Abstract
Morphine is commonly used in neonates with hypothermic ischemic encephalopathy (HIE) during therapeutic hypothermia to provide comfort and analgesia. However, pharmacokinetic data to support morphine dosing in this vulnerable population are lacking. A prospective, two-center, clinical pharmacokinetic study of morphine was conducted in 20 neonates (birthweight 1.82 – 5.3 kg) with HIE receiving hypothermia. Morphine dosing was per standard of care at each center. Morphine and glucuronide metabolites (morphine-3-glucuronide and morphine-6-gluronide) were measured via a validated dried blood spot LC-MS/MS assay. From the available concentration data (n=106 for morphine; n=106 for each metabolite), a population pharmacokinetic model was developed using nonlinear mixed-effects modeling (NONMEM). The clearance of morphine and glucuronide metabolites were best predicted by birthweight allometrically scaled using an exponent of 1.23. In addition, the clearance of each glucuronide metabolite was influenced by serum creatinine. No other significant predictors of clearance or volume of distribution were found. For a 3.5 kg neonate, morphine clearance was 0.77 L/h (CV 48%) and the steady-state volume of distribution was 8.0 L (CV 49%). Compared to previous studies in full-term newborns without HIE, morphine clearance was markedly lower. Dosing strategies customized for this vulnerable population will be needed. Applying the final population pharmacokinetic model, repeated Monte Carlo simulations (n=1000 per simulation) were performed to evaluate various morphine dosing strategies that optimized achievement of morphine concentrations between 10-40 ng/ml. An optimized morphine loading dose of 50 μg/kg followed by a continuous infusion of 5 μg/kg/h was predicted across birthweight.
Keywords: Morphine, Population Pharmacokinetics, Neonates, Hypoxic Ischemic Encephalopathy, Hypothermia
INTRODUCTION
Current understanding of the dose needs of commonly used drugs in neonates with hypoxic ischemic encephalopathy (HIE) receiving hypothermia therapy is limited. Alterations in drug pharmacokinetics are expected due to frequent associated hypoxic-ischemic injury to organs important in drug elimination (i.e. liver and kidney)(1–5) and potential changes in organ physiology and blood flow during hypothermia.(6) Therefore, it is critical to consider the unique clinical pharmacologic needs of this vulnerable population. For example, a recent clinical study examined the pharmacokinetics of gentamicin in neonates with HIE receiving hypothermia which demonstrated a marked reduction in gentamicin clearance and the need for extended dosing intervals.(7,8) Careful inquiry into the pharmacokinetics of other drugs used in the HIE population can help further advance customized, evidenced based dose recommendations.(9)
Morphine is commonly used in neonates with HIE to provide comfort and analgesia during therapeutic hypothermia and/or assisted ventilation. Morphine is metabolized in the liver by UDP glucuronosyltransferase 2B7 (UGT2B7) to morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G).(10) Delayed maturation of UGT2B7 in the neonate results in enzymatic activity <10% of adults and subsequent decreased morphine clearance and metabolite formation.(11) After formation, the metabolites are subsequently eliminated via the kidney, and kidney maturation and function influence metabolite pharmacokinetics.(12) In addition, M6G is an active metabolite and contributes to analgesia and sedation.(10) Understanding the pharmacokinetics of both morphine and its metabolites will therefore be necessary to develop a therapeutic framework for morphine in neonates with HIE receiving hypothermia.
Current pharmacokinetic data to support morphine dosing in neonates with HIE receiving hypothermia are limited. A small clinical study demonstrated neonates with HIE receiving hypothermia and standard morphine infusion rates frequently (n=6/7) had elevated morphine concentrations >300 ng/ml(13), which are exposures associated with toxicity in neonates including respiratory depression.(14) Morphine metabolites were not measured. Due to study design limitations of this secondary analysis, a quantitative pharmacokinetic framework was not established and no alternative dosing strategies were recommended. Nonetheless, this study clearly demonstrates the need for further inquiry. The objective of the current prospective clinical pharmacokinetic study was to evaluate the pharmacokinetics of morphine in neonates with HIE receiving hypothermia and use the information gained to develop a customized morphine dosing strategy in this population.
METHODS
Study Design
The Stanford University Institutional Review Board and University of California San Francisco (UCSF) Committee on Human Research approved the study. This was a prospective, open-label, clinical pharmacokinetic study of morphine in 20 neonates with HIE receiving hypothermia. It was conducted at two-centers: UCSF Benioff Children’s Hospital and Lucile Packard Children’s Hospital at Stanford University (LPCH). Both centers are an academic level III neonatal intensive care unit (NICU) with expertise in caring for neonates with HIE including therapeutic hypothermia. Informed parental consent was obtained for each patient.
Patients
Neonates with moderate to severe HIE treated with whole-body therapeutic hypothermia (33.5°C) and receiving morphine as part of standard of care were eligible for enrollment. Patients at each center meeting inclusion and exclusion criteria were enrolled consecutively. Eligibility criteria for hypothermia were based on the CoolCap(2) (at UCSF) or National Institute of Child Health and Human Development (at LPCH) studies.(15) Additional study exclusion criteria included: known major structural or chromosomal abnormality; requirement of continuous renal replacement therapy (CRRT), peritoneal or hemodialysis; requirement of extracorporeal membrane oxygenation (ECMO); or decision made to withhold full support.
Morphine Administration
Morphine was administered intravenously as morphine sulfate at both centers. The decision to prescribe morphine and the dosing regimen were per the treating clinical team. At the time of study, the recommended starting morphine dose at UCSF was 20 μg/kg/h via continuous intravenous infusion with a planned dose reduction to 10 μg/kg/h at 24 hours after the onset of hypothermia treatment. The recommended starting morphine dose at LPCH for neonates with HIE receiving hypothermia was 40 μg/kg every 6 hours given over 10-minutes. This dose was lower than the standard morphine dose of 50-100 μg/kg every 4 h used in full-term neonates without HIE at LPCH and was reduced based on the concern for elevated morphine concentrations at standard doses in neonates with HIE receiving hypothermia.(13) At LPCH, morphine could alternatively be given as a continuous intravenous infusion of 10 to 20 μg/kg/h. At both centers, the dose was adjusted based on assessment of clinical need (i.e. pain, discomfort, and shivering) as determined by the treating clinical team. Additional intermittent bolus doses of morphine (50 – 100 μg/kg) were given as needed for pain, discomfort, and shivering.
Pharmacokinetic sampling
Dried blood spot (DBS) samples for pharmacokinetic analysis were collected during hypothermia. Samples were collected during two different time periods for each patient while receiving morphine. Period 1 was between 12 and 48 hours after the start of hypothermia, and Period 2 was between 48 and 72 hours after the start of hypothermia. Flexibility in the timing of pharmacokinetic samples was allowed. For patients receiving a continuous infusion, two DBS samples at least 4 hours apart were collected during Period 1 and Period 2 (i.e. total of 4 DBS samples). For patients receiving intermittent dosing, DBS samples were collected at the following times relative to a morphine dose: Period 1 = pre-dose, 0.25-0.75h, 1-2h, 3-4h, and 5-6h; Period 2 = 0.25-3h and 4-6h. Samples were collected around the same dose for a given period.
For each DBS sample, ~100 μL whole blood was collected on Whatman 903 filter paper cards (~50 μL whole blood/spot × 2 spots) from an in-dwelling catheter already in place for clinical care. After allowing to air dry for 4-24 hours, the DBS sample was then stored at less than −80° C until analysis. The concentrations of morphine, M3G, and M6G in whole blood were measured using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) DBS assay by iC42 (Denver, CO).(16) The lower limit of quantification was 1 ng/ml for morphine and 5 ng/ml for M3G and M6G. The calibration curves were linear from 1–1000 ng/mL for morphine and 5–1000 ng/mL for M3G and M6G. The within-run and between-run coefficients of variation were ≤ 11% for morphine, ≤ 11% for M3G and ≤ 15% for M6G. The median duration of time DBS samples were stored at −80° C prior to LC-MS/MS analysis was 5.5 months (range 2.3-8.2 months). A previous study has shown morphine to be stable in DBS for at least 6 months at −20° C.(17)
Population Pharmacokinetic Analysis
A population pharmacokinetic model was developed from the morphine, M3G, and M6G concentration time data using the nonlinear mixed-effects modeling program NONMEM (Version VII, Icon Development Solutions). The first order conditional estimation method with interaction was used throughout the model building and evaluation process. Morphine and both metabolites were simultaneously fitted. The dose of morphine sulfate in μg was converted to morphine in μg according to morphinedose = FMW × morphine sulfatedose, where FMW is the fractional difference in molecular mass between morphine and morphine sulfate equal to 0.853. Similarly, concentrations of M3G and M6G were converted to ng morphine units per ml using a FMW of 0.618.
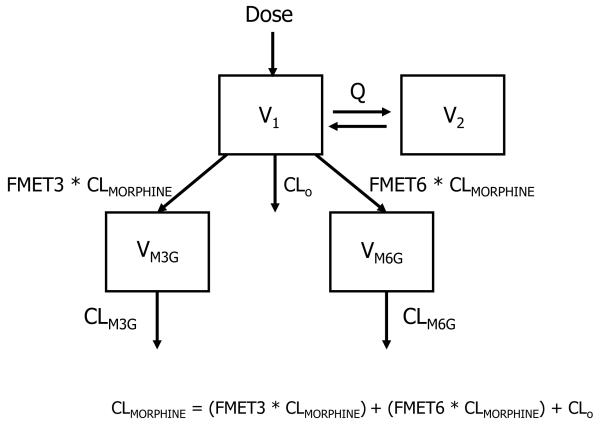
The pharmacokinetic structural model implemented is shown in Figure 1. For morphine, both one- and two-compartment models with first-order elimination were examined, parameterized in terms of total clearance (CLMORPHINE), inter-compartmental clearance (Q), central (V1) and peripheral (V2) volume of distribution. Formation clearances of M3G and M6G were represented as fractions (FMET3 and FMET6) of morphine total clearance. FMET3 and FMET6 were estimated in the model. Morphine elimination by other routes was calculated as CLO = CLMORPHINE × (1- FMET3 - FMET6). The pharmacokinetics of the metabolites were described by a one-compartment model parameterized in terms of elimination clearances (CLM3G and CLM6G) and distribution volumes (VM3G and VM6G). VM3G and VM6G cannot be identified with the present study design and were fixed to 1.2 L and 1.5 L, respectively, based on reported adult literature values after scaling linearly for weight.(18,19)
Figure 1.
Schematic representation of the structural pharmacokinetic model for morphine and its glucuronides. CLMORPHINE, total morphine clearance; Q, intercompartment morphine clearance; V1, central volume of morphine; V2, peripheral volume of morphine; FMET3, fraction metabolized to morphine-3-glucuronide; FMET6, fraction metabolized to morphine-6-glucuronide; ClO, morphine clearance by routes other than glucuronidation (calculated); CLM3G, elimination clearance of morphine-3-glucuronide; CLM6G, elimination clearance of morphine-6-glucuronide; VM3G, volume of morphine-3-glucuronide (fixed); VM6G, volume of morphine-6-glucuronide (fixed).
Interindividual variability in pharmacokinetic parameters was evaluated using an exponential error model. To model the residual (or intraindividual) variability, a proportional error model was implemented. Selection between structural models was based on the difference in the NONMEM objective function value (OFV) and visual comparison of standard diagnostic plots. The difference in OFV between two models has an approximate χ2 distribution with degrees of freedom equal to the difference in the number of parameters between models. Significance was set at a decrease in OFV larger than 10.83, corresponding to a p<0.001.
Once the structural pharmacokinetic model was established, the covariate model was developed. Based on previous population pharmacokinetic models for a range of compounds in neonates and children, an allometric model of birthweight was first implemented to account for the influence of body size on elimination clearances (CLMORPHINE, CLM3G, and CLM6G) and volume of distribution (V1 and V2).(20) The exponent defining the relationship of birthweight and clearance was examined both as a fixed (0.75) and an estimated parameter (k). The exponent defining the relationship of birthweight and volume was fixed to one. Next, the potential effect for maturational changes of UGT2B7 in neonates was explored using gestational age (GA) as a predictor of CLMORPHINE applying a sigmoid Emax maturation function. Due to the relatively limited range of GA in the study population (interquartile range [IQR]: 37.2 to 40.4 wks), the sigmoid Emax maturation function was not identifiable. Therefore, parameters were fixed to those reported by Holford et al. (maturation half-life [TM50] = 58.1 weeks; slope parameter for the sigmoid Emax model [Hill] = −3.58).(21)
Additional biologically and/or clinically plausible covariates were also evaluated for their influence on pharmacokinetic parameters. Continuous covariates investigated included postnatal age, serum creatinine (SCr), and urine output. Biomarkers of severity of hypoxic-ischemic injury at birth were also evaluated (e.g. lowest blood pH, highest blood base deficit, APGAR score at five minutes). Categorical covariates evaluated included concomitant medications (dopamine, lorazepam, gentamicin, phenobarbital), ‘severe’ HIE diagnosis (based on the modified Sarnat classification scale(22)), diagnosis of pulmonary hypertension, need for assisted ventilation, and alanine aminotransferase ≥ 100 U/L. The effect of a continuous covariate on a parameter was modeled using a power function. Continuous covariates were scaled to their median values. Categorical covariates were modeled proportionally, i.e. the fractional change in clearance when the categorical covariate was true.
The covariate model was built using a standard forward addition backward deletion procedure. Covariates were added in a stepwise manner to the model in the order of their reduction in the OFV. During forward stepwise addition, a covariate was allowed to enter the model as long as the decrease in OFV due to its addition was larger than 3.84, corresponding to a p<0.05. After the stepwise addition terminates, the model is pruned using backward elimination. Covariates were eliminated one at a time, until the removal of a covariate results in an OFV increase of more than 10.83, corresponding to a p<0.001.
To evaluate the accuracy and stability of the final pharmacokinetic model, a non-parametric bootstrap re-sampling method was performed using the NONMEM support software Perl-speaks-NONMEM (PsN, Version 3.6.2). A total of 1000 bootstrap datasets were generated from the original data set by repeated sampling with replacement, and the final pharmacokinetic model was used to estimate model parameters for each data set. In addition, the final pharmacokinetic model was assessed using an internal evaluation procedure by computing the normalized prediction distribution errors (NPDE) of 5000 simulated datasets compared to the observed dataset.(23,24)
Dose-Exposure Relationships
Simulations were conducted to evaluate morphine sulfate dosing regimens in neonates with HIE receiving hypothermia. Using the final population pharmacokinetic model parameter estimates, the pharmacokinetic profiles of 1,000 neonates were repeatedly simulated. Morphine sulfate doses given as a continuous intravenous infusion (range, 2.5 to 20 μg/kg/h) and intermittent dose (range, 20 to 100 μg /kg) every 6 h were examined. Birthweights evaluated ranged from 2.5 to 4.5 kg. The predicted morphine, M3G, and M6G concentrations at steady-state were examined for each simulation. While morphine concentrations needed in neonates for analgesia are not firmly established (and may vary by patient depending on age, disease, concomitant medications, pain sources, etc.), effective concentrations in the range of 10 – 40 ng/ml have been reported.(25–28) Therefore, ‘optimized’ morphine sulfate doses were selected that maximized achievement of morphine concentrations between 10 – 40 ng/ml across simulated neonates. In addition, a sensitivity analysis was conducted to evaluate the influence of serum creatinine on metabolite concentrations. Simulations were conducted as described above, however, the dose was held constant while the serum creatinine was varied from 0.4 to 1.6 mg/dL between simulations.
RESULTS
Patients and Morphine Concentrations
Twenty neonates (LPCH n=13; UCSF n=7) with HIE treated with hypothermia were enrolled and had morphine data available for analysis. Patient characteristics are shown in Table 1. Morphine was given as intermittent dosing in 10 neonates and continuous infusion in 10 neonates. The median (range) intermittent dose was 40 μg/kg (40-60 μg/kg) every 6 h during Period 1. No dose changes were made between Period 1 and Period 2. The median (range) continuous infusion was 15 μg/kg/h (10-20 μg/kg/h) during Period 1 and 10 μg/kg/h (10-20 μg/kg/h) during Period 2.
Table 1.
Patient Demographics (n = 20)
| Median or No. |
IQR | Min, Max | |
|---|---|---|---|
|
| |||
| Gestational Age, wks | 39.1 | 37.2 – 40.4 | 36.0, 41.3 |
|
| |||
| Birthweight, kg | 3.50 | 3.03 – 3.71 | 1.82, 5.3 |
|
| |||
| Female, n (%) | 13 (65%) | ||
|
| |||
| Race / Ethnicity, n (%) | |||
| White | 9 (45%) | ||
| Hispanic | 8 (40%) | ||
| Asian | 3 (15%) | ||
|
| |||
| APGAR | |||
| 5 min | 4 | 2 – 6 | 0, 8 |
| 10 min | 6 | 5 – 7 | 0, 8 |
|
| |||
| First arterial or capillary pH | 6.9 | 6.9 – 7.1 | 6.6, 7.2 |
|
| |||
| Base Deficit, mmol/L | −18 | −15 – −20 | −12, −25 |
|
| |||
| Crmax, mg/dL | 1.1 | 0.8 – 1.3 | 0.5, 2.0 |
|
| |||
| ALTmax, U/L | 32 | 18 – 78 | 15, 558 |
|
| |||
| ALTmax >100 U/L, n (%) | 3 (15%) | ||
|
| |||
| Temperature core, °C | 33.5 | 33.4 – 33.7 | 33.1, 33.7 |
|
| |||
| Assisted Ventilation, n (%) | 14 (70%) | - | - |
|
| |||
| Seizures, n (%) | 4 (20%) | - | - |
|
| |||
| Dopamine, n (%) | 6 (30%) | - | - |
|
| |||
| Death, n (%) | 1 (5%) | - | - |
IQR, Interquartile range; Crmax, maximum serum creatinine during hypothermia; ALTmax, maximum alanine aminotransferse during hypothermia; Temperature core, esophageal temperature at time of pharmacokinetic sampling.
All patients completed pharmacokinetic sampling as scheduled except one neonate who had pharmacokinetic sampling only during Period 1 (intermittent dosing schedule) due to redirection of the goals of care and early discontinuation of therapeutic hypothermia. No morphine, M3G, or M6G concentrations were below the limit of quantification. Two DBS samples from one patient were considered outliers (morphine concentrations 1560 ng/ml and 454 ng/ml) and were not included in the analysis. All other morphine concentrations for this patient were < 31 ng/ml. Therefore, a total of 106 concentrations were available each for morphine, M3G, and M6G.
Population Pharmacokinetic Analysis
The time course of morphine whole blood concentrations were best described by a two-compartment model with first-order elimination and an exponential error model for interindividual variability on morphine clearance (CLMORPHINE) and central volume (V1). The peripheral volume of morphine (V2) was not found to be significantly different than V1. Therefore, V2 was fixed to equal V1, which improved the precision of model parameter estimates and reduced interindividual variability.
The whole blood concentrations of the M3G and M6G metabolites were each adequately described in terms of formation clearance represented as a fraction (FMET3 and FMET6, respectively) of total morphine clearance followed by first-order elimination from a one-compartment model. Interindividual variability in the elimination clearance of M3G (CLM3G) and M6G (CLM6G) were not found to be significantly different, and a single exponential error model was used to describe the interindividual variability for both metabolite clearances. This is congruent with both metabolites having the same route of elimination (i.e. renal). After accounting for the fraction of morphine metabolized to M3G and M6G, morphine clearance by other routes (ClO) accounted for 62% of morphine clearance. The residual (or intra-individual) variability was best described by a proportional error model with different errors for morphine, M3G and M6G.
Once the structural model was established, the covariate model was developed. Birthweight was highly predictive of CLMORPHINE, CLM3G, and CLM6G (ΔOFV −15; p<0.001). The exponent (kwt) defining the allometric relationship of birthweight and clearances was estimated to be 1.23. Fixing kwt = 0.75 resulted in a 2.7 increase in objective function. Given a previous report that found kwt = 1.44 for morphine pharmacokinetics in neonates, k was allowed to be estimated in the final model.(12) Estimating different values of kwt for CLMORPHINE, CLM3G, and CLM6G did not improve the model. No significant relationship between birthweight and volume was identified (ΔOFV +2.2; p> 0.05), and therefore birthweight as a covariate on volume was not included in the final model.
After incorporating the allometric model of weight on morphine and metabolite clearances, serum creatinine was identified as a significant predictor of M3G and M6G clearance (ΔOFV −52; p<0.001). In addition, GA (ΔOFV −4.7; p<0.05) and dopamine (ΔOFV −6.4; p<0.05) were found to significantly impact morphine clearance during forward stepwise addition. However, during backward elimination, neither met the statistical criteria of p<0.001 for remaining in the final model and were removed. No other significant covariates were identified. Of note, a model implementing both a fixed allometric weight exponent of 0.75 and GA on clearance was not significantly better compared to our final model having only an estimated allometric weight exponent (ΔOFV −2.6; p>0.05).
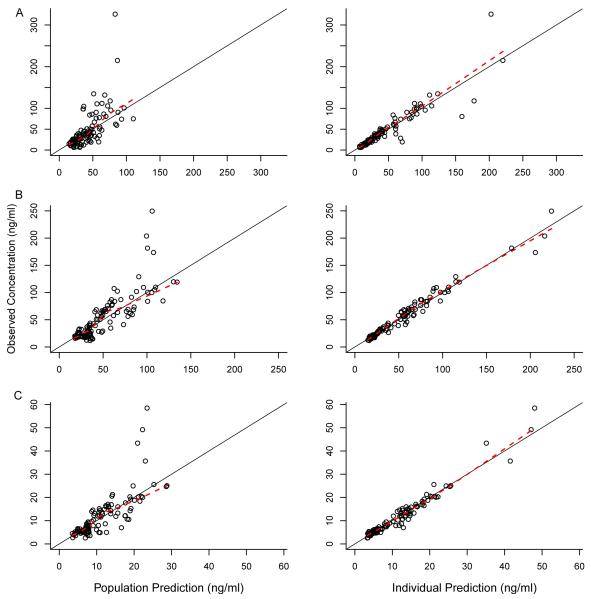
The final population pharmacokinetic model parameter estimates are presented in Table 2. In general, observed versus population and individual predicted concentrations showed no systemic bias (Figure 2), and the weighted residuals were homogeneously scattered for both parent and metabolites (data not shown). The parameter estimates as found by bootstrap were in agreement with those obtained by the final population pharmacokinetic model (Table 2), indicating reliability of the final model estimates. Internal model evaluation also demonstrated that the final model performed well in describing the observed data. The mean (variance) NPDE was 0.01 (0.95) for morphine, 0.02 (1.0) for M3G, −0.01 (0.93) for M6G. The theoretical mean NPDE is zero with a variance 1.0. The percentage of observations that fell inside the theoretical 90% prediction interval were 92%, 91%, and 90% for morphine, M3G, and M6G, respectively. In addition, there were no major trends in NPDE across time, birthweight, or GA (Figure 3).
Table 2.
Final population PK model parameter estimates and bootstrap results.
| Final Model | Bootstrap (n=1000) | |||
|---|---|---|---|---|
| Parameter | Estimate | %SE | Median | 95% CI |
| Fixed Effects | ||||
| kWT | 1.23 | 18.9 | 1.24 | 0.58– 1.93 |
| CLMORPHINE (L/h) | 0.765 | 10.9 | 0.759 | 0.609 – 0.930 |
| V1 = V2 (L) | 4.01 | 22.2 | 3.89 | 2.57 – 5.41 |
| Q (L) | 3.47 | 28.3 | 3.58 | 0.769 – 5.51 |
| FMET3 | 0.367 | 21.4 | 0.359 | 0.222 – 0.537 |
| FMET6 | 0.085 | 18.2 | 0.082 | 0.054 – 0.114 |
| CLM3G (L/h) | 0.188 | 29.9 | 0.180 | 0.086 – 0.303 |
| CLM6G (L/h) | 0.197 | 22.4 | 0.186 | 0.103 – 0.284 |
| kSCr | 0.536 | 39.6 | 0.549 | 0.318 – 0.940 |
| VM3G (L) | 1.15 (fixed) | - | - | - |
| VM6G (L) | 1.5 (fixed) | - | - | - |
| Interindividual variability | ||||
| CLMORPHINE, %CV | 48.2 | 35.8 | 47.1 | 29.8 – 65.6 |
| V1, %CV | 49.5 | 53.9 | 47.1 | 11.9 – 78.9 |
| CLM3G / CLM6G, %CV | 34.2 | 35.1 | 33.6 | 20.8 – 46.7 |
| Residual Error | ||||
| Morphine, %CV | 22.1 | 37.4 | 21.8 | 14.3 – 28.8 |
| M3G, %CV | 13.2 | 17.1 | 12.8 | 10.3 – 14.9 |
| M6G, %CV | 15.7 | 20.6 | 15.4 | 12.4 – 18.8 |
kWT exponent scaling factor for weight on clearances; CLMORPHINE, total morphine clearance; V1, central volume of morphine; V2, peripheral volume of morphine; Q, intercompartment morphine clearance; FMET3, fraction metabolized to morphine-3-glucuronide; FMET6, fraction metabolized to morphine-6-glucuronide; CLM3G, elimination clearance of M3G; CLM6G, elimination clearance of M6G; kSCr exponent scaling factor for serum creatinine on metabolite elimination clearances; VM3G, volume of M3G; VM6G, volume of M6G; M3G, morphine-3-glucuronide; M6G, morphine-6-glucuronide; %CV, coefficient of variation × 100; %SE, relative standard error × 100; 95% CI, Bootstrap parameter estimate at the 2.5th and 97.5th percentiles.
Figure 2.
Observed vs. population predicted concentrations and observed vs. individual predicted concentrations for (A) morphine, (B) morphine-3-glucuronide, and (C) morphine-6-glucuronide. Solid line indicates the line of unity. Dashed line indicates loess smooth.
Figure 3.
Normalized prediction distribution errors (NPDE) of the final pharmacokinetic model for (A) morphine, (B) morphine-3-glucuronide, and (C) morphine-6-glucuronide. Histograms show the kernel density plot of NPDE with a normal, Gaussian distribution overlaid for comparative purposes. The distribution of NPDE versus time since first dose and birthweight are also shown. Dotted lines represent the 5% and 95% of a standard normal distribution (i.e. 90% of npde should fall between this range). Dashed line indicates loess smooth of NPDE.
Dose-Exposure Relationships
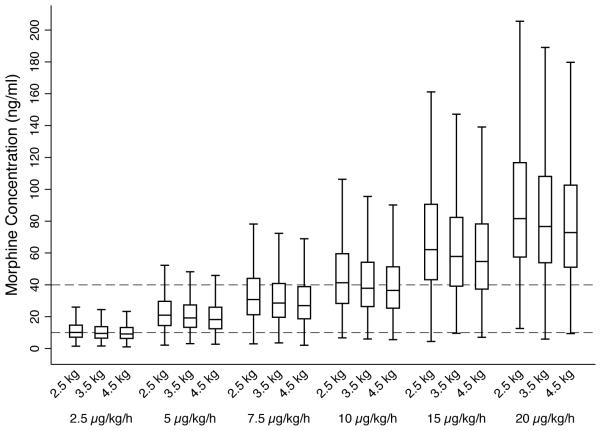
Morphine exposure after various morphine sulfate dosing regimens in neonates with HIE receiving hypothermia were simulated using the final pharmacokinetic model and Monte Carlo methods. A continuous morphine infusion of 5 μg/kg/h was predicted to maximize achievement of target morphine concentrations of 10 – 40 ng/ml (Figure 4). At a continuous morphine infusion of 5 μg/kg/h, 81% of simulated neonates weighing 3.5 kg achieved a concentration within the target morphine concentration range. At a morphine infusion of 10 μg/kg/h, a dose that is routinely used for analgesia and sedation in full-term neonates without HIE, only 54% of neonates with HIE receiving hypothermia achieved a concentration within the target range and 46% had a morphine concentration >40 ng/ml.
Figure 4.
Predicted morphine concentrations at different continuous morphine infusion rates by birthweight. Each boxplot represents steady-state concentrations from 1000 simulated neonates with HIE receiving hypothermia. Outliers are not presented. Dashed lines reference target concentrations between 10 to 40 ng/ml.
Overall, variability in morphine exposure was high between neonates with >4-fold variation in concentrations at a given dose and weight. For example, the 90% range of morphine steady-state concentrations in simulated neonates was 9.6 to 37.4 ng/ml at an infusion of 5 μg/kg/h and birthweight 3.5 kg. The impact of birthweight on the achieved morphine concentration for a given dose in μg/kg was small and likely not clinically relevant in terms of starting dose selection (Figure 4). Similar trends were seen for intermittent q6h dosing. An intermittent dose of 40 to 50 μg/kg every 6 h was predicted to maximize achievement of target trough morphine concentrations (Supplemental Figure S1).
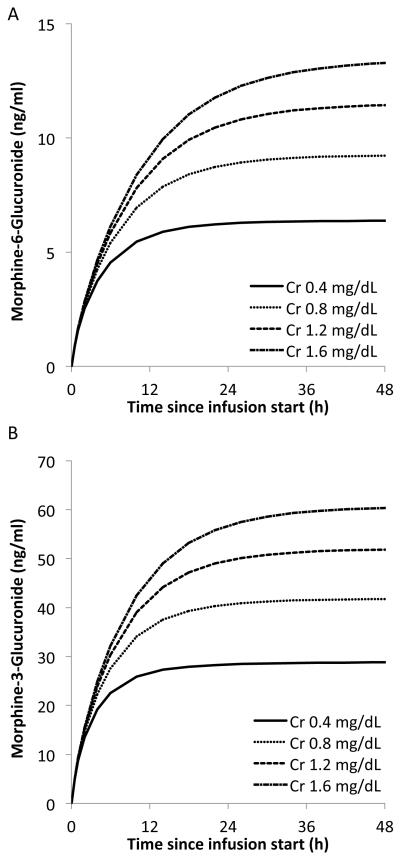
Based on the final model, metabolite concentrations were impacted by serum creatinine. M6G clearance was reduced by approximately 30 % (from 0.26 to 0.18 L/h), while serum creatinine increases from 0.6 to 1.2 mg/ml for a 3.5 kg neonate. Figure 5A shows the time-course of the active metabolite M6G at different serum creatinine levels in the median neonate weighing 3.5kg after a 50 μg/kg loading dose followed by a continuous morphine infusion of 5 μg/kg/h. As serum creatinine increased, higher M6G concentrations were predicted. In addition, metabolite concentrations continued to accumulate over the first 12-24 hours of dosing, which may be clinically relevant given M6G is an active metabolite resulting in an increased effect over time despite a stable dose. A similar trend was seen for the metabolite M3G (Figure 5B).
Figure 5.
Impact of serum creatinine (Cr) on the accumulation of the morphine metabolites: (A) morphine-6-glucuroinde and (B) morphine-3-glucuroinde. Each line represents the concentration time-course of the metabolite by serum creatinine level after a 50 μg/kg loading dose followed by a continuous morphine infusion of 5 μg/kg/h in a neonate weighing 3.5kg.
DISCUSSION
This is the first study to describe the population pharmacokinetics of morphine and its glucuronide metabolites in neonates with HIE receiving hypothermia. Similar to previous morphine population pharmacokinetic models in neonates without HIE and young children, morphine pharmacokinetics were adequately described by a two compartment model for morphine and one compartment model for each of the metabolites.(12,29,30) The major study finding was that morphine clearance was markedly lower in neonates with HIE receiving hypothermia compared to reports in full-term normothermic neonates <7 day old without HIE.(12,21,30) Accordingly, lower morphine doses will be needed in this vulnerable population of neonates to achieve exposures suggested for analgesia in neonates and children without hypothermia.
Understanding the unique drug dose needs of neonates with HIE receiving hypothermia will be essential to continuing the advancement of their clinical care.(6) Extrapolation of general pharmacologic understanding from non-asphyxiated, normothermic neonates can be a useful starting point. For example, the pharmacokinetics of morphine in neonates is already known to be highly variable,(12,21,30) and therefore variation in morphine pharmacokinetics in neonates with HIE receiving hypothermia is expected. In addition, kidney function is known to impact elimination of the active M6G metabolite.(29,31,32) However, neonates with HIE receiving hypothermia represent a distinct and vulnerable population from a pharmacologic perspective and extrapolation of dose strategies from non-asphyxiated, normothermic neonates are likely inappropriate.(6) For example, when standard gentamicin doses of 4-5 mg/kg every 24 h were given to neonates with HIE receiving hypothermia, potentially toxic trough concentrations frequently occurred.(7,33) In contrast, a customized every 36 h dosing strategy that was developed based on an in-depth quantitative pharmacokinetic understanding of gentamicin in neonates with HIE receiving hypothermia, reliably achieved target drug exposures.(8) Similarly, a detailed examination of morphine pharmacokinetics in neonates with HIE receiving hypothermia is essential to help understand optimal dosing strategies of morphine in this population.
In the final population pharmacokinetic model, the clearance of morphine and its glucuronide metabolites in neonates with HIE receiving hypothermia were significantly influenced by birthweight. Body size is a fundamental scalar of clearance in neonates, and weight has been previously described as a predictor of clearance in morphine and its metabolites in non-asphyxiated neonates.(12,30) In these previous pharmacokinetic reports in neonates, the allometric exponent describing the relationship between weight and morphine clearance has been both estimated (kwt=1.44)(12) and fixed (kwt =0.75)(30). It is unknown what impact hypothermia may have on the theory-based relationship between size and clearance that has been used to justify fixing the exponent to 0.75. For example, hypothermia may impact clearance to a greater degree in lower weight children than higher weight children. Therefore, in the current population pharmacokinetic model, the exponent was estimated and found to be 1.23.
After incorporating weight, the addition of a maturation function on morphine clearance using gestational age did not improve the model. Our study was limited by the narrow range of GA studied, which is inherent to the criteria utilized to qualify for hypothermia therapy (GA ≥ 36 weeks) in neonates with HIE as based on the original randomized controlled trials. Therefore, our study likely had low power to find a developmental age effect on clearance. However, the lack of impact of developmental age on morphine clearance was also found in a larger population of non-asphyxiated neonates when the allometric weight exponent was estimated as in the current study.(12) Extrapolation of our pharmacokinetic model to neonates outside the range of GA and birthweight studied is not recommended.
Biomarkers of injury to organs important in drug elimination such as the liver and kidney may be helpful in identifying neonates with altered drug clearance. For example, we examined whether neonates with an elevated ALT had reduced clearance of morphine, which is metabolized by UGT2B7 in the liver. A significant impact of ALT on morphine clearance was not found. In contrast, serum creatinine as a measure of renal function was a significant predictor of the clearance of both metabolites, which are eliminated almost exclusively via the kidney. The ability to characterize the impact of serum creatinine on metabolite clearance but not the impact of ALT on morphine clearance in our study is likely multi-factorial. Serum creatinine is a specific biomarker of kidney function while ALT indicates only hepatocyte injury and not function directly. In addition, while serum creatinine during the first days of life can be confounded by placental transfer, in neonates with HIE serum creatinine is frequently elevated beyond what would be expected from placental transfer in pregnant women (serum creatinine reference range 0.4–0.9 mg/dl)(3,4,34,35). In our current study, nine (45%) neonates had a serum creatinine ≥ 1.2 mg/dl. Therefore, when serum creatinine is elevated beyond what is expected from placental transfer, it may provide predictive information of underlying poor kidney function in neonates even during the first days of life. Serum creatinine was similarly shown to be predictive of gentamicin pharmacokinetics (a renal eliminated drug) in neonates with HIE receiving hypothermia.(7) Conversely, few neonates in our study had an elevated ALT (three neonates had ALT ≥ 100 U/L; only one neonate had ALT ≥ 500 U/L), and there was likely low power to uncover an effect of ALT on morphine clearance in our study. Consideration of liver injury may still be helpful in understanding morphine pharmacokinetics in neonate with HIE receiving hypothermia. For example, the lowest morphine clearance (scaled for weight) in our study population was observed in a neonate with the greatest liver injury (ALT >500 U/L). This neonate also had other clinical surrogates indicating multi-organ failure and likely altered physiology including mechanical ventilation need, inotropic support, pulmonary hypertension, and low urine output.
The independent effect of hypothermia on the pharmacokinetics of morphine was not able to be directly examined in the current study since all neonates received hypothermia. Since hypothermia is now firmly established as standard of care in neonates with HIE, similar limitations will face all future pharmacokinetic studies in this population.(36) Analyses applied to investigate the effect of hypothermia have included comparing a drug’s pharmacokinetics in the same group of neonates with HIE during hypothermia (first 72 hours of life) versus after the neonate is rewarmed and normothermic (after 72 hours of life).(37) However, neonates with HIE have changing physiology over the first days of life independent of hypothermia. For example due to organ maturation, drug clearance will increase during the first week of life.(38) In addition, hypoxic injury to the liver and kidney will also recover concurrently during this same time period. Therefore, isolating the independent effect of hypothermia will be challenging and will require large, robust, and longitudinal data that provide enough power to model for the independent effect of maturation, organ dysfunction, and hypothermia.
Compared to reports in normothermic neonates without HIE, morphine clearance was reduced in neonates with HIE receiving hypothermia. The predicted morphine clearance in our study for a 3.5 kg neonate with HIE receiving hypothermia was 0.765 L/h, which is almost 50% lower than that reported for a normothermic neonate without HIE of 1.42 L/h(12) and 1.53 L/h(30). Our results are in line with previous pharmacokinetic studies in neonates with HIE receiving hypothermia, which have found reductions in drug clearance for gentamicin(7), phenobarbital(39) and topiramate(40). The volume of distribution for a 3.5 kg neonate in our study (V1 + V2 = 8.0 L) was smaller than predicted based on a two-compartment model in neonates without HIE (V1 + V2 = 12.7 L).(12) Decreases in volume during hypothermia have been reported for other drugs including for morphine in dogs.(41,42)
Due to the reduced clearance in neonates with HIE receiving hypothermia, typical recommended morphine sulfate doses for full-term neonates will be inappropriate. To develop optimized morphine sulfate dosing regimens for neonates with HIE receiving hypothermia, the final pharmacokinetic model was used to perform Monte Carlo simulations and examine different dosing strategies. A morphine sulfate loading dose of 50 μg/kg followed by a continuous infusion of 5 μg/kg/h was predicted to maximize target morphine drug concentrations of 10-40 ng/L. If intermittent dosing is used, a dose of 40 to 50 μg/kg every 6 hours was predicted.
A limitation to our exposure matching approach is that morphine concentrations needed for analgesia in neonates with HIE receiving hypothermia are not established. It is also unclear how to account for the concentration of the active metabolite (M6G) in the therapeutic strategy since available pediatric pharmacodynamic data in the literature examined only morphine concentrations. However, the morphine exposure target utilized was extrapolated from previous studies in infants and neonates based on morphine concentrations shown to be effective and safe.(25–28) The modeling and simulation approach applied provides an evidenced based pharmacokinetic framework and starting point for the typical dose needs in neonates with HIE receiving hypothermia. In addition, the understanding gained can shed light on therapeutic considerations during morphine treatment in neonates with HIE receiving hypothermia. For example, accumulation of the active metabolite M6G is expected over the first 12-24 hours after starting an infusion, which may be clinically relevant resulting in an increased (side) effect over time. Future pharmacodynamic studies of morphine in neonates will benefit by incorporation of both morphine and M6G concentrations in determining exposure-response. Lastly, due to the large variability in pharmacokinetics between neonates with HIE receiving hypothermia, further individualization in the therapeutic strategy for a given neonate will be necessary.
Performing clinical pharmacokinetic trials in critically ill neonatal populations is challenging due to limitations in clinical trial infrastructure, low consent rates, and restrictions in blood sampling volumes.(43) The current study was facilitated by enrolling from two centralized, multidisciplinary neonatal neurocritical care units that emphasize continued practice improvement through evidenced based clinical research.(44) We also were able to utilize micro-volume pharmacokinetic sampling using DBS to minimize blood volume requirements.(16) Morphine concentrations in blood and plasma are known to be equivalent and allowed direct comparison of our study results to previous studies that measured drug concentrations in plasma.(45,46) Lastly, our study design took advantage of medication administered as standard of care. Taken together, our study was completed in <12 months and our consent rate approached over 95%. The current study design may serve as a model for future drug trials in critically ill neonates.
Conclusions
In neonates with HIE receiving hypothermia, morphine clearance is markedly decreased compared to previous studies in full-term normothermic newborns without HIE. To avoid excessive accumulation of morphine, dosing strategies customized for this vulnerable population are needed. A continuous infusion of 5 μg/kg/h is predicted to optimize achievement of target concentrations associated with analgesia. For intermittent dosing, a dose of 40 to 50 μg/kg every 6 hours is predicted to be needed. Due to large variation between neonates with HIE in pharmacokinetics and pathophysiology, further individualization of the therapeutic strategy based on clinical considerations will be required.
Supplementary Material
Acknowledgements
We would like to thank Bethany Ball, May Zepeda, Jaspreet Sangha, MD, and members of the leadership and nursing teams in the LPCH Stanford NeuroNICU and UCSF Neuro-Intensive Care Nursery for helping support this project.
Funding
The work was supported by the Child Health Research Institute, Lucile Packard Foundation for Children’s Health; the Stanford Clinical Translational Science Award program funded by the National Center for Advancing Translational Sciences at the National Institutes of Health [grant number UL1TR001085]; and the Eunice Kennedy Shriver National Institute of Child Health and Human Development [grant number K23HD079557].
Footnotes
Declaration of Conflicting Interests
The authors have no conflict of interest, real or perceived, to report.
Previously Presented
Findings of this work in part were submitted as an abstract for the 2016 Pediatric Academic Societies Annual Meeting, Baltimore, MD, May 2016.
REFERENCES
- 1.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009 Oct 1;361(14):1349–58. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 2.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005 Feb 19;365(9460):663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 3.Róka A, Vásárhelyi B, Bodrogi E, Machay T, Szabó M. Changes in laboratory parameters indicating cell necrosis and organ dysfunction in asphyxiated neonates on moderate systemic hypothermia. Acta Paediatr. 2007 Aug;96(8):1118–21. doi: 10.1111/j.1651-2227.2007.00361.x. [DOI] [PubMed] [Google Scholar]
- 4.Shah P, Riphagen S, Beyene J, Perlman M. Multiorgan dysfunction in infants with post-asphyxial hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2004 Mar;89(2):F152–155. doi: 10.1136/adc.2002.023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarcan A, Tiker F, Güvenir H, Gürakan B. Hepatic involvement in perinatal asphyxia. J Matern Fetal Neonatal Med. 2007 May;20(5):407–10. doi: 10.1080/14767050701287459. [DOI] [PubMed] [Google Scholar]
- 6.Zanelli S, Buck M, Fairchild K. Physiologic and pharmacologic considerations for hypothermia therapy in neonates. J Perinatol. 2011 Jun;31(6):377–86. doi: 10.1038/jp.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frymoyer A, Meng L, Bonifacio SL, Verotta D, Guglielmo BJ. Gentamicin Pharmacokinetics and Dosing in Neonates with Hypoxic Ischemic Encephalopathy Receiving Hypothermia. Pharmacotherapy. 2013 Apr 1; doi: 10.1002/phar.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frymoyer A, Lee S, Bonifacio SL, et al. Every 36-hour Gentamicin Dosing in Neonates with Hypoxic Ischemic Encephalopathy Receiving Hypothermia. J Perinatol. 2013 doi: 10.1038/jp.2013.59. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.IOM (Institute of Medicine) Safe and Effective Medicines for Children: Pediatric Studies Conducted Under the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act. The National Academies Press; Washington, DC: 2012. [PubMed] [Google Scholar]
- 10. [cited 2013 Sep 26];Codeine and Morphine Pathway, Pharmacokinetics [PharmGKB] [Internet] Available from: http://www.pharmgkb.org/pathway/PA146123006.
- 11.de Wildt SN, Kearns GL, Leeder JS, van den Anker JN. Glucuronidation in humans. Pharmacogenetic and developmental aspects. Clin Pharmacokinet. 1999 Jun;36(6):439–52. doi: 10.2165/00003088-199936060-00005. [DOI] [PubMed] [Google Scholar]
- 12.Knibbe CAJ, Krekels EHJ, van den Anker JN, et al. Morphine glucuronidation in preterm neonates, infants and children younger than 3 years. Clin Pharmacokinet. 2009;48(6):371–85. doi: 10.2165/00003088-200948060-00003. [DOI] [PubMed] [Google Scholar]
- 13.Róka A, Melinda KT, Vásárhelyi B, Machay T, Azzopardi D, Szabó M. Elevated morphine concentrations in neonates treated with morphine and prolonged hypothermia for hypoxic ischemic encephalopathy. Pediatrics. 2008 Apr;121(4):e844–849. doi: 10.1542/peds.2007-1987. [DOI] [PubMed] [Google Scholar]
- 14.Chay PC, Duffy BJ, Walker JS. Pharmacokinetic-pharmacodynamic relationships of morphine in neonates. Clin Pharmacol Ther. 1992 Mar;51(3):334–42. doi: 10.1038/clpt.1992.30. [DOI] [PubMed] [Google Scholar]
- 15.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005 Oct 13;353(15):1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 16.Clavijo CF, Hoffman KL, Thomas JJ, et al. A sensitive assay for the quantification of morphine and its active metabolites in human plasma and dried blood spots using high-performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011 May;400(3):715–28. doi: 10.1007/s00216-011-4775-z. [DOI] [PubMed] [Google Scholar]
- 17.Saussereau E, Lacroix C, Gaulier JM, Goulle JP. On-line liquid chromatography/tandem mass spectrometry simultaneous determination of opiates, cocainics and amphetamines in dried blood spots. J Chromatogr B Analyt Technol Biomed Life Sci. 2012 Feb 15;:885–886. 1–7. doi: 10.1016/j.jchromb.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 18.Hanna MH, Peat SJ, Knibb AA, Fung C. Disposition of morphine-6-glucuronide and morphine in healthy volunteers. Br J Anaesth. 1991 Jan;66(1):103–7. doi: 10.1093/bja/66.1.103. [DOI] [PubMed] [Google Scholar]
- 19.Penson RT, Joel SP, Clark S, Gloyne A, Slevin ML. Limited phase I study of morphine-3-glucuronide. J Pharm Sci. 2001 Nov;90(11):1810–6. doi: 10.1002/jps.1131. [DOI] [PubMed] [Google Scholar]
- 20.Anderson BJ, Holford NHG. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–32. doi: 10.1146/annurev.pharmtox.48.113006.094708. [DOI] [PubMed] [Google Scholar]
- 21.Holford NHG, Ma SC, Anderson BJ. Prediction of morphine dose in humans. Paediatr Anaesth. 2012 Mar;22(3):209–22. doi: 10.1111/j.1460-9592.2011.03782.x. [DOI] [PubMed] [Google Scholar]
- 22.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976 Oct;33(10):696–705. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- 23.Brendel K, Comets E, Laffont C, Mentré F. Evaluation of different tests based on observations for external model evaluation of population analyses. J Pharmacokinet Pharmacodyn. 2010 Feb;37(1):49–65. doi: 10.1007/s10928-009-9143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Comets E, Brendel K, Mentré F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed. 2008 May;90(2):154–66. doi: 10.1016/j.cmpb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Olkkola KT, Maunuksela EL, Korpela R, Rosenberg PH. Kinetics and dynamics of postoperative intravenous morphine in children. Clin Pharmacol Ther. 1988 Aug;44(2):128–36. doi: 10.1038/clpt.1988.127. [DOI] [PubMed] [Google Scholar]
- 26.Bouwmeester NJ, van den Anker JN, Hop WCJ, Anand KJS, Tibboel D. Age- and therapy-related effects on morphine requirements and plasma concentrations of morphine and its metabolites in postoperative infants. Br J Anaesth. 2003 May;90(5):642–52. doi: 10.1093/bja/aeg121. [DOI] [PubMed] [Google Scholar]
- 27.Krekels EHJ, Tibboel D, de Wildt SN, et al. Evidence-based morphine dosing for postoperative neonates and infants. Clin Pharmacokinet. 2014 Jun;53(6):553–63. doi: 10.1007/s40262-014-0135-4. [DOI] [PubMed] [Google Scholar]
- 28.Kart T, Christrup LL, Rasmussen M. Recommended use of morphine in neonates, infants and children based on a literature review: Part 2--Clinical use. Paediatr Anaesth. 1997;7(2):93–101. doi: 10.1111/j.1460-9592.1997.tb00488.x. [DOI] [PubMed] [Google Scholar]
- 29.Elkomy MH, Drover DR, Glotzbach KL, et al. Pharmacokinetics of Morphine and Its Metabolites in Infants and Young Children After Congenital Heart Surgery. AAPS J. 2016 Jan;18(1):124–33. doi: 10.1208/s12248-015-9826-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouwmeester NJ, Anderson BJ, Tibboel D, Holford NHG. Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. Br J Anaesth. 2004 Feb;92(2):208–17. doi: 10.1093/bja/aeh042. [DOI] [PubMed] [Google Scholar]
- 31.Milne RW, Nation RL, Somogyi AA, Bochner F, Griggs WM. The influence of renal function on the renal clearance of morphine and its glucuronide metabolites in intensive-care patients. Br J Clin Pharmacol. 1992 Jul;34(1):53–9. doi: 10.1111/j.1365-2125.1992.tb04107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolff J, Bigler D, Christensen CB, Rasmussen SN, Andersen HB, Tønnesen KH. Influence of renal function on the elimination of morphine and morphine glucuronides. Eur J Clin Pharmacol. 1988;34(4):353–7. doi: 10.1007/BF00542435. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Borooah M, Stone J, Chakkarapani E, Thoresen M. Serum gentamicin concentrations in encephalopathic infants are not affected by therapeutic hypothermia. Pediatrics. 2009 Jul;124(1):310–5. doi: 10.1542/peds.2008-2942. [DOI] [PubMed] [Google Scholar]
- 34.Abbassi-Ghanavati M, Greer LG, Cunningham FG. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol. 2009 Dec;114(6):1326–31. doi: 10.1097/AOG.0b013e3181c2bde8. [DOI] [PubMed] [Google Scholar]
- 35.Gupta BD, Sharma P, Bagla J, Parakh M, Soni JP. Renal failure in asphyxiated neonates. Indian Pediatr. 2005 Sep;42(9):928–34. [PubMed] [Google Scholar]
- 36.Perlman JM, Wyllie J, Kattwinkel J, et al. Part 11: Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010 Oct 19;122(16 Suppl 2):S516–538. doi: 10.1161/CIRCULATIONAHA.110.971127. [DOI] [PubMed] [Google Scholar]
- 37.van den Broek MPH, Groenendaal F, Toet MC, et al. Pharmacokinetics and clinical efficacy of phenobarbital in asphyxiated newborns treated with hypothermia: a thermopharmacological approach. Clin Pharmacokinet. 2012 Oct;Jan;51(10):671–9. doi: 10.1007/s40262-012-0004-y. [DOI] [PubMed] [Google Scholar]
- 38.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med. 2003 Sep 18;349(12):1157–67. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 39.Filippi L, la Marca G, Cavallaro G, et al. Phenobarbital for neonatal seizures in hypoxic ischemic encephalopathy: A pharmacokinetic study during whole body hypothermia. Epilepsia. 2011 Apr;52(4):794–801. doi: 10.1111/j.1528-1167.2011.02978.x. [DOI] [PubMed] [Google Scholar]
- 40.Filippi L, la Marca G, Fiorini P, et al. Topiramate concentrations in neonates treated with prolonged whole body hypothermia for hypoxic ischemic encephalopathy. Epilepsia. 2009 Nov;50(11):2355–61. doi: 10.1111/j.1528-1167.2009.02302.x. [DOI] [PubMed] [Google Scholar]
- 41.Bansinath M, Turndorf H, Puig MM. Influence of hypo and hyperthermia on disposition of morphine. J Clin Pharmacol. 1988 Sep;28(9):860–4. doi: 10.1002/j.1552-4604.1988.tb03229.x. [DOI] [PubMed] [Google Scholar]
- 42.van den Broek MPH, Groenendaal F, Egberts ACG, Rademaker CMA. Effects of hypothermia on pharmacokinetics and pharmacodynamics: a systematic review of preclinical and clinical studies. Clin Pharmacokinet. 2010 May 1;49(5):277–94. doi: 10.2165/11319360-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 43.Laughon MM, Benjamin DK, Jr, Capparelli EV, et al. Innovative clinical trial design for pediatric therapeutics. Expert Rev Clin Pharmacol. 2011 Sep;4(5):643–52. doi: 10.1586/ecp.11.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glass HC, Bonifacio SL, Peloquin S, et al. Neurocritical care for neonates. Neurocrit Care. 2010 Jun;12(3):421–9. doi: 10.1007/s12028-009-9324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hand CW, Moore RA, Sear JW. Comparison of whole blood and plasma morphine. J Anal Toxicol. 1988 Aug;12(4):234–5. doi: 10.1093/jat/12.4.234. [DOI] [PubMed] [Google Scholar]
- 46.Jantos R. Comparison of the determination of drugs with influence on driving performance in serum, whole blood and dried blood spots. Toxichem Krimtech. 2013;80(1):49–59. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.