Abstract
Transfer RNAs (tRNAs) are fundamental adapter components of translational machinery. tRNAs can further serve as a source of tRNA-derived noncoding RNAs that play important roles in various biological processes beyond translation. Among all species of tRNAs, tRNAHisGUG has been known to uniquely contain an additional guanosine residue at the −1 position (G−1) of its 5′-end. To analyze this −1 nucleotide in detail, we developed a TaqMan qRT-PCR method that can distinctively quantify human mature cytoplasmic tRNAHisGUG containing G−1, U−1, A−1, or C−1 or lacking the −1 nucleotide (starting from G1). Application of this method to the mature tRNA fraction of BT-474 breast cancer cells revealed the presence of tRNAHisGUG containing U−1 as well as the one containing G−1. Moreover, tRNA lacking the −1 nucleotide was also detected, thus indicating the heterogeneous expression of 5′-tRNAHisGUG variants. A sequence library of sex hormone-induced 5′-tRNA halves (5′-SHOT-RNAs), identified via cP-RNA-seq of a BT-474 small RNA fraction, also demonstrated the expression of 5′-tRNAHisGUG halves containing G−1, U−1, or G1 as 5′-terminal nucleotides. Although the detected 5′-nucleotide species were identical, the relative abundances differed widely between mature tRNA and 5′-half from the same BT-474 cells. The majority of mature tRNAs contained the −1 nucleotide, whereas the majority of 5′-halves lacked this nucleotide, which was biochemically confirmed using a primer extension assay. These results reveal the novel identities of tRNAHisGUG molecules and provide insights into tRNAHisGUG maturation and the regulation of tRNA half production.
Keywords: tRNA, tRNAHisGUG, tRNA half, SHOT-RNA, −1 nucleotide
INTRODUCTION
Transfer RNAs (tRNAs) are noncoding RNAs (ncRNAs) with lengths of 60- to 90-nucleotide (nt) that play central roles as adapter molecules in the translational machinery. Although tRNA molecules are stable and abundant, the expression profiles of individual tRNAs vary dynamically among different cells and tissues (Dittmar et al. 2006; Pavon-Eternod et al. 2009; Zhou et al. 2009; Mahlab et al. 2012) and this variation has been implicated in the translational regulation of mRNA expression (Gingold et al. 2014), animal development (Marshall et al. 2012; Rideout et al. 2012; Schmitt et al. 2014), and disease (Daly et al. 2005; Pavon-Eternod et al. 2010; Zhou et al. 2012; Clarke et al. 2016). Accumulating evidence regarding tRNA-derived ncRNAs has further increased the complexity of tRNA biology. In many organisms, tRNAs are not always end products but are processed further into smaller ncRNAs, many of which are known to be functional molecules with roles in various biological processes beyond translation (Garcia-Silva et al. 2012; Gebetsberger and Polacek 2013; Anderson and Ivanov 2014; Saikia and Hatzoglou 2015; Shigematsu and Kirino 2015; Telonis et al. 2015; Diebel et al. 2016). These tRNA-derived ncRNAs are in general classified into two groups: tRNA halves that range either from the 5′-end to the anticodon loop (5′-half) or from the anticodon loop to the 3′-end (3′-half) of a mature tRNA, and shorter tRNA-derived fragments (tRFs) that originate from various regions of mature tRNAs or their precursor transcripts (pre-tRNAs).
To date, two distinct classes of tRNA halves have been identified: tRNA-derived stress-induced RNAs (tiRNAs) (Thompson et al. 2008; Fu et al. 2009; Hsieh et al. 2009; Yamasaki et al. 2009; Saikia et al. 2012) and sex hormone-dependent tRNA-derived RNAs (SHOT-RNAs) (Honda et al. 2015). Although both tiRNAs and SHOT-RNAs are produced from mature tRNAs via angiogenin (ANG)-mediated cleavage of the anticodon loop (Fu et al. 2009; Yamasaki et al. 2009; Honda et al. 2015), the molecular factors that trigger their production are different. The expression of tiRNAs is triggered by a variety of stress stimuli, including oxidative stress, heat/cold shock, and UV irradiation (Shigematsu et al. 2014; Saikia and Hatzoglou 2015). The accumulation of tiRNAs has been implicated in stress granule formation (Emara et al. 2010; Lyons et al. 2016), translational regulation (Yamasaki et al. 2009; Ivanov et al. 2011), and the pathogenesis of neurodevelopmental disorders (Blanco et al. 2014). In contrast, the expression of SHOT-RNAs is promoted by signaling pathways associated with sex hormones (e.g., estrogen and androgen) and their receptors (e.g., estrogen receptor [ER] and androgen receptor [AR]). SHOT-RNAs are specifically expressed in ER- or AR-positive breast and prostate cancers and have functional significance in cell proliferation (Honda et al. 2015).
Because ANG leaves a 2′,3′-cyclic phosphate (cP) on its 5′-cleavage products (Shapiro et al. 1986), ANG-generated 5′-tRNA halves contain a cP at the 3′-end (Honda et al. 2015). These cP-containing RNAs cannot be captured accurately by standard RNA-seq methods because they are not ligated to a 3′-adapter during library preparation procedure. We circumvented the issue by developing a cP-RNA-seq method that can exclusively sequence cP-containing RNAs (Honda et al. 2015, 2016) and used this method to determine the expression repertoire of 5′-SHOT-RNAs (5′-tRNA halves) in human BT-474 ER-positive breast cancer cells; accordingly, we identified eight cytoplasmic (cyto) tRNA species as the major sources of SHOT-RNAs (Honda et al. 2015). Although 5′-SHOT-RNAs from cyto tRNALysCUU and tRNAHisGUG were particularly enriched, the molecular mechanism by which specific tRNAs are selectively cleaved for SHOT-RNA production remains elusive.
Among all species of tRNAs, tRNAHisGUG is unique in that it contains an additional guanosine residue at the −1 position (G−1) of its 5′-end (Sprinzl et al. 1998). This G−1 residue is conserved across phyla and has been observed in bacteria (Singer and Smith 1972; Orellana et al. 1986), yeast (Keith and Pixa 1984), fruit fly (Altwegg and Kubli 1980), and mammals (Boisnard and Petrissant 1981; Rosa et al. 1983). In Escherichia coli, this G−1 residue is genome-encoded, and anomalous RNase P cleavage of pre-tRNAHisGUG between positions −1 and −2 yields G−1-containing tRNAHisGUG (Orellana et al. 1986; Burkard et al. 1988). In yeast, G−1 is not derived from the genomic sequence; instead, tRNAHis guanylyltransferase (Thg1) post-transcriptionally adds this residue to the 5′-end (Gu et al. 2003). The conservation of G−1 residue addition via different mechanisms in different organisms implies the functional significance of the G−1 residue. Indeed, the G−1 residue is a critical determinant for the aminoacylation of tRNAHisGUG by the cognate histidyl-tRNA synthetase (HisRS) in both E. coli (Himeno et al. 1989) and yeast (Rudinger et al. 1994; Rosen et al. 2006). In yeast, the loss of this G−1 residue consequent to the depletion of Thg1 or its polymerase activity causes a severe reduction in the tRNAHisGUG aminoacylation levels, resulting in growth impairment (Gu et al. 2005; Jackman and Phizicky 2008; Preston and Phizicky 2010). The G−1 residue is also implicated in post-transcriptional nucleotide modification because yeast lacking this residue has been shown to acquire additional 5-methylcytidine (m5C) modifications (Gu et al. 2005; Preston et al. 2013), although a biological role for the interplay between the absence of G−1 and the presence of m5C is unknown. In contrast to the presence and significance of G−1 in these studies, some organisms such as α-proteobacteria (Wang et al. 2007; Jackman et al. 2012), Acanthamoeba (Rao et al. 2013), and Trypanosoma (Rao and Jackman 2015) lack G−1. HisRS does not require G−1 for aminoacylation in these species.
Despite the advent of next-generation sequencing (NGS) technologies and their widespread use in RNA-seq for transcriptome analyses, the −1 nucleotide of tRNAHisGUG has not been investigated in RNA-seq-based studies. This could be partly attributable to the expectation that tRNAHisGUG contains post-transcriptional modifications that would interfere with reverse transcription (Kellner et al. 2010), such as a 1-methyl-guanosine at nucleotide position 37 (m1G37) (Boisnard and Petrissant 1981) (nucleotide position [np] is based on the tRNA numbering system [Sprinzl et al. 1998]). Indeed, human and Bombyx 3′-haves of cyto tRNAHisGUG, possessing G37 (likely modified to m1G37), could not be amplified by RT-PCR despite being successfully detected by Northern blot (Honda et al. 2015). The presence of the RT-interfering modification would lead to underrepresentation and bias in the tRNAHisGUG sequence information generated from RNA-seq data. While analyzing a cP-RNA-seq library of 5′-SHOT-RNAs from BT-474 cells (Honda et al. 2015), we reasoned that this library would be useful for observing the −1 nucleotide on human cyto tRNAHisGUG for the following reasons. First, 5′-SHOT-RNAHisGUG (5′-half of cyto tRNAHisGUG) does not contain RT-inhibitory modifications, and therefore sequence analyses should not be biased by modifications. Second, 5′-SHOT-RNAHisGUG is produced from mature aminoacylated cyto tRNAHisGUG, and therefore information about the −1 nucleotide in mature tRNA might be retained in this 5′-half. Third, 5′-SHOT-RNAHisGUG was the second most abundant species in the 5′-SHOT-RNA library from BT-474 cells, and thereby sufficient sequence reads of the 5′-half are available for an estimation of the −1 nucleotide state.
In this study, we investigated the 5′-terminal nucleotides of 5′-SHOT-RNAHisGUG expressed in human BT-474 cells and observed an unexpected level of variation that was not limited to G−1. Furthermore, we developed a TaqMan qRT-PCR-based method that could distinctively quantify each tRNA variant containing a different 5′-terminal nucleotide and thus clarified a 5′-terminal nucleotide variation of the mature cyto tRNAHisGUG expressed in the same BT-474 cells. This identification and comparison of the 5′-terminal nucleotides and their variations among mature cyto tRNAHisGUG and 5′-half molecules have yielded insights into tRNAHisGUG identities and the regulatory mechanisms underlying tRNAHisGUG maturation and cleavage.
RESULTS AND DISCUSSION
The majority of 5′-SHOT-RNAHisGUG molecules lack the −1 nucleotide
The human nuclear genome contains 11 cyto tRNAHisGUG genes that encode three different isodecoders (Supplemental Fig. S1). In the 5′-SHOT-RNA sequence repertoire of BT-474 cells, which was previously identified using cP-RNA-seq (Honda et al. 2015, 2016), 5′-SHOT-RNAHisGUG sequences constituted approximately 7.9 million reads, comprising 27.5% of the total reads of 5′-SHOT-RNAs. Accordingly, 5′-SHOT-RNAHisGUG is the second most abundant 5′-SHOT-RNA species in BT-474 cells, after 5′-SHOT-RNALysCUU. Almost all of the identified 5′-SHOT-RNAHisGUG sequences corresponded to a single major isodecoder of cyto tRNAHisGUG (Fig. 1A) that is encoded by nine of the 11 genes (Supplemental Fig. S1), suggesting that this isodecoder is the major cyto tRNAHisGUG molecule expressed in the cells. Almost all (98%) of the 5′-SHOT-RNAHisGUG had a 3′-terminal position at np 34 (Fig. 1B), indicating a focal pattern of ANG cleavage between the anticodon first (G34) and second (U35) letters during the production of 5′-SHOT-RNAHisGUG. In contrast to the consistent 3′-termini, six 5′-terminal variations were observed among the sequenced 5′-SHOT-RNAHisGUG (Fig. 1C). In contrast to previous reports of a major presence of the 5′-terminal G−1 in tRNAHisGUG, a majority (>75%) of 5′-SHOT-RNAHisGUG lacked the −1 nucleotide and initiated at np 1 (G1) (Fig. 1D). The second most abundant class (>12%) of 5′-SHOT-RNAHisGUG contained the −1 nucleotide; here, both guanosine (G−1: 44.3%) and uridine (U−1: 44.2%) were frequently present at the −1 nucleotide. The other two nucleotides (A−1: 9%; C−1: 3%) were also detected as minor −1 nucleotide species, and a 5′-SHOT-RNAHisGUG initiating from np 2 (C2: 11%) was also identified.
FIGURE 1.
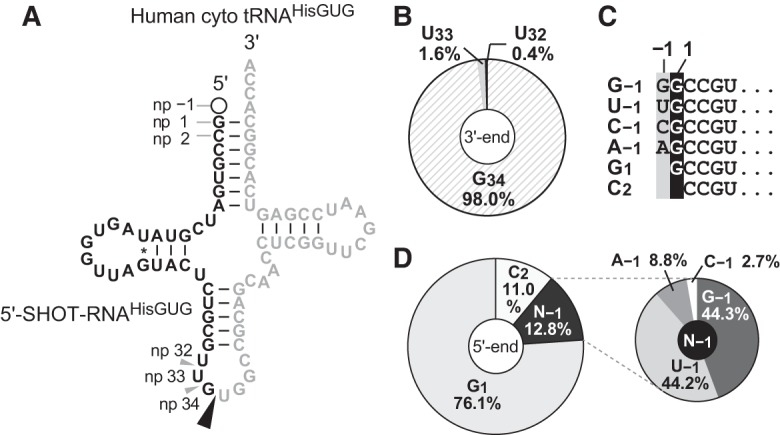
Terminal nucleotide analyses of BT-474 5′-SHOT-RNAHisGUG identified by cP-RNA-seq. (A) The cloverleaf secondary structure of the major isodecoder of human cyto tRNAHisGUG encoded by nine genes (Supplemental Fig. S1) on the genome. Nucleotide positions (np) are indicated according to the tRNA nucleotide numbering systems (Sprinzl et al. 1998). The ANG-cleavage sites for SHOT-RNA production, predicted by the 3′-terminal position of 5′-SHOT-RNAHisGUG, are indicated by arrowheads. Regions from which 5′-SHOT-RNAHisGUG molecules were derived are shown in black; other regions are shown in gray. (B) Pie chart indicating the 3′-terminal position of 5′-SHOT-RNAHisGUG. (C) The six 5′-terminal variations identified in 5′-SHOT-RNAHisGUG. (D) Pie charts showing the 5′-terminal positions of 5′-SHOT-RNAHisGUG.
Experimental validation of the predominant expression of 5′-SHOT-RNAHisGUG lacking the −1 nucleotide
Because our cP-RNA-seq scheme includes several chemical and enzymatic RNA treatments (Honda et al. 2015, 2016), unexpected variations of the 5′-termini of 5′-SHOT-RNAHisGUG might have resulted from undesired procedural RNA damage. To exclude this possibility and confirm that our cP-RNA-seq results reflect the cellular state of RNA expression, we conducted a primer extension assay for 5′-SHOT-RNAHisGUG. In this assay, a radiolabeled DNA primer complementary to np 6−25 of cyto tRNAHisGUG (Fig. 2A) was specifically hybridized to 5′-SHOT-RNAHisGUG present in gel-purified small RNA fractions (20−50 nt) from BT-474 cells; subsequently, reverse transcription was carried out from the primer. When using synthetic tRNAHisGUG initiating from G1 as a template, the 5-nt primer extension was detected as a 25-nt band (Fig. 2B). In contrast, the use of synthetic tRNAHisGUG containing G−1 yielded an additional extension of 1 nt and a 26-nt band that was clearly distinct from the above-mentioned 25-nt band. An equal mix of these two synthetic tRNAs yielded two bands of equal abundance, indicating the ability of this assay to estimate the presence or absence of the −1 nucleotide. By performing reactions using dideoxynucleotides, we confirmed that the reverse transcription was correctly run on tRNAHisGUG in both synthetic RNA and cellular RNA samples (Supplemental Fig. S2). Analyses of BT-474 small RNA fractions revealed the marked and more abundant presence of the 25-nt band in comparison with the 26-nt band (Fig. 2B). Quantification of the band intensities suggested that ∼70% of the 5′-SHOT-RNAHisGUG lacks the −1 nucleotide (Supplemental Fig. S3), which is consistent with the cP-RNA-seq-based analyses shown in Figure 1. These results indicate that the majority of the 5′-SHOT-RNAHisGUG molecules expressed in BT-474 cells lack the −1 nucleotide and initiate from G1. In the primer extension assay, we did not observe a clear 24-nt band corresponding to 5′-SHOT-RNAHisGUG initiating from C2; therefore, the presence of such RNA in cP-RNA-seq data might result from undesired procedural RNA damage.
FIGURE 2.
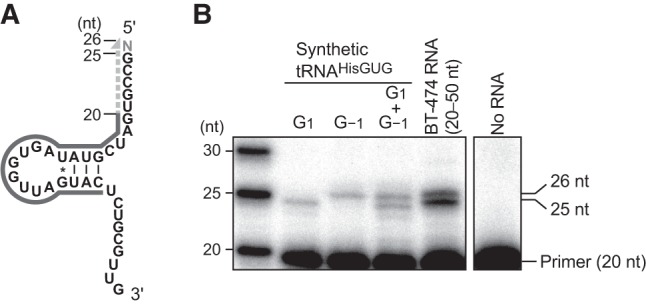
Primer extension assay to determine the 5′-terminal position of 5′-SHOT-RNAHisGUG. (A) The cloverleaf secondary structure of 5′-SHOT-RNAHisGUG used as a primer extension template. The 5′-end-labeled 20-nt primer, which was hybridized to the D-arm of tRNA, is shown as a black solid line; nascent cDNA synthesized from the primer is indicated as a gray dotted line. Reverse transcription from the primer terminates at np 1 or −1 to yield a cDNA band with a length of 25 or 26 nt, respectively. (B) Synthetic mature tRNAHisGUG containing either G1 or G−1, or a 30- to 50-nt small RNA fraction of BT-474 cells were subjected to a primer extension assay for an analysis of the 5′-terminal position of 5′-SHOT-RNAHisGUG. An assay without template RNA was also performed as a negative control experiment.
Development of a TaqMan qRT-PCR-based method for the discriminative quantification of 5′-terminal variants of mature cyto tRNAHisGUG
SHOT-RNAs originate from mature aminoacylated tRNAs (Honda et al. 2015); accordingly, we reasoned that the 5′-terminal variations of 5′-SHOT-RNAHisGUG would mirror those of mature cyto tRNAHisGUG, although these variations did not match the canonical, reported variations in mature tRNA. Because RNA-seq data are not appropriate for analyses of mature tRNAHisGUG sequences, we developed a TaqMan qRT-PCR-based method that can discriminatively quantify each 5′-terminal variant of mature cyto tRNAHisGUG containing G−1, U−1, A−1, C−1, or G1 as the 5′-terminal nucleotide. We focused on a single major isodecoder encoded by nine of 11 genes in the human genome (Supplemental Fig. S1).
In the developed method, mature tRNA fractions (70−90 nt) were first gel-purified from total RNA, after which an acceptor-stem disrupter (AS-disrupter), a DNA oligo complementary to np 55−76 (3′-end) of the cyto tRNAHisGUG, was hybridized to the purified fractions (Fig. 3A,B; Supplemental Fig. S4). Subsequently, a DNA/RNA chimeric 5′-adapter was ligated to the 5′-ends of mature tRNAHisGUG, and the ligation product was amplified and quantified by TaqMan qRT-PCR to eventually generate an 86- (tRNA starting from G1) or 87-bp cDNA (tRNA containing −1 nucleotide). The AS-disrupter was utilized to disrupt mature tRNAHisGUG structure; this disruption was expected to enhance the accessibility of the adapter, primer, and enzymes to the tRNA and thereby increase the reaction efficiencies following hybridization. In addition, dimethyl sulfoxide (DMSO) and polyethylene glycol (PEG) 8000, both of which enhance RNA ligation efficiency, were added to the adapter ligation reaction. Indeed, the combined inclusion of the AS-disrupter hybridization step and addition of PEG/DMSO to the ligation reaction increased the detection efficiency of synthetic tRNAHisGUG with G−1 by more than 95-fold and prevented the synthesis of nonspecific cDNA bands (Fig. 3C). The TaqMan probe was designed to target the boundary of the adapter and the 5′-end of mature tRNAHisGUG, thus allowing an exclusive analysis of the tRNA 5′-end in the ligation product. Indeed, we were unable to detect an amplification signal in the absence of T4 RNA ligase (Fig. 3C). Because the TaqMan probe has a single-nucleotide resolution (Ranade et al. 2001; Honda and Kirino 2015), our design scheme was expected to distinctively quantify each 5′-terminal variant of tRNAHisGUG without cross-reactivity with other variant species. We confirmed the exclusive specificity of our TaqMan probes to quantify perfectly matched target sequences without cross-reactivity from other variants (Supplemental Table S1). To examine the quantification ability, our method was applied to different amounts of synthetic mature tRNAHisGUG (0.1–100 fmol). To mimic tRNA quantification using a total tRNA fraction, an E. coli tRNA fraction was mixed with synthetic RNA as a carrier; we confirmed that the E. coli tRNA fraction did not yield an amplification signal in our system. For all five synthetic 5′-terminal variants, the quantifications demonstrated clear linearity between the log of tRNA input and the Ct value (Supplemental Fig. S5), indicating that this method has a dynamic range of at least three orders of magnitude, and discriminately quantifies the 5′-terminal variants. We further validated our method by quantifying a mixture of different synthetic tRNA variants. Since 5′-SHOT-RNAsHisGUG starting from G1, G−1, and U−1 were mainly detected (Fig. 1D), corresponding mature synthetic tRNAsHisGUG were mixed at several different ratios and subjected to the method with an E. coli tRNA carrier. The amount of each detected tRNA was calculated based on the standard curves (Supplemental Fig. S5). As shown in Figure 3D, the resultant relative abundances of detected tRNAs well reflected those of the tRNAs added to the reactions, allowing us to conclude that our method can estimate the relative abundance of 5′-terminal variants of mature tRNAHisGUG.
FIGURE 3.
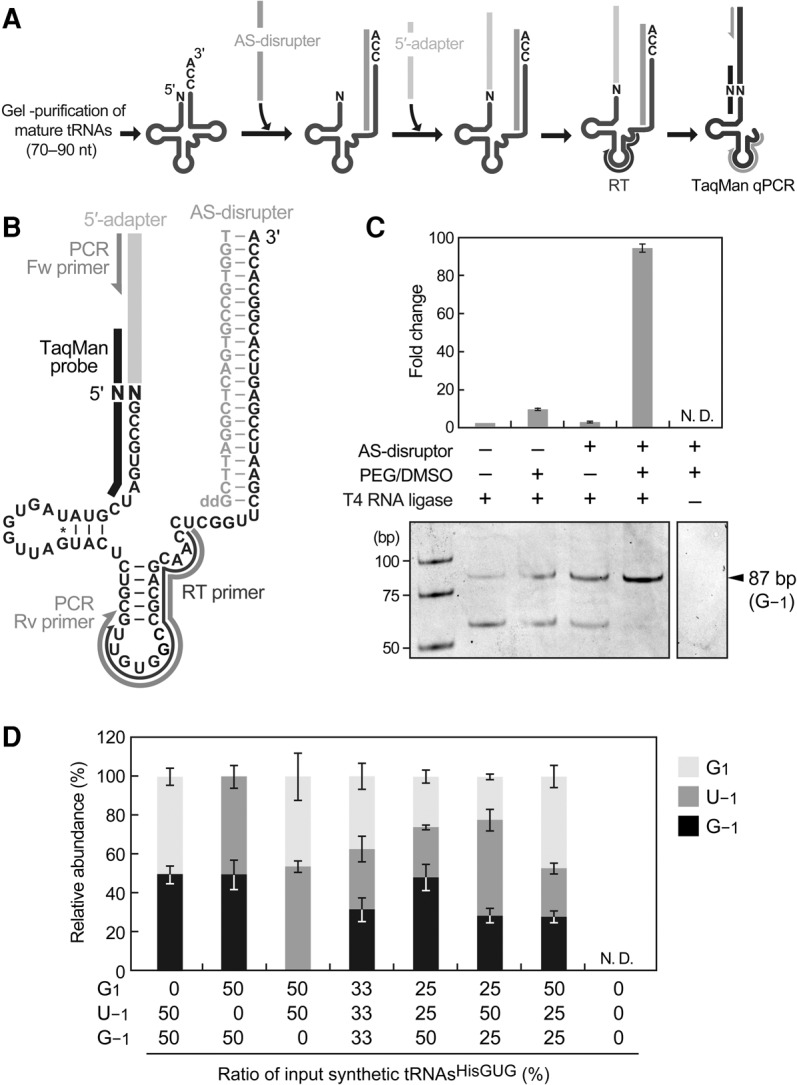
TaqMan qRT-PCR method to analyze the 5′-terminal nucleotide of mature tRNAHisGUG. (A) Schematic representation of the TaqMan qRT-PCR analysis used to quantify each 5′-terminal variant of mature tRNAHisGUG. (B) Sequences and/or positions of the mature tRNAHisGUG and the following TaqMan qPCR components: adapter, AS-disrupter, primers, and TaqMan probe. (C) Under the indicated conditions, this method was applied to synthetic mature tRNAHisGUG containing G−1. The reaction containing only T4 RNA ligase (far left) was set to one, and fold changes relative to this reference are shown; bars indicate SD from three independent experiments. Amplified cDNA bands observed in native PAGE after 40 cycles of PCR are also shown. (D) Synthetic mature tRNAsHisGUG starting from G1, G−1, and U−1 were mixed at the indicated ratios and quantified by the TaqMan qRT-PCR. Detected amounts were calculated using standard curves (Supplemental Fig. S5), and the relative abundances of detected tRNAs containing each 5′-terminal nucleotide are shown. Bars indicate SD from three independent experiments. N.D. indicates that the reaction did not amplify detectable cDNA signals.
The majority of the mature tRNAHisGUG molecules contain the −1 nucleotide
Given the high specificity and quantification ability of our TaqMan qRT-PCR method, we utilized this method to determine the relative abundances of the 5′-terminal variants of endogenous mature cyto tRNAHisGUG expressed in BT-474 cells. Using our synthetic tRNA results as standards, we determined the relative abundances of the five potential 5′-terminal variants of tRNAHisGUG. In contrast to the 5′-terminal variations of 5′-SHOT-RNAHisGUG, which were dominated by G1 (Fig. 1C), ∼60% of the mature tRNAHisGUG contained G−1 as a 5′-terminal nucleotide (Fig. 4A). A significant proportion of tRNAs contained U−1 (∼20%), and a similar proportion lacked the –1 nucleotide. We could not detect amplification signals from tRNAs containing A−1 and C−1, likely because those tRNA species were not expressed at sufficient levels to allow detection by our system. The predominance of the –1 nucleotide-containing tRNAHisGUG was validated by a primer extension assay using the mature tRNA fractions from BT-474 cells. In contrast to the 5′-SHOT-RNAHisGUG analyses, in which the 25-nt band was more abundant than the 26-nt band (Fig. 2B), mature tRNA analyses revealed a predominance of the 26-nt band (Fig. 4B). Because of the low resolution of this method for mature tRNA, no clear band around 25 nt appeared, and thereby it was difficult to estimate the relative abundance of the –1 nucleotide lacking tRNA. However, this result at least validated our TaqMan qRT-PCR result that the –1 nucleotide-containing tRNAHisGUG is the major mature species in BT-474 cells.
FIGURE 4.
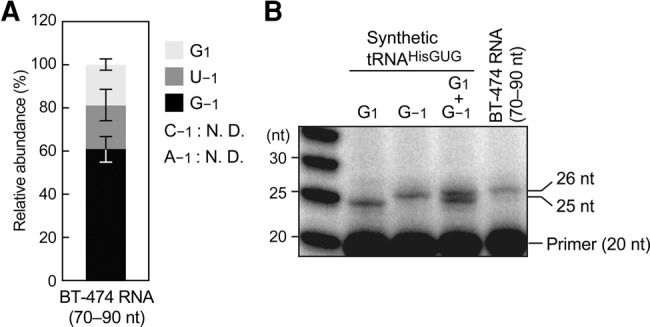
Variations in 5′-terminal nucleotide from mature tRNAHisGUG expressed in BT-474 cells. (A) Of note, 70- to 90-nt mature tRNA fractions of BT-474 cells were subjected to TaqMan qRT-PCR quantification of each 5′-terminal variant of mature tRNAHisGUG. Expression levels were estimated using standard curves from synthetic tRNAs (Supplemental Fig. S5), and the relative abundances of mature tRNAs containing each 5′-terminal nucleotide are shown. Bars indicate SD from three independent experiments. N.D. indicates that the reaction did not amplify detectable cDNA signals. (B) A primer extension assay to analyze the 5′-terminal positions of mature tRNAHisGUG was performed using the 70- to 90-nt mature tRNA fraction from BT-474 cells.
Potential mechanisms by which distinct 5′-terminal variations are formed in mature cyto tRNAHisGUG molecules and 5′-halves
This study identified the 5′-terminal variations of human mature cyto tRNAHisGUG in BT-474 cells. Although more than half of the tRNAs contained G−1, substantial amounts of previously unreported mature tRNA species either containing U−1 or lacking the –1 nucleotide were also identified. In earlier studies, mammalian cyto tRNAHisGUG from HeLa cells and sheep liver were analyzed using chromatography, and neither U−1-containing nor –1 nucleotide-lacking tRNAs were detected (Boisnard and Petrissant 1981; Rosa et al. 1983). This difference might be attributable to differences in the detection method sensitivities. During the course of this study, an advanced tRNA sequencing method has been reported in which methylations, including m1G, were removed from tRNAs via engineered AlkB demethylase prior to reverse transcription, thereby reducing sequencing bias from these methylations (Zheng et al. 2015). We investigated the –1 nucleotide variations of tRNAHisGUG in the reported less-biased tRNA sequencing data set from HEK293T cells (Supplemental Table S2). As a result, we observed a major population of mature tRNAHisGUG containing G−1 (47%), and substantial populations of mature tRNAs containing U−1 (18%) or lacking the –1 nucleotide (30%); these results were similar to the 5′-variations that we observed in mature tRNA from BT-474 cells. These results suggest the universality of the presence of mature tRNAHisGUG containing U−1 or lacking the –1 nucleotide, as well as molecules containing G−1, among human cultured cell lines. The mechanism underlying the formation of these 5′-variations in mature tRNA and the functional significance remain to be determined. Because Thg1 or a Thg1-like protein (TLP) from Bacillus, archaea and yeast can attach not only to guanosine, but also to uridine, to the −1 position of the tRNA in vitro (Jackman and Phizicky 2006; Rao et al. 2011), Thg1 might incorporate both G−1 and U−1 into human mature tRNAHisGUG. It will be intriguing to analyze the efficiency of human HisRS aminoacylation toward each mature tRNAHisGUG 5′-variant to determine whether these variations affect the regulation of aminoacylation. Because human Thg1 is associated with cell cycle regulation (Guo et al. 2004), the biological significance of these 5′-variations in mature tRNAHisGUG might also include cell growth regulation.
The 5′-terminal variations of 5′-SHOT-RNAHisGUG exhibited a pattern distinct from that of mature tRNA; specifically, 5′-SHOT-RNAHisGUG molecules mostly lack the –1 nucleotide. This inconsistency might be attributable to ANG cleavage activity to generate SHOT-RNAs. ANG might selectively cleave –1 nucleotide-lacking tRNAs, resulting in a considerable accumulation of –1 nucleotide-lacking SHOT-RNA molecules. However, ANG is a small protein, and thus selective cleavage should be facilitated by cofactors. Alternatively, ANG might cleave tRNA irrespective of the 5′-terminal nucleotide; SHOT-RNA lacking the –1 nucleotide might then be more stable within the cells than SHOT-RNA containing G−1, or an unknown ribonuclease might trim the –1 nucleotide from SHOT-RNAs. The generative mechanism and biological significance of these 5′-terminal variations in mature tRNAHisGUG molecules 5′-halves remain to be elucidated.
MATERIALS AND METHODS
Bioinformatics analyses of 5′-SHOT-RNAHisGUG
Human cyto tRNAHisGUG sequences were identified using the tRNAscan-SE program (Lowe and Eddy 1997) and are shown in Supplemental Figure S1. The 5′-SHOT-RNA library was previously obtained by cP-RNA-seq of gel-purified 30- to 50-nt RNAs from BT-474 cells (Honda et al. 2015) and can be found in the Gene Expression Omnibus Database (GEO accession no. SRX1060214). Reads previously mapped to mature cyto tRNAHisGUG sequences (Honda et al. 2015) were extracted and used for this study.
Cell culture
BT-474 cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Life Technologies) containing 10% (v/v) FBS.
In vitro synthesis of tRNAHisGUG
Templates for the in vitro synthesis of human cyto tRNAHisGUG (with or without N−1 nucleotide) were prepared by annealing two ssDNAs (5′-GCTTAATACGACTCACTATAGCCGTGATCGTATAGTGGTTAGTACTCTGCGTTGTGGC-3′ and 5′- mUmGGTGCCGTGACTCGGATTCGAACCGAGGTTGCTGCGGCCACAACGC-3′) in a solution containing 10 mM Tris–HCl (pH 8.0) and 20 mM MgCl2. After blunting the formed duplex using sequenase (Affymetrix), the resultant dsDNAs were used as templates for transcription with T7 RNA polymerase (New England Biolabs). Synthesized RNAs were gel-purified using denaturing PAGE.
5′-End identification of tRNAHisGUG by TaqMan qRT-PCR
The sequences of the adapter, AS-disrupter, primers, and TaqMan probes for TaqMan qRT-PCR are shown in Supplemental Table S4. The 70- to 90-nt RNA fraction, which contained mature tRNAs, was initially gel-purified from total RNA using denaturing PAGE. To ligate the 5′-adapter, to the 5′-end of cyto tRNAHisGUG, 500 ng of the tRNA fraction were incubated with 100 pmol of AS-disrupter in a 4-µL reaction mixture at 90°C for 2 min and subsequently incubated at 37°C. RNA was then added immediately to a ligation reaction mixture (total volume: 10 µL) containing 200 pmol of 5′-adapter, T4 RNA ligase 1 (New England Biolabs), 10% (v/v) DMSO, and 5% PEG8000 and incubated at 37°C for 1 h, followed by an overnight incubation at 4°C. Next, 1 µL of the ligation mixture was subjected to cDNA synthesis with 1 µM of RT primer and SuperScript III (Invitrogen). For TaqMan qPCR quantification, the cDNA product (0.5 µL of the RT mixture) was added to a reaction mixture (total volume: 10 µL) containing 5 µL of qPCR Master Mix (TaKaRa), 0.2 µM each of reverse and forward primers, and 0.1 µM of TaqMan probe. Using a StepOne Plus Real-time PCR machine (Applied Biosystems), the reaction mixture was incubated at 95°C for 20 sec, followed by 40 cycles of 95°C for 1 sec and 65°C for 20 sec.
Primer extension assay
To detect 5′-SHOT-RNAHisGUG, 30- to 50-nt RNAs were first gel-purified from BT-474 total RNA. Subsequently, 50 ng of gel-purified RNA or 0.1 pmol of synthetic tRNAHisGUG were incubated with SuperScript III, the corresponding reaction buffer (Invitrogen), and 0.1 pmol of 5′-32P-labeled DNA primer (5′-GTACTAACCACTATACGATC-3′) at 55°C for 30 min. The reaction mixtures were developed using denaturing PAGE containing 7 M rea and 20% formamide. To analyze mature tRNAHisGUG, 70- to 90-nt mature tRNAs were gel-purified from the total RNAs extracted from BT-474 cells. Gel-purified RNAs (1 µg) were then subjected to a primer extension assay as described above.
SUPPLEMENTAL MATERIAL
Supplemental material is available for this article.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Dr. Shozo Honda for helpful discussions. This study was supported by National Institutes of Health, National Institute of General Medical Sciences grant GM106047 (to Y.K.).
Footnotes
Article is online at http://www.rnajournal.org/cgi/doi/10.1261/rna.058024.116.
REFERENCES
- Altwegg M, Kubli E. 1980. The nucleotide sequence of histidine tRNA γ of Drosophila melanogaster. Nucleic Acids Res 8: 3259–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P, Ivanov P. 2014. tRNA fragments in human health and disease. FEBS Lett 588: 4297–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco S, Dietmann S, Flores JV, Hussain S, Kutter C, Humphreys P, Lukk M, Lombard P, Treps L, Popis M, et al. 2014. Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders. EMBO J 33: 2020–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisnard M, Petrissant G. 1981. The nucleotide sequence of sheep liver histidine-tRNA (anticodon Q-U-G). FEBS Lett 129: 180–184. [DOI] [PubMed] [Google Scholar]
- Burkard U, Willis I, Söll D. 1988. Processing of histidine transfer RNA precursors. Abnormal cleavage site for RNase P. J Biol Chem 263: 2447–2451. [PubMed] [Google Scholar]
- Clarke CJ, Berg TJ, Birch J, Ennis D, Mitchell L, Cloix C, Campbell A, Sumpton D, Nixon C, Campbell K, et al. 2016. The initiator methionine tRNA drives secretion of type II collagen from stromal fibroblasts to promote tumor growth and angiogenesis. Curr Biol 26: 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly NL, Arvanitis DA, Fairley JA, Gomez-Roman N, Morton JP, Graham SV, Spandidos DA, White RJ. 2005. Deregulation of RNA polymerase III transcription in cervical epithelium in response to high-risk human papillomavirus. Oncogene 24: 880–888. [DOI] [PubMed] [Google Scholar]
- Diebel KW, Zhou K, Clarke AB, Bemis LT. 2016. Beyond the ribosome: extra-translational functions of tRNA fragments. Biomark Insights 11: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar KA, Goodenbour JM, Pan T. 2006. Tissue-specific differences in human transfer RNA expression. PLoS Genet 2: e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emara MM, Ivanov P, Hickman T, Dawra N, Tisdale S, Kedersha N, Hu GF, Anderson P. 2010. Angiogenin-induced tRNA-derived stress-induced RNAs promote stress-induced stress granule assembly. J Biol Chem 285: 10959–10968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Feng J, Liu Q, Sun F, Tie Y, Zhu J, Xing R, Sun Z, Zheng X. 2009. Stress induces tRNA cleavage by angiogenin in mammalian cells. FEBS Lett 583: 437–442. [DOI] [PubMed] [Google Scholar]
- Garcia-Silva MR, Cabrera-Cabrera F, Güida MC, Cayota A. 2012. Hints of tRNA-derived small RNAs role in RNA silencing mechanisms. Genes (Basel) 3: 603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebetsberger J, Polacek N. 2013. Slicing tRNAs to boost functional ncRNA diversity. RNA Biol 10: 1798–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingold H, Tehler D, Christoffersen NR, Nielsen MM, Asmar F, Kooistra SM, Christophersen NS, Christensen LL, Borre M, Sorensen KD, et al. 2014. A dual program for translation regulation in cellular proliferation and differentiation. Cell 158: 1281–1292. [DOI] [PubMed] [Google Scholar]
- Gu W, Jackman JE, Lohan AJ, Gray MW, Phizicky EM. 2003. tRNAHis maturation: an essential yeast protein catalyzes addition of a guanine nucleotide to the 5′ end of tRNAHis. Genes Dev 17: 2889–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Hurto RL, Hopper AK, Grayhack EJ, Phizicky EM. 2005. Depletion of Saccharomyces cerevisiae tRNAHis guanylyltransferase Thg1p leads to uncharged tRNAHis with additional m5C. Mol Cell Biol 25: 8191–8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Hu K, Lei Y, Wang Y, Ma T, He D. 2004. Identification and characterization of a novel cytoplasm protein ICF45 that is involved in cell cycle regulation. J Biol Chem 279: 53498–53505. [DOI] [PubMed] [Google Scholar]
- Himeno H, Hasegawa T, Ueda T, Watanabe K, Miura K, Shimizu M. 1989. Role of the extra G-C pair at the end of the acceptor stem of tRNAHis in aminoacylation. Nucleic Acids Res 17: 7855–7863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S, Kirino Y. 2015. Dumbbell-PCR: a method to quantify specific small RNA variants with a single nucleotide resolution at terminal sequences. Nucleic Acids Res 43: e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S, Loher P, Shigematsu M, Palazzo JP, Suzuki R, Imoto I, Rigoutsos I, Kirino Y. 2015. Sex hormone-dependent tRNA halves enhance cell proliferation in breast and prostate cancers. Proc Natl Acad Sci 112: E3816–E3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S, Morichika K, Kirino Y. 2016. Selective amplification and sequencing of cyclic phosphate-containing RNAs by the cP-RNA-seq method. Nat Protoc 11: 476–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh LC, Lin SI, Shih AC, Chen JW, Lin WY, Tseng CY, Li WH, Chiou TJ. 2009. Uncovering small RNA-mediated responses to phosphate deficiency in Arabidopsis by deep sequencing. Plant Physiol 151: 2120–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov P, Emara MM, Villen J, Gygi SP, Anderson P. 2011. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol Cell 43: 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman JE, Phizicky EM. 2006. tRNAHis guanylyltransferase catalyzes a 3′-5′ polymerization reaction that is distinct from G-1 addition. Proc Natl Acad Sci 103: 8640–8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman JE, Phizicky EM. 2008. Identification of critical residues for G-1 addition and substrate recognition by tRNAHis guanylyltransferase. Biochemistry 47: 4817–4825. [DOI] [PubMed] [Google Scholar]
- Jackman JE, Gott JM, Gray MW. 2012. Doing it in reverse: 3′-to-5′ polymerization by the Thg1 superfamily. RNA 18: 886–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith G, Pixa G. 1984. The nucleotide sequence of asparagine tRNA from brewer's yeast. Biochimie 66: 639–643. [DOI] [PubMed] [Google Scholar]
- Kellner S, Burhenne J, Helm M. 2010. Detection of RNA modifications. RNA Biol 7: 237–247. [DOI] [PubMed] [Google Scholar]
- Lowe TM, Eddy SR. 1997. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res 25: 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons SM, Achorn C, Kedersha NL, Anderson PJ, Ivanov P. 2016. YB-1 regulates tiRNA-induced stress granule formation but not translational repression. Nucleic Acids Res 44: 6949–6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlab S, Tuller T, Linial M. 2012. Conservation of the relative tRNA composition in healthy and cancerous tissues. RNA 18: 640–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Rideout EJ, Grewal SS. 2012. Nutrient/TOR-dependent regulation of RNA polymerase III controls tissue and organismal growth in Drosophila. EMBO J 31: 1916–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana O, Cooley L, Söll D. 1986. The additional guanylate at the 5′ terminus of Escherichia coli tRNAHis is the result of unusual processing by RNase P. Mol Cell Biol 6: 525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavon-Eternod M, Gomes S, Geslain R, Dai Q, Rosner MR, Pan T. 2009. tRNA over-expression in breast cancer and functional consequences. Nucleic Acids Res 37: 7268–7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavon-Eternod M, Wei M, Pan T, Kleiman L. 2010. Profiling non-lysyl tRNAs in HIV-1. RNA 16: 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston MA, Phizicky EM. 2010. The requirement for the highly conserved G-1 residue of Saccharomyces cerevisiae tRNAHis can be circumvented by overexpression of tRNAHis and its synthetase. RNA 16: 1068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston MA, D'Silva S, Kon Y, Phizicky EM. 2013. tRNAHis 5-methylcytidine levels increase in response to several growth arrest conditions in Saccharomyces cerevisiae. RNA 19: 243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade K, Chang MS, Ting CT, Pei D, Hsiao CF, Olivier M, Pesich R, Hebert J, Chen YD, Dzau VJ, et al. 2001. High-throughput genotyping with single nucleotide polymorphisms. Genome Res 11: 1262–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao BS, Jackman JE. 2015. Life without post-transcriptional addition of G-1: two alternatives for tRNAHis identity in Eukarya. RNA 21: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao BS, Maris EL, Jackman JE. 2011. tRNA 5′-end repair activities of tRNAHis guanylyltransferase (Thg1)-like proteins from Bacteria and Archaea. Nucleic Acids Res 39: 1833–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao BS, Mohammad F, Gray MW, Jackman JE. 2013. Absence of a universal element for tRNAHis identity in Acanthamoeba castellanii. Nucleic Acids Res 41: 1885–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout EJ, Marshall L, Grewal SS. 2012. Drosophila RNA polymerase III repressor Maf1 controls body size and developmental timing by modulating tRNAiMet synthesis and systemic insulin signaling. Proc Natl Acad Sci 109: 1139–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa MD, Hendrick JP, Lerner MR, Steitz JA, Reichlin M. 1983. A mammalian tRNAHis-containing antigen is recognized by the polymyositis-specific antibody anti-Jo-1. Nucleic Acids Res 11: 853–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen AE, Brooks BS, Guth E, Francklyn CS, Musier-Forsyth K. 2006. Evolutionary conservation of a functionally important backbone phosphate group critical for aminoacylation of histidine tRNAs. RNA 12: 1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudinger J, Florentz C, Giegé R. 1994. Histidylation by yeast HisRS of tRNA or tRNA-like structure relies on residues −1 and 73 but is dependent on the RNA context. Nucleic Acids Res 22: 5031–5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikia M, Hatzoglou M. 2015. The many virtues of tRNA-derived stress-induced RNAs (tiRNAs): discovering novel mechanisms of stress response and effect on human health. J Biol Chem 290: 29761–29768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikia M, Krokowski D, Guan BJ, Ivanov P, Parisien M, Hu GF, Anderson P, Pan T, Hatzoglou M. 2012. Genome-wide identification and quantitative analysis of cleaved tRNA fragments induced by cellular stress. J Biol Chem 287: 42708–42725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt BM, Rudolph KL, Karagianni P, Fonseca NA, White RJ, Talianidis I, Odom DT, Marioni JC, Kutter C. 2014. High-resolution mapping of transcriptional dynamics across tissue development reveals a stable mRNA-tRNA interface. Genome Res 24: 1797–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R, Riordan JF, Vallee BL. 1986. Characteristic ribonucleolytic activity of human angiogenin. Biochemistry 25: 3527–3532. [DOI] [PubMed] [Google Scholar]
- Shigematsu M, Kirino Y. 2015. tRNA-derived short non-coding RNA as interacting partners of argonaute proteins. Gene Regul Syst Bio 9: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigematsu M, Honda S, Kirino Y. 2014. Transfer RNA as a source of small functional RNA. J Mol Biol Mol Imaging 1: 8. [PMC free article] [PubMed] [Google Scholar]
- Singer CE, Smith GR. 1972. Histidine regulation in Salmonella typhimurium. 13. Nucleotide sequence of histidine transfer ribonucleic acid. J Biol Chem 247: 2989–3000. [PubMed] [Google Scholar]
- Sprinzl M, Horn C, Brown M, Ioudovitch A, Steinberg S. 1998. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res 26: 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telonis AG, Loher P, Honda S, Jing Y, Palazzo J, Kirino Y, Rigoutsos I. 2015. Dissecting tRNA-derived fragment complexities using personalized transcriptomes reveals novel fragment classes and unexpected dependencies. Oncotarget 6: 24797–24822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DM, Lu C, Green PJ, Parker R. 2008. tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA 14: 2095–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Sobral BW, Williams KP. 2007. Loss of a universal tRNA feature. J Bacteriol 189: 1954–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S, Ivanov P, Hu GF, Anderson P. 2009. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J Cell Biol 185: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G, Qin Y, Clark WC, Dai Q, Yi C, He C, Lambowitz AM, Pan T. 2015. Efficient and quantitative high-throughput tRNA sequencing. Nat Methods 12: 835–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Goodenbour JM, Godley LA, Wickrema A, Pan T. 2009. High levels of tRNA abundance and alteration of tRNA charging by bortezomib in multiple myeloma. Biochem Biophys Res Commun 385: 160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou DH, Lee J, Frankenberger C, Geslain R, Rosner M, Pan T. 2012. Anti-tumor effects of an engineered “killer” transfer RNA. Biochem Biophys Res Commun 427: 148–153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


