975–3990. doi: 10.1021/bi00131a013. [DOI] [PubMed] [Google Scholar]
- Cullinane C., Wickham G., McFadyen W. D., Denny W. A., Palmer B. D., Phillips D. R. The use of bidirectional transcription footprinting to detect platinum-DNA crosslinks by acridine-tethered platinum diamine complexes and cisplatin. Nucleic Acids Res. 1993 Feb 11;21(3):393–400. doi: 10.1093/nar/21.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtinger-Schepman A. M., van Oosterom A. T., Lohman P. H., Berends F. cis-Diamminedichloroplatinum(II)-induced DNA adducts in peripheral leukocytes from seven cancer patients: quantitative immunochemical detection of the adduct induction and removal after a single dose of cis-diamminedichloroplatinum(II). Cancer Res. 1987 Jun 1;47(11):3000–3004. [PubMed] [Google Scholar]
- Fichtinger-Schepman A. M., van der Veer J. L., den Hartog J. H., Lohman P. H., Reedijk J. Adducts of the antitumor drug cis-diamminedichloroplatinum(II) with DNA: formation, identification, and quantitation. Biochemistry. 1985 Jan 29;24(3):707–713. doi: 10.1021/bi00324a025. [DOI] [PubMed] [Google Scholar]
- Govan H. L., 3rd, Valles-Ayoub Y., Braun J. Fine-mapping of DNA damage and repair in specific genomic segments. Nucleic Acids Res. 1990 Jul 11;18(13):3823–3830. doi: 10.1093/nar/18.13.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A., Brown R. Human N-ras: cDNA cloning and gene structure. Nucleic Acids Res. 1985 Jul 25;13(14):5255–5268. doi: 10.1093/nar/13.14.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawalt P. C. Preferential repair of damage in actively transcribed DNA sequences in vivo. Genome. 1989;31(2):605–611. doi: 10.1139/g89-113. [DOI] [PubMed] [Google Scholar]
- Hartley J. A., Bingham J. P., Souhami R. L. DNA sequence selectivity of guanine-N7 alkylation by nitrogen mustards is preserved in intact cells. Nucleic Acids Res. 1992 Jun 25;20(12):3175–3178. doi: 10.1093/nar/20.12.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. A., Gibson N. W., Kohn K. W., Mattes W. B. DNA sequence selectivity of guanine-N7 alkylation by three antitumor chloroethylating agents. Cancer Res. 1986 Apr;46(4 Pt 2):1943–1947. [PubMed] [Google Scholar]
- Jennerwein M. M., Eastman A. A polymerase chain reaction-based method to detect cisplatin adducts in specific genes. Nucleic Acids Res. 1991 Nov 25;19(22):6209–6214. doi: 10.1093/nar/19.22.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinowski D. P., Illenye S., Van Houten B. Analysis of DNA damage and repair in murine leukemia L1210 cells using a quantitative polymerase chain reaction assay. Nucleic Acids Res. 1992 Jul 11;20(13):3485–3494. doi: 10.1093/nar/20.13.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn K. W., Hartley J. A., Mattes W. B. Mechanisms of DNA sequence selective alkylation of guanine-N7 positions by nitrogen mustards. Nucleic Acids Res. 1987 Dec 23;15(24):10531–10549. doi: 10.1093/nar/15.24.10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunala S., Brash D. E. Excision repair at individual bases of the Escherichia coli lacI gene: relation to mutation hot spots and transcription coupling activity. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11031–11035. doi: 10.1073/pnas.89.22.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993 Apr 22;362(6422):709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- Lommel L., Hanawalt P. C. Increased UV resistance of a xeroderma pigmentosum revertant cell line is correlated with selective repair of the transcribed strand of an expressed gene. Mol Cell Biol. 1993 Feb;13(2):970–976. doi: 10.1128/mcb.13.2.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrot L., Leng M. Chemical probes of the conformation of DNA modified by cis-diamminedichloroplatinum(II). Biochemistry. 1989 Feb 21;28(4):1454–1461. doi: 10.1021/bi00430a005. [DOI] [PubMed] [Google Scholar]
- Mattes W. B., Hartley J. A., Kohn K. W. DNA sequence selectivity of guanine-N7 alkylation by nitrogen mustards. Nucleic Acids Res. 1986 Apr 11;14(7):2971–2987. doi: 10.1093/nar/14.7.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon I., Hanawalt P. C. Induction of the Escherichia coli lactose operon selectively increases repair of its transcribed DNA strand. Nature. 1989 Nov 2;342(6245):95–98. doi: 10.1038/342095a0. [DOI] [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987 Oct 23;51(2):241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Mueller P. R., Wold B. In vivo footprinting of a muscle specific enhancer by ligation mediated PCR. Science. 1989 Nov 10;246(4931):780–786. doi: 10.1126/science.2814500. [DOI] [PubMed] [Google Scholar]
- Murray V., Motyka H., England P. R., Wickham G., Lee H. H., Denny W. A., McFadyen W. D. An investigation of the sequence-specific interaction of cis-diamminedichloroplatinum(II) and four analogues, including two acridine-tethered complexes, with DNA inside human cells. Biochemistry. 1992 Dec 1;31(47):11812–11817. doi: 10.1021/bi00162a020. [DOI] [PubMed] [Google Scholar]
- Murray V., Motyka H., England P. R., Wickham G., Lee H. H., Denny W. A., McFadyen W. D. The use of Taq DNA polymerase to determine the sequence specificity of DNA damage caused by cis-diamminedichloroplatinum(II), acridine-tethered platinum(II) diammine complexes or two analogues. J Biol Chem. 1992 Sep 15;267(26):18805–18809. [PubMed] [Google Scholar]
- Pfeifer G. P., Steigerwald S. D., Mueller P. R., Wold B., Riggs A. D. Genomic sequencing and methylation analysis by ligation mediated PCR. Science. 1989 Nov 10;246(4931):810–813. doi: 10.1126/science.2814502. [DOI] [PubMed] [Google Scholar]
- Ponti M., Forrow S. M., Souhami R. L., D'Incalci M., Hartley J. A. Measurement of the sequence specificity of covalent DNA modification by antineoplastic agents using Taq DNA polymerase. Nucleic Acids Res. 1991 Jun 11;19(11):2929–2933. doi: 10.1093/nar/19.11.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. J., Friedlos F. Quantitative aspects of the formation and loss of DNA interstrand crosslinks in Chinese hamster cells following treatment with cis-diamminedichloroplatinum(II) (cisplatin). I. Proportion of DNA-platinum reactions involved in DNA crosslinking. Biochim Biophys Acta. 1981 Sep 28;655(2):146–151. doi: 10.1016/0005-2787(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Selby C. P., Sancar A. Molecular mechanism of transcription-repair coupling. Science. 1993 Apr 2;260(5104):53–58. doi: 10.1126/science.8465200. [DOI] [PubMed] [Google Scholar]
- Shwartz H., Shavitt O., Livneh Z. The role of exonucleolytic processing and polymerase-DNA association in bypass of lesions during replication in vitro. Significance for SOS-targeted mutagenesis. J Biol Chem. 1988 Dec 5;263(34):18277–18285. [PubMed] [Google Scholar]
- Tessier D. C., Brousseau R., Vernet T. Ligation of single-stranded oligodeoxyribonucleotides by T4 RNA ligase. Anal Biochem. 1986 Oct;158(1):171–178. doi: 10.1016/0003-2697(86)90606-8. [DOI] [PubMed] [Google Scholar]
- Wassermann K., Kohn K. W., Bohr V. A. Heterogeneity of nitrogen mustard-induced DNA damage and repair at the level of the gene in Chinese hamster ovary cells. J Biol Chem. 1990 Aug 15;265(23):13906–13913. [PubMed] [Google Scholar]
- Zhen W., Link C. J., Jr, O'Connor P. M., Reed E., Parker R., Howell S. B., Bohr V. A. Increased gene-specific repair of cisplatin interstrand cross-links in cisplatin-resistant human ovarian cancer cell lines. Mol Cell Biol. 1992 Sep;12(9):3689–3698. doi: 10.1128/mcb.12.9.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]