Abstract
Tuberous sclerosis (TSC) is an inherited tumor syndrome caused by mutations in TSC1 or TSC2 that lead to aberrant activation of mTOR and development of tumors in multiple organs including the kidneys. The mTOR inhibitors rapamycin and everolimus (rapalogs) have demonstrated clinical efficacy in treating TSC-associated tumors including renal angiomyolipomas. However, tumor responses are usually only partial, and regrowth occurs after drug withdrawal. TSC-associated tumors are highly vascular, and TSC patients with renal angiomyolipomas have elevated levels of circulating vascular endothelial growth factor (VEGF) A and VEGFD. Sorafenib inhibits multiple kinases including VEGF receptors and has been used to treat metastatic epithelioid angiomyolipoma in one case, but formal trials have not been undertaken. In this study, we investigated tumor angiogenesis and the therapeutic efficacy of everolimus in combination with sorafenib for renal tumors in Tsc2+/− mice. We found that these tumors exhibited remarkably variable angiogenesis despite consistent aberrant activation of mTOR and increased expression of HIF1α and VEGFA. Treatment of 11-month-old Tsc2+/− mice for 2 months with a combination of everolimus and sorafenib significantly reduced the number and size of solid renal tumors, whereas everolimus or sorafenib alone did not. These results suggest that inhibition of mTOR and multiple kinases including VEGF receptors using combination therapy could hold promise for the treatment of TSC-associated tumors that have responded inadequately to a rapalog alone.
Introduction
Tuberous sclerosis (TSC) is a tumor syndrome caused by mutations in TSC1 or TSC2 that lead to aberrant activation of mTOR and development of tumors in multiple organs. More than 80% of TSC patients develop renal manifestations, usually multiple and bilateral angiomyolipomas (AMLs) that are the leading cause of adult deaths from the disease. Single and multiple renal cysts are also frequently observed, and renal cell carcinoma (RCC) is found in around 2% of TSC patients [1]. Treatment with the mTOR inhibitor sirolimus (rapamycin) or its derivative everolimus significantly reduces the size of renal AML in TSC patients [2], [3], [4]. Everolimus has also demonstrated clinical efficacy in TSC-associated renal carcinoma [5]. However, AML and other TSC-associated tumor responses to mTOR inhibitors are partial, and tumors that initially respond to treatment usually regrow after drug withdrawal. TSC-associated tumors are highly vascular [6], and TSC patients with renal AMLs have elevated levels of circulating vascular endothelial growth factor (VEGF) A and VEGFD [7]. The angiogenesis inhibitors sunitinib and sorafenib have been used to treat TSC-associated RCC and epithelioid AML in a limited number of cases [5], [8], [9]. Combination therapy using these multiple kinase inhibitors together with rapalogs may improve therapeutic efficacy for TSC-associated tumors.
Mouse models heterozygous for Tsc1 or Tsc2 have been described previously and develop lesions in multiple organs [10], [11]. Renal lesions are prominent and include cysts, papillary adenomas, solid adenomas, and carcinomas. These lesions are associated with somatic loss of function mutations of the corresponding second Tsc1 or Tsc2 allele and aberrant activation of the mTOR signaling pathway [12]. Expression of HIF1 and VEGFA is increased in Tsc2−/− mouse embryo fibroblasts, but rapamycin only partially downregulates VEGFA. Serum levels of VEGFA are increased and appear to be associated with the extent of tumor development in Tsc2−/− mice [13].
In this study, we investigated angiogenesis in renal lesions and the antitumor efficacy of everolimus in combination with sorafenib in the kidneys of Tsc2+/− mice. We show that combination of everolimus with sorafenib is superior to everolimus alone for treating solid renal tumors in Tsc2+/− mice.
Materials and Methods
Animal Procedures
Animal procedures were performed in accordance with the UK Home Office guidelines and approved by the Ethical Review Group of Cardiff University. Tsc2+/− balb/c mice were described previously [11]. To test whether combination of everolimus with sorafenib could improve antitumor efficacy, we first determined the combined maximum tolerated dose in the Tsc2+/− mice in a 2-week pilot treatment study. Tsc2+/− littermates were randomly allocated into 4 groups of 10, balanced for gender and of the same age. Animals were treated from the age of 11 months with vehicle, everolimus (10 mg/kg), sorafenib (42 mg/kg), or everolimus (6 mg/kg) plus sorafenib (30 mg/kg). All mice were treated five times a week via gavage for 2 months and then sacrificed for assessment of tumor burden and analysis of protein expression and phosphorylation in the kidneys. Vehicle and everolimus were supplied by Novartis Pharma AG, Basel, Switzerland. Sorafenib was supplied by Bayer Schering Pharma AG, Leverkusen, Germany.
Histology
Assessment of tumor burden in the kidneys of mice was performed as described previously [14]. Mouse kidneys were fixed in 10% buffered formalin saline (Thermo Scientific, Runcorn, UK) for 24 hours. Fixed kidneys were processed and paraffin embedded according to standard procedures. A series of 5-μm coronal kidney sections was prepared at 200-μm intervals from each kidney. Kidney sections were hematoxylin and eosin (HE)–stained and scanned to create virtual HE slides using an Aperio system (http://www.aperio.com/?gclid = CNXN-8by4a UCFcINfAods3eg1w). Virtual slides were used for lesion quantification. Maximum cross-sectional whole area and cellular area of each renal lesion were measured, respectively, using ImageJ (http://rsbweb.nih.gov/ij). Cellular areas of renal lesions were obtained from parenchyma and stroma. To estimate average size of tumor cells, cysts and papillary and solid tumors on HE-stained kidney sections were randomly chosen using ImageJ. Average size of tumor cells was determined from parenchyma (not stroma) through total area of all tumor cells divided by the number of tumor cells examined. Analysis was conducted blindly with respect to treatment status.
Immunohistochemistry (IHC)
Primary antibodies against VEGFR2, PDGFRα, PDGFRβ, ABCB1, and phosphorylated S6 ribosomal protein at S235/236, Akt at S473, and Erk1/2 at T202/Y204 were supplied by Cell Signaling Technology (Danvers, MA). Antibodies against VEGFA, VEGFR1, CD34, HIF1α, Ki67, and phosphorylated RAF1 at S259 were supplied by Abcam (Cambridge, UK). Antibody against ABCC1 was supplied by St John's laboratory Ltd. (London, UK). Antibody against ABCG2 was supplied by Santa Cruz Biotechnology, Inc. (Dallas, TX). Antibody against RALBP1 was supplied by Proteintech Europe (Manchester, UK). SignalStain Boost Rabbit specific IHC Detection Reagent (Cell Signaling Technology, Danvers, MA) was used to stain antigens.
Western Blot
In addition to primary antibodies described above, primary antibody against β-actin and horseradish peroxidase–conjugated secondary antibody against rabbit were purchased from Cell Signaling Technology (Danvers, MA) for Western blot. Extracts of tumor samples were prepared using AllPrep DNA/RNA/Protein Mini Kit (QIAGEN Ltd. UK, Crawley, UK). Proteins were purified according to the kit supplier's instruction. Twenty micrograms of protein per sample was separated on NuPAGE 4% to 12% Bis-Tris Gels (Fisher Scientific UK Ltd., Loughborough, UK) and transferred onto Hybond ECL Membranes (GE Healthcare UK Ltd., Little Chalfont, UK). Blots were analyzed with ECL Select Western Detection Kit (GE Healthcare UK Ltd.), and signals were detected using Autochemi Imaging System (UVP, Upland, CA).
Statistical Analysis
Wilcoxon rank-sum test was performed for comparisons of treatment efficacy on mouse renal lesions. Fisher's exact test was used to assess massive cell death in mouse solid tumors. P < .05 was considered to be statistically significant. Analyses were performed using STATA 13.
Results
Variations in Angiogenesis of Renal Lesions in Tsc2+/− Mice
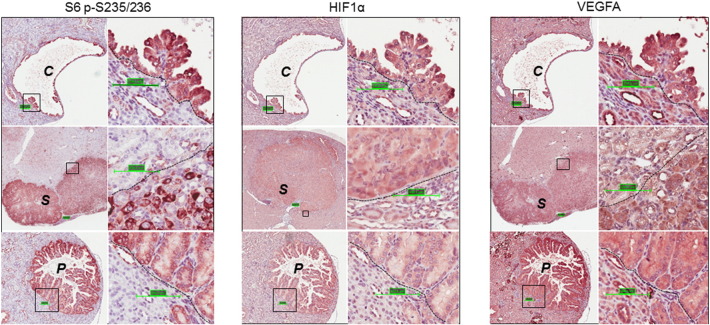
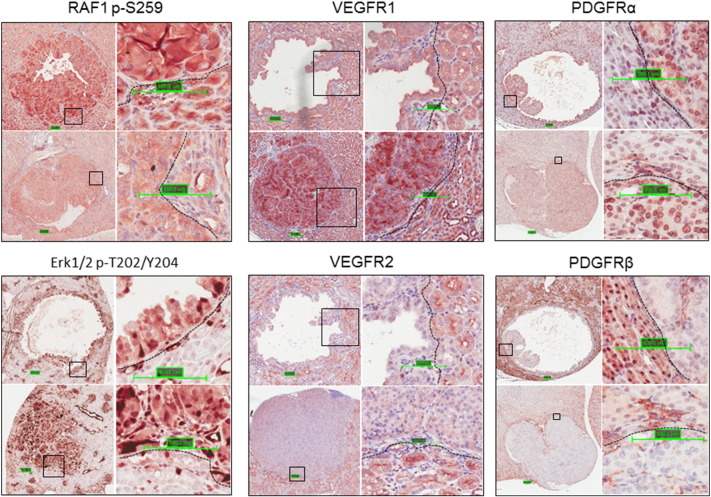
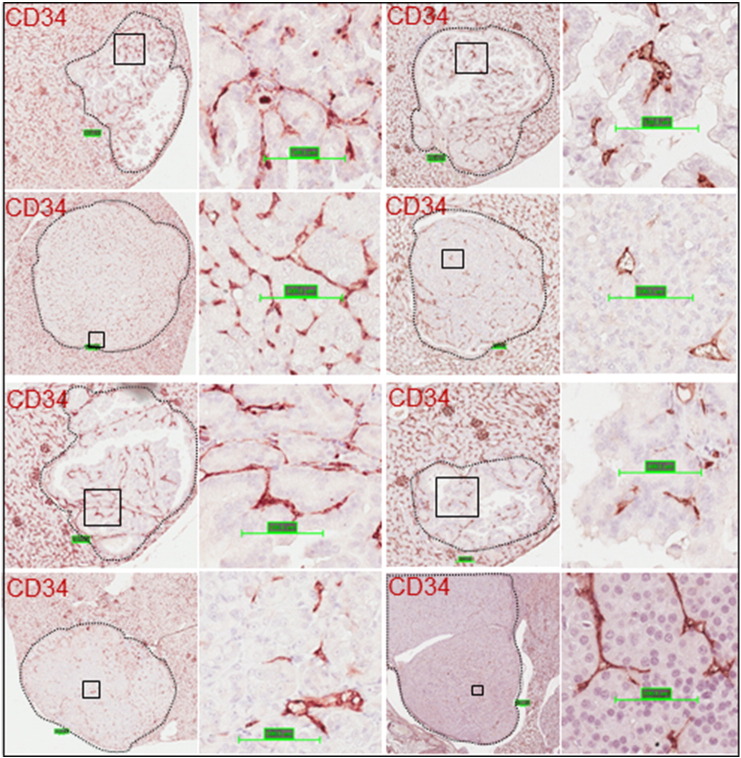
We investigated the expression of the drivers of tumor angiogenesis HIF1α and VEGFA in kidney sections by IHC. Consistent with activation of mTORC1, these proteins were highly expressed in these lesions as reported previously (Figure 1) [15]. RAF1 and Erk1/2 are positive regulators of tumor angiogenesis. As observed previously [12], RAF1 and Erk1/2 were highly phosphorylated in kidney lesions (Figure 2). We also investigated the expression of several receptor kinases that contribute to tumor angiogenesis using IHC. More VEGFR1 and PDGFRα but less VEGFR2 and PDGFRβ were detected in the lesions than in adjacent normal tissues (Figure 2). We used CD34 to assess angiogenesis and found that tumor angiogenesis in the kidneys of the Tsc2+/− mice was remarkably variable and showed no apparent association with lesion size or type (Figure 3).
Figure 1.
mTORC1 activation and angiogenesis signaling in renal lesions of Tsc2+/− mice.
Kidney sections prepared from 13-month-old Tsc2+/− mice were used for IHC analysis. Kidney sections were stained with antibodies against S6 p-S235/236, HIF1α, and VEGFA. Representative sections were shown demonstrating increased phosphorylation of S6 at S235/236 and increased expression of HIF1α and VEGFA in renal lesions including cysts (C), papillary adenomas (P) and solid tumors (S). Scale bars are 100.1 μm.
Figure 2.
MAPK signaling and expression of angiogenesis-contributing receptor kinases in renal lesions of Tsc2+/− mice.
Kidney sections prepared from 13-month-old Tsc2+/− mice were used for IHC analysis. Kidney sections were stained with antibodies against RAF1 p-S259 and Erk1/2 p-T202/Y204, VEGFR1, VEGFR2, PDGFRα, and PDGFRβ. Representative sections were shown demonstrating increased phosphorylation of RAF1 at S259 and Erk1/2 at T202/Y204, increased expression of VEGFR1 and PDGFRα, and diminished expression of VEGFR2 and PDGFRβ in renal lesions. Scale bars are 100.1 μm.
Figure 3.
Angiogenesis in renal lesions of Tsc2+/− mice.
Kidney sections prepared from 13-month-old Tsc2+/− mice were used for IHC analysis. Kidney sections were stained with antibody against CD34, an angiogenesis marker. Representative sections were shown demonstrating remarkable variation in angiogenesis in renal lesions. Scale bars are 100.1 μm.
Better Efficacy of Combination of Everolimus with Sorafenib than Everolimus Alone for Solid Renal Tumors in Tsc2+/− Mice
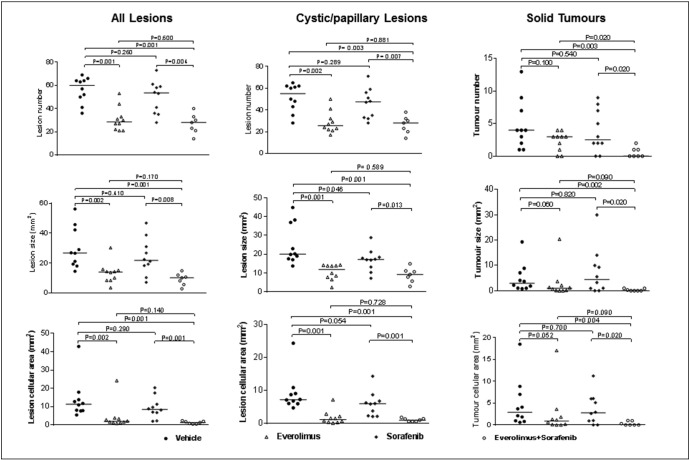
To test whether combination of everolimus with sorafenib could improve antitumor efficacy, Tsc2+/− mice were randomly allocated to 4 groups of 10 mice each: vehicle, everolimus, sorafenib, and everolimus plus sorafenib (Supplemental Table 1). We treated animals for 2 months from the age of 11 months. Three mice from the everolimus plus sorafenib group were euthanized due to significant loss of body weight within the first month of treatment and were excluded from further analysis in this study. We also noticed that two animals treated with everolimus plus sorafenib moved slowly and suffered from diarrhea with sore eyes in the last 2 weeks of treatment. Drug doses were then reduced from 6 to 3 mg/kg for everolimus and from 30 to 15 mg/kg for sorafenib to treat these two mice until the end of the experiment. When all renal lesions were analyzed, everolimus alone and everolimus plus sorafenib both significantly reduced lesion number, size, and cellular area (P ≤ .002). The reductions were slightly greater for the combination of everolimus with sorafenib than everolimus alone, but the difference was not statistically significant (Figure 4; Supplemental Table 2). Sorafenib alone appeared to reduce lesion number, size, and cellular area but not significantly when all lesions were analyzed (Figure 4; Supplemental Table 2). Similar results were obtained when cystic/papillary lesions were analyzed except that sorafenib alone slightly reduced lesion size (P = .046; Figure 4, Supplemental Table 3). For solid lesions, however, everolimus in combination with sorafenib significantly reduced total number, size, and cellular area (P ≤ .004), whereas everolimus or sorafenib alone did not (Figure 4; Supplemental Table 4).
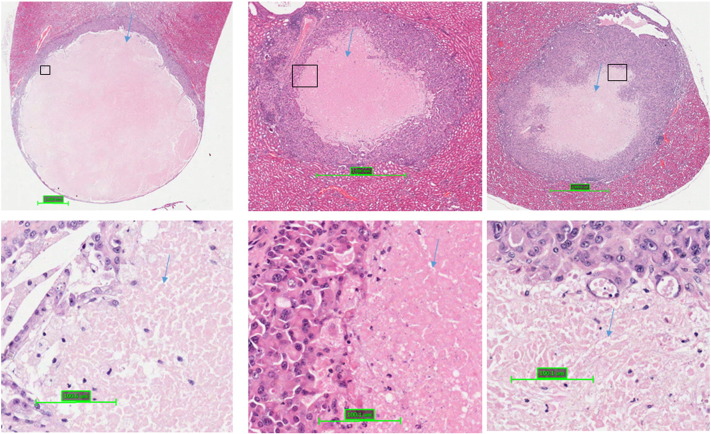
Supplemental Figure 1.
Massive cell death in solid renal tumors in Tsc2+/− mice treated with sorafenib. Kidney sections were prepared from 13-month-old Tsc2+/− mice treated with sorafenib and HE-stained. Representative solid tumors showing massive cell death as indicated by blue arrows.
Figure 4.
Treatment efficacy on renal lesions of Tsc2+/− mice.
Tsc2+/− mice were treated from 11 months old for 2 months (n = 10 each group). Mice were sacrificed for tumor burden assessment at the age of 13 months. Three mice from the everolimus plus sorafenib group were euthanized due to significant loss of body weight within the first month of treatment and excluded from further analysis in this study. Dosages are described in methods. Kidney sections were prepared for histological assessment of treatment efficacy. (Left panel) Comparison of total number and size (area) as well as cellular area of all lesions (cystic, papillary and solid). (Middle panel) Comparison of total number and size (area) as well as cellular area of cystic/papillary lesions. (Right panel) Comparison of total number and size (area) as well as cellular area of solid lesions. Horizontal bars indicate a median. Anucleate ghost cells (dead cells) were not included for calculating cellular area of any lesions. For detailed statistical analysis see Supplemental Figure 2, Supplemental Table 3, Supplemental Table 4.
Tsc2+/− mice were treated from 11 months old for 2 months (n = 10 each group). Mice were sacrificed for tumor burden assessment at the age of 13 months. Three mice from the everolimus plus sorafenib group were euthanized due to significant loss of body weight within the first month of treatment and excluded from further analysis in this study. Dosages are described in methods. Kidney sections were prepared for histological assessment of treatment efficacy. (Left panel) Comparison of total number and size (area) as well as cellular area of all lesions (cystic, papillary and solid). (Middle panel) Comparison of total number and size (area) as well as cellular area of cystic/papillary lesions. (Right panel) Comparison of total number and size (area) as well as cellular area of solid lesions. Horizontal bars indicate a median. Anucleate ghost cells (dead cells) were not included for calculating cellular area of any lesions. For detailed statistical analysis see Supplemental Tables 2, 3, and 4.
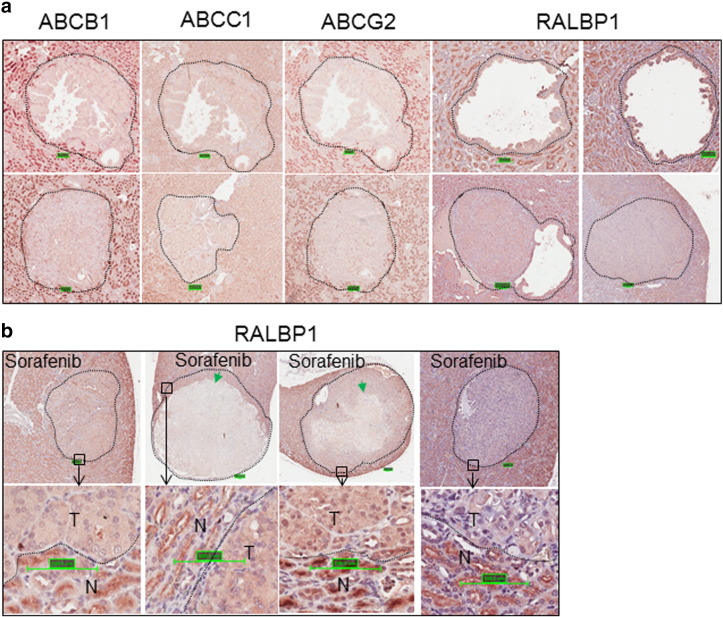
Supplemental Figure 2.
Expression of ABC transporters and RALBP1 in renal lesions of Tsc2+/− mice. Kidney sections from 13-month-old Tsc2+/− mice were prepared and stained by IHC with antibodies against ABCB1, ABCC1, ABCG2, and RALBP1. (A) Expression of ABC transporter and RALBP1 in untreated tumors. Representative kidney sections were presented to show suppressed expression of ABC transporters and variation in RALBP1 expression. (B) Expression of RALBP1 in sorafenib-treated solid tumors. Representative kidney sections were presented to show expression of RALBP1 in tumors (T) and normal tissues (N). Green arrows indicate massive cell death.
Effects of Everolimus or Sorafenib Alone and in Combination on Growth and Proliferation of Renal Tumor Cells from Tsc2+/− Mice
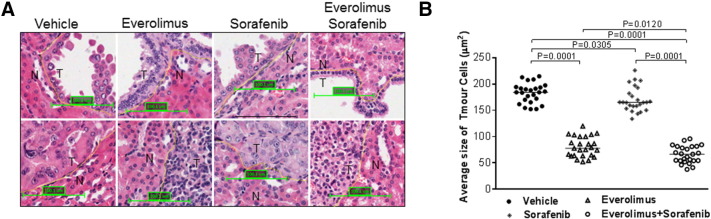
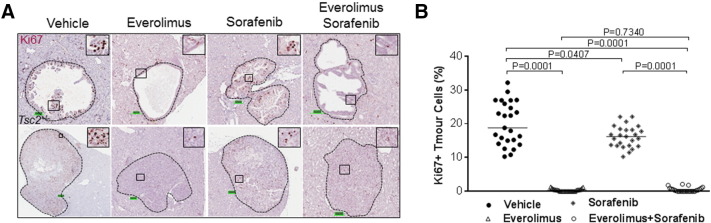
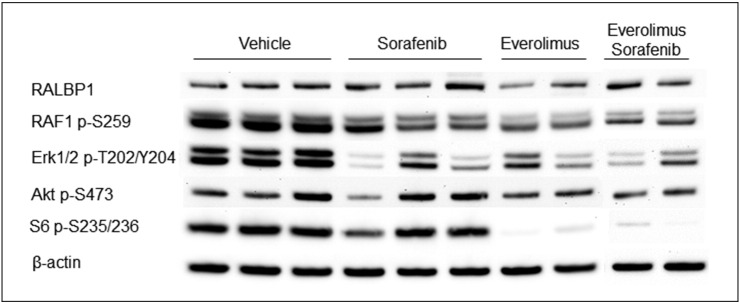
To assess effect of treatment on growth of tumor cells, average cell size was determined using HE-stained kidney sections. We also used IHC-stained Ki67 to determine the effect of treatment on proliferation of tumor cells. To determine average cell size and cell proliferation, 25 lesions were randomly chosen from each treatment group including 15 cysts and 10 papillary/solid lesions. At least 1000 cells from an area randomly chosen from each lesion or all cells from a lesion that had no more than 1000 cells were examined. We found that everolimus and everolimus plus sorafenib reduced the median cross-sectional area of tumor cells from 184.15 to 77.84 and 66.55 μm2, respectively (P = .0001) and also reduced median percentage of Ki67-positive tumor cells from 18.78% to 0.33% and 0.17%, respectively (P = .0001) (Figure 5, Figure 6; Supplemental Table 5). Sorafenib alone also reduced the median cross-sectional area of tumor cells (P = .0305) and median percentage of Ki67-positive tumor cells (P = .0407) but to a much smaller extent (Figure 5, Figure 6; Supplemental Table 5). These results were consistent with the decreased phosphorylation of RAF1 and Erk1/2 in tumors treated by everolimus, sorafenib, or both but decreased phosphorylation of S6 ribosomal protein only in tumors treated with everolimus or everolimus plus sorafenib as detected by Western blot analysis (Figure 7). No significant increase or decrease of phosphorylation of Akt was observed in everolimus-, sorafenib-, or everolimus plus sorafenib–treated tumors (Figure 7).
Figure 5.
Effect of treatment on size of renal tumor cells in Tsc2+/− mice.
Kidney sections were prepared from 13-month-old Tsc2+/− mice after treatment and HE stained for estimation of average size of tumor cells. (A) Tumor (T) and adjacent normal (N) cells. Representative sections were presented to show size of tumor cells after 2-month treatment with vehicle, everolimus, sorafenib, and everolimus plus sorafenib. Scale bars are 100.1 μm. (B) Average size of tumor cells. Twenty-five lesions randomly chosen from each group after 2-month treatment were used to estimate average size of tumor cells using ImageJ. Everolimus alone or everolimus plus sorafenib significantly reduced average size of tumor cells. Sorafenib alone significantly reduced average size of tumor cells but to a smaller extent.
Figure 6.
Effect of treatment on proliferation of renal tumor cells in Tsc2+/− mice.
Kidney sections were prepared from 13-month-old Tsc2+/− mice after treatment and stained with antibody against Ki67 by IHC to assess proliferation of tumor cells. (A) Expression of Ki67 in renal tumors. Representative sections were presented to show expression of Ki67 in tumor cells after 2-month treatment with vehicle, everolimus, sorafenib, and everolimus plus sorafenib. Scale bars are 100.1 μm. (B) Proliferation of tumor cells. Twenty-five lesions randomly chosen from each group after 2-month treatment were used to detect Ki67-positive tumor cells using ImageJ. Everolimus alone or everolimus plus sorafenib significantly inhibited proliferation of tumor cells. Sorafenib alone significantly inhibited proliferation of tumor cells but to a smaller extent.
Figure 7.
Effect of treatment on mTOR/MAPK signaling in renal lesions of Tsc2+/− mice.
Western blot was used to analyze mTOR/MAKP signaling in renal tumors. Proteins were prepared from solid renal tumors of Tsc2+/− mice treated for 1 month with vehicle, everolimus, sorafenib, and everolimus plus sorafenib, respectively. β-Actin was used as a loading control. Representative Western blots were presented to show that sorafenib, everolimus alone, or both attenuated phosphorylation of RAF1 and Erk1/2, and everolimus alone or everolimus plus sorafenib decreased phosphorylation of S6 but sorafenib alone did not in solid renal tumors.
Massive Cell Death Induced by Sorafenib in Large Solid Renal Tumors and RALBP1 Expression in Tsc2+/− Mice
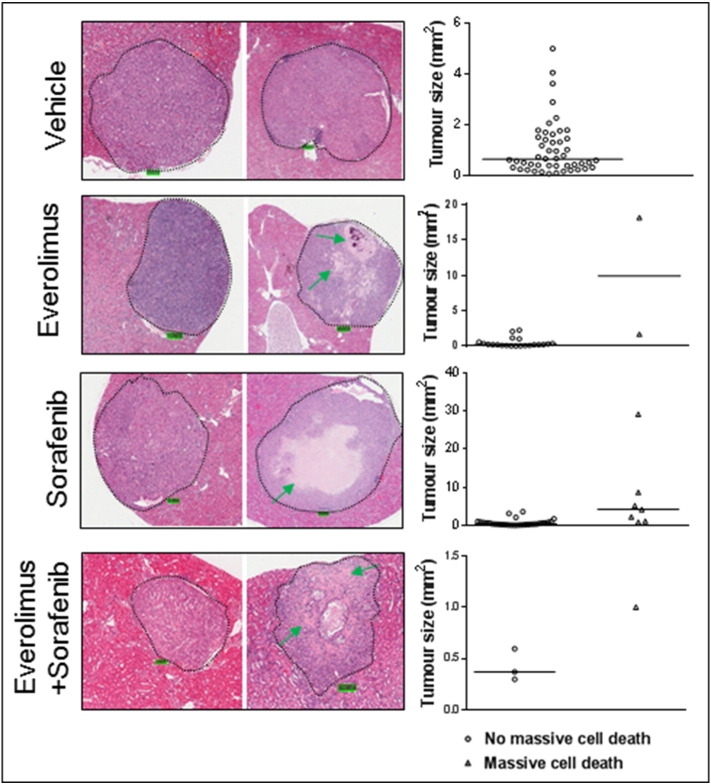
In some large solid tumors of the kidneys in Tsc2+/− mice treated by sorafenib, massive cell death was observed (Figure 8, Supplemental Figure 1). Everolimus or everolimus plus sorafenib also caused massive cell death but to a much smaller extent (Figure 8). The dying or dead cells exhibited features of anucleate ghost cells characteristic of necrosis [16]. Greatest cell death was found for the sorafenib-only group (Table 1) (P = .002). However, the solid tumor count was low in the combination therapy group, and it is unsurprising that although their results were comparable to those of the sorafenib only group, they did not reach statistical significance.
Figure 8.
Massive cell death in solid renal tumors of Tsc2+/− mice.
Kidney sections were prepared from 13-month-old Tsc2+/− mice after treatment and HE stained for examination of massive cell death in solid tumors. (Left panel) Representative solid tumors treated with vehicle, everolimus, sorafenib, and everolimus plus sorafenib. Green arrows indicate massive cell death. Scale bars are 100.1 μm. (Right panel) Solid tumors with or without massive cell death. All solid tumors detected were presented. Relatively large solid tumors treated with sorafenib showed massive cell death.
Table 1.
Massive Cell Death in Solid Renal Tumors of Tsc2+/− Mice after Treatment
| Treatment | Number of Solid Tumors Without Massive Cell Death | Number of Solid Tumors with Massive Cell Death | P⁎ (Compared with VEHICLE) | P⁎ (Compared with Sorafenib) |
|---|---|---|---|---|
| Vehicle | 48 | 0 | ||
| Everolimus | 21 | 2 | .102 | .462 |
| Sorafenib | 31 | 7 | .002 | |
| Everolimus + sorafenib | 3 | 1 | .077 | 1.000 |
Fisher's exact test for massive cell death in solid tumors.
To explore the possible mechanisms of massive cell death caused by sorafenib, we examined by IHC the expression of some ABC transporters and RALBP1 that was reported to be involved in sorafenib resistance in renal cancer [17]. We found that expression of RALBP1 was variable, whereas the ABC transporters were consistently suppressed in all untreated tumors examined (Supplemental Figure 2). We then investigated the protein level of RALBP1 in sorafenib-treated tumors. We did not find a consistent reduction of RALBP1 in sorafenib-treated tumors with massive cell death (Supplemental Figure 2).
Discussion
We investigated angiogenesis in renal lesions of Tsc2+/− mice and tested the antitumor efficacy of everolimus in combination with sorafenib on these lesions. We found that tumor angiogenesis is very variable, although HIF1 and VEGFA are consistently highly expressed in all lesions. Renal lesions, mainly, AMLs, in TSC patients are also very variable in vascularity. This variability may affect the response to therapy with angiogenesis inhibitors. We have shown that the combination therapy of everolimus with sorafenib represents an effective strategy to treat solid tumors, whereas everolimus alone is effective on cystic/papillary lesions in Tsc2+/− mice. This suggests that advanced lesions may be more dependent on tumor angiogenesis than early ones. In clinical settings, rapalogs alone have been used to treat renal AML in TSC patients [2], [3], [4]. Angiogenesis inhibitors and rapalogs have occasionally been used sequentially to treat TSC-associated RCC and epithelioid AML [5], [9]. No combined therapy has been reported in the treatment of TSC-associated renal lesions in patients. This study suggests that rapalogs in combination with multiple kinase inhibitors may improve therapeutic efficacy for TSC-associated solid tumors. The challenge of combination therapy is the increased toxicity as observed in this study and in sporadic RCC patients treated with both rapalogs and angiogenesis inhibitors together [18]. Lenvatinib is a kinase inhibitor targeting multiple kinases including VEGFR1, VEGFR2, and VEGFR3 and was first approved by the US Food and Drug Administration in 2015 for the treatment of locally recurrent or metastatic thyroid cancer [19]. Recently, combination therapy of lenvatinib with everolimus has been reported to significantly prolong progression-free survival in RCC with manageable toxicity and approved for the treatment of advanced RCC by the US Food and Drug Administration [20], [21]. Lenvatinib plus everolimus may also be a promising combination therapy for TSC patients with renal tumors that do not respond adequately to rapalogs and possibly for people with sporadic RCC containing TSC1 or TSC2 mutations.
We demonstrated that everolimus or everolimus plus sorafenib reduced tumor burden by dramatically shrinking tumor cell size and by preventing cell proliferation through inhibiting mTORC1 and the mitogen-activated protein kinase (MAPK) pathway. In contrast, sorafenib suppressed tumor cell growth and proliferation although to a smaller extent through inhibiting the MAPK pathway but not mTORC1. TSC-associated tumors are characterized by the presence of “giant” or grossly enlarged cells [22]. Our observations on the huge effect of mTOR inhibition on tumor cell size and proliferation suggest that much of the tumor response to mTOR inhibitors observed in the clinical setting, and the rapid regrowth of tumors on drug withdrawal, may be attributable to changes in tumor cell size as well as through effects on cell proliferation. We did not consistently see reduction or elevation of Akt phosphorylation by either everolimus or sorafenib or both. In some xenograft models of malignancy, everolimus plus sorafenib treatment reduces phosphorylation of Akt [23], [24]. This discrepancy may reflect the difference in tumor cell types and other factors such as tumor microenvironments.
We have found that sorafenib causes massive cell death with typical “ghost” cells in some large solid tumors [16]. Everolimus or everolimus plus sorafenib also causes massive cell death but to a much smaller extent. Necrosis and apoptosis in tumors caused by sorafenib have been documented in preclinical studies [25], but the mechanisms of sorafenib-induced massive tumor cell death are not fully understood. To better understand mechanisms of massive cell death caused by sorafenib, we examined the expression of drug transporters including ABCB1, ABCC1, ABCG2, and RALBP1. Protein levels of these transporters were remarkably reduced in renal tumors, although RALBP1 expression was variable. RALBP1 is suggested to be an efficient transporter of sorafenib, and its expression levels are correlated with drug resistance in patients with renal carcinoma [17]. However, in the current study, there was no obvious inverse correlation between expression of RALBP1 and massive cell death. Different levels of angiogenesis in these renal lesions may contribute to differences in massive cell death associated with sorafenib treatment. AMLs with large aneurysmal vessels cause most problems in TSC patients. Inhibition of mTORC1 reduces size of TSC-associated AMLs and prevents hemorrhage, but tumors regrow after drug withdrawal. Angiogenesis inhibitors or combination of rapalogs with angiogenesis inhibitors might prevent tumor regrowth by causing similar massive cell death in highly vascularized tumors, and this approach warrants further investigations in both preclinical and clinical settings.
We conclude that everolimus in combination with sorafenib is superior to everolimus alone for treating solid tumors in the kidneys of Tsc2+/− mice and that new agents for combination therapy with less toxicity may significantly improve therapy for TSC-associated solid tumors.
The following are the supplementary data related to this article.
Summary of Animal Treatment
Comparison of Tumor Burden in Tsc2+/− Mice by Histological Analysis (Wilcoxon Rank-Sum Test)
Comparison of Cystic/Papillary Lesions in Tsc2+/− Mice by Histological Analysis (Wilcoxon Rank-Sum Test)
Comparison of Solid Tumors in Tsc2+/− Mice by Histological Analysis (Wilcoxon Rank-Sum Test)
Effect of Treatment on Size and Proliferation of Renal Tumor Cells in Tsc2+/− Mice (Wilcoxon Rank-Sum Test)
Conflict of Interest Statement
The authors declare no conflict of interest.
Acknowledgements
We would like to thank Dr. David Kwiatkowski for providing the Tsc2+/− mouse model. Everolimus was provided by Novartis and Sorafenib by Bayer. This project was supported by the Wales Gene Park, UK, and the Tuberous Sclerosis Association, UK.
Footnotes
Financial support: This project was supported by the Wales Gene Park and the Tuberous Sclerosis Association.
References
- 1.Yang P, Cornejo KM, Sadow PM, Cheng L, Wang M, Xiao Y, Jiang Z, Oliva E, Jozwiak S, Nussbaum RL. Renal cell carcinoma in tuberous sclerosis complex. Am J Surg Pathol. 2014;38:895–909. doi: 10.1097/PAS.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, Schmithorst VJ, Laor T, Brody AS, Bean J. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies DM, Johnson SR, Tattersfield AE, Kingswood JC, Cox JA, McCartney DL, Doyle T, Elmslie F, Saggar A, de Vries PJ. Sirolimus therapy in tuberous sclerosis or sporadic lymphangioleiomyomatosis. N Engl J Med. 2008;358:200–203. doi: 10.1056/NEJMc072500. [DOI] [PubMed] [Google Scholar]
- 4.Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, Sauter M, Nonomura N, Brakemeier S, de Vries PJ. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2013;381:817–824. doi: 10.1016/S0140-6736(12)61767-X. [DOI] [PubMed] [Google Scholar]
- 5.Kim HS, Kim ST, Kang SH, Sung DJ, Kim CH, Shin SW, Kim YH, Cho WY, Park KH. The use of everolimus to target carcinogenic pathways in a patient with renal cell carcinoma and tuberous sclerosis complex: a case report. J Med Case Rep. 2014;8:95. doi: 10.1186/1752-1947-8-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arbiser JL, Brat D, Hunter S, D'Armiento J, Henske EP, Arbiser ZK, Bai X, Goldberg G, Cohen C, Weiss SW. Tuberous sclerosis-associated lesions of the kidney, brain, and skin are angiogenic neoplasms. J Am Acad Dermatol. 2002;46:376–380. doi: 10.1067/mjd.2002.120530. [DOI] [PubMed] [Google Scholar]
- 7.Siroky BJ, Yin H, Dixon BP, Reichert RJ, Hellmann AR, Ramkumar T, Tsuchihashi Z, Bunni M, Dillon J, Bell PD. Evidence for pericyte origin of TSC-associated renal angiomyolipomas and implications for angiotensin receptor inhibition therapy. Am J Physiol Renal Physiol. 2014;307:F560–F570. doi: 10.1152/ajprenal.00569.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DW, Chang H, Kim YJ, Kim KM, Lee HJ, Lee JS. Sorafenib induced tumour response in a patient with metastatic epithelioid angiomyolipoma. J Clin Oncol. 2014;32:e42–e45. doi: 10.1200/JCO.2012.48.1960. [DOI] [PubMed] [Google Scholar]
- 9.Citak EC, Yilmaz EB, Yaman E, Kaya S, Taskinlar H, Arpaci RB, Apaydin D. Malignant epitheloid angiomyolipoma of the kidney in a child treated with sunitinib, everolimus and axitinib. Can Urol Assoc J. 2015;9:E542–E545. doi: 10.5489/cuaj.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson C, Idziaszczyk S, Parry L, Guy C, Griffiths DF, Lazda E, Bayne RA, Smith AJ, Sampson JR, Cheadle JP. A mouse model of tuberous sclerosis 1 showing background specific early post-natal mortality and metastatic renal cell carcinoma. Hum Mol Genet. 2005;14:1839–1850. doi: 10.1093/hmg/ddi190. [DOI] [PubMed] [Google Scholar]
- 11.Onda H, Lueck A, Marks PW, Warren HB, Kwiatkowski DJ. Tsc2(+/-) mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. J Clin Invest. 1999;104:687–695. doi: 10.1172/JCI7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Kalogerou M, Samsel PA, Zhang Y, Griffiths DF, Gallacher J, Sampson JR, Shen MH. Renal tumours in a Tsc2(+/-) mouse model do not show feedback inhibition of Akt and are effectively prevented by rapamycin. Oncogene. 2015;34:922–931. doi: 10.1038/onc.2014.17. [DOI] [PubMed] [Google Scholar]
- 13.El-Hashemite N, Walker V, Zhang H, Kwiatkowski DJ. Loss of Tsc1 or Tsc2 induces vascular endothelial growth factor production through mammalian target of rapamycin. Cancer Res. 2003;63:5173–5177. [PubMed] [Google Scholar]
- 14.Kalogerou M, Zhang Y, Yang J, Garrahan N, Paisey S, Tokarczuk P, Stewart A, Gallacher J, Sampson JR, Shen MH. T2 weighted MRI for assessing renal lesions in transgenic mouse models of tuberous sclerosis. Eur J Radiol. 2012;81:2069–2074. doi: 10.1016/j.ejrad.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 15.Dodd KM, Yang J, Shen MH, Sampson JR, Tee AR. mTORC1 drives HIF-1α and VEGF-A signalling via multiple mechanisms involving 4E-BP1,S6K1 and STAT3. Oncogene. 2015;34:2239–2250. doi: 10.1038/onc.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gkogkou C, Frangia K, Saif MW, Trigidou R, Syrigos K. Necrosis and apoptotic index as prognostic factors in non-small cell lung carcinoma: a review. Springerplus. 2014;3:120. doi: 10.1186/2193-1801-3-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singhal SS, Sehrawat A, Sahu M, Singhal P, Vatsyayan R, Rao Lelsani PC, Yadav S, Awasthi S. Rlip76 transports sunitinib and sorafenib and mediates drug resistance in kidney cancer. Int J Cancer. 2010;126:1327–1338. doi: 10.1002/ijc.24767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buonerba C, Di Lorenzo G, Sonpavde G. Combination therapy for metastatic renal cell carcinoma. Ann Transl Med. 2016;4:100. doi: 10.21037/atm.2016.01.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frampton JE. Lenvatinib: A Review in Refractory Thyroid Cancer. Target Oncol. 2016;11:115–122. doi: 10.1007/s11523-015-0416-3. [DOI] [PubMed] [Google Scholar]
- 20.Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T, Jassem J, Zolnierek J, Maroto JP, Mellado B. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised,phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16:1473–1482. doi: 10.1016/S1470-2045(15)00290-9. [DOI] [PubMed] [Google Scholar]
- 21.Motzer RJ, Hutson TE, Ren M, Dutcus C, Larkin J. Independent assessment of lenvatinib plus everolimus in patients with metastatic renal cell carcinoma. Lancet Oncol. 2016;17:e4–e5. doi: 10.1016/S1470-2045(15)00543-4. [DOI] [PubMed] [Google Scholar]
- 22.Kwiatkowski DJ, Manning BD. Molecular basis of giant cells in tuberous sclerosis complex. N Engl J Med. 2014;371:778–780. doi: 10.1056/NEJMcibr1406613. [DOI] [PubMed] [Google Scholar]
- 23.Gulhati P, Zaytseva YY, Valentino JD, Stevens PD, Kim JT, Sasazuki T, Shirasawa S, Lee EY, Weiss HL, Dong J. Sorafenib enhances the therapeutic efficacy of rapamycin in colorectal cancers harboring oncogenic KRAS and PIK3CA. Carcinogenesis. 2012;33:1782–1790. doi: 10.1093/carcin/bgs203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pignochino Y, Dell'Aglio C, Basiricò M, Capozzi F, Soster M, Marchiò S, Bruno S, Gammaitoni L, Sangiolo D, Torchiaro E. The Combination of Sorafenib and Everolimus Abrogates mTORC1 and mTORC2 upregulation in osteosarcoma preclinical models. Clin Cancer Res. 2013;19:2117–2131. doi: 10.1158/1078-0432.CCR-12-2293. [DOI] [PubMed] [Google Scholar]
- 25.Murphy DA, Makonnen S, Lassoued W, Feldman MD, Carter C, Lee WM. Inhibition of tumor endothelial ERK activation, angiogenesis, and tumor growth by sorafenib(BAY43-9006) Am J Pathol. 2006;169:1875–1885. doi: 10.2353/ajpath.2006.050711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of Animal Treatment
Comparison of Tumor Burden in Tsc2+/− Mice by Histological Analysis (Wilcoxon Rank-Sum Test)
Comparison of Cystic/Papillary Lesions in Tsc2+/− Mice by Histological Analysis (Wilcoxon Rank-Sum Test)
Comparison of Solid Tumors in Tsc2+/− Mice by Histological Analysis (Wilcoxon Rank-Sum Test)
Effect of Treatment on Size and Proliferation of Renal Tumor Cells in Tsc2+/− Mice (Wilcoxon Rank-Sum Test)