Abstract
Apical expression of the large-conductance, calcium- and voltage-activated potassium (MaxiK) channel in the cortical collecting duct is responsible for flow-stimulated potassium secretion. Here, we identify two cytoplasmic regions controlling apical expression of the MaxiK channel. Disruption of the proximal region results in the intracellular retention of the MaxiK channel without affecting channel assembly, thereby reducing surface expression. Coexpression of the WT channel with this mutant results in a reduction of WT MaxiK channel at the cell surface. Our data indicate that this proximal region is necessary for export of the MaxiK channel from the endoplasmic reticulum as a way to assess the final assembly of the channel. Deletion of a more distal region disrupts apical sorting, resulting in a nonpolarized distribution of the channel without impairing its surface delivery. In summary, we have found that sequences of amino acids in the C terminus of the MaxiK channel operate after the channel is assembled into a multimer and play a role in its expression, movement to the cell surface, and apical localization.
Large-conductance, calcium- and voltage-activated (MaxiK) channels are broadly distributed in many excitable and nonexcitable tissues, where they function in regulating neurotransmitter release, vascular tone, electric tuning, cell immunity, and volume decrease after hypotonic stress (1-5). In the polarized epithelial cells of the cortical collecting duct (CCD) of the kidney, calcium-activated potassium channels are located in the apical cell membrane, where they play a role in potassium secretion (6, 7). This secretory role of Ca2+-activated K+ channels depends not only on the biophysical properties of single channels, but also on the number of channels expressed by the cell and the proper sorting of mature channels to the apical membrane. A growing body of evidence shows that the endoplasmic reticulum (ER), early in the biosynthetic secretory pathway, acts as an important determinant of the amount of a protein that reaches the plasma membrane (8), whereas the Golgi complex acts as a sorting center to direct proteins to the plasma membrane (9). Although some nonspecific forward trafficking signals out of the ER may occur for plasma membrane proteins (10), protein sequences that encode intrinsic forward trafficking signals may modulate the movement of proteins out of the ER, thereby determining surface expression (11). For example, specific sequence elements for surface expression have been identified for the ionotropic glutamate receptor δ2, the inwardly rectifying potassium channel (Kir2.1), the dopamine D1 receptor, the vesicular stomatitis virus G protein, and the sulfonylurea receptor (11-15). The trafficking elements found in these proteins are required for their efficient ER export. Mutations in the sequences result in protein retention in the ER without affecting assembly and folding. Furthermore, these forward trafficking elements determine the steady-state surface density of these proteins in the cell.
Once they pass through the ER quality control machinery, plasma membrane proteins such as ion channels are forwarded to the Golgi complex for further maturation and delivery to the cell surface (8, 9). Segregated expression to the apical and basolateral membranes is required for vectorial cell function in highly polarized cells. This polarization can be accomplished by either direct targeting of proteins from the trans-Golgi network to the desired region or through a mechanism whereby proteins are first distributed to the plasma membrane in a nonpolarized fashion and then selectively endocytosed to achieve polarized expression (9). Most basolateral sorting signals found to date are located in the cytoplasmic domains of membrane proteins and often overlap with tyrosine-based and dihydrophobic endocytosis signals (9). These basolateral sorting signals have led to the identification of a heterotrimeric adaptor complex that functions as a basolateral sorting mechanism (16). In contrast, apical sorting signals are more complex and less well characterized. Apical sorting signals are encoded not only in the amino acid sequence of extracellular, transmembrane, and cytoplasmic domains but also in carbohydrate moieties including N- and O-glycosylations and glycosylphosphatidylinositol anchors (9). Previously we examined the differences in subcellular localization of two splicing variants and showed that the cytoplasmic tail of the renal MaxiK channel is necessary for its cell-surface expression (17). We also found that the renal MaxiK channel is predominantly localized to the apical membrane domain in polarized renal epithelial cells (17). Because sequence information of MaxiK channels that confer cell-surface expression and sorting to the apical domain have not been identified and are based on the vastly different fate of two different splice variants, we wanted to examine whether specific sequences in the C terminus determine both export from the ER and apical cell sorting.
Here we describe a proximal region in the cytoplasmic tail of the MaxiK channel that is necessary for its cell-surface expression. Deletion of the proximal region exclusively retains the MaxiK channel in the ER, thereby reducing the steady-state surface expression in both nonpolarized and polarized cells without affecting channel assembly. Moreover, when coexpressed with a mutant showing markedly reduced surface expression, the surface expression of the WT channel was significantly reduced. This finding not only validates that the mutation does not affect channel assembly but also demonstrates that the missing region is critical for the MaxiK channel to exit from the ER.
In addition, we have identified the distal region required for apical expression of the MaxiK channel. Deletion of the distal region in the MaxiK channel results in loss of polarized distribution without affecting surface delivery, indicating that the deleted region is required for the apical distribution of the MaxiK channel. Failures in trafficking of plasma membrane proteins often lead to human diseases including cystic fibrosis (18) and nephrogenic diabetes insipidus (19). To date, single-nucleotide polymorphisms of the MaxiK channel have not been associated with human disease. However, our study suggests that disruption of the trafficking determinants may result in human channelopathies.
Materials and Methods
DNA Constructs. The plasmids encoding myc-tagged rbSlo1 (GeneBank accession no. AF201702) and rbslo2 are described in ref. 17. Truncation mutations were generated either by overlap extension PCR technique or by using the QuikChange Site-Directed Mutagenesis kit (Stratagene). All constructs were verified by automated DNA sequence analysis.
Cell Culture and Transient Transfection. COS-7 cells were cultured in DMEM supplemented with 10% FBS, 50 units/ml penicillin, and 50 μg/ml streptomycin. M-1 CCD cells were cultured in DMEM/F12 supplemented with 10% FBS, 50 units/ml penicillin, 50 μg/ml streptomycin, and 5 μM dexamethasone (37°C and 5% CO2). COS-7 and M-1 cells were transiently transfected by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Luminometric Cell-Surface Protein Quantification. The assay was as described by Zerangue et al. (15). Briefly, 1 day after transfection, cells were twice washed with Dulbecco's PBS (DPBS) containing Ca2+ and Mg2+, fixed with 4% formaldehyde in DPBS, blocked with 5% nonfat milk in DPBS, and labeled with mouse anti-myc antibody (1:500, Santa Cruz Biotechnology) followed by horseradish peroxidase-conjugated secondary antibody (1:3,000, Amersham Pharmacia). After incubation with 600 μl of SuperSignal ELISA Femto Maximum Sensitivity substrate (Pierce) signals were quantified with a TD20/20 luminometer (Turner). Extensive washing was performed between antibody labeling steps, and rbslo2-transfected cells were included in all experiments to assess potential permeabilization (17). For each construct, three 35-mm dishes were prepared. Relative luminometric units were obtained from three independent experiments and analyzed by using origin 6.0 (Origin Lab, Northampton, MA).
Cell-Surface Immunoprecipitation (CS-IP). One day after transfection, cells were washed with DPBS containing Ca2+ and Mg2+, blocked with 5% nonfat milk in DPBS, labeled with rabbit polyclonal anti-myc antibody (1:500, Santa Cruz Biotechnology), and extensively washed. All steps were performed on ice to prevent endocytosis and degradation. Cells were collected and lysed for 30 min at 4°C in 20 mM Hepes, pH 7.5/120 mM NaCl/5 mM EDTA/1% Triton X-100/EDTA-free Complete Protease Inhibitor (Roche Diagnostics). Channel-antibody complexes in the cleared lysates were immunoprecipitated with immobilized protein G, eluted with 1× SDS sample buffer, and immunoblotted with mouse monoclonal myc-antibody (1:500, Santa Cruz Biotechnology). Signals on the blot were captured and analyzed by using the Intelligent Dark Box LAS-1000 plus system and the Image Gauge (Fuji).
Immunostaining. One day after transfection, COS-7 cells on glass coverslips were washed with DPBS containing Ca2+ and Mg2+, fixed with 4% paraformaldehyde in DPBS (20 min), and permeabilized with 0.05% Triton X-100 in DPBS (10 min). After blocking with 3% milk and 2% donkey serum in DPBS (DMS), cells were incubated with the indicated primary antibodies (1:300) followed by fluorescent-labeled secondary antibodies (1:200). After staining, the coverslips were mounted by using VECTASHIELD (Vector Laboratories). Fluorescence labeling was visualized with Ultraview confocal microscopy by using temporal software (PerkinElmer). For cell-surface labeling experiments, cells were washed with DPBS containing Ca2+ and Mg2+, blocked with DMS (1 h), and incubated with rabbit anti-myc antibody (1:300) (1 h). All steps before permeabilization were performed on ice. Subsequent intracellular labeling steps were performed as described above. For M-1 cell immunostaining, cells were grown to confluence on clear permeable supports (Becton Dickinson). Three days after transfection, immunostaining was performed as described in this section. For 3D image reconstructions, the volocity program (PerkinElmer) was used.
Results
Identification of a Key Region for the MaxiK Channel Surface Delivery. We previously demonstrated that a splice variant of the MaxiK channel rbslo2, missing the cytoplasmic tail, has greatly reduced both total protein and plasma membrane expression (17) compared with WT channels. As shown in Fig. 1B, cells transfected with the truncation mutant 839X, which is similar to the natural splice variant rbslo2, expressed less total protein than cells transfected with the WT channel. To determine which portion of the C terminus of MaxiK is involved and to ask whether the C-terminal domain plays a role in both total protein and surface expression, we generated various truncation mutants tagged with myc at the extreme N terminus and transiently transfected them into COS-7 cells (Fig. 1 A). We showed previously that the myc tag does not affect MaxiK channel gating or trafficking (17). Compared with the WT channel, removal of residues beyond 1061X significantly reduced total expression levels similar to that observed with the 839X. Further truncations, including 966X and 921X, did not produce proteins that migrated at the expected size by SDS/PAGE (data not shown).
Fig. 1.
Total and surface expression of MaxiK channel truncation mutants in COS-7 cells. (A Upper) Topology of the MaxiK channel. An extracellular myc tag was used to quantify surface and total expression levels. (A Lower) Truncation mutants used in this study. (B) Immunoblot of total myc-tagged proteins in cleared lysates from cells transfected with indicated constructs. (C) Quantification of surface expression of mutants in fixed cells. Surface expression was measured by chemiluminescence. RLU, relative luminometric unit. Results are means and SD of three dishes each for the three independent experiments. Note that the 839X protein is intracellular (17) and was included in this analysis to control for potential cell permeabilization during the assay.
To determine the effect of the mutations on cell-surface expression, we took advantage of the position of the myc tag, which is exposed on the extracellular side of the channel. With a luminometric assay, we observed that truncation before residue 1105 completely abolished the surface expression of the channels, whereas smaller truncations were without effect (Fig. 1C). To evaluate whether the lack of surface expression was simply caused by a reduction in total protein expression, we examined cells by immunostaining. Cells were labeled with rabbit anti-myc antibodies before permeabilization to identify extracellular pools of channels and then permeabilized and labeled with mouse anti-myc antibodies. This labeling allowed us to discriminate between the intracellular and cell-surface pools of channels in the same cells (Fig. 2). Consistent with our results when using the luminometric assay shown in Fig. 1C, immunostaining showed that deletion of the last 67 amino acids (1105X, 1081X, 1061X, and 839X) resulted in the loss of cell-surface expression. In the mutants that had lost their ability to traffic to the cell surface, the MaxiK protein that was expressed was retained within the cell. Again consistent with the luminomentric assay, deletion of the last 61 amino acids (1111X, 1117X, and 1123X) did not affect cell-surface expression. Taken together, these results indicated that the residues between 1105 and 1110 are critical for channel surface delivery.
Fig. 2.
1105X, 1081X, 1061X, and 839X were expressed but not on the cell surface. Surface and intracellular expression in the same cells for each of the truncation mutants is shown. Intact cells were labeled with rabbit anti-myc antibody, permeabilized, and labeled with mouse anti-myc antibody to discriminate surface and intracellular channels. Red (Cy3-anti-rabbit antibody) represents cell-surface expression, and green (FITC-anti-mouse antibody) represents intracellular expression.
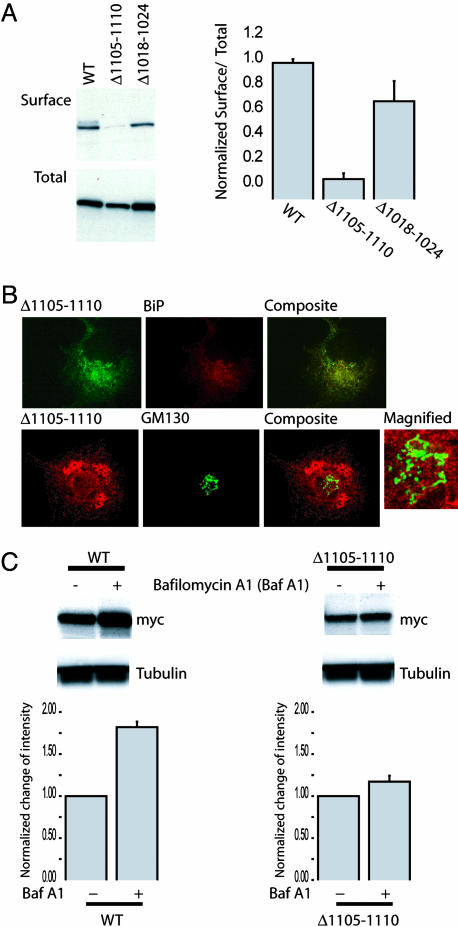
To examine this critical region more closely, we created a deletion mutant lacking only residues 1105-1110 (Δ1105-1110). To determine the effect of this mutant on surface expression, we performed a CS-IP assay. In this assay, cell-surface channels in intact cells were labeled with anti-myc antibodies, and cells were extensively washed to remove excessive antibodies before a pull-down with immobilized protein G. To quantify the total channel expression, aliquots of the cell lysates were saved for immunoblotting. In this manner, CS-IP analysis allowed quantification of both the cell-surface expression and the total expression of the channel. All steps were performed on ice to prevent potential endocytosis or degradation of channel-antibody complexes. The results showed that total expression of the Δ1105-1110 mutant decreased somewhat relative to WT and the Δ1018-1124 mutant (Fig. 3A). Notably, the reduction in the relative ratio of surface to total expression of Δ1105-1110 could not be accounted for by a simple decrease in total expression. To verify that the disruption was not due to local destabilization of the protein, we created and included a Δ1018-1024 mutant lacking seven amino acids in a nearby region; this Δ1018-1024 mutant indeed was expressed at the cell surface (Fig. 3A Right).
Fig. 3.
Deletion of residues 1105-1110 traps the MaxiK channel in the ER, thereby specifically reducing the ratio of surface to total cell expression. (A Left) Representative CS-IP (surface) and immunoblot (total) with COS-7 cells transfected with the indicated constructs. (A Right) The relative ratio of surface expression to total expression from three independent experiments was calculated as (pixel intensity of CS-IP)/(pixel intensity of total) and normalized to the value calculated for the 1171X construct. Deletion of residues 1105-1110 markedly reduced the relative ratio of surface to total expression. (B) Δ1105-1110 channels are retained in the ER. (B Upper) COS-7 cells transfected with myc Δ1105-1110 were immunostained with antibodies against myc (green) and the ER marker BiP (red). The composite image indicates colocalization (yellow) of Δ1105-1110 with the ER. (B Lower) COS-7 cells transfected with myc Δ1105-1110 were immunostained with antibodies against myc (red) and Golgi marker GM130 (green). Composite and magnified micrographs indicate minimal colocalization of Δ1105-1110 with Golgi apparatus. (C) One day after transfection with the indicated constructs, 0.5 μM of the lysosome inhibitor bafilomycin A1 was treated for 16 h at 37°C. Lysates were subjected to 5% SDS/PAGE followed by immunoblotting with mouse anti-myc and anti-β-tubulin antibodies. Protein expression change was quantified after the bafilomycin treatment. Plots are presented as the ratio change from nontreated cells. The data were shown as mean and SD. Note that steady-state amounts of WT, not Δ1105-1110, channels were greatly increased in the presence of bafilomycin A1.
To determine where Δ1105-1110 was trapped in the biosynthetic and secretory pathways, we performed immunostaining with ER and Golgi markers. When expressed in COS-7 cells, Δ1105-1110 predominantly colocalized with the ER marker, BiP, but showed little overlap with the cis- and medial-Golgi marker, GM130 (Fig. 3B). Only a negligible amount of Δ1105-1110 colocalized with the early endosome antigen 1, also demonstrating that this mutant is not processed in early endosomal compartments (data not shown). To eliminate the possibility that the mutant channels are processed by the Golgi apparatus and enter lysosomes through postGolgi compartments, Δ1105-1110-expressing cells were treated with bafilomycin A1, which specifically inhibits the vacuolar type H+-ATPase and thereby affects acidic proteases by disturbing the pH of endocytic organelles, including lysosomes (20). As shown in Fig. 3C, steady-state amounts of WT but not Δ1105-1110 channels were greatly increased in the presence of bafilomycin A1. These results provide additional evidence that the Δ1105-1110 mutant is largely trapped in the ER.
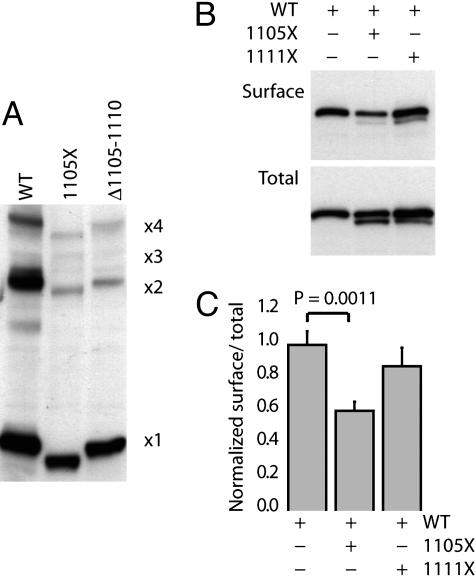
Residues 1105-1110 in the Cytoplasmic Tail of the MaxiK Channel Are Assessed by the ER After Channel Assembly into Multimers. To assess whether mutant channels could assemble into homomers or heteromers, we first examined whether mutant channels could form homomeric complexes. Multimerization was assessed by using a nonreducing SDS/PAGE assay (17). As shown in Fig. 4A, both the WT MaxiK and the mutants 1105X and Δ1105-1110 migrated as expected for monomers, dimers, and tetramers. This result clearly indicates that neither the deletion of the C terminus beyond residue 1105 nor loss of residues 1105-1110 affects the ability of the channel to self-assemble into homomers.
Fig. 4.
Deletion of residues 1105-1110 does not affect the MaxiK channel assembly. (A) 1105X and Δ1105-1110 multimerize similar to the WT channel. Cell lysates from cells transfected with the indicated constructs were separated by 5% SDS/PAGE under nonreducing conditions and immunoblotted with anti-myc antibody. Numbers at right indicate the calculated migration position of multimers of 1171X (17) and correlate well with mutant protein migration. (B) 1105X specifically reduces the surface expression of the MaxiK channel. Mutant channels on the cell surface were immunoprecipitated from COS-7 cells cotransfected with an equal amount of the indicated DNA constructs. Shown are representative CS-IP (Surface) and immunoblot (Total). Total 1105X and 1111X expression levels were similar, and total expression of the WT construct was not significantly affected by coexpression. (C) Quantification of surface and total expression from three independent experiments. The ratios were calculated as for data in Fig. 3, and data are shown as mean and SD. The relative ratio of surface to total expression for the WT construct coexpressed with 1105X was markedly reduced compared with the coexpression of 1111X and WT constructs and WT alone. Statistical significance was determined by a two-tailed, paired Student's t test.
Next we examined whether mutant channels could interfere with the trafficking of WT channels to the cell surface. To accomplish this, cells were transfected with WT or cotransfected in either of two combinations: WT and 1105X or WT and 1111X. Because of its similar size to the WT channel, Δ1105-1110 was excluded from this assay. As shown in Fig. 4B, the expression of WT channels in the plasma membrane was reduced by coexpression with 1105X but not 1111X. This finding is shown clearly in the ratio of surface to total expression of WT channels in each experiment normalized to the ratio measured when WT channels were expressed alone (Fig. 4C). When the WT channel is coexpressed with the 1105X mutant, relative surface expression of the WT channel is reduced. On the other hand, coexpression with 1111X is without significant effect. It is important to note that although there is lower total expression of the mutant 1105X compared with WT, it is still effective in reducing surface expression of the WT channel by 40%. These data are consistent with the notion that a heteromer of the WT and the 1105X mutant traffics poorly to the plasma membrane. Taken together, these data indicate that residues 1105-1110 contain sequence information that is required for the ER exit of MaxiK channels after assembly into multimers.
Residues 1117-1123 in the Cytoplasmic Tail of the MaxiK Channel Are Required for Its Apical Sorting. Because the MaxiK channel is in the apical membrane of renal epithelial cells, we asked whether the C-terminal tail also plays a role in sorting of the channel to the apical cell membrane. Mouse CCD cells (M-1) were grown to confluence on clear permeable supports, and cell-surface expression of constructs was evaluated by immunostaining (Fig. 5 and Fig. 6, which is published as supporting information on the PNAS web site). We examined whether Δ1105-1110 failed to appear at the surface in the polarized M-1 cells as with COS-7 cells. As the micrographs (Fig. 5, Bottom) indicate, Δ1105-1110 showed a weak and diffused staining pattern, representing intracellular expression only, and did not achieve a polarized distribution. The mutant 1105X showed virtually the same staining pattern (data not shown). This finding indicates that the 1105-1111 region of the MaxiK channel is functional in polarized M-1 cells as well as in nonpolarized COS-7 cells.
Fig. 5.
Residues 1117-1123 contain an apical sorting determinant of the MaxiK channel. M-1 cells expressing the indicated constructs were immunostained with a FITC-conjugated anti-ZO-1 antibody as an apical marker (green) and an anti-myc antibody (red). A stack of series of z-axis images was captured, and the acquired data were volumized for 3D reconstruction. We started with 3D images with a bird's-eye view of the apical side of a layer of cells. The top edges of images were then tilted along the z axis such that the apical side of the cells was tilted 15° toward the end of this page (Left) or toward the beginning of the page (Center) to help visualize the apical and basolateral sides, respectively. (Scale bar, 10 μm.)
With these data in mind, the question is whether the remaining portions of the tail play a role in epithelial cells. WT and 1123X constructs were predominantly expressed in the apical membrane. However, deletion of the last 55 or 61 amino acids (1117X and 1111X) resulted in a loss of polarized expression. This result suggests that the cytoplasmic tail, especially residues 1117-1123, contains residues that allow the MaxiK channel to be localized to the apical membrane. In summary, our results show that the cytoplasmic tail of the MaxiK channel contains multiple and hierarchical trafficking elements involved in overall MaxiK channel expression, movement to the cell surface, and apical localization.
Discussion
The MaxiK channel is present in the apical cell membrane of the renal collecting duct, where it is thought to play a role in flow-dependent K+ secretion (6, 21). To learn more about precisely how the MaxiK channel is targeted to the apical cell membrane, we took advantage of two isoforms of the MaxiK channels that we previously identified in the rabbit kidney, rbslo1 and rbslo2 (17). rbslo1 is expressed in the apical cell membrane, whereas rbslo2 is primarily intracellular. Alternate splicing of the rbslo gene yields a truncated C-terminal tail with several unique amino acids at the C-terminal end of the molecule. The deletion of these unique amino acids does not affect the trafficking of rbslo2, suggesting that the information for the apical location of rbso1 must be present in the portion missing in rbslo2. Indeed, we identified separate regions that are important for ER export and apical localization.
In this study we identified a region in the C terminus, encompassing residues 1105-1110 (DLIFCL) that is required for exit from the ER both in nonpolarized COS-7 cells as well as in polarized M-1 cells. Without these six residues, the MaxiK channel does not exit the ER. One possible explanation for this result is that the six residues are recognized by the ER quality control mechanism as a final check of the status of the protein before it continues on to the Golgi apparatus. A unique property of the MaxiK channel is the presence of a multimeric assembly domain in the cytoplasmic tail, rather than at the N terminus, that is more common for many voltage-dependent potassium channels (22). Because of this unique location, we argue that detecting the status of the MaxiK channel once the entire protein is properly folded would be advantageous. To assess this, we showed that the formation of multimers in the mutant constructs missing these six amino acids is preserved, as in the WT. In addition, when the WT MaxiK channel is coexpressed with mutants missing the six amino acids, the ratio of surface to total expression of the WT protein is markedly decreased, suggesting that the MaxiK channels missing the six amino acids oligomerized with WT channels and trapped them in the ER. Taken together, our data suggest that the C-terminal amino acids between 1105 and 1110 are critical for the MaxiK channel to move forward out of the ER after its assembly.
What is special about these amino acids? One possibility is that the six amino acids represent a sequence recognized by the ER machinery as a signal for ER export. The amino acids 1105-1110 are conserved in a paralogue, slo3 (23), and orthologues of the MaxiK channel. However, there is no similarity at the primary sequence level with previously identified ER forward trafficking determinants. Interestingly, however, Sevier et al. (24) showed that the secondary structure of a short sequence of amino acids involved in ER export in vesicular stomatitis virus G protein forms a short α-helix. A secondary structure prediction by using the PredictProtein server of the European Molecular Biology Laboratory (Heidelberg) indicates that residues 1105-1110, along with several neighboring residues of the MaxiK channel, are also predicted to form a short α-helix. Further analysis of other short sequences of amino acids involved in efficient ER export of other proteins such as the ionotropic glutamate receptor δ2, the inwardly rectifying potassium channel (Kir2.1), the dopamine D1 receptor, the vesicular stomatitis virus G protein, and the sulfonylurea receptor (11-15) show an interesting consensus of secondary structure. Although there is no consensus sequence among these proteins for a critical ER export region, each portion identified to be involved in ER export is predicted to form a short α-helix. It is possible that the ER machinery detects a critical secondary structure to assess the quality of all of these proteins.
As previously mentioned, the apical expression of the renal MaxiK channel was indispensable for net potassium secretion in the CCD (6, 21). We have identified a sequence of amino acids located in the C terminus that is necessary for MaxiK apical expression in polarized CCD cells. The WT protein localizes to the apical membrane in M-1 cells, consistent with a role for the renal MaxiK channels in potassium secretion. Although surface delivery of the tested mutants was not affected as in nonpolarized COS-7 cells, polarized distribution to the apical domain was abolished when the protein was truncated after residue 1117 but not after residue 1123. This result clearly indicates that residues 1117-1123 contain information necessary for apical MaxiK channel expression. To our knowledge, the identified sequence (NAGQSRA) in the MaxiK channel does not share sequence similarity with any previously described trafficking and sorting determinants, including endocytosis signals. Therefore, this finding indicates either that the MaxiK channel undergoes direct targeting to the apical membrane or that this sequence represents a previously uncharacterized apical membrane retention signal. In summary, we have identified two cytoplasmic trafficking determinants responsible for the apical and surface expression of the MaxiK channel in CCD cells.
Supplementary Material
Acknowledgments
We thank Dr. Sandra Guggino and Dr. Peying Fong for critical reading of the manuscript. This work was supported by National Institutes of Health Grant 32753.
Author contributions: S.-H.K. and W.G. designed research; S.-H.K. performed research; S.-H.K. and W.G. analyzed data; and S.-H.K. and W.G. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CCD, cortical collecting duct; CS-IP, cell-surface immunoprecipitation; DPBS, Dulbecco's PBS; MaxiK, large-conductance, calcium- and voltage-activated potassium; ER, endoplasmic reticulum.
References
- 1.Wang, Z. W., Saifee, O., Nonet, M. L. & Salkoff, L. (2001) Neuron 32, 867-881. [DOI] [PubMed] [Google Scholar]
- 2.Amberg, G. C., Bonev, A. D., Rossow, C. F., Nelson, M. T. & Santana, L. F. (2003) J. Clin. Invest. 112, 717-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramanathan, K., Michael, T. H., Jiang, G. J., Hiel, H. & Fuchs, P. A. (1999) Science 283, 215-217. [DOI] [PubMed] [Google Scholar]
- 4.Ahluwalia, J., Tinker, A., Clapp, L. H., Duchen, M. R., Abramov, A. Y., Pope, S., Nobles, M. & Segal, A. W. (2004) Nature 427, 853-858. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Sheader, E. A., Brown, P. D. & Best, L. (2001) Mol. Cell. Endocrinol. 181, 179-187. [DOI] [PubMed] [Google Scholar]
- 6.Woda, C. B., Bragin, A., Kleyman, T. R. & Satlin, L. M. (2001) Am. J. Physiol. 280, F786-F793. [DOI] [PubMed] [Google Scholar]
- 7.Giebisch, G., Hebert, S. C. & Wang, W. H. (2003) Pflügers Arch. 446, 289-297. [DOI] [PubMed] [Google Scholar]
- 8.Ellgaard, L., Molinari, M. & Helenius, A. (1999) Science 286, 1882-1888. [DOI] [PubMed] [Google Scholar]
- 9.Yeaman, C., Grindstaff, K. K. & Nelson, W. J. (1999) Physiol. Rev. 79, 73-98. [DOI] [PubMed] [Google Scholar]
- 10.Pfeffer, S. R. & Rothman, J. E. (1987) Annu. Rev. Biochem. 56, 829-852. [DOI] [PubMed] [Google Scholar]
- 11.Ma, D., Zerangue, N., Lin, Y. F., Collins, A., Yu, M., Jan, Y. N. & Jan, L. Y. (2001) Science 291, 316-319. [DOI] [PubMed] [Google Scholar]
- 12.Kalandadze, A., Wu, Y., Fournier, K. & Robinson, M. B. (2004) J. Neurosci. 24, 5183-5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bermak, J. C., Li, M., Bullock, C. & Zhou, Q. Y. (2001) Nat. Cell Biol. 3, 492-498. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura, N. & Balch, W. E. (1997) Science 277, 556-558. [DOI] [PubMed] [Google Scholar]
- 15.Zerangue, N., Schwappach, B., Jan, Y. N. & Jan, L. Y. (1999) Neuron 22, 537-548. [DOI] [PubMed] [Google Scholar]
- 16.Folsch, H., Ohno, H., Bonifacino, J. S. & Mellman, I. (1999) Cell 99, 189-198. [DOI] [PubMed] [Google Scholar]
- 17.Wang, S. X., Ikeda, M. & Guggino, W. B. (2003) J. Biol. Chem. 278, 2713-2722. [DOI] [PubMed] [Google Scholar]
- 18.Moyer, B. D., Denton, J., Karlson, K. H., Reynolds, D., Wang, S., Mickle, J. E., Milewski, M., Cutting, G. R., Guggino, W. B., Li, M., et al. (1999) J. Clin. Invest. 104, 1353-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamsteeg, E. J., Bichet, D. G., Konings, I. B., Nivet, H., Lonergan, M., Arthus, M. F., van Os, C. H. & Deen, P. M. (2003) J. Cell Biol. 163, 1099-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drose, S. & Altendorf, K. (1997) J. Exp. Biol. 200, 1-8. [DOI] [PubMed] [Google Scholar]
- 21.Woda, C. B., Miyawaki, N., Ramalakshmi, S., Ramkumar, M., Rojas, R., Zavilowitz, B., Kleyman, T. R. & Satlin, L. M. (2003) Am. J. Physiol. 285, F629-F639. [DOI] [PubMed] [Google Scholar]
- 22.Quirk, J. C. & Reinhart, P. H. (2001) Neuron 32, 13-23. [DOI] [PubMed] [Google Scholar]
- 23.Schreiber, M., Wei, A., Yuan, A., Gaut, J., Saito, M. & Salkoff, L. (1998) J. Biol. Chem. 273, 3509-3516. [DOI] [PubMed] [Google Scholar]
- 24.Sevier, C. S., Weisz, O. A., Davis, M. & Machamer, C. E. (2000) Mol. Biol. Cell 11, 13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.