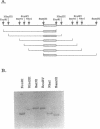
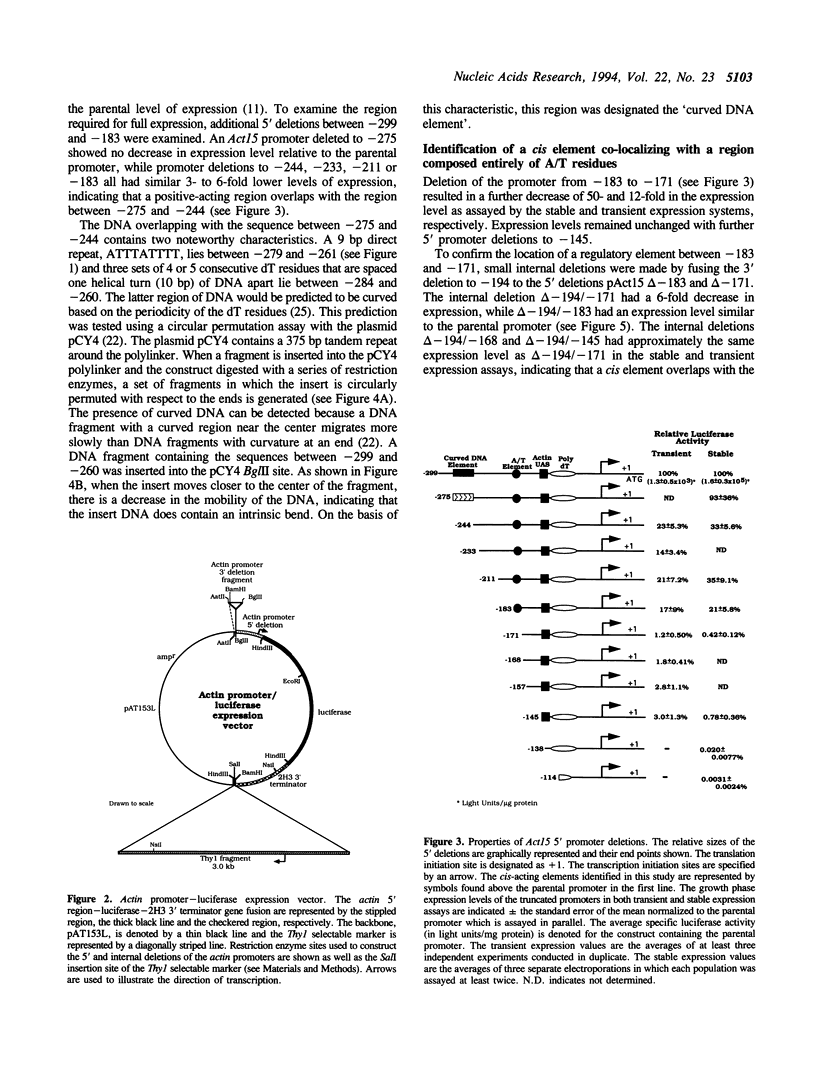
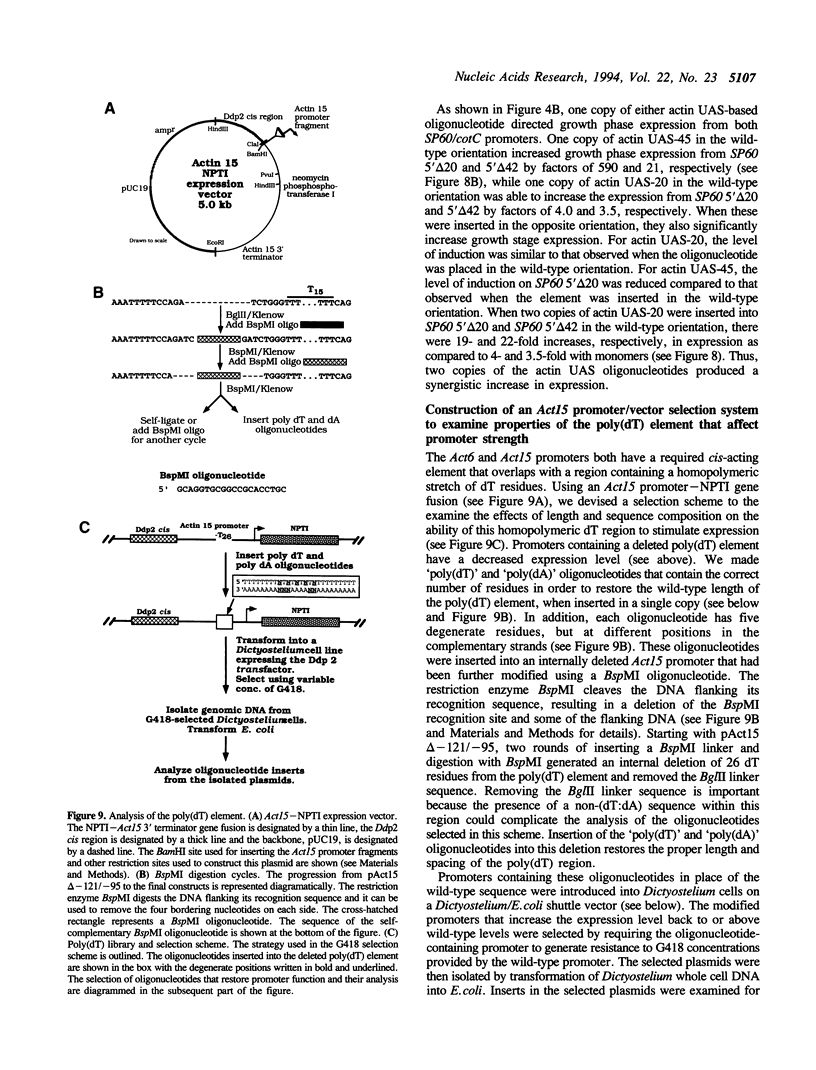
Abstract
The promoter elements in the Dictyostelium actin 15 and actin 6 genes required for full growth phase expression were identified by assaying promoter/luciferase reporter constructs. We find that these promoters contain common cis-acting elements, an actin upstream activating sequence (UAS) and sequences proximal to the transcription start site that overlap with a poly(dT) region. The actin 15 promoter has two additional cis-acting elements not present in the actin 6 promoter that may account for the higher level of expression from the actin 15 promoter. All of the identified promoter elements are unusual for Dictyostelium in that they are all A/T-rich. Two cis-acting elements, the actin UAS and the poly(dT) domain were studied in greater detail. The actin UAS was tested on a heterologous promoter from the prespore-specific gene SP60 and shown to have the ability to confer growth phase expression. The actin UAS also exhibited the ability to function in a distance- and orientation-independent manner and activate expression synergistically when present in two copies. The poly(dT) domain of the actin 15 promoter was studied in greater detail by using a genetic selection scheme to define parameters that effect the strength of this element. This element is comprised of 45 consecutive dT residues immediately upstream of the putative TATA box. We show that the length of the homopolymer dT region correlates with the expression level of the promoter. The poly(dT) element is also shown to function to promote wild-type levels of expression with small deviations in the sequence, indicating that the element is not required to be homopolymeric to function.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton T. H., Lodish H. F. Developmental changes in messenger RNAs and protein synthesis in Dictyostelium discoideum. Dev Biol. 1977 Oct 1;60(1):180–206. doi: 10.1016/0012-1606(77)90118-x. [DOI] [PubMed] [Google Scholar]
- Bracco L., Kotlarz D., Kolb A., Diekmann S., Buc H. Synthetic curved DNA sequences can act as transcriptional activators in Escherichia coli. EMBO J. 1989 Dec 20;8(13):4289–4296. doi: 10.1002/j.1460-2075.1989.tb08615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli A., Mahbubani H., Williams J. G. Positively and negatively acting signals regulating stalk cell and anterior-like cell differentiation in Dictyostelium. Cell. 1991 Jun 14;65(6):983–989. doi: 10.1016/0092-8674(91)90550-i. [DOI] [PubMed] [Google Scholar]
- Cohen S. M., Knecht D., Lodish H. F., Loomis W. F. DNA sequences required for expression of a Dictyostelium actin gene. EMBO J. 1986 Dec 1;5(12):3361–3366. doi: 10.1002/j.1460-2075.1986.tb04651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crothers D. M., Haran T. E., Nadeau J. G. Intrinsically bent DNA. J Biol Chem. 1990 May 5;265(13):7093–7096. [PubMed] [Google Scholar]
- Dynes J. L., Firtel R. A. Molecular complementation of a genetic marker in Dictyostelium using a genomic DNA library. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7966–7970. doi: 10.1073/pnas.86.20.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch R. K., Howard P. K., Firtel R. A. Regulation of the Dictyostelium cAMP-induced, prestalk-specific DdrasD gene: identification of cis-acting elements. Nucleic Acids Res. 1992 Mar 25;20(6):1325–1332. doi: 10.1093/nar/20.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S., Harley V. R., Pontiggia A., Goodfellow P. N., Lovell-Badge R., Bianchi M. E. SRY, like HMG1, recognizes sharp angles in DNA. EMBO J. 1992 Dec;11(12):4497–4506. doi: 10.1002/j.1460-2075.1992.tb05551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firtel R. A., Lodish H. F. A small nuclear precursor of messenger RNA in the cellular slime mold Dictyostelium discoideum. J Mol Biol. 1973 Sep 15;79(2):295–314. doi: 10.1016/0022-2836(73)90007-7. [DOI] [PubMed] [Google Scholar]
- Firtel R. A. Signal transduction pathways controlling multicellular development in Dictyostelium. Trends Genet. 1991 Nov-Dec;7(11-12):381–388. doi: 10.1016/0168-9525(91)90260-w. [DOI] [PubMed] [Google Scholar]
- Galas D. J., Eggert M., Waterman M. S. Rigorous pattern-recognition methods for DNA sequences. Analysis of promoter sequences from Escherichia coli. J Mol Biol. 1985 Nov 5;186(1):117–128. doi: 10.1016/0022-2836(85)90262-1. [DOI] [PubMed] [Google Scholar]
- Haberstroh L., Firtel R. A. A spatial gradient of expression of a cAMP-regulated prespore cell-type-specific gene in Dictyostelium. Genes Dev. 1990 Apr;4(4):596–612. doi: 10.1101/gad.4.4.596. [DOI] [PubMed] [Google Scholar]
- Hadwiger J. A., Firtel R. A. Analysis of G alpha 4, a G-protein subunit required for multicellular development in Dictyostelium. Genes Dev. 1992 Jan;6(1):38–49. doi: 10.1101/gad.6.1.38. [DOI] [PubMed] [Google Scholar]
- Howard P. K., Ahern K. G., Firtel R. A. Establishment of a transient expression system for Dictyostelium discoideum. Nucleic Acids Res. 1988 Mar 25;16(6):2613–2623. doi: 10.1093/nar/16.6.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel A. R., Firtel R. A. Sequence organization in Dictyostelium: unique structure at the 5'-ends of protein coding genes. Nucleic Acids Res. 1983 Jan 25;11(2):541–552. doi: 10.1093/nar/11.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht D. A., Cohen S. M., Loomis W. F., Lodish H. F. Developmental regulation of Dictyostelium discoideum actin gene fusions carried on low-copy and high-copy transformation vectors. Mol Cell Biol. 1986 Nov;6(11):3973–3983. doi: 10.1128/mcb.6.11.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A., Pupillo M., Gundersen R., Miake-Lye R., Devreotes P. N., Firtel R. A. Regulation and function of G alpha protein subunits in Dictyostelium. Cell. 1989 Apr 21;57(2):265–275. doi: 10.1016/0092-8674(89)90964-1. [DOI] [PubMed] [Google Scholar]
- Kunkel G. R., Martinson H. G. Nucleosomes will not form on double-stranded RNa or over poly(dA).poly(dT) tracts in recombinant DNA. Nucleic Acids Res. 1981 Dec 21;9(24):6869–6888. doi: 10.1093/nar/9.24.6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiting B., Lindner I. J., Noegel A. A. The extrachromosomal replication of Dictyostelium plasmid Ddp2 requires a cis-acting element and a plasmid-encoded trans-acting factor. Mol Cell Biol. 1990 Jul;10(7):3727–3736. doi: 10.1128/mcb.10.7.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue N. F., Buchman A. R., Kornberg R. D. Activation of yeast RNA polymerase II transcription by a thymidine-rich upstream element in vitro. Proc Natl Acad Sci U S A. 1989 Jan;86(2):486–490. doi: 10.1073/pnas.86.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod C., Firtel R. A., Papkoff J. Regulation of actin gene expression during spore germination in Dictyostelium discoideum. Dev Biol. 1980 May;76(2):263–274. doi: 10.1016/0012-1606(80)90378-4. [DOI] [PubMed] [Google Scholar]
- May T., Blusch J., Sachse A., Nellen W. A cis-acting element responsible for early gene induction by extracellular cAMP in Dictyostelium discoideum. Mech Dev. 1991 Feb;33(2):147–155. doi: 10.1016/0925-4773(91)90081-g. [DOI] [PubMed] [Google Scholar]
- McKeown M., Firtel R. A. Differential expression and 5' end mapping of actin genes in Dictyostelium. Cell. 1981 Jun;24(3):799–807. doi: 10.1016/0092-8674(81)90105-7. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Nellen W., Silan C., Firtel R. A. DNA-mediated transformation in Dictyostelium discoideum: regulated expression of an actin gene fusion. Mol Cell Biol. 1984 Dec;4(12):2890–2898. doi: 10.1128/mcb.4.12.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellen W., Silan C., Saur U., Firtel R. A. Regulatory sequences in the promoter of the Dictyostelium Actin 6 gene. EMBO J. 1986 Dec 1;5(12):3367–3372. doi: 10.1002/j.1460-2075.1986.tb04652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovic J., Haribabu B., Dottin R. P. Identification of a signal transduction response sequence element necessary for induction of a Dictyostelium discoideum gene by extracellular cyclic AMP. Mol Cell Biol. 1989 Nov;9(11):4660–4669. doi: 10.1128/mcb.9.11.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell-Coffman J. A., Firtel R. A. Characterization of a novel Dictyostelium discoideum prespore-specific gene, PspB, reveals conserved regulatory sequences. Development. 1994 Jun;120(6):1601–1611. doi: 10.1242/dev.120.6.1601. [DOI] [PubMed] [Google Scholar]
- Prentki P., Chandler M., Galas D. J. Escherichia coli integration host factor bends the DNA at the ends of IS1 and in an insertion hotspot with multiple IHF binding sites. EMBO J. 1987 Aug;6(8):2479–2487. doi: 10.1002/j.1460-2075.1987.tb02529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunell A. Nucleosome reconstitution on plasmid-inserted poly(dA) . poly(dT). EMBO J. 1982;1(2):173–179. doi: 10.1002/j.1460-2075.1982.tb01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R., Gorman C. M., Howard B. Minichromosome assembly of non-integrated plasmid DNA transfected into mammalian cells. Nucleic Acids Res. 1985 May 24;13(10):3599–3615. doi: 10.1093/nar/13.10.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romans P., Firtel R. A. Organization of the Dictyostelium discoideum actin multigene family. Flanking sequences show subfamily homologies and unusual dyad symmetries. J Mol Biol. 1985 Jun 5;183(3):311–326. doi: 10.1016/0022-2836(85)90003-8. [DOI] [PubMed] [Google Scholar]
- Romans P., Firtel R. A., Saxe C. L., 3rd Gene-specific expression of the actin multigene family of Dictyostelium discoideum. J Mol Biol. 1985 Nov 20;186(2):337–355. doi: 10.1016/0022-2836(85)90109-3. [DOI] [PubMed] [Google Scholar]
- Russell D. W., Smith M., Cox D., Williamson V. M., Young E. T. DNA sequences of two yeast promoter-up mutants. Nature. 1983 Aug 18;304(5927):652–654. doi: 10.1038/304652a0. [DOI] [PubMed] [Google Scholar]
- Struhl K. Molecular mechanisms of transcriptional regulation in yeast. Annu Rev Biochem. 1989;58:1051–1077. doi: 10.1146/annurev.bi.58.070189.005155. [DOI] [PubMed] [Google Scholar]
- Struhl K. Naturally occurring poly(dA-dT) sequences are upstream promoter elements for constitutive transcription in yeast. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8419–8423. doi: 10.1073/pnas.82.24.8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang A. S., Mahbubani H., Williams J. G. Cell-type-specific actin mRNA populations in dictyostelium discoideum. Cell. 1982 Dec;31(2 Pt 1):375–382. doi: 10.1016/0092-8674(82)90131-3. [DOI] [PubMed] [Google Scholar]