Abstract
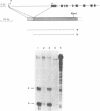
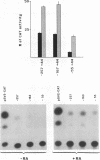
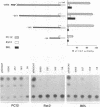
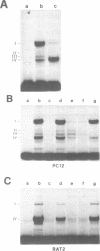
The FE65 gene encodes a nuclear protein of unknown function that is expressed in several areas of the rat nervous system during development and in the adult animal, particularly in somatic and visceral ganglia. FE65 mRNA is abundant in neuronal cell lines, whereas it is barely detectable in non-neuronal cells. We identified the two transcription start sites of the FE65 gene and we isolated the rat genomic fragment containing one of these two transcriptional start sites. We demonstrate that this fragment contains a promoter able to direct an efficient transcription of a reporter gene in PC12 cells and in NTERA2 cells upon their differentiation with retinoic acid, whereas it functions poorly in non-neuronal cells, such as Rat2 fibroblasts and BRL hepatocytes. This promoter is composed of two regions. The first includes a cis-element whose removal greatly decreases the transcriptional efficiency in all cells examined and which forms similar complexes with proteins from PC12 and Rat2 cells. This cis-element binds Sp1 or another GC-binding factor. The second cis-element encompasses the transcription start site and is still able to direct transcription only in neuronal cells. The DNA-protein complexes formed by this cis-element in neuronal cells differ from those formed in non-neuronal cells. The analysis of point mutations in this region indicates that the proteins that bind to this cis-element interact with both overlapping and distinct nucleotide sequences.
Full text
PDF

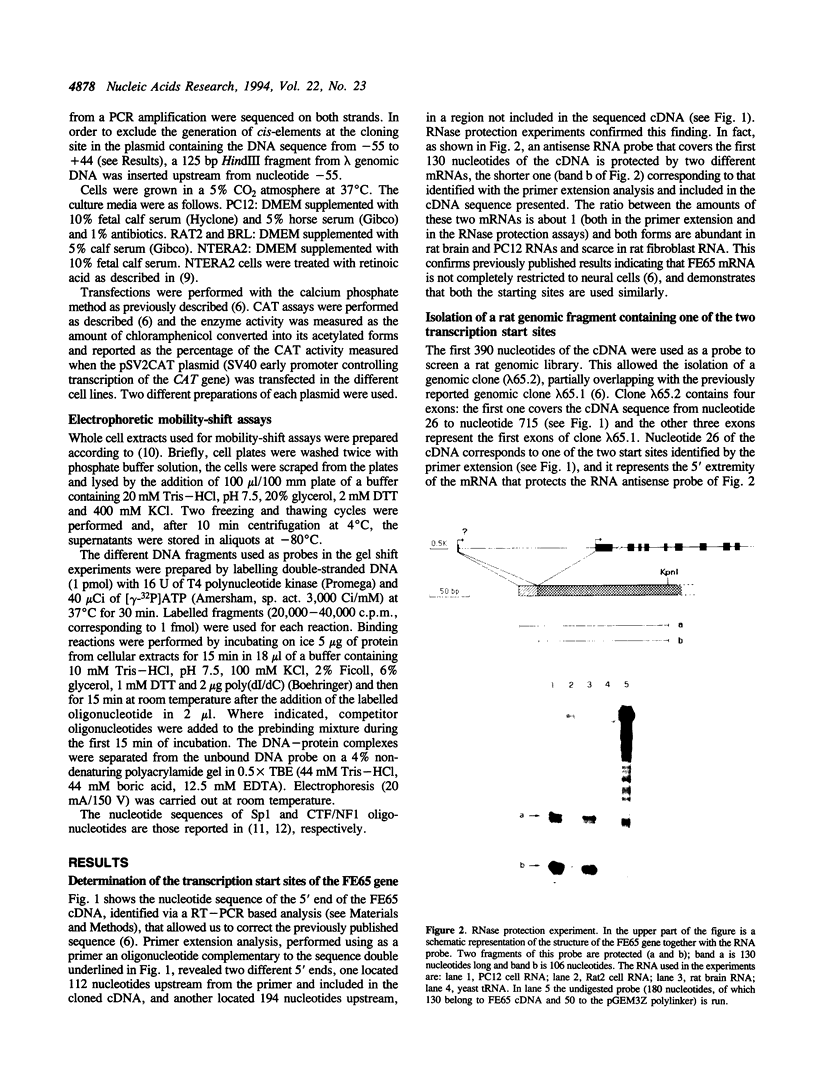
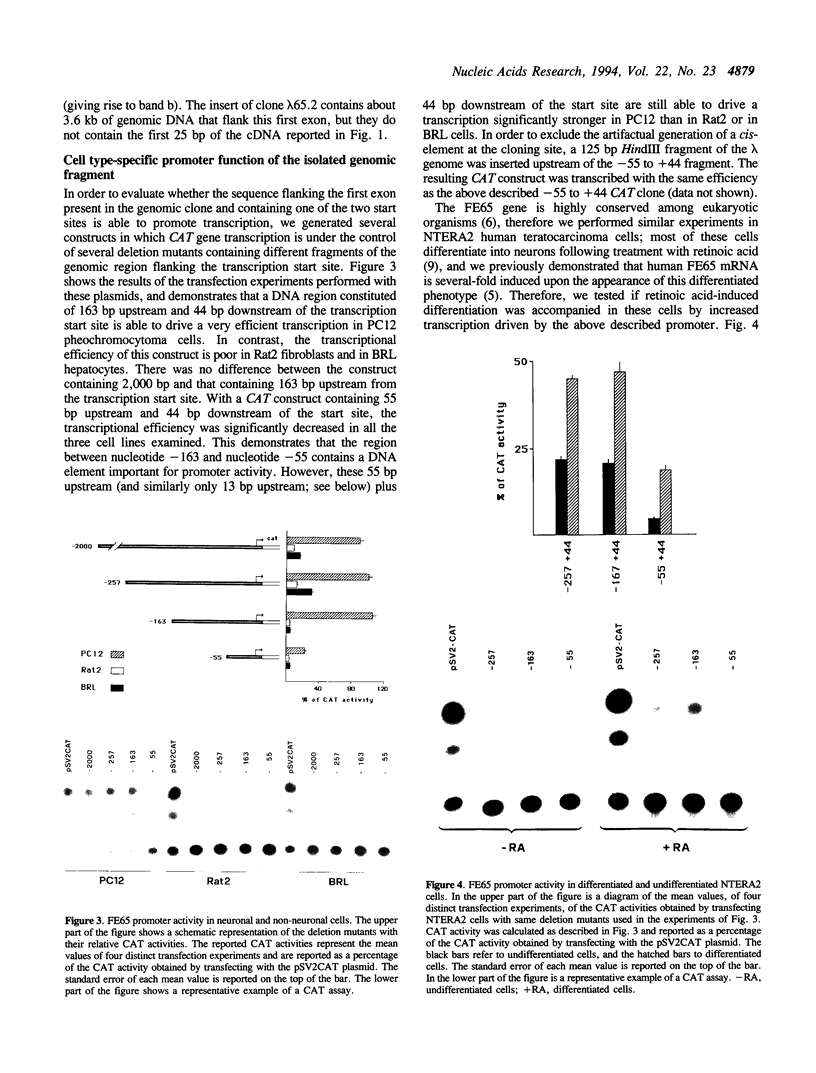

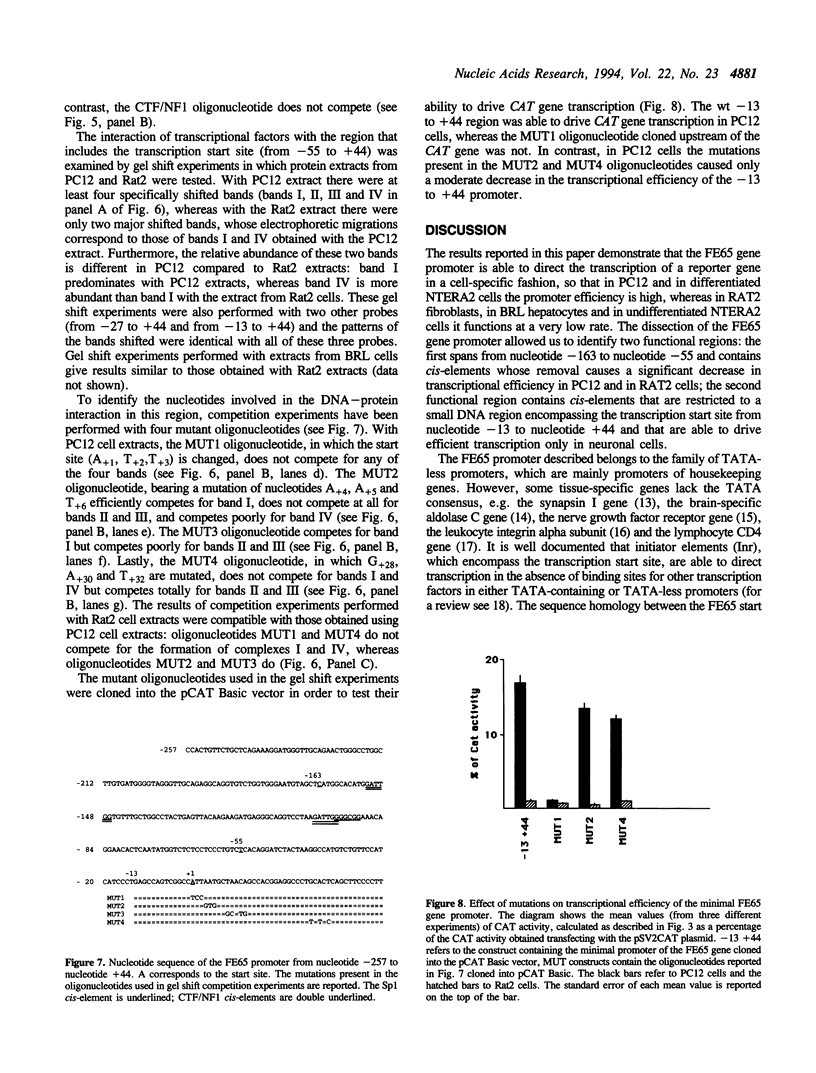


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buono P., de Conciliis L., Olivetta E., Izzo P., Salvatore F. Cis-acting elements in the promoter region of the human aldolase C gene. FEBS Lett. 1993 Aug 16;328(3):243–249. doi: 10.1016/0014-5793(93)80936-o. [DOI] [PubMed] [Google Scholar]
- Duilio A., Zambrano N., Mogavero A. R., Ammendola R., Cimino F., Russo T. A rat brain mRNA encoding a transcriptional activator homologous to the DNA binding domain of retroviral integrases. Nucleic Acids Res. 1991 Oct 11;19(19):5269–5274. doi: 10.1093/nar/19.19.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F., Ammendola R., Duilio A., Costanzo F., Giordano M., Zambrano N., D'Agostino P., Russo T., Cimino F. Isolation of cDNA fragments hybridizing to rat brain-specific mRNAs. Dev Neurosci. 1990;12(6):373–381. doi: 10.1159/000111865. [DOI] [PubMed] [Google Scholar]
- He X., Rosenfeld M. G. Mechanisms of complex transcriptional regulation: implications for brain development. Neuron. 1991 Aug;7(2):183–196. doi: 10.1016/0896-6273(91)90257-z. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Tjian R. Affinity purification of sequence-specific DNA binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovesdi I., Reichel R., Nevins J. R. Identification of a cellular transcription factor involved in E1A trans-activation. Cell. 1986 Apr 25;45(2):219–228. doi: 10.1016/0092-8674(86)90386-7. [DOI] [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Galvin K. M., Shi Y. Evidence for physical interaction between the zinc-finger transcription factors YY1 and Sp1. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6145–6149. doi: 10.1073/pnas.90.13.6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenormand J. L., Guillier M., Leibovitch S. A. Identification of a cis acting element responsible for muscle specific expression of the c-mos protooncogene. Nucleic Acids Res. 1993 Feb 11;21(3):695–702. doi: 10.1093/nar/21.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Cabrera M., Nueda A., Vara A., García-Aguilar J., Tugores A., Corbí A. L. Characterization of the p150,95 leukocyte integrin alpha subunit (CD11c) gene promoter. Identification of cis-acting elements. J Biol Chem. 1993 Jan 15;268(2):1187–1193. [PubMed] [Google Scholar]
- Mandel G., McKinnon D. Molecular basis of neural-specific gene expression. Annu Rev Neurosci. 1993;16:323–345. doi: 10.1146/annurev.ne.16.030193.001543. [DOI] [PubMed] [Google Scholar]
- Means A. L., Farnham P. J. Transcription initiation from the dihydrofolate reductase promoter is positioned by HIP1 binding at the initiation site. Mol Cell Biol. 1990 Feb;10(2):653–661. doi: 10.1128/mcb.10.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paonessa G., Gounari F., Frank R., Cortese R. Purification of a NF1-like DNA-binding protein from rat liver and cloning of the corresponding cDNA. EMBO J. 1988 Oct;7(10):3115–3123. doi: 10.1002/j.1460-2075.1988.tb03178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A. L., Malik S., Meisterernst M., Roeder R. G. An alternative pathway for transcription initiation involving TFII-I. Nature. 1993 Sep 23;365(6444):355–359. doi: 10.1038/365355a0. [DOI] [PubMed] [Google Scholar]
- Roy A. L., Meisterernst M., Pognonec P., Roeder R. G. Cooperative interaction of an initiator-binding transcription initiation factor and the helix-loop-helix activator USF. Nature. 1991 Nov 21;354(6350):245–248. doi: 10.1038/354245a0. [DOI] [PubMed] [Google Scholar]
- Salmon P., Giovane A., Wasylyk B., Klatzmann D. Characterization of the human CD4 gene promoter: transcription from the CD4 gene core promoter is tissue-specific and is activated by Ets proteins. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7739–7743. doi: 10.1073/pnas.90.16.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerwald A., Hoesche C., Oschwald R., Kilimann M. W. The 5'-flanking region of the synapsin I gene. A G+C-rich, TATA- and CAAT-less, phylogenetically conserved sequence with cell type-specific promoter function. J Biol Chem. 1990 Sep 5;265(25):14932–14937. [PubMed] [Google Scholar]
- Sehgal A., Patil N., Chao M. A constitutive promoter directs expression of the nerve growth factor receptor gene. Mol Cell Biol. 1988 Aug;8(8):3160–3167. doi: 10.1128/mcb.8.8.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Seto E., Chang L. S., Shenk T. Transcriptional repression by YY1, a human GLI-Krüppel-related protein, and relief of repression by adenovirus E1A protein. Cell. 1991 Oct 18;67(2):377–388. doi: 10.1016/0092-8674(91)90189-6. [DOI] [PubMed] [Google Scholar]
- Simeone A., Acampora D., Nigro V., Faiella A., D'Esposito M., Stornaiuolo A., Mavilio F., Boncinelli E. Differential regulation by retinoic acid of the homeobox genes of the four HOX loci in human embryonal carcinoma cells. Mech Dev. 1991 Mar;33(3):215–227. doi: 10.1016/0925-4773(91)90029-6. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Baltimore D. The "initiator" as a transcription control element. Cell. 1989 Apr 7;57(1):103–113. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Schmidt M. C., Berk A. J., Baltimore D. Transcriptional activation by Sp1 as directed through TATA or initiator: specific requirement for mammalian transcription factor IID. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4509–4513. doi: 10.1073/pnas.87.12.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeller W. C., Poole S. J., Kornberg T. In vitro transcription of the Drosophila engrailed gene. Genes Dev. 1988 Jan;2(1):68–81. doi: 10.1101/gad.2.1.68. [DOI] [PubMed] [Google Scholar]
- Takahashi Y. Gene expression in cells of the central nervous system. Prog Neurobiol. 1992 Jun;38(6):523–569. doi: 10.1016/0301-0082(92)90041-c. [DOI] [PubMed] [Google Scholar]
- Tamura T., Sumita K., Hirose S., Mikoshiba K. Core promoter of the mouse myelin basic protein gene governs brain-specific transcription in vitro. EMBO J. 1990 Oct;9(10):3101–3108. doi: 10.1002/j.1460-2075.1990.tb07507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis L., Reinberg D. Transcription by RNA polymerase II: initiator-directed formation of transcription-competent complexes. FASEB J. 1992 Nov;6(14):3300–3309. doi: 10.1096/fasebj.6.14.1426767. [DOI] [PubMed] [Google Scholar]