Abstract
Background
Clinical experiences suggest that breast cancer (BC) and thyroid cancer (TC) occur metachronously or synchronously in a patient more frequently than it would by chance. This study was conducted to investigate the clinicopathological characteristics and survival of these double primary malignancies.
Methods
18732 patients with first primary BC and 12877 female patients with first primary TC were performed in this retrospective case-controlled study. The control groups were matched with both age at diagnosis and time of surgery (±2 years). The clinicopathological factors, Overall survival (OS), and HRs were evaluated by SPSS.
Results
There were 91(0.49%) BC patients developed metachronous second primary TC (B-T group), and 117 (0.91%) TC patients developed metachronous second primary BC (T-B group). The expression of estrogen and progesterone receptors, and the value of Ki-67, were significantly higher in the B-T group than control. The median value of thyroid globulin antibody (TGAb) and thyroid peroxidase antibody (TPOAb) were higher in T-B group than control (p <0.05). The duration before second primary cancer was shorter for the B-T group than the T-B group (4.09 years vs. 5.82 years, p<0.001). B-T group patients showed poorer survival than BC only patients (p=0.044).
Conclusions
In general, the overall risk of the occurrence of a second primary TC or BC elevated highly in patients with BC or TC. Detailed mechanisms need to be studied to explore the association between these two cancers. Early detection and effective prevention for the first primary BC or TC patients are necessities for reducing the incidence of the second primary cancer and improving the OS.
Keywords: breast cancer, thyroid cancer, double primary malignancies, clinicopathologic characteristics, prognosis
INTRODUCTION
Globally, breast cancer (BC) and thyroid cancer (TC) are two of the most common malignancies among women. The incidences of BC and TC are increasing in China. With the advances of cancer treatment and early detection, patients' survival has improved. However, more and more patients acquire multiple primary cancers (MPCs) because of varied reasons, such as environmental modifications, genetic predisposition, therapy, increased surveillance, or prolonged survival.
The criteria for diagnosing multiple primary tumors are as follows: 1) each tumor must present a definite picture of malignancy; 2) each tumor must be distinct; and 3) the probability that one tumor is a metastatic lesion originating from the other must be excluded. Patients with metachronous cancer are defined as those diagnosed with a secondary cancer 6 months or more after their primary cancer diagnosis; patients with synchronous cancer are defined as those diagnosed with a secondary cancer half a year after their primary cancer diagnosis [1].
Clinical experiences suggest that BC and TC occur synchronously or metachronously in a patient more frequently than it would by chance. Some studies have reported an increased risk of TC among BC patients [2, 3], whereas others have found an increased risk of BC in TC patients [4–6]. It seems that TC and BC may have some possible associations in terms of genesis and development, such as hormonal, genetic, environmental, or therapeutic factors. It is important to know how much the risk of additional tumors increases after a primary tumor (BC or TC), as well as the differences in the clinical, pathological, and treatment characteristics among patients with and without the second primary cancer (BC or TC).
PATIENTS AND METHODS
Population
A retrospective case-controlled study was performed at Tianjin Medical University Cancer Institute and Hospital, Tianjin, China. The study included 18732 patients with first primary BC who underwent curative surgery and 12877 female patients with first primary TC who underwent total thyroidectomy between January 2001 and December 2010. Patients with medullary or anaplastic type of TC and those with follow-ups of less than two years were excluded. And this study conforms to the STROBE (The Strengthening of Reporting of Observational Studies in Epidemiology) statement.
During the follow up, the BC followed by metachronous second primary TC was defined as B-T group. TC followed by metachronous second primary BC was defined as T-B group. The synchronous double primary BC and TC was defined as B = T group. The control groups, all female, were matched with a ratio of 1:4 B-T and T-B groups. Matching was based on both age at diagnosis and time of surgery (±2 years). These groups were selected on a case-by-case basis from the BC or TC patients who had not been diagnosed with second primary cancer, i.e., defined as B only and T only group separately.
Clinicopathological characteristics
We collected the clinicopathological characteristics of BC and TC, such as age at diagnosis, family history, pathologic types, tumor size, lymph nodes (LN) metastasis, distant metastasis, histological grading, estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), molecular classification, Ki-67, p53, multifocality, extrathyroidal extension, thyroid stimulating hormone (TSH), thyroglobulin (TG), thyroid globulin antibody (TGAb), and thyroid peroxidase antibody (TPOAb). Immunohistochemistry was performed using the avidin-biotin-immunoperoxidase technique for ER, PR, HER2, Ki-67, and p53 in the formalin-fixed paraffin embedded representative tumor sections of each case.
The ER, PR, and HER2 status was determined using the criteria of the American Society of Clinical Oncology/College of American Pathologists [7, 8]. For ER and PR, nuclear staining in ≥ 1% of the tumor cells was considered positive. HER2 immunoreactivity was evaluated on a standardized scale from 0 to 3 based on the intensity of membranous staining and the proportion of tumor cells stained, wherein a strong complete membranous staining in > 10% of tumor cells (3+) was considered positive. Ki-67 and p53 immunoreactions were presented through nuclear staining (Figure 1). Molecular classification of tumor was performed using the established criteria [9, 10].
Figure 1. Immunohistochemistry(IHC) Images of ER, PR, Her-2, Ki67 and P53 (×100; ×400).
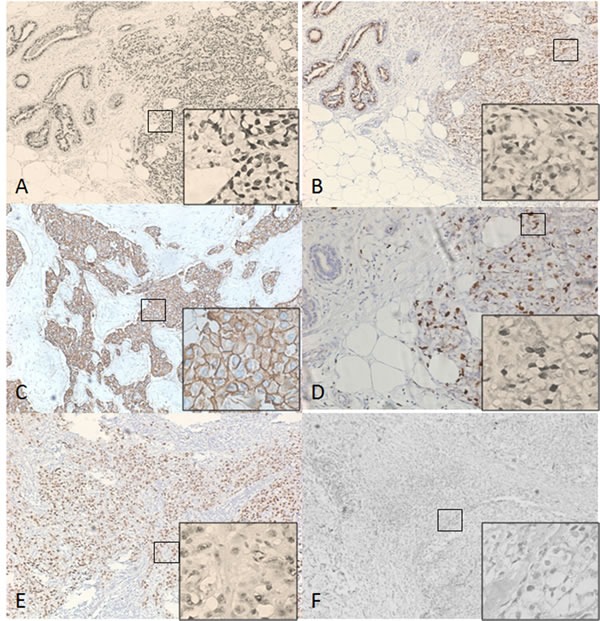
A. Estrogen receptor (ER) positive; B. Progesterone receptor (PR) positive; C. Human epidermal growth factor receptor 2 (HER2) positive; D., E. Immunoreactions of Ki-67 and p53 were presented through nuclear staining; F. Negative Immunoreactions.
Statistical analysis
SPSS 22.0 software was used for statistical analyses. To compare the clinicopathological characteristics of patients, chi square was used for dichotomous variables. Continuous variables were compared using the independent two-sample t-test. Nonparametric test was used to analyze the ranked data and continuous data not normal distributed. The standardized incidence ratio (SIR) was calculated to assess the risk of second primary malignancies by comparing the number of patients with subsequent cancer to the number of cancers that would be expected based on incidence rates for the general Chinese population [11]. Associations of different groups and the relevant tumor characteristics were explored using ANOVA and post-hoc test (LSD). Overall survival (OS) was calculated from the date of surgery. Survival curves were plotted using the Kaplan-Meier method, and group differences in survival curve were investigated by the log-rank test. A Cox proportional hazard model was used to identify variables that were independently associated with OS. All statistical tests were two-sided and a p-value < 0.05 was considered statistically significant.
RESULTS
Incidence of co-existing BC and TC
Among 18732 patients with BC, 99(0.53%) were diagnosed with synchronous double primary cancers (SDPCs) and 217(1.1%) were diagnosed with metachronous double primary cancers(MDPCs). The top five cancer types of the double primary cancers (DPCs) after BC were thyroid cancer, endometrial cancer, cervical cancer, stomach cancer and lung cancer. The incidence of thyroid cancer (0.49%) is obviously higher than other types (Table 1).
Table 1. The top five distribution of synchronous and metachronous double primary cancers in 18732 cases of breast cancer patients.
| DPC type | No.of MDPCs (%) (Total BC, n=18732) |
No.of SDPCs (%) (Total BC, n=18732) |
|---|---|---|
| Total DPC | 217(1.1) | 99(0.53) |
| Thyroid cancer | 91(0.49) | 53(0.28) |
| Endometrial cancer | 22(0.12) | 12(0.06) |
| Cervical cancer | 19(0.10) | 7(0.04) |
| Stomach cancer | 17(0.09) | 7(0.04) |
| Lung cancer | 12(0.06) | 5(0.03) |
Abbreviations: DPC: Double primary cancer, MDPCs: Metachronous double primary cancers, SDPCs: Synchronous double primary cancers
Among 12877 patients with TC, 83(0.64%) were diagnosed with SDPCs and 232(1.8%) were diagnosed with MDPCs. The top five cancer types of DPCs after TC were breast cancer, stomach cancer, endometrial cancer, ovarian cancer, cervical cancer. The incidence of breast cancer (0.91%) is obviously higher than other types (Table 2).
Table 2. The top five distribution of synchronous and metachronous double primary cancer in 12877 cases of thyroid cancer patients.
| DPC type | No.of MDPCs (%) (Total TC, n=12877) |
No.of SDPCs (%) (Total TC, n=12877) |
|---|---|---|
| Total DPC | 232(1.8) | 83(0.64) |
| Breast cancer | 117(0.91) | 53(0.41) |
| Stomach cancer | 25(0.19) | 11(0.09) |
| Endometrial cancer | 21 (0.16) | 4(0.03) |
| Ovarian cancer | 15(0.11) | 3(0.02) |
| Cervical cancer | 11(0.09) | 3(0.02) |
Abbreviations: DPC: Double primary cancer, MDPCs: Metachronous double primary cancers, SDPCs: Synchronous double primary cancers
In our study, there were 91(0.49%) patients in B-T group, 117 (0.91%) patients in T-B group, and 53 cases in B = T group. During the entire follow-up period, there were 261(0.83%) patients diagnosed with co-existing BC and TC.
The estimated incidence of BC was 37.86/100,000 and TC was 10.32/100,000 in China, 2011 [11]. In patients with BC, the incidence of TC development (0.49%)was increased compared to that of the general population; the SIR for developing second primary TC was 4.75 [confidence interval (CI) 3.83-5.96]. The incidence of BC (0.91%) also increased in TC patients; the SIR for developing second primary BC was 2.40(CI 1.87-3.01).
Clinicopathological characteristics of BC in B-T group and B only group
The clinicopathological characteristics of BC were compared between the 91 patients in the B-T group and the 364 matched controls in the B only group (Table 3). The respective mean age and menopausal status at diagnosis of BC, family history, pathologic types, histological grading, tumor size, LN metastasis, distant metastasis, Molecular Classification, and p53 expression were similar between the two groups. The expression of both the ER and PR was significantly higher in the tumors from individuals in the B-T group compared with those from the B only group (ER+ 76.9% vs. 65.1%, p = 0.034; PR+ 74.7% vs. 62.1%; p = 0.027). The median value of Ki-67 was significantly higher (30 vs. 15, p = 0.036) in B-T group compared with B only group (Table 3).
Table 3. Clinicopathological Characteristics of the B-T group.
| Characteristics | B-T group (n=91) |
B only group (n=364) |
t/X2/z | P value |
|---|---|---|---|---|
| Age at first primary cancer (year, mean±s) | 47.88±9.55 | 47.94±9.53 | 0.054* | 0.957 |
| Post-menopause, n(%) | 33(36.3) | 157(43.1) | 1.412# | 0.284 |
| Family history, n (%) | 7(7.7) | 23(6.3) | 0.223# | 0.638 |
| Pathologic types (%) | −0.195** | 0.845 | ||
| IDC/DCIS/other | 84.6/11.5/3.9 | 86.2/11.3/2.5 | ||
| Maximal tumor size, n(%) | −0.610** | 0.542 | ||
| ≤2cm | 39(42.9) | 151(41.5) | ||
| 2-5cm | 38(41.8) | 140(38.5) | ||
| >5cm | 14(15.4) | 73(20.1) | ||
| LN metastasis, n(%) | 25(27.5) | 109(29.9) | 0.214# | 0.701 |
| Distant metastasis, n (%) | 2(2.2) | 8(2.2) | 0.000# | 1.000 |
| Histological grading (%) | −0.107** | 0.915 | ||
| Nottingham I/ II/ III | 8.8/73.6/17.6 | 8.2/75.3/16.5 | ||
| ER+, n (%) | 70(76.9) | 237(65.1) | 4.629# | 0.034 |
| PR+, n (%) | 68(74.7) | 226(62.1) | 5.085# | 0.027 |
| HER2+, n (%) | 25(27.5) | 113(31.0) | 0.439# | 0.610 |
| Molecular classification, n(%) | −0.042** | 0.967 | ||
| Luminal A | 55(60.4) | 208(57.1) | ||
| Luminal B | 15(16.5) | 89(24.5) | ||
| HER2 positive | 10(11.0) | 25(6.9) | ||
| Triple negative | 11(12.1) | 42(11.5) | ||
| Ki-67(median, InterQuartile Range) | 30(5~50) | 15(5~40) | −2.323** | 0.036 |
| P53 (median, InterQuartile Range) | 15(0~35) | 10(0~30) | −0.419** | 0.342 |
compared using the independent two-sample t-test
compared using chi square test
compared using nonparametric test
Abbreviations: BC, breast cancer; TC, thyroid cancer; B-T group, BC followed by TC metachronously; B only group, BC patients without other second primary cancers(1:4 matched with the B-T groups); IDC, infiltrative ductal carcinoma; DCIS, ductal carcinoma in situ;
Clinicopathological characteristics of TC in T-B and T only groups
The clinicopathological characteristics of TC were compared between the 117 patients in the T-B group and the 468 matched controls in the T only group (Table 4). The values of FT3, FT4, TSH, TG, TGAb, TPOAb were record preoperatively. The mean age, age at diagnosis of TC, family history, pathologic types, tumor size, lymph node (LN) metastasis, distant metastasis, the proportion of multifocality, extrathyroidal extension, FT3, and FT4 were similar between the two groups. The median value of TGAb and TPOAb were higher in T-B group compared with that in T only group (TGAb 5.25 vs. 2.65, p = 0.008; TPOAb 7.69 vs. 3.54, p = 0.022) (Table 4).
Table 4. Clinicopathological Characteristics of the T-B group.
| Characteristics | T-B group (n=117) |
T only group (n=468) |
t/X2/z | P value |
|---|---|---|---|---|
| Age at first primary cancer (year, mean ± SD) | 46.38±9.66 | 46.70±9.61 | 0.313* | 0.755 |
| Post-menopause, n (%) | 52(44.4) | 214(45.7) | 0.062# | 0.836 |
| Family history, n (%) | 13 (11.1) | 39(8.3) | 0.892# | 0.364 |
| Histologic type (%) | 0.266# | 0.747 | ||
| PTC/ FTC | 89.7/10.3 | 88.0/12.0 | ||
| Maximal tumor size, n (%) | −1.315** | 0.188 | ||
| ≤2cm | 51(43.6) | 177(37.8) | ||
| 2-4cm | 42(35.9) | 172(36.8) | ||
| >4cm | 24(17.9) | 119(15.2) | ||
| LN metastasis, n(%) | 37(31.6) | 174(37.3) | 1.287# | 0.283 |
| Distant metastasis, n (%) | 1(0.9) | 7(1.5) | 0.285# | 1.000 |
| Multifocality, n (%) | 30(25.6) | 110(23.5) | 0.235# | 0.629 |
| Extrathyroidal extension, n (%) | 34(29.1) | 121(25.9) | 0.494# | 0.484 |
| FT3 (pmol/L, median, InterQuartile Range) | 7.23(5.24~9.66) | 7.67(5.12~9.92) | −1.634** | 0.102 |
| FT4 (pmol/L, median, InterQuartile Range) | 10.12(8.21~12.13) | 10.37(7.91~12.34) | −1.516** | 0.130 |
| TSH (mIU/L, median, InterQuartile Range) | 2.67(1.96~3.42) | 2.88(2.12~3.45) | −1.645** | 0.101 |
| TG (μg/L, median, InterQuartile Range) | 59.23(29.89~70.25) | 57.11(28.21~69.35) | −1.034** | 0.218 |
| TGAb (IU/ml, median, InterQuartile Range) | 5.25 (2.68~8.38) | 2.65 (1.95~5.34) | −2.672** | 0.008 |
| TPOAb (IU/ml, median, InterQuartile Range) | 7.69(3.52~10.24) | 3.54(1.12~5.94) | −2.124** | 0.022 |
compared using the independent two-sample t-test
compared using chi square test
compared using nonparametric test
Abbreviations: BC, breast cancer; TC, thyroid cancer; T-B group, TC followed by BC metachronously; T only, TC patients without other second primary cancers(1:4 matched with the T-B groups); PTC, papillary thyroid carcinoma; FTC, follicular thyroid carcinoma.
Difference of characteristics among co-existing BC and TC
Among the 18732 BC and 12877 TC patients, 261 were diagnosed with co-existing BC and TC (B-T: 34.9%, T-B: 44.8%, B = T: 20.3%) after the follow-up period (Table 5). The age at first primary cancer is significantly different among the three groups (B = T: 43.66 vs. T-B: 47.88, T-B: 46.38). Analyzed by post-hoc test, patients in B = T group were younger than B-T groups (p = 0.012). The mean interval time before second primary cancer is shorter for the B-T group than the T-B group (4.09 years vs. 5.82 years, p < 0.001) (Figure 2). The BC and TC characteristics, such as ER, PR, Her-2, Ki-67, P53, FT3, FT4, TSH, TG, TGAb, and TPOAb, were similar among B-T, T-B, and B = T groups (Table 5).
Table 5. Difference of characteristics among co-existing BC and TC.
| Characteristics | Co-existing BC and TC (n=261) | P value | ||
|---|---|---|---|---|
| B-T n=91 (34.9%) |
T-B n=117 (44.8%) |
B=T n=53 (20.3%) |
||
| Age at first primary cancer (year, mean ± SD) | 47.88±9.55* | 46.38±9.66 | 43.66±10.03* | 0.044 |
| Duration before second primary cancer (years, mean± SD) | 4.09±3.13 | 5.82±3.04 | — | <0.001 |
| BC characteristics | ||||
| ER+, n(%) | 70(76.9) | 95(81.2) | 40(75.5) | 0.629 |
| PR+, n (%) | 68(74.7) | 93(79.5) | 38(71.7) | 0.497 |
| HER2 (%) | 25(27.5) | 31(26.5) | 19(35.8) | 0.434 |
| Ki-67 | 30(5~50) | 30(5~50) | 25(5~45) | 0.234 |
| P53 | 10(0~35) | 15(0~40) | 10(0~35) | 0.901 |
| TC characteristics | ||||
| FT3 (pmol/L, median InterQuartile Range) | 7.13(5.44~9.64) | 7.23(5.24~9.66) | 7.25(5.34~9.56) | 0.643 |
| FT4 (pmol/L, median InterQuartile Range) | 10.10(8.54~12.45) | 10.12(8.21~12.13) | 10.64(8.41~13.03) | 0.223 |
| TSH (mIU/L, median InterQuartile Range) | 2.34(1.33~3.34) | 2.67(1.96~3.42) | 2.87(1.90~3.32) | 0.742 |
| TG (μg/L, median InterQuartile Range) | 60.77(29.78~71.24) | 59.23(29.89~70.25) | 59.44(26.89~70.66) | 0.256 |
| TGAb (IU/ml, median InterQuartile Range) | 5.87 (1.92~8.33) | 5.25 (1.98~8.38) | 5.23 (2.18~8.88) | 0.811 |
| TPOAb (IU/ml, median InterQuartile Range) | 7.33(3.23~10.22) | 7.69(3.52~10.24) | 7.67(3.77~10.74) | 0.245 |
analyzed by post-hoc test, p = 0.012.
Abbreviations: BC, breast cancer; TC, thyroid cancer; B-T, BC followed by TC metachronously; B=T, BC and TC occurred synchronously ; T-B, TC followed by BC metachronously; Co-existing BC and TC, total cases of B-T group, B=T group and T-B group.
Figure 2. Time interval between breast cancer and thyroid cancer.
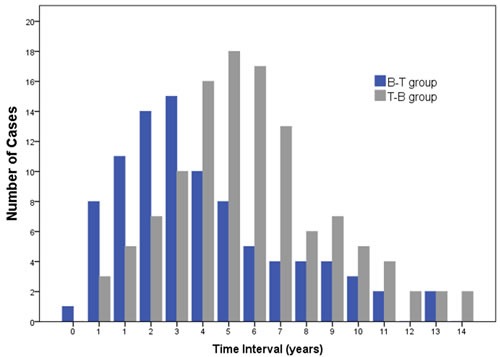
The mean interval time before second primary cancer is shorter for the B-T group than the T-B group (4.09 years vs. 5.82 years, p < 0.001)
Survival analysis
The survival curves of B-T and B = T, B-T and B only groups are shown in Figure 3. We did not report the survival data of T-B group because we will obtain a worse OS compared to that of T only group with no controversy.
Figure 3. Comparison of OS between B-T group, B = T and B only group.
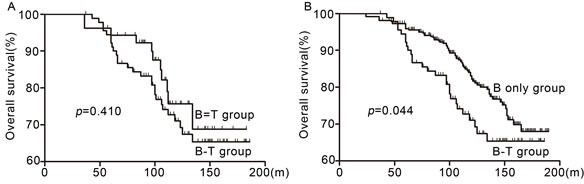
A. B-T group showed similar survival with B = T group (p = 0.410). B. B-T group showed poorer survival than the B only group (p = 0.043).
The median OS of B-T group, B = T and B only group were 110, 112 and 118 months separately. Five year OS rates were 88.8%, 89.2% and 96.7%, whereas 10 year OS rates were 69.7%, 73.1% and 78.5%. B-T group patients showed similar survival with B = T group (p = 0.410) (Figure 3A), but showed poorer survival than the B only group (p = 0.044) (Figure 3B). The survival curve of B-T, B = T and B only group showed obvious decline at about 60, 80 and 100 months separately after diagnosis.
In multivariate Cox regression analysis, after adjusting the factors of maximal tumor size, LN metastasis, and distant metastasis at diagnosis of breast cancer, patients in B-T group showed a significant increase in the risk of death compared with B only group (HR 2.261, 95% CI 1.378-3.710, p = 0.001) (Table 6).
Table 6. Multivariate Cox regression prognostic analysis of OS.
| Factors | Hazard ratio | 95% confidence interval | p value |
|---|---|---|---|
| Maximal tumor size | |||
| ≤2cm | Ref. | ||
| 2-4cm | 0.445 | 0.260-0.760 | 0.003 |
| >4cm | 0.446 | 0.253-0.787 | 0.005 |
| LN metastasis | |||
| Negative | Ref. | ||
| Positive | 0.096 | 0.057-0.160 | <0.001 |
| Distant metastasis | |||
| Negative | Ref. | ||
| Positive | 0.309 | 0.146-0.651 | 0.002 |
| Groups | |||
| B-T | Ref. | ||
| B only | 2.261 | 1.378-3.710 | 0.001 |
Abbreviations: LN, lymph nodes
DISCUSSION
In our study, 0.49% of the BC and 0.91% of the TC patients were diagnosed with metachronous TC and BC, respectively. In patients with BC, the incidence of TC development increased compared with the general population. The SIR for developing second primary TC was 4.75. The incidence of BC also increased in TC patients with a SIR for developing second primary BC of 2.40. These revealed that patients with TC or BC have a higher risk of developing second primary BC or TC. The SIR value was calculated according to the estimated incidence of BC (37.86/100,000) and TC (10.32/ 100,000) in China in 2011 [11]. The SIR value of TC was higher compared with the value obtained in previous studies (from 1.2 to 4.6) [12–15]. The increased incidence of TC followed by the occurrence of BC might be attributed to the incidental detection of TC during follow-up of BC or to the frequent health check-ups following BC. Besides, obvious differences existed in incident area or time. Across U.S. counties, incidence of TC ranged widely, from 0 to 29.7 per 100,000 [16], whereas the incidence was 10.32 per 100,000 in China 2011 [11].
In the B-T group, the expression of ER and PR was significantly higher, and the median Ki-67 labeling was higher than that of B only group. In accordance with our result, some previous studies have also suggested for the role of hormone receptors in the molecular pathogenesis of TC. Sex steroid receptors were found in human thyroid tissue, several TC cell lines, and ER levels were significantly higher in TC compared with normal thyroid tissue [17–19], which have shown the possibility that ER or PR signaling might represent common etiological factors in the development of TC and BC. Other studies have shown that estrogen could up-regulate the expression of cell cycle-related genes and proto-oncogene in thyroid cells, which were likely to contribute to the development of TC [20]. Proliferative activity of tumor tissue is commonly measured by Ki67, which is a well-established prognostic and a predictive marker [21]. Higher expression of Ki67 could be detected in breast-ovarian cancer syndrome (BOCS) [22]. In our study, the incidence of TC was increased in BC patients with higher Ki-67.
Compared with T-B and T only group, we found that the mean value of TGAb and TPOAb were higher in T-B group. Previous studies showed that serum levels of TPOAb and TGAb were significantly higher in patients with BC than in healthy people [23, 24]. Another study showed a poor prognosis in BC with higher TPOAb [25]. However, other studies found that TPOAb positivity was associated with a lower incidence of metastasis in BC patients [26]. Our study indicated that not only the healthy population but also the TC patients should pay more attention to the increase of TGAb and TPOAb. Regular breast examination is important for TC patients.
Among the 18732 BC patients and the 12877 TC patients, 261 were diagnosed with co-existing BC and TC over the follow-up period. The mean interval time before second primary is shorter for B-T group than T-B group (4.09 years vs. 5.82 years, p < 0.001). Close monitoring for the detection of TC development might be necessary for patients with BC, especially 4-5 years after primary diagnosis. Moreover, regular breast examination should be emphasized among TC patients, especially 5-6 years after primary diagnosis. The age at first primary cancer is significantly different among the three groups. Patients in B = T group were younger than B-T groups. The mean age was 44 years old. Other studies showed that the mean duration before the occurrence of second primary cancer was different for different types of cancers and areas(3.1-8.5 years) [27–30]. In our study, results indicated that co-existing BC and TC patients, whether synchronous or metachronous, possessed similar characteristics of ER, PR, Her-2, Ki-67, P53, FT3, FT4, TSH, TG, TGAb, and TPOAb values.
There are limited data on the effect of second primary cancer of thyroid on the survival of BC patients. In the M.D. Anderson Cancer Center, a study on 4198 patients subjected to breast conservation therapy showed that the patients with MPCs showed worse OS than those without MPCs [31]. Similarly, patients with MPCs in another study demonstrated worse DFS and OS [32]. In our study, more deaths due to cancer occurred in the B-T group than in the B only group. Although thyroid cancer usually has better prognosis, it increased the death rate of patients with BC as a second primary cancer. BC patients with metachronous and synchronous double primary TC showed similar survival. We did not report the data of survival on T-B group because we will obviously obtain OS statistics that is worse than T only group with no controversy.
In conclusion, this study has identified that that the overall risk of having a second primary TC or BC is increased in patients with BC or TC. BC patients with higher expression of ER, PR, or Ki-67 should pay more attention to the development of TC, especially 4-5 years after breast surgery. TC patients with higher TGAb and TPOAb have a higher risk of obtaining a second primary BC. Thus, regular breast examination should be emphasized among these patients, especially 5-6 years after thyroid surgery. Patients with co-existing BC and TC usually exhibited worse survival than those with only BC or TC. Therefore, further efforts are needed to explore the mechanism, develop early detection, and administer effective prevention for patients with second primary cancers.
Acknowledgments
Research supported by grants from the National Science & Technology Pillar Program of China (2015BAI12B15), National Natural Science Foundation of China (Grant No. 81202101) and Natural Science Foundation of Tianjin City (Grant No. 15JCQNJC45300).
Footnotes
CONFLICTS OF INTEREST
There is no conflict of interest.
REFERENCES
- 1.Warren S. Multiple primary malignant tumors: a survey of the literature and a statistical study. Am J Cancer. 1932;16:1358–414. [Google Scholar]
- 2.Nielsen SM, White MG, Hong S, Aschebrook-Kilfoy B, Kaplan EL, Angelos P, Kulkarni SA, Olopade OI, Grogan RH. The Breast-Thyroid Cancer Link: A Systematic Review and Meta-analysis. Cancer Epidemiol Biomarkers Prev. 2016;25:231–8. doi: 10.1158/1055-9965.EPI-15-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans HS, Lewis CM, Robinson D, Bell CM, Moller H, Hodgson SV. Incidence of multiple primary cancers in a cohort of women diagnosed with breast cancer in southeast England. Br J Cancer. 2001;84:435–40. doi: 10.1054/bjoc.2000.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Consorti F, Di Tanna G, Milazzo F, Antonaci A. Nulliparity enhances the risk of second primary malignancy of the breast in a cohort of women treated for thyroid cancer. World J Surg Oncol. 2011;9:88. doi: 10.1186/1477-7819-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown AP, Chen J, Hitchcock YJ, Szabo A, Shrieve DC, Tward JD. The risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab. 2008;93:504–15. doi: 10.1210/jc.2007-1154. [DOI] [PubMed] [Google Scholar]
- 6.Subramanian S, Goldstein DP, Parlea L, Thabane L, Ezzat S, Ibrahim-Zada I, Straus S, Brierley JD, Tsang RW, Gafni A, Rotstein L, Sawka AM. Second primary malignancy risk in thyroid cancer survivors: a systematic review and meta-analysis. Thyroid. 2007;17:1277–88. doi: 10.1089/thy.2007.0171. [DOI] [PubMed] [Google Scholar]
- 7.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version) Arch pathol lab med. 2010;134:e48–72. doi: 10.1043/1543-2165-134.7.e48. [DOI] [PubMed] [Google Scholar]
- 8.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J clin oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 9.Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, Perou CM, Ellis MJ, Nielsen TO. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–50. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altaf FJ, Mokhtar GA, Emam E, Bokhary RY, Mahfouz NB, Al AS, Al-Gaithy ZK. Metaplastic carcinoma of the breast: an immunohistochemical study. Diagn pathol. 2014;9:139. doi: 10.1186/1746-1596-9-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen W, Zheng R, Zeng H, Zhang S. The updated incidences and mortalities of major cancers in China, 2011. Chin J Cancer. 2015;34:53. doi: 10.1186/s40880-015-0042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown AP, Chen J, Hitchcock YJ, Szabo A, Shrieve DC, Tward JD. The risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab. 2008;93:504–15. doi: 10.1210/jc.2007-1154. [DOI] [PubMed] [Google Scholar]
- 13.Marti JL, Jain KS, Morris LG. Increased risk of second primary malignancy in pediatric and young adult patients treated with radioactive iodine for differentiated thyroid cancer. Thyroid. 2015;25:681–7. doi: 10.1089/thy.2015.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canchola AJ, Horn-Ross PL, Purdie DM. Risk of second primary malignancies in women with papillary thyroid cancer. Am j epidemiol. 2006;163:521–7. doi: 10.1093/aje/kwj072. [DOI] [PubMed] [Google Scholar]
- 15.An JH, Hwangbo Y, Ahn HY, Keam B, Lee KE, Han W, Park DJ, Park IA, Noh DY, Youn YK, Cho BY, Im SA, Park YJ. A Possible Association Between Thyroid Cancer and Breast Cancer. Thyroid. 2015;25:1330–8. doi: 10.1089/thy.2014.0561. [DOI] [PubMed] [Google Scholar]
- 16.Morris LG, Sikora AG, Tosteson TD, Davies L. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid. 2013;23:885–91. doi: 10.1089/thy.2013.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manole D, Schildknecht B, Gosnell B, Adams E, Derwahl M. Estrogen promotes growth of human thyroid tumor cells by different molecular mechanisms. J Clin Endocrinol Metab. 2001;86:1072–7. doi: 10.1210/jc.86.3.1072. [DOI] [PubMed] [Google Scholar]
- 18.Clark OH, Gerend PL, Davis M, Goretzki PE, Hoffman PJ. Estrogen and thyroid-stimulating hormone (TSH) receptors in neoplastic and nonneoplastic human thyroid tissue. J surg res. 1985;38:89–96. doi: 10.1016/0022-4804(85)90012-5. [DOI] [PubMed] [Google Scholar]
- 19.Tan W, Li Q, Chen K, Su F, Song E, Gong C. Estrogen receptor beta as a prognostic factor in breast cancer patients: A systematic review and meta-analysis. Oncotarget. 2016;7:10373–85. doi: 10.18632/oncotarget.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teng CJ, Hu YW, Chen SC, Yeh CM, Chiang HL, Chen TJ, Liu CJ. Use of Radioactive Iodine for Thyroid Cancer and Risk of Second Primary Malignancy: A Nationwide Population-Based Study. J Natl Cancer Inst. 2015;108 doi: 10.1093/jnci/djv314. [DOI] [PubMed] [Google Scholar]
- 21.Esposito A, Criscitiello C, Curigliano G. Highlights from the 14(th) St Gallen International Breast Cancer Conference 2015 in Vienna: Dealing with classification, prognostication, and prediction refinement to personalize the treatment of patients with early breast cancer. Ecancermedicalscience. 2015;9:518. doi: 10.3332/ecancer.2015.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senkus E, Szade J, Pieczynska B, Zaczek A, Swierblewski M, Biernat W, Jassem J. Are bilateral breast cancers and breast cancers coexisting with ovarian cancer different from solitary tumors? A pair-matched immunohistochemical analysis aimed at intrinsic tumor phenotype. Pathol int. 2014;64:508–17. doi: 10.1111/pin.12202. [DOI] [PubMed] [Google Scholar]
- 23.Shi XZ, Jin X, Xu P, Shen HM. Relationship between breast cancer and levels of serum thyroid hormones and antibodies: a meta-analysis. Asian Pac J Cancer Prev. 2014;15:6643–7. doi: 10.7314/APJCP.2014.15.16.6643. [DOI] [PubMed] [Google Scholar]
- 24.Muller I, Zhang L, Giani C, Dayan CM, Ludgate ME, Grennan-Jones F. The sodium iodide symporter is unlikely to be a thyroid/breast shared antigen. J endocrinol invest. 2016;39:323–31. doi: 10.1007/s40618-015-0368-6. [DOI] [PubMed] [Google Scholar]
- 25.Szychta P, Szychta W, Gesing A, Lewinski A, Karbownik-Lewinska M. TSH receptor antibodies have predictive value for breast cancer - retrospective analysis. Thyroid Res. 2013;6:8. doi: 10.1186/1756-6614-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kemal Y, Demirag G, Ekiz K, Yucel I. Antithyroid peroxidase antibody positivity is associated with lower incidence of metastasis in breast cancer. Mol Clin Oncol. 2015;3:629–32. doi: 10.3892/mco.2015.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raymond JS, Hogue CJ. Multiple primary tumours in women following breast cancer, 1973-2000. Br J Cancer. 2006;94:1745–50. doi: 10.1038/sj.bjc.6603172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung YS, Lim J, Jung KW, Ryu J, Won YJ. Metachronous Second Primary Malignancies after Head and Neck Cancer in a Korean Cohort (1993-2010) Plos one. 2015;10:e134160. doi: 10.1371/journal.pone.0134160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JY, Song HS. Metachronous double primary cancer after treatment of breast cancer. Cancer res treat. 2015;47:64–71. doi: 10.4143/crt.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korc-Grodzicki B, Holmes HM, Shahrokni A. Geriatric assessment for oncologists. Cancer Biol Med. 2015;12:261–74. doi: 10.7497/j.issn.2095-3941.2015.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yi M, Cormier JN, Xing Y, Giordano SH, Chai C, Meric-Bernstam F, Vlastos G, Kuerer HM, Mirza NQ, Buchholz TA, Hunt KK. Other primary malignancies in breast cancer patients treated with breast conserving surgery and radiation therapy. Ann surg oncol. 2013;20:1514–21. doi: 10.1245/s10434-012-2774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J, Park S, Kim S, Kim J, Ryu J, Park HS, Kim SI, Park BW. Characteristics and Survival of Breast Cancer Patients with Multiple Synchronous or Metachronous Primary Cancers. Yonsei med j. 2015;56:1213–20. doi: 10.3349/ymj.2015.56.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]


