Abstract
Background
Early developmental insults can cause dysfunction within parvalbumin (PVB)-containing interneurons in the prefrontal cortex. The neuropsychiatric disorders associated with such dysfunction might involve neuroinflammatory processes. Cyclooxygenase-2 (COX-2) is a key mediator of inflammation and is therefore a potential target for preventive treatment. Here, we investigated whether the developmental trajectories of PVB expression and COX-2 induction in the prelimbic region of the prefrontal cortex are altered after maternal separation stress in male rats.
Methods
Male rat pups were separated from their mother and littermates for 4 hours/day between postnatal Days 2 and 20. Western blotting and immunohistochemistry were used to analyze PVB and COX-2 expression in the prefrontal cortex and hippocampus. A separate cohort of animals was treated with a COX-2 inhibitor during preadolescence and analyzed for PVB, COX-2, and working memory performance.
Results
We demonstrate that maternal separation causes a reduction of PVB and an increase in COX-2 expression in the prefrontal cortex in adolescence, with concurrent working memory deficits. Parvalbumin was not affected earlier in development. Prophylactic COX-2 inhibition preadolescence prevents PVB loss and improves working memory deficits induced by maternal separation.
Conclusions
These data are the first to show a preventive pharmacological intervention for the delayed effects of early life stress on prefrontal cortex interneurons and working memory. Our results suggest a possible mechanism for the relationship between early life stress and interneuron dysfunction in adolescence.
Keywords: Adolescence, COX-2, development, inflammation, parvalbumin, stress, working memory
Early life exposure to stressful situations impairs cognitive and motivational processes later in life and leads to significant increases in vulnerability to psychiatric disorders, including depression, drug abuse, and schizophrenia (1–5). Due to the delayed age of emergence of these disorders—often adolescence or young adulthood (6–8)—the role that early adversity plays in these diseases has been difficult to determine because of a host of intervening and moderating variables found in clinical studies. Animal studies can help clarify the causality within the early adversity literature through the use of experimental manipulations of exposure to early life stress. Daily repeated removal of rat pups from their mothers (e.g., maternal separation [MS]) during the neonatal period is an ethologically relevant rodent model of early life stress (9).
Exposure to early life stress might yield a vulnerable population with neurodevelopmental deficits that could particularly benefit from intervention during a critical period. Many preclinical studies report effects of MS on the hippocampus (e.g., [10–14]), with recent attention turning also to biochemical and physiological effects of MS in the prefrontal cortex (PFC) (15–18). The PFC is a relatively late-maturing region (19), is extensively connected with subcortical brain regions, and subserves all higher-order cognitive and emotional functions. Notably, consequences of MS in the PFC typically manifest in adolescence (18,20) or adulthood (16,17) but not earlier in development. Many stress-induced changes in the PFC specifically involve the prelimbic region (plPFC) (21,22) as well as other PFC regions and might have delayed effects on the PFC due to its late and protracted developmental profile (8,19). This delay could provide an opportunistic window for treatment in a vulnerable population before the deleterious consequences of MS take hold. Although environmental enrichment might represent one promising avenue to reverse some neuroendocrine- or hippocampal-dependent effects of MS (23–25), to our knowledge there are no reports of pharmacological intervention to prevent developmental disturbances of PFC neurophysiology or behavior after MS.
One possible approach to reverse MS-related deficits might involve the inflammatory molecule cyclooxygenase-2 (COX-2), which is induced in the brain in response to stress (26), oxidative stress, inflammatory cytokines, and glutamatergic activity in the brain (27). Because several of these processes reportedly occur in the PFC after exposure to developmental stressors (28,29), COX-2 might play a role in the pathogenesis of early life stress exposure. Consistent with this hypothesis, COX-2 has been specifically linked to symptoms of psychotic disorders such as depression and schizophrenia (26,30), where early stress exposure might be a risk factor. However, the effects of COX-2 during development have never been examined. We aimed here to determine whether PFC dysfunction after MS involves COX-2 and whether COX-2 inhibition could offer protection from further damage.
Dysfunction or loss of γ-aminobutyric acid-ergic cells that express the calcium binding protein parvalbumin (PVB) is important, because these interneurons are implicated in a number of major psychiatric disorders (31–33). For example, decreases in PVB are reported in all subregions of the hippocampus (34) and the PFC (35,36) of postmortem tissue samples from schizophrenia patients. Preclinical studies suggest that PVB cells are often affected by early developmental insults (37–40), and deficits are observed during adolescence or adulthood. Proper functioning of PVB-containing interneurons is necessary for cognitive processes such as working memory (41), which is also impaired after various developmental insults in both animals (42,43) and humans (44). Very few studies to date have measured effects of PVB in the PFC after MS (18), and none have explored consequences of MS on PFC-mediated cognitive behaviors such as working memory during the crucial adolescent phase of development, when many psychopathologies first emerge.
Here, we investigated whether the developmental expression of PVB and COX-2 in the PFC are altered after MS in male rats. We tested the hypothesis that MS causes delayed PVB loss through a COX-2–mediated pathway and that this, in turn, impairs working memory. We report that prophylactic COX-2 inhibition during preadolescence prevents PVB loss and improves working memory deficits induced by MS.
Methods and Materials
Subjects
Pregnant female multiparous Sprague–Dawley rats (250–275 g) were obtained from Charles River Laboratories (Wilmington, Massachusetts) on Day 13 of gestation. The day of birth was designated as postnatal Day 0 (P0). At P2, litters were culled to 10 pups (7 males, and 3 females), and litters were randomly assigned to either a maternal separation group (MS Group) or animal facility reared control group (CON Group). Pups in the MS Group were isolated for 4 hours/day between P2 and P20 and kept at thermoneutral temperature. This procedure is identical to procedures used previously by this laboratory (12,45) and similar to others (46). Pups in the CON Group were not disturbed after P2, except for routine weekly changes in cage bedding, during which all pups were weighed. Rats were housed with food and water available ad libitum in constant temperature and humidity conditions on a 12-hour light/dark cycle (light period 7:00 AM–7:00 PM). Rats were weaned on P21–22 and group-housed with same-sex littermates with 3–4 rats/cage until experimentation. Only one rat/litter was used/age/condition to avoid litter effects. Rats were tested—as stated within each specific experimental section—during the juvenile stage (P25) or in adolescence (P40), ages that are based on achievement of milestones, including brain development and pubertal status (47). Only male rats were used in these studies.
These experiments were conducted in accordance with the 1996 Guide for the Care and Use of Laboratory Animals (National Institutes of Health) and were approved by the Institutional Animal Care and Use Committee at McLean Hospital.
Experiment 1: Effects of MS on PVB Expression in the PFC and Hippocampus Across Development
Western Blotting
Western immunoblots of both plPFC and hippocampus tissue were analyzed from juvenile and adolescent rats from each group (n=6–7/Age and Stress Group of MS and CON). The plPFC and whole hippocampus were dissected on ice, and tissue was sonicated in 1% sodium dodecylsulfate solution containing a protease inhibitor cocktail (Pierce, Rockford, Illinois). Protein content was determined by a Bradford assay (Bio-Rad, Hercules, California). Thirty micrograms of protein were loaded into a 15% Tris-hydrogen chloride polyacrylamide gel and subjected to sodium dodecylsulfate-polyacrylamide gel electrophoresis. After protein separation, the samples were transferred to a nitrocellulose membrane and probed for PVB protein with mouse monoclonal anti-PVB immunoglobulin G (IgG) (1:500; Sigma, St. Louis, Missouri) and actin (to control for loading protein) with mouse polyclonal antiactin IgG (1:10,000, MP Pharmaceuticals, Aurora Ohio). Membranes were then incubated with antimouse secondary antibodies conjugated with horseradish peroxidase (1:10,000; Sigma). Immunoreactivity was visualized by enhanced chemiluminescent detection (West Pico Kit; Pierce). Optical densities of the bands were measured with ImageJ software and normalized with actin. Antibody specificity for PVB is illustrated in Figure S1A in Supplement 1. Group differences were determined in each region with two-way (Age Stress×Group) analyses of covariance, with Western blot run as a covariate. Bonferroni post hoc t tests compared group means after interactions were found.
Immunohistochemistry
To confirm the observed changes in PVB protein content, we performed immunohistochemical analysis of the plPFC in juvenile and adolescent MS and CON rats (n=6/Age and Stress Group). At P25 or P40, rats were deeply anesthetized and intracardially perfused. Tissue was processed with standard immunohistochemical methods (48). Briefly, 40-μm frozen sections were incubated with a monoclonal mouse antibody raised against PVB (1:10,000; Sigma) and then with biotinylated anti-mouse secondary serum (1:500; Sigma) and streptavidin (1:4,000; Invitrogen, Camarillo, California). The antigen-antiserum complex was detected by incubation in diaminobenzidine and nickel sulfate in presence of hydrogen peroxide. All steps were preceded and followed by washes in phosphate-buffered saline–Triton X-100. Sections were mounted on gelatin-coated slides, dehydrated, clarified, and cover-slipped with Permount (Thermo Fisher Scientific, Inc., Waltham, Massachusetts). Stereo Investigator Image Analysis System (MBF BioScience, Williston, Vermont) was used to estimate the density of PVB cells. The plPFC in four serial coronal sections (intersection interval 240 μm)/animal were analyzed (49). In each section, the entire plPFC was outlined at 4× magnification, and the total number of PVB-immunoreactive (IR) cells were measured at 20× exclusively within the outlined area. Investigators were strictly blinded to the conditions for all analyses. Tracings of the plPFC boundaries were used for calculation of the surface area (a) in each section. The density of PVB-IR (cells/mm2) was based on the total number of PVB-IR cells divided by Σa for each subject (the sum of areas obtained from all outlined regions). Volume of the plPFC was calculated according to the Cavalieri principle (50) as v=z×i×Σa, where z is the thickness of the section (40 μm) and i is the section interval (i=24; i.e., number of serial sections between each section and the following one within a compartment). Group differences were determined by 2-way (Age×Stress Group) analysis of variance. Bonferroni post hoc t tests compared group means after interactions were found.
Experiment 2: Effects of MS on COX-2 Expression During Adolescence
To determine whether an induction of COX-2 was present at the time of PVB decreases, tissue samples (40 μg of protein) from the same adolescent MS and CON animals used in Experiment 1 (n=7) were analyzed for COX-2 expression with Western blotting, as described in the preceding text. Polyclonal rabbit anti-COX-2 IgG (1: 500; Millipore, Temecula, California) and antirabbit secondary antibodies (1:10,000; Sigma) were used. Antibody specificity for COX-2 is illustrated in Figure S1B in Supplement 1. Group differences were determined in each region with Student t tests.
Experiment 3: Effects of Preadolescent COX-2 Inhibition on PVB and COX-2 Expression After MS
The MS or CON subjects (n=6/Stress Group and Treatment) were treated with either the COX-2 inhibitor NS-398 (8 mg/kg, diluted in dimethyl sulfoxide [DMSO]) or vehicle (1 ml/kg DMSO) from P30 to 38 once/day every second day. This dose was chosen on the basis of previous studies showing that between 5 and 10 mg/kg NS-398 has neuroprotective effects from excitotoxic and neuroinflammatory damage (51–53). Subjects were dosed every second day to avoid unnecessary stress and discomfort of the animals, because DMSO is a mild irritant. On P40, subjects were killed, and brains were processed for Western blot analysis of PVB and COX-2 as described in the preceding text. Group differences were determined with a 2-way (Stress Group×Treatment) analysis of covariance, with run as a covariate. Finally, linear regression analysis evaluated the relationship between COX-2 and PVB protein levels in subjects where both proteins were measured.
Experiment 4: Effects of Preadolescent COX-2 Inhibition on Win-Shift Performance After MS
Effects of MS with and without COX-2 inhibition on working memory were assessed during late adolescence. Seven–ten animals/group were food-deprived to 85% of their free-feeding weight and habituated to an eight-radial-arm maze for 10 min/day on P43–P44. After each habituation session, rats were returned to their home cages and given eight Froot Loops/cage (Kellogg, Battle Creek, Michigan). Subsequent testing was performed as described previously (54,55), with the Froot Loops reward. Briefly, each session consisted of a training phase and test phase. During the training phase, four of the eight arms were blocked, and the remaining four were baited with a reward. During the test phase, all eight arms were accessible, but only the arms that were previously blocked contained a reward. Training and test phases were separated by a 5-min delay, until subjects reached a criterion of retrieving all four rewards in five or fewer choices (i.e., one or fewer errors) for 2 consecutive days (56). All animals reached criterion between P51 and P56. A within-phase error was recorded for every re-entry into an arm where the reward had already been retrieved, whereas a between-phase error was recorded during test phase for every entry into a nonbaited arm (an arm that had previously been baited in the training phase). After the criterion was reached, daily tests were given with increasing delays of 30, 180, and 360 min introduced between training and testing phases. Errors made between groups were compared across days with a mixed three-way analysis of variance (Stress Group×Treatment×[Delay]) with delay time as a repeated measure.
Results
Experiment 1: Effects of Early Life Stress on PVB-Expression in the PFC and Hippocampus Across Development
An Age×Group interaction [F(1,24)=4.86; p=.04] revealed that MS decreased PVB protein content in the plPFC of adolescents, compared with CON adolescents [post hoc Bonferroni correction: t (12)=2.57; p=.02] (Figure 1). In the hippocampus, no age-related or group-related differences in PVB expression were found (p=.402) (Figure 1), and therefore we focused further analyses on the plPFC only. When PVB-IR was analyzed with immunohistochemistry, an Age×Group interaction [F(1,14)=5.12; p=.04] once again showed that PVB is differentially expressed in the plPFC over development, depending on MS experience (Figure 2). Maternal separation decreased PVB-IR in adolescence compared with CON rats [post hoc t (8)= 2.46; p=.04]. The plPFC volumes did not differ at either age between MS (P25: 3.8±.06 mm3; P40: 4.1±.4 mm3) and CON groups (P25: 4.6±.26 mm3; P40: 4.4±.39 mm3).
Figure 1.
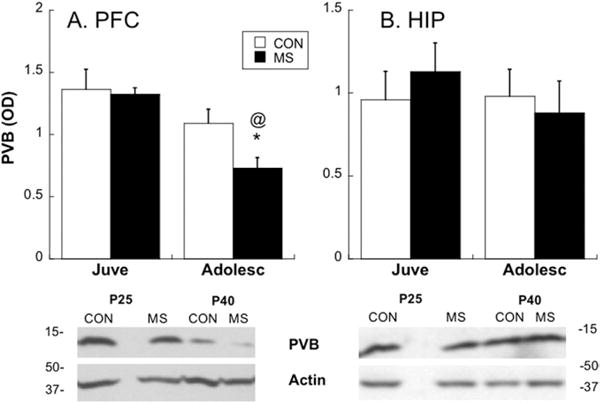
Measurement of parvalbumin (PVB) changes over development in control (CON) and maternal separation (MS) animals with Western blot analysis. Top: graphic representation of relative PVB at two ages (juvenile [Juve], postnatal day [P]25 and adolescent [Adolesc], P40) after CON or MS rearing in prefrontal cortex (PFC) (A) or hippocampus (HIP) (B) tissue; *p<.05 different from CON; @p<.05 different from juveniles. Bottom: representative Western blots from the PFC (A) or HIP (B) showing all groups tested. Means ± SE presented. OC, optical density.
Figure 2.
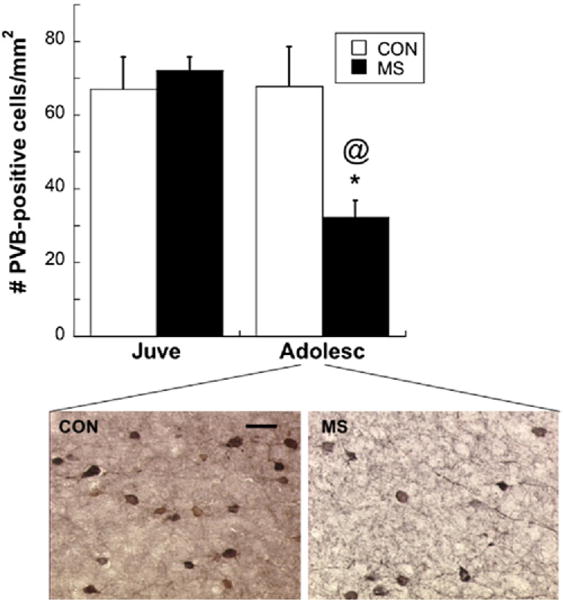
Top: graphic representation of PVB-immunoreactivity at two ages after CON or MS rearing; *p<.05 different from CON; @p<.05 different from Juve. Bottom: representative PVB-immunoreactive cells within the PFC of Adolesc. Both images taken from the same location and layer (5) within the PFC. 40×: bar: 33 μm. Means ± SE presented. Abbreviations as in Figure 1.
Experiment 2: Effects of MS on COX-2 Expression During Adolescence
In parallel with a loss of PVB during adolescence after MS, COX-2 expression increased 159±20% in the plPFC of MS rats compared with CON rats [t (12)=2.82; p=.015] (Figure 3). No difference in hippocampal COX-2 was found between MS and CON groups during adolescence (p=.911). Preliminary data (n=4) suggest that PFC COX-2 levels were already rising by P25 (49±27.6% higher than CON values) but did not significantly differ from control subjects.
Figure 3.
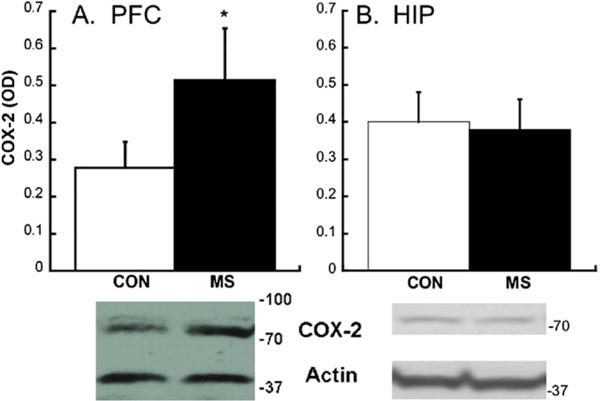
Cyclooxygenase-2 (COX-2) levels during adolescence after CON or MS conditions. (A) Western blot analysis of COX-2 in the prelimbic PFC of CON and MS animals; *p<.05. (B) Western blot analysis of COX-2 in the HIP of CON and MS animals. A representative Western blot from each region is shown below each graph. Due to different concentrations of proteins in HIP, COX-2 and actin required different exposure times, necessitating two separate images. Means ± SE presented. Abbreviations as in Figure 1.
Experiment 3: Effects of Preadolescent COX-2 Inhibition on PVB Expression After MS
Preadolescent treatment with NS-398 significantly reduced COX-2 expression in the plPFC of MS adolescents [Group×Treatment interaction: F(1,13)=10.79; p=.006] (Figure 4A). Similarly, preadolescent treatment with NS-398 significantly increased PVB in the plPFC of adolescent MS rats [Group×Treatment interaction: F(1,21)=1.97; p=.035] (Figure 4B). The COX-2 and PVB amounts were negatively correlated, such that animals with lower COX-2 displayed higher amounts of PVB (R=−.613; p=.02) (Figure 4C).
Figure 4.
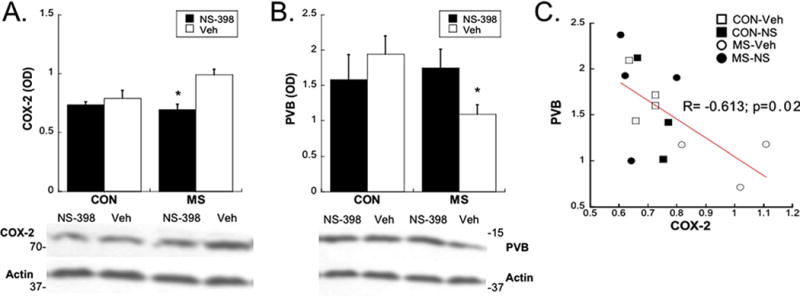
Effects of preadolescent treatment with NS-398 (NS) on PVB (A) and COX-2 (B) levels in the PFC during adolescence after CON or MS conditions. Means SE presented. A representative Western blot from each region is shown below each graph; *p<.05; (C) linear regression analysis shows a direct relationship between COX-2 and PVB levels; R=−.613; p=.02. Note: 2–3 subjects/group were not included in regression analysis, due to lack of within-subject measurement of both proteins. Veh, vehicle; other abbreviations as in Figures 1 and 3.
Experiment 4: Effects of Preadolescent COX-2 Inhibition on Win-Shift Performance After MS
Once criterion was reached, we observed a significant Group×Treatment interaction [F(1,27)=6.86; p=.014] for total errors made during the test phase, which was independent of delay (p=.17 for Group×Treatment×[Delay]). Post hoc analyses show that vehicle-treated MS subjects made significantly more total errors during the test phase than vehicle-treated CON subjects (p=.007 collapsed across delay), but this difference was reversed by treatment with NS-398 (p=.03 collapsed across delay; Figure 5). Group effects were likely driven by between-phase errors, because a trend-level Group×Treatment interaction was found for between-phase errors (p=.069) but not within-phase errors (p=.182). Neither stress experience nor treatment had an effect on the number of trials to reach criterion of no more than one error in the test phase for 2 consecutive days (p=.34). All rats required 9.5±.5 days to reach criterion, resulting in testing for longer delays occurring on approximately P55–P60 (late adolescence/early adulthood).
Figure 5.
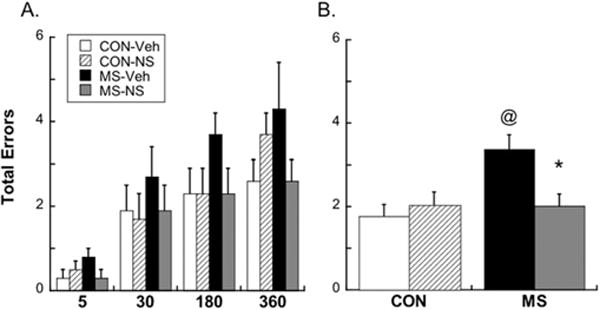
Problems with working memory in MS animals were detected with the win-shift test, regardless of delay. (A) Total errors made after each delay; (B) total errors made by each group collapsed across delay. @p<.05 different from CON; *p<.05 different from Veh. Means ± SE presented. Abbreviations as in Figures 1 and 4.
Discussion
We demonstrate that preadolescent treatment with a nonsteroidal anti-inflammatory drug after MS can prevent a delayed loss of PVB-IR in the plPFC. Maternal separation during the first 3 weeks of life decreased PVB-IR cell densities and relative protein amounts in the plPFC in adolescence but spared the hippocampus for these measures. This MS-induced decrease in PVB was further associated with an increase in COX-2 induction in the plPFC. The MS animals also displayed impaired performance in the win-shift maze, a test of working spatial memory that is dependent on the PFC (57). Early intervention with a COX-2 inhibitor prevented the decrease in PVB and improved delayed response performance on the win-shift working memory task in late adolescence.
The demonstration that early life stress produces a delayed loss of PVB in the PFC provides a mechanism through which stress—long considered a risk factor for many illnesses—manifests during adolescence. Reduced PVB in the plPFC during adolescence might highlight a vulnerability to neuropsychiatric disorders that is produced by early life stress. Previous investigations in animals have revealed structural alterations in the adult brain because of early life stress, including reduced dendrite length, dendritic branching, spine density, and suppression of neurogenesis in limbic structures and the PFC (12,58,59). In line with this, human studies examining the neuroanatomical correlates of childhood maltreatment in adults found decreased gray matter volume in the hippocampus and medial PFC (8,60–62). The current study has revealed changes in the PFC that occur during a critical time of development (adolescence), when altered inhibitory influence in the PFC could be the most deleterious.
Maternal separation also produced working memory impairment during late adolescence/early adulthood. Identified cases of prodromal schizophrenia and depression are often characterized by marked problems with working memory and social withdrawal. Adult levels of executive function emerge relatively late in the postnatal development of humans (63), nonhuman primates (19), and rodents (64). Preclinical studies show that this delay in achieving mature performance on executive function tasks correlates with the maturation of PVB-interneuronal networks throughout childhood and adolescence (41,65–67). Therefore, environmental insults affecting the development of this inhibitory network might lead to abnormal working memory performance, as we observed in late adolescence after MS.
Few studies have measured levels of PVB in the PFC and hippocampus after MS. However, our report of PVB changes in the adolescent rat after MS contrasts with prior studies in a more precocial rodent species, Octodon degus (18). Different schedules of MS or species differences might explain these contrasting results. Although MS reduces PVB in the dentate gyrus of degus weanlings and increases PVB in other subcortical regions (14), our results in the hippocampus are consistent with previous evidence that PVB neither changes between weaning and adulthood in normal rats (68) nor is altered by MS (69).
Early intervention in vulnerable individuals could help prevent maladaptive changes during adolescence and consequentially protect from resulting psychiatric disorders that begin during this phase of development. We have shown here that administration of a COX-2 inhibitor during preadolescence prevents the delayed loss of PFC PVB that occurs after MS. A target of nonsteroidal anti-inflammatory agents, COX-2 is also a key enzymatic mediator of oxidative stress and excitotoxicity. All three of these mechanisms play roles in neuropsychiatric disorders (30) and specifically target PVB-positive interneurons throughout the brain (70,71). Therefore, intervening with a COX-2 inhibitor during a critical time might be a promising prophylactic strategy after early life stress exposure.
The mechanism by which MS leads to COX-2 induction during adolescence remains to be examined. It is likely that abnormal development begins during stress exposure and is coordinated by both neuroendocrine and neurotransmitter systems (72). However, we show here that at least some of the deleterious effects of MS in the PFC do not manifest until adolescence, when the PFC begins to reach maturity. Maternal separation affects structures that project to the PFC, such as the hippocampus (11,12), amygdala (73), and nucleus accumbens (15). Projections from the amygdala (74) and to the nucleus accumbens (75) reportedly increase with age, such that the PFC is relatively “disconnected” until adolescence. Therefore, early life stress could initiate aberrant connectivity or activation that subsequently leads to COX-2-mediated injury in adolescence, when the PFC becomes more functionally integrated with subcortical structures. Indeed, neonatal damage to the hippocampus has delayed deleterious effects on PVB-positive PFC interneurons (38,40,76). It has also been proposed that MS stimulates hypothalamic-pituitary-axis–evoked proinflammatory processes that further sensitize stress and proinflammatory responses later in life (28). Although we observed a baseline increase in COX-2 expression after MS without any subsequent stress or proinflammatory exposure, it is possible that the transition through puberty itself could kindle an inflammatory response in previously sensitized subjects.
The importance of COX-2 in neuropathology is highlighted by recent findings demonstrating that the inflammatory enzyme mediates many of the central effects of psychologically relevant stressors (52,77). For example, NS-398 lowers stress-induced accumulation of free radicals in the cortex after acute restraint stress in rats (52). Clinically, pharmacological strategies directed toward COX-2 pathways are neuroprotective in stress-induced brain damage (78). Here, we show a delayed COX-2-mediated response to early life stress, which affected PVB interneurons in the plPFC.
A significant correlation was observed between COX-2 expression and PVB expression in the plPFC (R=−.613), with PVB being the greatest in MS animals treated with NS-398. The observed decrease of PVB-IR after MS in untreated animals could indicate an outright loss of PVB-expressing interneurons or, alternatively, that these interneurons lost their phenotype and no longer expressed PVB. Some neonatal pharmacological insults that result in PVB loss reportedly spare the interneurons themselves but reduce PVB expression via oxidative stress (79). Because COX-2 activity releases free radicals, MS-induced COX-2 expression might lead to decreased PVB expression through oxidative stress. Another possible mechanism of PVB loss could lie in the neuroinflammatory products of COX-2 action. Cyclooxygenase-2 catalyzes the conversion of arachidonic acid into prostaglandin, which can have multifaceted downstream effects, including induction of proinflammatory mediators and subsequent neuronal damage (80). Prostaglandins can also aggravate excitotoxic neurodegeneration (81) or induce apoptosis via inducing glutamate release from astrocytes (82). Whether PVB interneurons are rendered especially susceptible to these processes after MS is unknown.
Treatment aimed at reducing the negative sequelae of early life stress is sorely lacking. Clinically, we know that individuals that have been exposed to adverse events during development are relatively resistant to pharmacotherapy (e.g., fluoxetine) for depression (83). Thus, it might be the case that treatment-resistant schizophrenic persons might also have an underlying abuse history that renders them less sensitive to standard interventions. Our demonstration of prefrontal cortical PVB loss and working memory deficits that are reversible with COX-2 inhibition displays relationships among MS, cortical interneuron damage, and altered behavior with a proposed avenue for treatment of vulnerable individuals.
Supplementary Material
Acknowledgments
We wish to thank the National Alliance for Research on Schizophrenia and Depression and the Shine Initiative for their funding to HCB and DA-15403 to SLA. We would like to thank Sabina Beretta for her guidance with immunohistochemical analyses and Nadjia Freund, Britta Thompson, and Heather MacGivillary for technical assistance. Dr. Brenhouse reports having received financial support from Infinity Pharmaceuticals for studies unrelated to this report.
Footnotes
Dr. Andersen reported no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, et al. Environment and vulnerability to major psychiatric illness: A case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4:163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- 2.Kessler R, Davis C, Kendler K. Childhood adversity and adult psychiatric disorder in the US national comorbidity survey. Psychol Med. 1997;27:1101–1119. doi: 10.1017/s0033291797005588. [DOI] [PubMed] [Google Scholar]
- 3.Teicher MH, Tomoda A, Andersen SL. Neurobiological consequences of early stress and childhood maltreatment: Are results from human and animal studies comparable? Ann N Y Acad Sci. 2006;1071:313–323. doi: 10.1196/annals.1364.024. [DOI] [PubMed] [Google Scholar]
- 4.Kohut S, Roma P, Davis C, Zernig G, Saria A, Dominguez J, et al. The impact of early environmental rearing condition on the discriminative stimulus effects and Fos expression induced by cocaine in adult male and female rats. Psychopharmacology (Berl) 2009;203:383–397. doi: 10.1007/s00213-008-1368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci. 2004;19:1863–1874. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- 6.Kessler R, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: An epidemiologic perspective. Biol Psychiatry. 2001;49:1002–1014. doi: 10.1016/s0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- 7.Davey C, Yucel M, Allen N. The emergence of depression in adolescence: Development of the prefrontal cortex and the representation of reward. Neurosci Biobehav Rev. 2008;32:1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: Consistent or confusing? Rev Neurosci. 2000;11:383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- 10.Hsu FC, Zhang GJ, Raol YS, Valentino RJ, Coulter DA, Brooks-Kayal AR, et al. Repeated neonatal handling with maternal separation permanently alters hippocampal GABAA receptors and behavioral stress responses. Proc Natl Acad Sci U S A. 2003;100:12213–12218. doi: 10.1073/pnas.2131679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aisa B, Elizalde N, Tordera R, Lasheras B, Del Rio J, Ramirez MJ, et al. Effects of neonatal stress on markers of synaptic plasticity in the hippocampus: Implications for spatial memory. Hippocampus. 2009;19:1222–1231. doi: 10.1002/hipo.20586. [DOI] [PubMed] [Google Scholar]
- 12.Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29:1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- 13.Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci. 2007;25:3091–3098. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- 14.Seidel K, Helmeke C, Poeggel G, Braun K. Repeated neonatal separation stress alters the composition of neurochemically characterized interneuron subpopulations in the rodent dentate gyrus and basolateral amygdala. Dev Neurobiol. 2008;68:1137–1152. doi: 10.1002/dneu.20651. [DOI] [PubMed] [Google Scholar]
- 15.Monroy E, Hernandez-Torres E, Flores G. Maternal separation disrupts dendritic morphology of neurons in prefrontal cortex, hippocampus, and nucleus accumbens in male rat offspring. J Chem Neuroanat. 2010;40:93–101. doi: 10.1016/j.jchemneu.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Wilber AA, Southwood CJ, Wellman CL. Brief neonatal maternal separation alters extinction of conditioned fear and corticolimbic glucocorticoid and NMDA receptor expression in adult rats. Dev Neurobiol. 2009;69:73–87. doi: 10.1002/dneu.20691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevenson CW, Marsden CA, Mason R. Early life stress causes FG-7142-induced corticolimbic dysfunction in adulthood. Brain Res. 2008;1193:43–50. doi: 10.1016/j.brainres.2007.11.062. [DOI] [PubMed] [Google Scholar]
- 18.Helmeke C, Ovtscharoff WJ, Poeggel G, Braun K. Imbalance of immunohistochemically characterized interneuron populations in the adolescent and adult rodent medial prefrontal cortex after repeated exposure to neonatal separation stress. Neuroscience. 2008;152:18–28. doi: 10.1016/j.neuroscience.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 19.Alexander GE, Goldman PS. Functional development of the dorsolateral prefrontal cortex: An analysis utlizing reversible cryogenic depression. Brain Res. 1978;143:233–249. doi: 10.1016/0006-8993(78)90566-8. [DOI] [PubMed] [Google Scholar]
- 20.Chocyk A, Dudys D, Przyborowska A, Mackowiak M, Wedzony K. Impact of maternal separation on neural cell adhesion molecules expression in dopaminergic brain regions of juvenile, adolescent and adult rats. Pharmacol Rep. 2010;62:1218–1224. doi: 10.1016/s1734-1140(10)70385-6. [DOI] [PubMed] [Google Scholar]
- 21.Radley JJ, Gosselink KL, Sawchenko PE. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci Neurosci. 2009;29:7330–7340. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J Neurosci. 2002;22:7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hui JJ, Zhang ZJ, Liu SS, Xi GJ, Zhang XR, Teng GJ, et al. Hippocampal neurochemistry is involved in the behavioural effects of neonatal maternal separation and their reversal by post-weaning environmental enrichment: A magnetic resonance study. Behav Brain Res. 2011;217:122–127. doi: 10.1016/j.bbr.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Bredy TW, Zhang TY, Grant RJ, Diorio J, Meaney MJ. Peripubertal environmental enrichment reverses the effects of maternal care on hippocampal development and glutamate receptor subunit expression. Eur J Neurosci. 2004;20:1355–1362. doi: 10.1111/j.1460-9568.2004.03599.x. [DOI] [PubMed] [Google Scholar]
- 26.Guo JY, Li CY, Ruan YP, Sun M, Qi XL, Zhao BS, et al. Chronic treatment with celecoxib reverses chronic unpredictable stress-induced depressive-like behavior via reducing cyclooxygenase-2 expression in rat brain. Eur J Pharmacol. 2009;612:54–60. doi: 10.1016/j.ejphar.2009.03.076. [DOI] [PubMed] [Google Scholar]
- 27.Liang X, Wu L, Wang Q, Hand T, Bilak M, McCullough L, et al. Function of COX-2 and prostaglandins in neurological disease. J Mol Neurosci. 2007;33:94–99. doi: 10.1007/s12031-007-0058-8. [DOI] [PubMed] [Google Scholar]
- 28.Hennessy MB, Deak T, Schiml-Webb PA. Early attachment-figure separation and increased risk for later depression: Potential mediation by proinflammatory processes. Neurosci Biobehav Rev. 2010;34:782–790. doi: 10.1016/j.neubiorev.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fumagalli F, Pasini M, Frasca A, Drago F, Racagni G, Riva MA. Prenatal stress alters glutamatergic system responsiveness in adult rat prefrontal cortex. J Neurochem. 2009;109:1733–1744. doi: 10.1111/j.1471-4159.2009.06088.x. [DOI] [PubMed] [Google Scholar]
- 30.Muller N, Dursun SM. Schizophrenia genes, epigenetics and psychoneuroimmunology therapeutics: All make sense now? J Psychopharmacol. 2011;11:773–780. doi: 10.1177/0269881110364268. [DOI] [PubMed] [Google Scholar]
- 31.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 32.Sibille E, Morris HM, Kota RS, Lewis DA. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol. 2011;13:1–14. doi: 10.1017/S1461145710001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lisman J, Coyle J, Green R, Javitt D, Benes F, Heckers S, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z, Sun J, Reynolds GP. A selective reduction in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia patients. Chin Med J (Engl) 2002;115:819–823. [PubMed] [Google Scholar]
- 35.Beasley CL, Reynolds GP. Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophr Res. 1997;24:349–355. doi: 10.1016/s0920-9964(96)00122-3. [DOI] [PubMed] [Google Scholar]
- 36.Hashimoto T, Volk D, Eggan S, Mirnics K, Pierri J, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cabungcal J-H, Nicolas D, Kraftsik R, Cuenod M, Do K, Hornung J-P, et al. Glutathione deficit during development induces anomalies in the rat anterior cingulate GABAergic neurons: Relevance to schizophrenia. Neurobiol Dis. 2006;22:624–637. doi: 10.1016/j.nbd.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Tseng KY, Lewis DA, Hashimoto T, Sesack SR, Kloc M, Lewis DA, et al. A neonatal ventral hippocampal lesion causes functional deficits in adult prefrontal cortical interneurons. J Neurosci. 2008;28:12691–12699. doi: 10.1523/JNEUROSCI.4166-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coleman LG, Jr, Jarskog LF, Moy SS, Crews FT. Deficits in adult prefrontal cortex neurons and behavior following early post-natal NMDA antagonist treatment. Pharmacol Biochem Behav. 2009;93:322–330. doi: 10.1016/j.pbb.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Francois J, Ferrandon A, Koning E, Angst MJ, Sandner G, Nehlig A, et al. Selective reorganization of GABAergic transmission in neonatal ventral hippocampal-lesioned rats. Int J Neuropsychopharmacol. 2009;12:1097–1110. doi: 10.1017/S1461145709009985. [DOI] [PubMed] [Google Scholar]
- 41.Wilson FA, O’Scalaidhe SP, Goldman-Rakic PS. Functional synergism between putative gamma-aminobutyrate-containing neurons and pyramidal neurons in prefrontal cortex. Proc Natl Acad Sci U S A. 1994;91:4009–4013. doi: 10.1073/pnas.91.9.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brady AM, Saul RD, Wiest MK. Selective deficits in spatial working memory in the neonatal ventral hippocampal lesion rat model of schizophrenia. Neuropharmacology. 2010;59:605–611. doi: 10.1016/j.neuropharm.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 43.Mehta M, Schmauss C. Strain-specific cognitive deficits in adult mice exposed to early life stress. Behav Neurosci. 2011;125:29–36. doi: 10.1037/a0021952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Entringer S, Buss C, Kumsta R, Hellhammer DH, Wadhwa PD, Wust S, et al. Prenatal psychosocial stress exposure is associated with subsequent working memory performance in young women. Behav Neurosci Neurosci. 2009;123:886–893. doi: 10.1037/a0016265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andersen SL, Lyss PJ, Dumont NL, Teicher MH. Enduring neurochemical effects of early maternal separation on limbic structures. Ann N Y Acad Sci. 1999;877:756–759. doi: 10.1111/j.1749-6632.1999.tb09317.x. [DOI] [PubMed] [Google Scholar]
- 46.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 47.Andersen SL. Trajectories of brain development: Point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 48.Berretta S, Lange N, Bhattacharyya S, Sebro R, Garces J, Benes FM, et al. Long-term effects of amygdala GABA receptor blockade on specific subpopulations of hippocampal interneurons. Hippocampus. 2004;14:876–894. doi: 10.1002/hipo.20002. [DOI] [PubMed] [Google Scholar]
- 49.Sherwood N, Timeras P. A Stereotaxic Atlas of the Developing Rat Brain. Los Angeles: University of California Press; 1970. [Google Scholar]
- 50.Cavalieri B. Geometria degli Indivisibili. Unione Tipografico-Editrice Torinese [Original Work—Geometria Indivisilibus Continuorum. Torino: Bononi, Typus Clementis Feronji, 1635] 1966 [Google Scholar]
- 51.Salzberg-Brenhouse HC, Chen EY, Emerich DF, Baldwin S, Hogeland K, Ranelli S, et al. Inhibitors of cyclooxygenase-2, but not cyclooxygenase-1 provide structural and functional protection against quinolinic acid-induced neurodegeneration. J Pharmacol Exp Ther. 2003;306:218–228. doi: 10.1124/jpet.103.049700. [DOI] [PubMed] [Google Scholar]
- 52.Madrigal JL, Moro MA, Lizasoain I, Lorenzo P, Fernandez AP, Rodrigo J, et al. Induction of cyclooxygenase-2 accounts for restraint stress-induced oxidative status in rat brain. Neuropsychopharmacology. 2003;28:1579–1588. doi: 10.1038/sj.npp.1300187. [DOI] [PubMed] [Google Scholar]
- 53.Wu Chen R, Zhang Y, Rose ME, Graham SH. Cyclooxygenase-2 activity contributes to neuronal expression of cyclin D1 after anoxia/ ischemia in vitro and in vivo. Brain Res Mol Brain Res. 2004;132:31–37. doi: 10.1016/j.molbrainres.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 54.Enomoto T, Floresco S. Disruptions in spatial working memory, but not short-term memory, induced by repeated ketamine exposure. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:668–675. doi: 10.1016/j.pnpbp.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 55.Andersen SL, Greene-Colozzi EA, Sonntag KC. A novel, multiple symptom model of obsessive-compulsive-like behaviors in animals. Biol Psychiatry. 2010;68:741–747. doi: 10.1016/j.biopsych.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 56.Floresco S, Seamans J, Phillips A. A selective role for dopamine in the nucleus accumbens of the rat in random foraging but not delayed spatial win-shift-based foraging. Behav Brain Res. 1996;80:161–168. doi: 10.1016/0166-4328(96)00031-9. [DOI] [PubMed] [Google Scholar]
- 57.Seamans J, Floresco S, Phillips A. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 59.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 60.van Harmelen AL, van Tol MJ, van der Wee NJ, Veltman DJ, Aleman A, Spinhoven P, et al. Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biol Psychiatry. 2010;68:832–838. doi: 10.1016/j.biopsych.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 61.Tomoda A, Suzuki H, Rabi K, Sheu YS, Polcari A, Teicher MH. Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. Neuroimage. 2009;47(suppl 2):T66–T71. doi: 10.1016/j.neuroimage.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frodl T, Reinhold E, Koutsouleris N, Donohoe G, Bondy B, Reiser M, et al. Childhood stress, serotonin transporter gene and brain structures in major depression. Neuropsychopharmacology. 2010;35:1383–1390. doi: 10.1038/npp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Geier CF, Garver K, Terwilliger R, Luna B. Development of working memory maintenance. J Neurophysiol. 2009;101:84–99. doi: 10.1152/jn.90562.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ba A, Seri BV. Psychomotor functions in developing rats: Ontogenetic approach to structure-function relationships. Neurosci Biobehav Rev. 1995;19:413–425. doi: 10.1016/0149-7634(94)00042-y. [DOI] [PubMed] [Google Scholar]
- 65.Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Doischer D, Hosp JA, Yanagawa Y, Obata K, Jonas P, Vida I, et al. Postnatal differentiation of basket cells from slow to fast signaling devices. J Neurosci. 2008;28:12956–12968. doi: 10.1523/JNEUROSCI.2890-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Uhlhaas PJ, Roux F, Singer W, Haenschel C, Sireteanu R, Rodriguez E, et al. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc Natl Acad Sci U S A. 2009;106:9866–9871. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hayakawa N, Abe M, Eto R, Kato H, Araki T. Age-related changes of NGF, BDNF, parvalbumin and neuronal nitric oxide synthase immunoreactivity in the mouse hippocampal CA1 sector. Metab Brain Dis. 2008;23:199–211. doi: 10.1007/s11011-008-9084-7. [DOI] [PubMed] [Google Scholar]
- 69.Giachino C, Canalia N, Capone F, Fasolo A, Alleva E, Riva MA, et al. Maternal deprivation and early handling affect density of calcium binding protein-containing neurons in selected brain regions and emotional behavior in periadolescent rats. Neuroscience. 2007;145:568–578. doi: 10.1016/j.neuroscience.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 70.Schiavone S, Source S, Dubois-Dauphin M, Jaquet V, Colaianna M, Zotti M, et al. Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biol Psychiatry. 2009;66:384–392. doi: 10.1016/j.biopsych.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 71.Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, Belforte JE. GABAergic interneuron origin of schizophrenia pathophysiology [published online ahead of print January 26] Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Faturi CB, Tiba PA, Kawakami SE, Catallani B, Kerstens M, Suchecki D, et al. Disruptions of the mother-infant relationship and stress-related behaviours: Altered corticosterone secretion does not explain everything. Neurosci Biobehav Rev. 2010;34:821–834. doi: 10.1016/j.neubiorev.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 73.Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- 74.Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: Implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- 75.Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: Relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feleder C, Tseng KY, Calhoon GG, O’Donnell P. Neonatal intrahippocampal immune challenge alters dopamine modulation of prefrontal cortical interneurons in adult rats. Biol Psychiatry. 2010;67:386–392. doi: 10.1016/j.biopsych.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dhir A, Padi SS, Naidu PS, Kulkarni SK. Protective effect of naproxen (non-selective COX-inhibitor) or rofecoxib (selective COX-2 inhibitor) on immobilization stress-induced behavioral and biochemical alterations in mice. Eur J Pharmacol. 2006;535:192–198. doi: 10.1016/j.ejphar.2006.01.064. [DOI] [PubMed] [Google Scholar]
- 78.Munhoz CD, Garcia-Bueno B, Madrigal JL, Lepsch LB, Scavone C, Leza JC, et al. Stress-induced neuroinflammation: Mechanisms and new pharmacological targets. Braz J Med Biol Res. 2008;41:1037–1046. doi: 10.1590/s0100-879x2008001200001. [DOI] [PubMed] [Google Scholar]
- 79.Powell SB, Sejnowski TJ, Behrens MM. Behavioral and neurochemical consequences of cortical oxidative stress on parvalbumin-interneuron maturation in rodent models of schizophrenia [published online ahead of print February 17] Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ferri CC, Ferguson AV. Prostaglandin E2 mediates cellular effects of interleukin-1beta on parvocellular neurones in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2005;17:498–508. doi: 10.1111/j.1365-2826.2005.01336.x. [DOI] [PubMed] [Google Scholar]
- 81.Takadera T, Ohyashiki T. Prostaglandin E2 deteriorates N-methyl-D-aspartate receptor-mediated cytotoxicity possibly by activating EP2 receptors in cultured cortical neurons. Life Sci. 2006;78:1878–1883. doi: 10.1016/j.lfs.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 82.Takadera T, Yumoto H, Tozuka Y, Ohyashiki T. Prostaglandin E(2) induces caspase-dependent apoptosis in rat cortical cells. Neurosci Lett. 2002;317:61–64. doi: 10.1016/s0304-3940(01)02449-1. [DOI] [PubMed] [Google Scholar]
- 83.Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci U S A. 2003;100:14293–14296. doi: 10.1073/pnas.2336126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


