Abstract
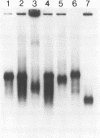
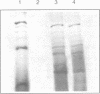
2'-Fluoro- and 2'-amino-2'-deoxynucleoside triphosphates have been used for in vitro transcription of 2'-modified luciferase mRNA. The 2'-modified deoxynucleoside-containing transcripts were tested for the expression of luciferase in X.Laevis oocytes as well as in rabbit reticulocyte lysate. Only 2'-fluoro-2'-deoxy-adenosine-modified mRNA gave rise to luciferase as shown by SDS gel as well as by enzyme activity measurements in vivo as well as in vitro. 2'-Fluoro-2'-deoxy-pyrimidine nucleoside-modified mRNA did not give rise to luciferase activity. However, they directed incorporation of 35S-labeled methionine into peptide fragments in rabbit reticulocyte lysate indicating premature termination of translation. No or only extremely little of such incorporation could be detected with 2'-amino modified transcripts.
Full text
PDF
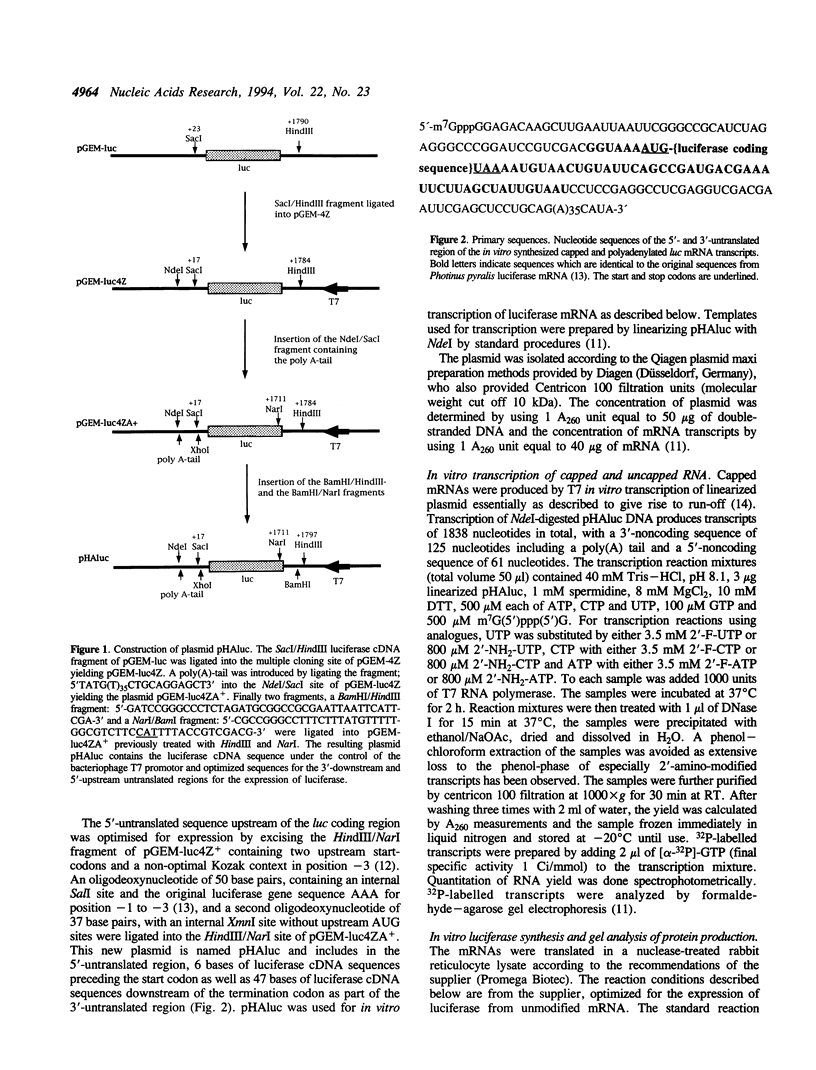




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aurup H., Tuschl T., Benseler F., Ludwig J., Eckstein F. Oligonucleotide duplexes containing 2'-amino-2'-deoxycytidines: thermal stability and chemical reactivity. Nucleic Acids Res. 1994 Jan 11;22(1):20–24. doi: 10.1093/nar/22.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurup H., Williams D. M., Eckstein F. 2'-Fluoro- and 2'-amino-2'-deoxynucleoside 5'-triphosphates as substrates for T7 RNA polymerase. Biochemistry. 1992 Oct 13;31(40):9636–9641. doi: 10.1021/bi00155a016. [DOI] [PubMed] [Google Scholar]
- CHAMBERLIN M., BERG P. MECHANISM OF RNA POLYMERASE ACTION: CHARACTERIZATION OF THE DNA-DEPENDENT SYNTHESIS OF POLYADENYLIC ACID. J Mol Biol. 1964 May;8:708–726. doi: 10.1016/s0022-2836(64)80120-0. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Dunlap B. E., Friderici K. H., Rottman F. 2'-O-Methyl polynucleotides as templates for cell-free amino acid incorporation. Biochemistry. 1971 Jun 22;10(13):2581–2587. doi: 10.1021/bi00789a026. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- Fu L. N., Ye R. Q., Browder L. W., Johnston R. N. Translational potentiation of messenger RNA with secondary structure in Xenopus. Science. 1991 Feb 15;251(4995):807–810. doi: 10.1126/science.1990443. [DOI] [PubMed] [Google Scholar]
- Fukui T., Kakiuchi N., Ikehara M. Protein synthesis using poly(2'-halogeno-2'-deoxyadenylic acids) as messenger. Biochim Biophys Acta. 1982 May 31;697(2):174–177. doi: 10.1016/0167-4781(82)90074-4. [DOI] [PubMed] [Google Scholar]
- Glass M. J., Jia X. Y., Summers D. F. Identification of the hepatitis A virus internal ribosome entry site: in vivo and in vitro analysis of bicistronic RNAs containing the HAV 5' noncoding region. Virology. 1993 Apr;193(2):842–852. doi: 10.1006/viro.1993.1193. [DOI] [PubMed] [Google Scholar]
- Goldin A. L. Maintenance of Xenopus laevis and oocyte injection. Methods Enzymol. 1992;207:266–279. doi: 10.1016/0076-6879(92)07017-i. [DOI] [PubMed] [Google Scholar]
- Goldin A. L., Sumikawa K. Preparation of RNA for injection into Xenopus oocytes. Methods Enzymol. 1992;207:279–297. doi: 10.1016/0076-6879(92)07018-j. [DOI] [PubMed] [Google Scholar]
- Groebe D. R., Uhlenbeck O. C. Characterization of RNA hairpin loop stability. Nucleic Acids Res. 1988 Dec 23;16(24):11725–11735. doi: 10.1093/nar/16.24.11725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B., Wickens M. P. The use of Xenopus oocytes for the expression of cloned genes. Methods Enzymol. 1983;101:370–386. doi: 10.1016/0076-6879(83)01028-9. [DOI] [PubMed] [Google Scholar]
- Heidenreich O., Benseler F., Fahrenholz A., Eckstein F. High activity and stability of hammerhead ribozymes containing 2'-modified pyrimidine nucleosides and phosphorothioates. J Biol Chem. 1994 Jan 21;269(3):2131–2138. [PubMed] [Google Scholar]
- Heidenreich O., Eckstein F. Hammerhead ribozyme-mediated cleavage of the long terminal repeat RNA of human immunodeficiency virus type 1. J Biol Chem. 1992 Jan 25;267(3):1904–1909. [PubMed] [Google Scholar]
- Hobbs J., Sternbach H., Sprinzl M., Eckstein F. Polynucleotides containing 2'-amino-2'-deoxyribose and 2'-azido-2'-deoxyribose. Biochemistry. 1973 Dec 4;12(25):5138–5145. doi: 10.1021/bi00749a018. [DOI] [PubMed] [Google Scholar]
- Hobbs J., Sternbach H., Sprinzl M., Eckstein F. Polynucleotides containing 2'-chloro-2'-deoxyribose. Biochemistry. 1972 Nov 7;11(23):4336–4344. doi: 10.1021/bi00773a021. [DOI] [PubMed] [Google Scholar]
- Ikehara M., Fukui T., Kakiuchi N. Polynucleotides. LII. Synthesis and properties of poly(2'-deoxy-2'-fluoroadenylic acid). Nucleic Acids Res. 1978 Jun;5(6):1877–1887. doi: 10.1093/nar/5.6.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janik B., Kotick M. P., Kreiser T. H., Reverman L. F., Sommer R. G., Wilson D. P. Synthesis and properties of poly 2'-fluoro-2'-deoxyuridylic acid. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1153–1160. doi: 10.1016/s0006-291x(72)80095-0. [DOI] [PubMed] [Google Scholar]
- Kakiuchi N., Marck C., Rousseau N., Leng M., De Clerq E., Guschlbauer W. Polynucleotide helix geometry and stability. Spectroscopic, antigenic and interferon-inducing properties of deoxyribose-, ribose-, or 2'-deoxy-2'-fluororibose-containing duplexes of poly(inosinic acid) . poly(cytidylic acid). J Biol Chem. 1982 Feb 25;257(4):1924–1928. [PubMed] [Google Scholar]
- Kawasaki A. M., Casper M. D., Freier S. M., Lesnik E. A., Zounes M. C., Cummins L. L., Gonzalez C., Cook P. D. Uniformly modified 2'-deoxy-2'-fluoro phosphorothioate oligonucleotides as nuclease-resistant antisense compounds with high affinity and specificity for RNA targets. J Med Chem. 1993 Apr 2;36(7):831–841. doi: 10.1021/jm00059a007. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova L. G., Romanova E. A., Volkov E. M., Tashlitskii V. N., Oretskaia T. S., Krynetskaia N. F., Shabarova Z. A. Oligodezoksiribonukleotidy, soderzhashchie 2'-amino-2'-dezoksipirimidinovye nukleozidy. Bioorg Khim. 1993 Apr;19(4):455–466. [PubMed] [Google Scholar]
- Ludwig J. A new route to nucleoside 5'-triphosphates. Acta Biochim Biophys Acad Sci Hung. 1981;16(3-4):131–133. [PubMed] [Google Scholar]
- Macdonald L. E., Zhou Y., McAllister W. T. Termination and slippage by bacteriophage T7 RNA polymerase. J Mol Biol. 1993 Aug 20;232(4):1030–1047. doi: 10.1006/jmbi.1993.1458. [DOI] [PubMed] [Google Scholar]
- Martin F. H., Tinoco I., Jr DNA-RNA hybrid duplexes containing oligo(dA:rU) sequences are exceptionally unstable and may facilitate termination of transcription. Nucleic Acids Res. 1980 May 24;8(10):2295–2299. doi: 10.1093/nar/8.10.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen D. B., Benseler F., Aurup H., Pieken W. A., Eckstein F. Study of a hammerhead ribozyme containing 2'-modified adenosine residues. Biochemistry. 1991 Oct 8;30(40):9735–9741. doi: 10.1021/bi00104a024. [DOI] [PubMed] [Google Scholar]
- Paolella G., Sproat B. S., Lamond A. I. Nuclease resistant ribozymes with high catalytic activity. EMBO J. 1992 May;11(5):1913–1919. doi: 10.1002/j.1460-2075.1992.tb05244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieken W. A., Olsen D. B., Benseler F., Aurup H., Eckstein F. Kinetic characterization of ribonuclease-resistant 2'-modified hammerhead ribozymes. Science. 1991 Jul 19;253(5017):314–317. doi: 10.1126/science.1857967. [DOI] [PubMed] [Google Scholar]
- SZER W., OCHOA S. COMPLEXING ABILITY AND CODING PROPERTIES OF SYNTHETIC POLYNUCLEOTIDES. J Mol Biol. 1964 Jun;8:823–834. doi: 10.1016/s0022-2836(64)80163-7. [DOI] [PubMed] [Google Scholar]
- Spirin A. S., Baranov V. I., Ryabova L. A., Ovodov S. Y., Alakhov Y. B. A continuous cell-free translation system capable of producing polypeptides in high yield. Science. 1988 Nov 25;242(4882):1162–1164. doi: 10.1126/science.3055301. [DOI] [PubMed] [Google Scholar]
- Stühmer W. Electrophysiological recording from Xenopus oocytes. Methods Enzymol. 1992;207:319–339. doi: 10.1016/0076-6879(92)07021-f. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Pieken W. A., Eckstein F. Function of specific 2'-hydroxyl groups of guanosines in a hammerhead ribozyme probed by 2' modifications. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):918–921. doi: 10.1073/pnas.89.3.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormington M. Preparation of synthetic mRNAs and analyses of translational efficiency in microinjected Xenopus oocytes. Methods Cell Biol. 1991;36:167–183. doi: 10.1016/s0091-679x(08)60277-0. [DOI] [PubMed] [Google Scholar]
- Yang J. H., Usman N., Chartrand P., Cedergren R. Minimum ribonucleotide requirement for catalysis by the RNA hammerhead domain. Biochemistry. 1992 Jun 2;31(21):5005–5009. doi: 10.1021/bi00136a013. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]