Significance
Skeletal muscle contractions are regulated by a process called excitation–contraction (EC) coupling, and defects in it are associated with numerous human myopathies. Recently, stac3 (SH3 and cysteine-rich domain 3) was identified as a key regulator of EC coupling and a STAC3 mutation as causal for the debilitating Native American myopathy (NAM). We now show that Stac3 controls EC coupling by regulating Ca2+ channels in muscles. Both the NAM mutation and a mutation that leads to the loss of Stac3 decrease the amount, organization, stability, and voltage sensitivity of Ca2+ channels. Furthermore, we find evidence that the NAM allele of STAC3 is linked to malignant hyperthermia, a common pharmacogenic disorder. These findings define critical roles for Stac3 in muscle contraction and human disease.
Keywords: zebrafish, skeletal muscle, excitation–contraction coupling, dihydropyridine receptor, Native American myopathy
Abstract
Skeletal muscle contractions are initiated by an increase in Ca2+ released during excitation–contraction (EC) coupling, and defects in EC coupling are associated with human myopathies. EC coupling requires communication between voltage-sensing dihydropyridine receptors (DHPRs) in transverse tubule membrane and Ca2+ release channel ryanodine receptor 1 (RyR1) in the sarcoplasmic reticulum (SR). Stac3 protein (SH3 and cysteine-rich domain 3) is an essential component of the EC coupling apparatus and a mutation in human STAC3 causes the debilitating Native American myopathy (NAM), but the nature of how Stac3 acts on the DHPR and/or RyR1 is unknown. Using electron microscopy, electrophysiology, and dynamic imaging of zebrafish muscle fibers, we find significantly reduced DHPR levels, functionality, and stability in stac3 mutants. Furthermore, stac3NAM myofibers exhibited increased caffeine-induced Ca2+ release across a wide range of concentrations in the absence of altered caffeine sensitivity as well as increased Ca2+ in internal stores, which is consistent with increased SR luminal Ca2+. These findings define critical roles for Stac3 in EC coupling and human disease.
Contraction of skeletal muscle is mediated by the sliding of myofilaments that is initiated by an increase in cytosolic Ca2+ released from the intracellular organelle, the sarcoplasmic reticulum (SR). Ca2+ release from the SR is a voltage-dependent process called excitation–contraction (EC) coupling that occurs at junctions between the SR and invaginations of the sarcolemma called transverse (T) tubules that project into the interior of the muscle fiber called triads (1). Defects in EC coupling are the cause of congenital muscle myopathies labeled “triadopathies” that are characterized by defects in Ca2+ homeostasis and muscle weakness, for which there are few effective therapies (2).
EC coupling in skeletal muscle is mediated by a triadic complex that includes the dihydropyridine receptor (DHPR) and ryanodine receptor 1 (RyR1), which are both Ca2+ channels (3, 4). DHPRs located in the T tubule are voltage-gated, L-type channels that act as the voltage sensor for EC coupling. DHPRs are thought to directly interact with RyR1s in the SR membrane to rapidly trigger Ca2+ release from the SR at triads upon depolarization of the T-tubule membrane (5–7). Despite a wealth of knowledge of how DHPRs and RyR1 interact, the precise mechanisms by which this protein interaction is coordinated and modulated are poorly understood (8). Several congenital myopathies and the pharmacogenic disorder malignant hyperthermia (MH), a potentially lethal response to volatile anesthesia that affects between 1:5,000 and 1:50,000 of the general population (9), are caused by defects in EC coupling. However, precisely how genetic defects in proteins of the EC coupling complex contribute to disease pathogenesis is incompletely understood.
Recently, the cytosolic protein Stac3 was identified as an essential component for skeletal muscle EC coupling in zebrafish (10) and mice (11). Stac3 also regulates hypertrophy and fiber-type composition, and mutations in which it is responsible for impaired contractility in mouse muscles (12). Stac3 is expressed selectively in skeletal muscle, colocalizes and biochemically associates with DHPR and RyR1 at triads, and is required for normal release of Ca2+ from the SR. Coexpression of Stac3 with DHPR in cultured nonmuscle cell lines promotes the trafficking of the channel to the membrane, suggesting a role for Stac3 in trafficking and/or stabilization of the DHPR in the membrane (13). Furthermore, a hereditary triadopathy called Native American myopathy (NAM) is caused by a missense mutation of STAC3 (10). NAM, an autosomal-recessive disorder found within the Lumbee Native American population, is characterized by clinical features including congenital onset of muscle weakness, multiple joint contractures, dysmorphic facial features, and susceptibility to MH, with 36% of afflicted individuals dying by the age of 18 (14). Analysis of the analogous mutation in zebrafish stac3 showed that stac3NAM leads to a partial loss of Ca2+ release in muscle fibers (10), yet the mechanism for how Stac3 and Stac3NAM modulate EC coupling has remained undefined. Because there are currently no effective therapeutic agents to treat congenital triadopathies, a better mechanistic understanding of how mutations in EC components result in myopathy could lead to the discovery of new therapies.
Results
Stac3 Is Necessary for Normal Levels of DHPRα.
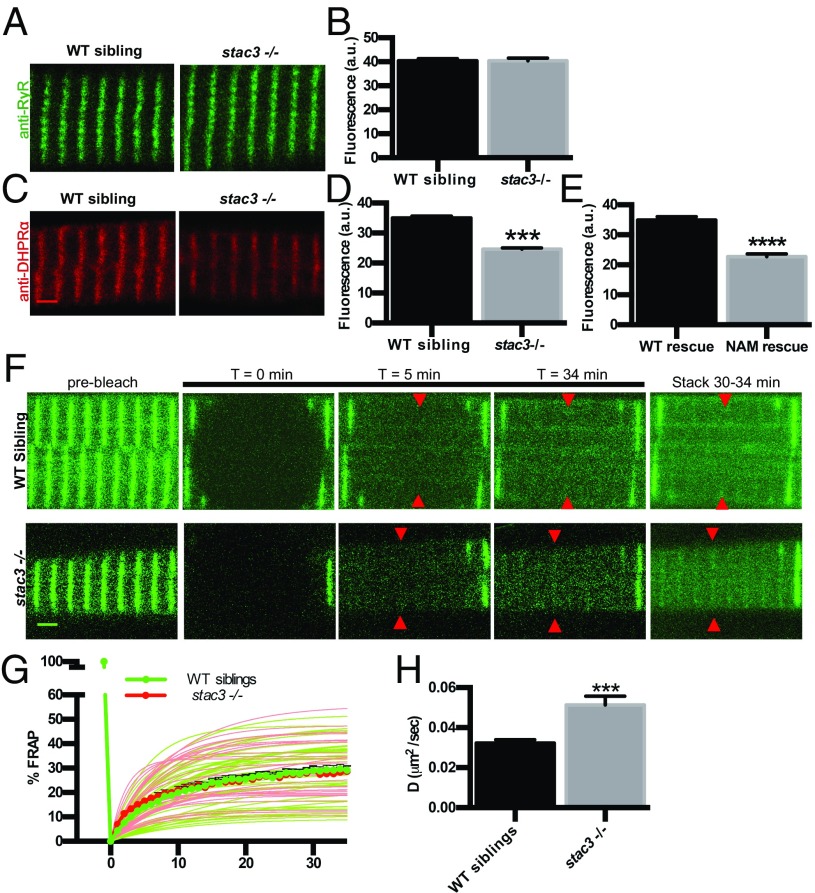
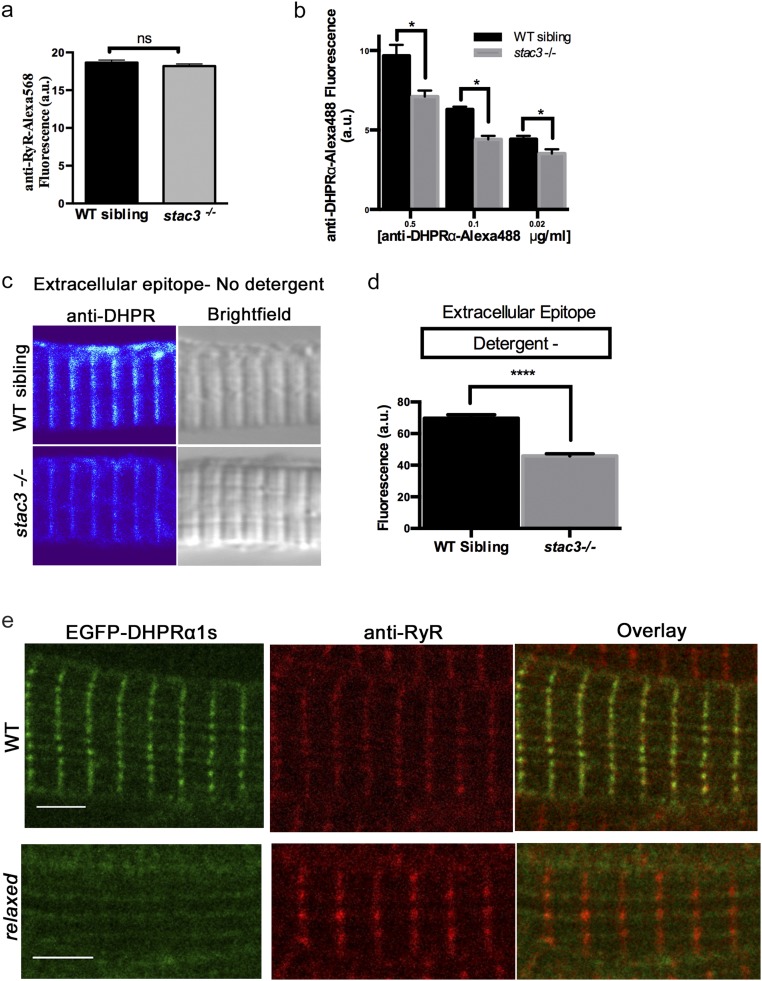
As a first step, the distribution of DHPRs and RyRs was assayed quantitatively in dissociated skeletal muscle fibers from wild-type (WT) and stac3−/− (null) embryos. Whereas there was no difference in RyR1 expression between WT and stac3−/− muscle (Fig. 1 A and B and Fig. S1A), a significant reduction in DHPRα was observed within triadic junctions when assayed with mAb1 (15), which recognizes a cytoplasmic epitope in DHPRα (30% reduction; Fig. 1 C and D, Left), fluorophore-conjugated antibodies to minimize potential steric effects of secondary antibodies within the compact triad (24% reduction; t test, P < 0.05, n > 35), and an anti-DHPRα that recognizes an extracellular epitope (34% reduction; t test, P < 0.0001, n > 70) without detergent (Fig. S1 C–E). DHPRα expression was also reduced in stac3−/− fibers expressing stac3NAM-EGFP in comparison with those expressing stac3WT-EGFP (35% reduction; t test, P < 0.0001, n > 53) (Fig. 1E). Hence, WT Stac3 was required for normal DHPRα, but not RyR1, expression within the triad.
Fig. 1.
DHPRα1 but not RyR1 is reduced in T-tubule striations of stac3 mutants. (A) Immunofluorescence labeling of WT sibling and stac3−/− disassociated myotubes with anti-pan RyR (34c). (B) Mean immunofluorescence intensity of anti-RyR in stac3−/− compared with WT siblings showing no difference in triadic RyR (t test, P = 0.89, n = 85 WT sibling, n = 50 stac3−/−). a.u., arbitrary units. (C) Immunofluorescence labeling of WT sibling and stac3−/− disassociated myotubes with mAb1 1A against a cytoplasmic region of DHPRα1S (15). (D) Mean mAb1 1A labeling in WT siblings and stac3−/− showing a decrease in triadic DHPRα1S (t test, ***P < 0.0001, n = 216 WT sibling, n = 264 stac3−/−). (E) Quantification of the mean of immunofluorescence labeling of anti-DHPRα1S in stac3−/− expressing stac3NAM (NAM rescue) at triads compared with stac3−/− expressing stac3WT (WT rescue) (n = 75 stac3−/−; stac3WT, n = 53 stac3−/−; stac3NAM, t test, ****P < 0.0001). (F) Time course for FRAP of EGFP-DHPRα1S expressed in WT siblings and stac3−/− myofibers. Shown are EGFP-DHPRα1S before (prebleach), after photobleaching (T = 0, 5, 34 min), and a maximum projection (stack) of T = 30 to 34 min (Right). (G) Mean quantification of the time course of FRAP in WT siblings (thick green line and circles) and stac3−/− (thick red line and circles). Thin lines represent nonlinear regressions from individual traces of FRAPs from WT siblings (green) and stac3−/− (red). The vertical thick green line depicts bleaching. (H) Histogram showing that the diffusion rate (D) of EGFP-DHPRα1S is higher in stac3−/− (t test, ***P < 0.0001, n = 33 stac3−/−, n = 45 WT siblings). SEMs are indicated. (Scale bars, 2 μm.)
Fig. S1.
Loss of Stac3 does not prevent trafficking of DHPRα to triads. (A) Quantification of the mean of immunofluorescence labeling (±SEM) of Alexa 568 directly coupled to anti-RyR in stac3−/− at triads compared with WT siblings (n = 60, t test, P = 0.29). (B) Quantification of the mean of immunofluorescence labeling of Alexa 488 directly coupled to anti-DHPRα1S at different concentrations in stac3−/− at triads compared with WT siblings (n = 40 WT sibling, n = 40 stac3−/−, t test, *P < 0.0001). (C) Immunofluorescence (Left) and bright-field images (Right) of WT sibling and stac3−/− disassociated myotubes labeled without detergent with anti-DHPRα1S that recognizes an extracellular epitope. (D) Histogram showing that there is a decrease in T-tubule triadic DHPRα1S in stac3−/− dissociated myotubes (n = 160 WT sibling, n = 125 stac3−/−, t test, ****P < 0.0001). (E) Anti-Ryr immunolabeling of a fixed muscle fiber expressing EGFP-DHPRα1S showing that EGFP-DHPRα1S does not localize to triads in a relaxed mutant fiber, whereas it does in a WT fiber. SEMs are indicated. n.s., not significant. (Scale bars, 4 μm.)
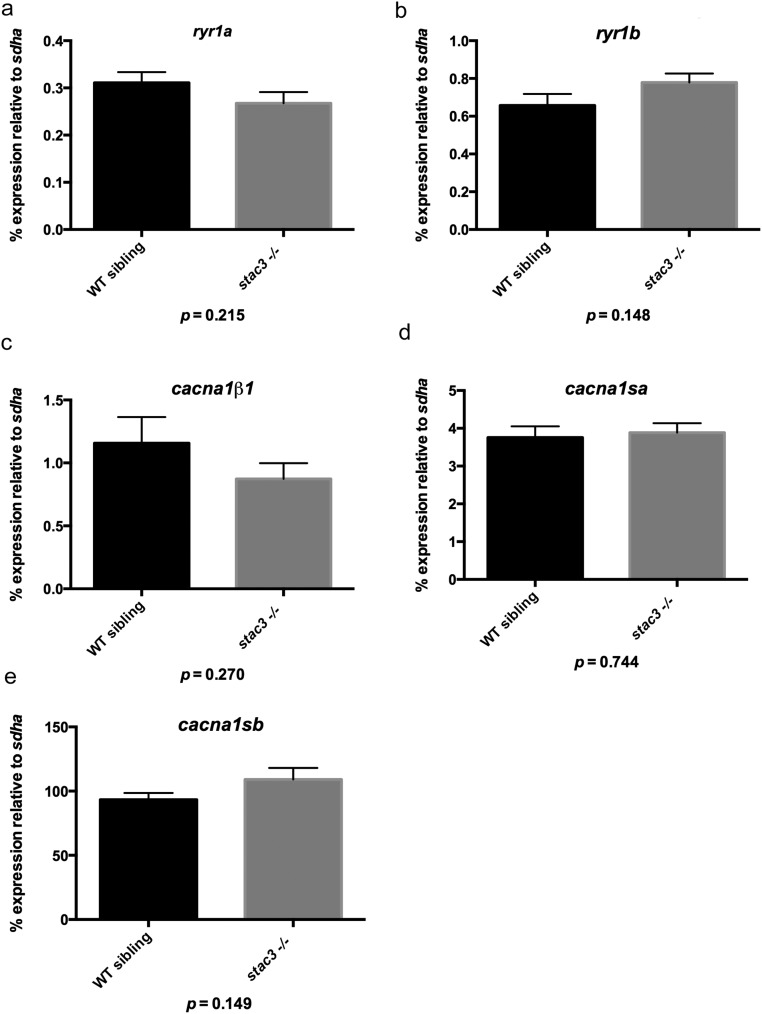
To assay whether the decrease in triadic DHPRα was due to decreased synthesis of DHPRα, transcription of DHPR subunits was examined by quantitative PCR. No differences were detected in transcription levels of genes encoding for DHPRα1, DHPRβ1, and RyR1 [cacna1sa, cacna1sb, cacnb1, ryr1a, and ryr1b (16)] between stac3−/− embryos and WT siblings (Fig. S2). Thus, although Stac3 was required for normal levels of DHPR expression, this was not due to regulation of DHPRα1 or DHPRβ1 transcription.
Fig. S2.
Transcription of EC coupling genes is normal in stac3−/−. Histograms showing quantitative PCR for target mRNAs relative to the housekeeping mRNA sdha. (A) ryr1a message (stac3−/− n = 6, WT sibling n = 6, t test, P = 0.215). (B) ryr1b message (stac3−/− n = 6, WT sibling n = 6, t test, P = 0.148). (C) cacna1b1 message (stac3−/− n = 6, WT sibling n = 6, t test, P = 0.270). (D) cacna1sa message (stac3−/− n = 6, WT sibling, n = 6, t test, P = 0.744). (E) cacna1sb message (stac3−/− n = 12, WT sibling n = 12, t test, P = 0.149). SEMs are indicated.
A defect in DHPR trafficking to the T tubule is one potential mechanism for the observed reduced triadic DHPR levels in stac3 mutants. In fact, a recent study showed that Stac3 was sufficient to promote DHPR trafficking to the plasma membrane in nonmuscle cell lines (13). To more directly test this mechanism, we examined the role of Stac3 for trafficking of DHPRα with fluorescence recovery after photobleaching (FRAP) analysis of EGFP-DHPRα–expressing WT and stac3−/− muscles. In dhprβ1-null (relaxed) fibers, which exhibit defective DHPRα trafficking (17), EGFP-DHPRα was not localized to triads as expected (Fig. S1E). However, in stac3−/− muscle fibers, EGFP-DHPRα localized to triads just as it does in WT sibling fibers (Fig. 1E, Left). Traces of fluorescence from WT siblings and stac3−/− muscles showed that the recovery of fluorescence was temperature-sensitive and occurred in the first 30 min after bleaching with about 30% of the fluorescence being mobile at room temperature (Fig. 1F and Fig. S3). Although the mobile fraction was not different between stac3−/− and WT siblings (t test, P = 0.88) (Fig. 1F), the diffusion rate of EGFP-DHPRα fluorescence to the plateau was significantly higher in triads of stac3−/− muscle (Fig. 1 G and H). Thus, the loss of Stac3 appears not to prevent trafficking of DHPRα to the triad in skeletal muscles.
Fig. S3.
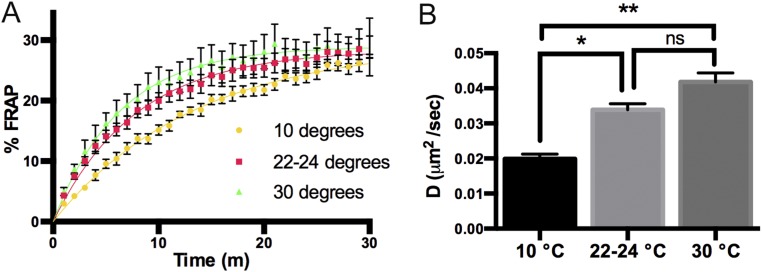
DHPR trafficking is temperature-dependent. (A) Time course for FRAP of EGFP-DHPRα in WT sibling embryos incubated at 30 °C (n = 6), 22 to 24 °C (n = 45), and 10 °C (n = 6); 22 to 24 °C data are the same data as in Fig. 1. (B) Histogram showing that the diffusion rate of EGFP-DHPRα increases as a function of temperature (30 °C versus 10 °C, ANOVA, **P < 0.01) and decreases at low temperature (10 °C versus 22 to 24 °C, ANOVA, *P < 0.05). SEMs are indicated. ns, not significant.
Stac3 Is Required for Normal Triadic DHPR but Not for Linkage to RyRs.
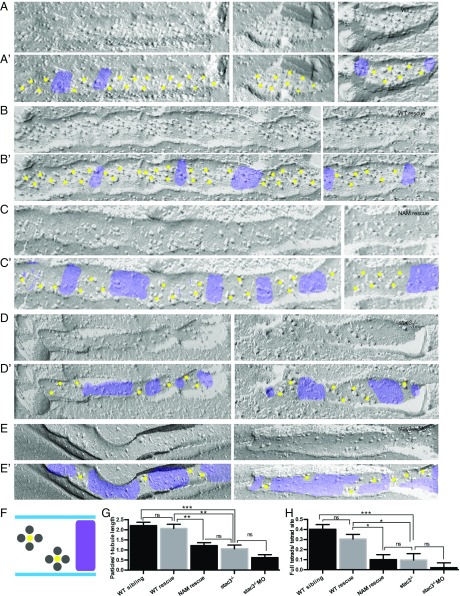
The positioning of DHPRs within surface membrane/T tubules of calcium release units (CRUs) in skeletal muscle is determined by their interaction with RyR1 in the adjoining SR (18). Each DHPR occupies one corner of the four subunits of the RyR1 homotetramer. In freeze-fracture images of mature CRUs, the four corners of most RyRs are fully occupied by DHPR particles identified by their characteristic size signature (18) such that the DHPRs are arranged in groups of four particles called tetrads. The tetrads are aligned along the T-tubule axis, as dictated by their linkage to RyRs, yet interestingly positioned over alternate RyR1.
In freeze-fracture images of T tubules in tails of ∼96-h postfertilization (hpf) larvae (Fig. 2 A and A′), the locations of tetrads were confirmed because the distance between the centers of tetrads was twice the distance between the feet (RyRs) that occupied the junctional SR membrane facing the T tubules (Fig. S4A). Tetrads were often incomplete, with one or more DHPR particles missing. Only 62% of tetrads were complete in WT larvae. Furthermore, T tubules contained junctional segments with tetrads and tetrad-free segments (purple in Fig. 2). In WT, the tetrad-free segments were directly related to discontinuities in the junctional SR membrane at triads, as the junctional SR membranes do not cover the entire T-tubule length (Fig. S4A). On the average, the junctional T-tubule segments made up ∼65% of the total T-tubule length (Table 1) and the average number of particles per arbitrary length of T tubule was 2.2 in WT larvae (Fig. 2G). The number of complete tetrads for the same length of T tubule was 0.4 (Fig. 2H).
Fig. 2.
DHPR tetrads are reduced and incomplete in stac3 mutants. (A–E) Freeze-fracture electron micrographs of 4-d postfertilization larvae showing DHPR particles in triadic clusters of WT (A), stac3−/− expressing stac3WT-EGFP (WT rescue) (B), stac3−/− expressing stac3NAM-mKate2 (NAM rescue) (C), stac3−/− (D), and stac3−/− injected with an antisense morpholino oligonucleotide against stac3 (stac3−/− + MO) (E). (A′–E′) Same as A–E, with yellow dots and purple shading added for clarity to denote, respectively, segments of T tubules with tetrad sites of DHPRs and segments of T tubules with no tetrad sites in muscle fibers of WT (A′), WT rescue (B′), NAM rescue (C′), stac3−/− (D′), and stac3−/− + MO (E′). (F) Illustration showing stereotypical DHPR particles in tetrad sites (labeled with yellow dots) along a T tubule and gaps with no tetrad sites (purple) as seen above. (G) Histogram showing that the particles per T-tubule length are decreased in NAM rescue, stac3−/−, and stac3−/− + MO muscles compared with WT and WT rescue. (ANOVA Tukey's; ***P < 0.001, **P < 0.01.) (H) Histogram showing that full tetrads per tetrad site are decreased in NAM rescue, stac3−/−, and stac3−/− + MO muscles compared with WT and WT rescue. ns, not significant. SEMs are indicated. (ANOVA Tukey's; ***P < 0.001, *P < 0.05.)
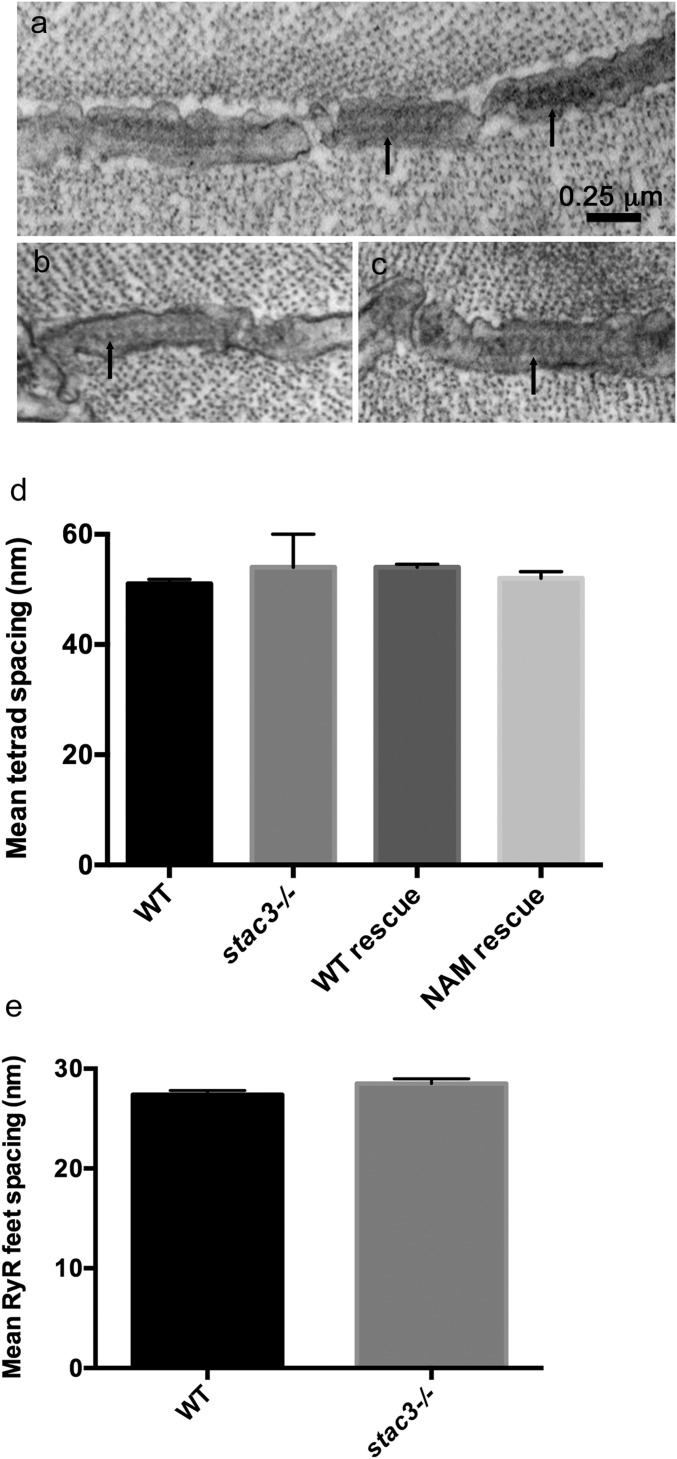
Fig. S4.
DHPR particles and tetrads are reduced in stac3 mutants but Ryr feet are unaffected. (A) Transverse EM section showing the RyR feet (arrows) at triads in a WT muscle fiber (96 hpf). (B and C) Examples of transverse EM sections showing that the distribution of RyR feet (arrows) in stac3−/− muscles is comparable to that in WT muscles. (D) Histogram showing that the spacing of tetrads was comparable in fibers from WT (n = 39) and stac3−/− (n = 25). stac3−/− expressing Stac3WT (WT rescue, n = 45) and stac3−/− expressing Stac3NAM (NAM rescue, n = 44) larvae (ANOVA, P = 0.07). (E) Histogram showing that the spacing of RyR feet was comparable in WT (n = 27) and stac3−/− (n = 39) fibers (t test, P = 0.11). SEMs are indicated.
Table 1.
Particles, tetrads, and functionality statistics
| Type | No. of particles per tetrad site | No. of complete tetrads per tetrad site | Junctional T tubule/total T-tubule length | Qmax, nC/μF | ΔF/Fmax | ΔF/F V1/2, mV | KF, mV |
| WT | 3.40 ± 0.52 (n = 84) | 0.62 ± 0.36 | 0.65 ± 0.13 (n = 42) | 8.4 | 3.87 ± 0.37 | −14.6 ± 2.3 | 6.2 ± 0.5 |
| WT rescue | 3.04 ± 0.70** (n = 72) | 0.45 ± 0.31* | 0.68 ± 0.13** (n = 40) | 9.5* | 2.30 ± 0.21* | −21.2 ± 1.8* | 7.2 ± 0.8 |
| NAM rescue | 2.76 ± 0.44**,ns (n = 43) | 0.23 ± 0.25*,ns | 0.44 ± 0.13**,ns (n = 38) | 0.9*,ns | 0.28 ± 0.04*,nd | −2.3 ± 6.3*,nd | 17.2 ± 2.8*,nd |
| stac3−/− | 2.52 ± 0.54** (n = 31) | 0.23 ± 0.41** | 0.42 ± 0.14** (n = 31) | 1.1* | 0* | nd | nd |
| stac3−/− MO | 2.37 ± 0.44ns (n = 35) | 0.08 ± 0.14# | 0.26 ± 0.14# (n = 28) | nd | nd | nd | nd |
Tukey post hoc comparisons: WT rescue vs. stac3−/−. NAM rescue vs. WT rescue (first P value) and NAM rescue vs. stac3−/− (second P value). stac3−/− vs. WT. ANOVA, **P < 0.001, *P < 0.01. t test was used for stac3−/− MO vs. stac3−/−. #t test, P < 0.05. nd, not determined.
In stac3−/−, T tubules contained junctional and tetrad-free segments as in WT but the overall frequency of DHPR particles in the T tubules was reduced (51% reduction; Fig. 2 D, D′, and G). Nevertheless, the structural relationship of DHPRs with RyRs was essentially unaltered in stac3−/− as the remaining particles were grouped in tetrads, albeit with many more incomplete tetrads (Table 1). However, within the junctional T-tubule segments, the spacing between the tetrads in stac3−/− was the same as in WT (Fig. S4D; ANOVA, P = 0.07), reflecting unaltered underlying spacing between the RyR feet in stac3−/− embryos (t test, P = 0.11; Fig. S4 A–C and E). The major difference between WT and stac3−/− T tubules was an increase in tetrad-free segments (Fig. 2D′) and thus a decrease in the ratio of junctional to total T-tubule length (Table 1), resulting in a significant reduction in the average frequency of particles per unit of T-tubule length (Fig. 2G). Furthermore, there were fewer complete tetrads in stac3−/−, which further depressed the frequency of tetrads per tetrad site (Fig. 2H).
Previous analyses showed that stac3−/− muscles exhibited a clear decrease in contraction but were not immotile due to maternal message in stac3−/− embryos, and that knocking down maternal Stac3 rendered the stac3−/− embryos immotile (10). To see how the loss of both maternal and zygotic stac3 affects DHPR particle distribution, an antisense oligonucleotide (MO) against stac3 that effectively knocks down Stac3 in the absence of off-target effects (9) was injected into stac3−/− embryos. The frequency of DHPR particles, complete tetrads, and proportion of T-tubule length containing clusters of particles in MO-injected stac3−/− larvae were all decreased even more than seen in stac3−/− larvae (Fig. 2 D–H and Table 1). Furthermore, complete tetrads were extremely rare. Note, however, that vestiges of particle grouping were still visible even in MO-treated stac3−/− larvae (Fig. 2E′), indicating that the link of DHPRs to RyR subunits persists when Stac3 is nearly or completely absent.
Expression of WT Stac3 in stac3−/− muscles fully restored DHPR particle (Fig. 2 B, B′, and G) and tetrad (Fig. 2H) frequency to WT levels, confirming that Stac3 promotes DHPR particle localization and organization within the junctional T tubule. To examine how Stac3NAM affects particle and tetrad frequencies, the distribution of DHPRs in tetrads was examined in stac3−/− muscles expressing stac3NAM. In stac3NAM fibers, the number and grouping of DHPR particles and complete tetrads and the proportion of T tubule that contained groups of particles were similar to that in stac3−/− fibers (Fig. 2 C–D′, G, and H and Fig. S4) and significantly lower than in stac3−/− fibers expressing stac3WT (Fig. 2 B–C′, G, and H and Table 1). As was the case with the stac3−/− fibers, particle groupings and spacing were unaltered in stac3NAM-expressing muscles, consistent with a normal relationship and spacing of underlying RyRs despite the reduction in DHPR particle frequency (Fig. S4D). These findings indicate that the NAM W-to-S substitution in the first SH3 domain of Stac3 (10) decreases the number of DHPR particles within the transverse tubule membrane but does not affect the positioning of DHPRs into tetrads above the underlying RyR1 array.
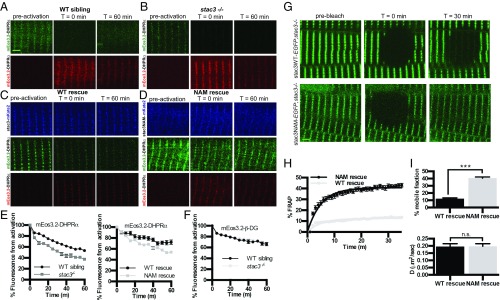
Loss of Stac3 Destabilizes the DHPR Complex at Triads.
The reduced levels of DHPRs in triadic junctions of stac3−/− muscle (Fig. 1) coupled with their continued association with RyRs raised the possibility that stac3 may regulate the stability of DHPRs at triads. To test this hypothesis, we developed an optical pulse-labeling method in which the gene encoding the DHPRα subunit (cacna1sa) was tagged with a photoconvertible protein, mEos3.2, that irreversibly switches spectral properties (green to red) when exposed to short-wavelength light (405 nm) (19). Using time-lapse confocal microscopy, we monitored loss of the photoconverted signal in triads as an index of the stability of triadic DHPRα. After photoconversion, the red fluorescence of mEos3.2-DHPRα at triads gradually decreased over the course of an hour in both WT sibling and stac3−/− embryos. The decrease in fluorescence was natural log-transformed and fit to a linear regression, and a decay (β) coefficient was calculated (Table S1). Fluorescence decay of triadic mEos3.2-DHPRα in stac3−/− fibers occurred at a significantly faster rate than that observed in WT siblings (Fig. 3 A, B, and E). Relative fluorescence of triadic mEos3.2-DHPRα in stac3−/− fibers expressing Stac3NAM also decreased faster than that of triadic mEos3.2-DHPRα in stac3−/− fibers expressing Stac3WT (Fig. 3 C–E). These results suggest that DHPRα was destabilized in triads of stac3−/−- and stac3NAM-expressing stac3−/− muscles. In contrast, the stability of photoconverted mEos3.2-β-DG, a protein found at triads not thought to be involved with EC coupling (20), expressed in stac3−/− fibers was equivalent to that of WT fibers (Fig. 3F), suggesting that Stac3 selectively stabilizes DHPR at triads. Thus, Stac3 is necessary for normal stability of DHPRα at triads, which may in part explain the reduction in DHPR particles per T-tubule length and tetrads per tetrad site (Fig. 2 G and H) observed in stac3-null and Stac3NAM-expressing muscle.
Table S1.
β-Coefficients from linear regressions of decay comparisons
| Protein | mEos3.2-DHPRα | mEos3.2-DHPRα | mEos3.2-β-DG | |||
| Genotype | WT sibling | stac3−/− | WT rescue | NAM rescue | WT sibling | stac3−/− |
| −0.0100 | −0.0200 | −0.0113 | −0.0194 | −0.0094 | −0.0053 | |
| −0.0099 | −0.0192 | −0.0078 | −0.0224 | −0.0086 | −0.0049 | |
| −0.0099 | −0.0199 | −0.0075 | −0.0193 | −0.0084 | −0.0062 | |
| −0.0074 | −0.0143 | −0.0126 | −0.0080 | −0.0032 | −0.0046 | |
| −0.0068 | −0.0110 | −0.0126 | −0.0080 | −0.0041 | −0.0042 | |
| −0.0069 | −0.0132 | −0.0070 | −0.0081 | −0.0042 | −0.0043 | |
| −0.0107 | −0.0115 | −0.0042 | −0.0096 | −0.0045 | −0.0048 | |
| −0.0083 | −0.0114 | −0.0034 | −0.0054 | −0.0044 | −0.0052 | |
| −0.0102 | −0.0100 | −0.0027 | −0.0092 | −0.0045 | −0.0042 | |
| −0.0130 | −0.0115 | −0.0031 | −0.0090 | |||
| −0.0134 | −0.0112 | −0.0023 | −0.0103 | |||
| −0.0130 | −0.0133 | −0.0044 | −0.0140 | |||
| −0.0089 | −0.0132 | −0.0074 | −0.0155 | |||
| −0.0094 | −0.0093 | −0.0047 | −0.0133 | |||
| −0.0096 | −0.0075 | −0.0020 | −0.0089 | |||
| −0.0036 | −0.0174 | −0.0025 | −0.0068 | |||
| −0.0058 | −0.0173 | −0.0030 | −0.0064 | |||
| −0.0044 | −0.0176 | −0.0056 | −0.0068 | |||
| −0.0114 | −0.0159 | −0.0051 | −0.0067 | |||
| −0.0113 | −0.0144 | −0.0047 | −0.0079 | |||
| −0.0119 | −0.0132 | −0.0075 | ||||
| −0.0123 | −0.0268 | −0.0056 | ||||
| −0.0121 | −0.0250 | −0.0038 | ||||
| −0.0106 | −0.0200 | −0.0055 | ||||
| −0.0147 | −0.0145 | |||||
| −0.0134 | −0.0110 | |||||
| −0.0102 | −0.0075 | |||||
| −0.0056 | ||||||
| −0.0038 | ||||||
| −0.0055 | ||||||
| −0.0165 | ||||||
| −0.0140 | ||||||
| −0.0157 | ||||||
| −0.0172 | ||||||
| −0.0102 | ||||||
| −0.0100 | ||||||
| Mean | −0.0100 | −0.0152 | −0.00570 | −0.0103 | −0.00570 | −0.00486 |
| SE | 0.000547 | 0.0009923 | 0.000742 | 0.000797 | 0.000791 | 0.000216 |
Fig. 3.
DHPRα is less stable in stac3 mutants. (A and B) Time course for optical pulse-labeling assay of mEos3.2-DHPRα1S expressed in the myofibers of WT sibling (A) and stac3−/− (B). Green channel (Top) and red channel (Bottom) before photoconversion (Left), immediately following photoconversion (Middle), and 60 min after photoconversion (Right). (C and D) Time course for optical pulse-labeling assay of mEos3.2-DHPRα1S in stac3−/− muscles expressing stac3WT-mKate2 (C) or stac3NAM-mKate2 (D). Blue channel representing the far-red mKate2 fluorescence (Top), green channel (Middle), and red channel (Bottom) for mEos3.2-DHPRα1S fluorescence as in A and B. (E) Time course of decay of photoconverted mEos3.2-DHPRα1S shows that fluorescence decays faster in stac3−/− (n = 24) compared with WT siblings (n = 24) (t-permutation test, P < 0.001) (Left) and that photoconverted mEos3.2-DHPRα1S decays faster in myofibers of stac3−/− expressing stac3NAM (n = 20) compared with expressing stac3WT (n = 20) (t-permutation test, P < 0.001) (Right). (F) Time course of decay of photoconverted mEos3.2-β-dystroglycan in WT siblings (n = 9) and stac3−/− (n = 9) shows that fluorescence decays at the same rate in WT and stac3−/− (t-permutation test, P = 0.86). (G) FRAP analysis of stac3−/− myofibers expressing stac3WT-EGFP (Top) or stac3NAM-EGFP (Bottom) before photobleaching (Left), immediately after photobleaching (Middle), and 30 min after photobleaching (Right). (H) Mean time course of FRAP of stac3−/− myofibers expressing stac3WT (n = 18) and stac3NAM (n = 36). (I, Top) Histogram showing the percentage of the mobile fraction is larger in stac3−/− myofibers expressing stac3NAM compared with stac3WT (t test, P < 0.0001). (I, Bottom) Histogram showing that the rate of recovery following photobleaching is unchanged between stac3−/− myofibers expressing stac3WT and stac3NAM (t test, ***P = 0.9). SEMs are indicated. n.s., not significant. (Scale bars, 2 μm.)
Although stac3NAM expression destabilized DHPRα compared with stac3WT, Stac3NAM protein nevertheless localized to the triad (Fig. 3 C and D). To understand how Stac3NAM increased DHPRα instability, the stability of Stac3NAM at triads was assayed by FRAP analysis of Stac3NAM-GFP– and Stac3WT-GFP–expressing stac3−/− muscle fibers. The mobile fraction of Stac3NAM-GFP expressed in stac3−/− fibers was significantly greater than that of Stac3WT-GFP expressed in stac3−/− fibers, despite the fact that the diffusion rates of Stac3NAM-GFP and Stac3WT-GFP were not different (Fig. 3 G–I). Therefore, the Stac3NAM protein is less stable at triads than the Stac3WT protein, which correlates with the decreased stability of triadic DHPRα in stac3−/− expressing Stac3NAM, although the trafficking rate of Stac3NAM to the triad was unchanged.
Stac3 Enhances DHPR Intramembrane Charge Movement and Voltage-Gated SR Ca2+ Release.
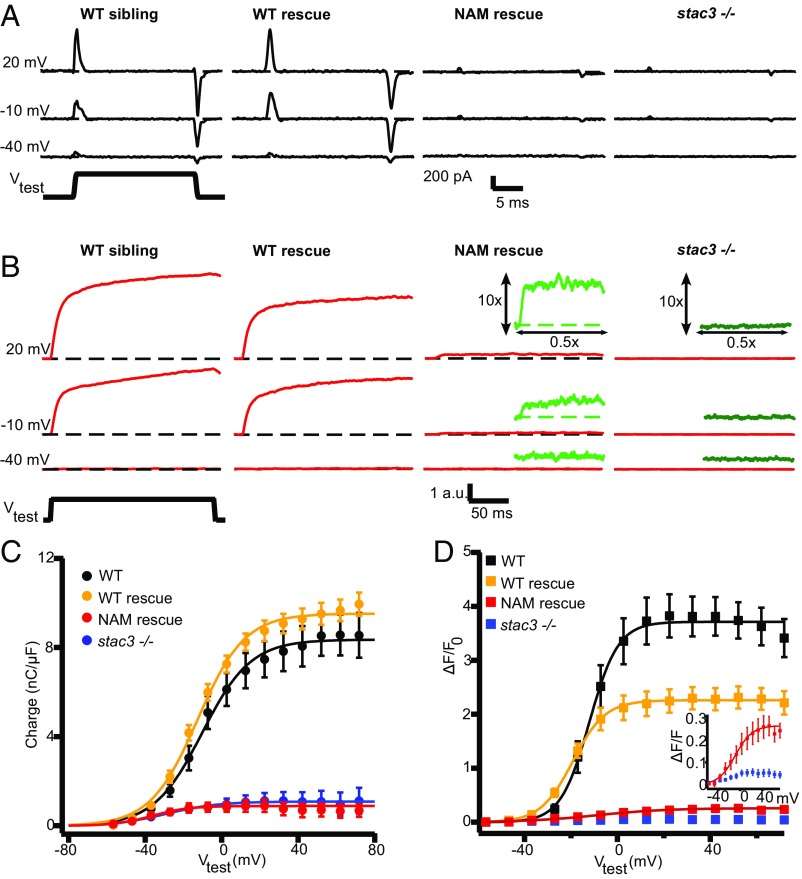
Because DHPRs serve as the voltage sensor for EC coupling, we examined how Stac3 affects the voltage dependence of DHPR charge movements and Ca2+ release from the SR. In zebrafish muscles, DHPRs do not pass ionic Ca2+ current, as is observed for DHPRs in mammalian skeletal muscle (16). However, DHPR functionality in fish can be assayed by measuring the magnitude and voltage dependence of intramembrane charge movement. Electrophysiological recordings from disassociated myotubes indicated an almost complete absence of intramembrane charge movement (Q) in stac3−/− fibers compared with that observed for WT fibers (Fig. 4 A and C and Table 1).
Fig. 4.
DHPR charge movement and SR Ca2+ release are dramatically reduced in stac3 mutants. (A) Representative DHPR gating currents elicited by test pulses (Vtest) to +20 mV, −10 mV, and −40 mV from a holding potential of −80 mV in myofibers from WT siblings, stac3−/− expressing stac3WT-EGFP (WT rescue), stac3−/− expressing stac3NAM-EGFP (NAM rescue), and stac3−/− zebrafish. Gating currents in WT rescue fibers were comparable to WT fibers but dramatically decreased in stac3−/− and NAM rescue fibers. (B) Representative Fluo-4 fluorescence traces elicited by 200-ms test pulses to −40, −10, and +20 mV in fibers from WT siblings, WT rescue, NAM rescue, and stac3−/−. Fluo-4 transients in WT rescue myofibers were comparable to WT fibers but dramatically decreased in stac3−/− and NAM rescue fibers. (Insets) Magnifications (10×) of the Fluo-4 transients (green) in NAM rescue and stac3−/− fibers. (C) The voltage dependence of the integrated ON component of intramembrane DHPR charge movement was comparable between myofibers from WT siblings (n = 10) and WT rescue (n = 14) (ANOVA, ns) but dramatically decreased in fibers from stac3−/− (n = 19) and NAM rescue (n = 12) fibers. Data from stac3−/− fibers were too small to be accurately fit (SI Materials and Methods). (D) Voltage dependence of Fluo-4 transients in fibers from WT siblings (n = 6), WT rescue (n = 13), NAM rescue (n = 8), and stac3−/− (n = 6) (ANOVA Tukey’s, P < 0.01). (Inset) Fluo-4 transients were small but clearly detectable in NAM rescue fibers but not in stac3−/− fibers. SEMs are indicated.
To test the effect of the stac3NAM mutation on the DHPR charge movement, stable muscle actin:stac3NAM-EGFP; stac3−/− transgenic lines were generated and compared with muscle actin:stacWT-EGFP; stac3−/− transgenic embryos. Expression of muscle actin:stac3WT-EGFP in stac3−/− fibers restored maximum charge movement (Qmax) back to WT sibling levels (Fig. 4 A and C and Table 1). However, in fibers expressing muscle actin:stac3NAM-EGFP myotubes, Qmax was significantly decreased compared with muscle actin:stac3WT-EGFP–expressing stac3−/− and WT sibling fibers, and comparable to that of stac3−/− fibers. These data indicate that Stac3, and more specifically the W residue in the first SH3 domain of Stac3 that is replaced by S in stac3NAM, is required for normal DHPR charge movement in zebrafish muscle.
We previously showed that stac3−/− muscle fibers expressing stac3NAM-mCherry exhibited a partial release of Ca2+ from the SR with slower kinetics than usual (10). To get a more comprehensive characterization of the magnitude, kinetics, and voltage dependence of Ca2+ release, depolarization-induced changes in intracellular Ca2+ were assessed using Fluo-4 AM recorded simultaneously with charge movement in myofibers of WT, stac3−/−, and stable transgenic stac3−/− embryos expressing Stac3WT or Stac3NAM. In stac3−/− fibers, Ca2+ release was nearly absent in response to voltage-clamp depolarizations of the muscle plasma membrane, whereas expression of muscle actin:stac3WT-EGFP restored robust Ca2+ release, exhibiting a sigmoidal voltage dependence (Fig. 4 B and D and Table 1). Despite the near absence of detectable charge movement (Fig. 4 A and C), stac3−/− fibers expressing muscle actin:stac3NAM-EGFP exhibited a low level of voltage-dependent Ca2+ release that was significantly higher than that observed in stac3−/− fibers (Fig. 4 B and D, Insets), indicating that muscle actin:stac3NAM-EGFP expression restored some Ca2+ release. In addition, voltage-gated Ca2+ release in myofibers from muscle actin:stac3NAM-EGFP was significantly right-shifted (V1/2max −2.3 ± 6.3 mV) and exhibited a dramatically shallower slope (slope 17.2 ± 2.8 mV) compared with that of muscle actin:stac3WT-EGFP (V1/2max −21.2 ± 1.8 mV, slope 7.2 ± 0.8 mV) (Table 1). Similar decreases in Ca2+ release were observed when delivering field stimulation of 10 Hz to fibers to mimic trains of action potentials exhibited by muscles during evoked swimming (10, 21) (Fig. S5). Thus, Stac3 was required for both normal DHPR charge movement and voltage-dependent SR Ca2+ release.
Fig. S5.
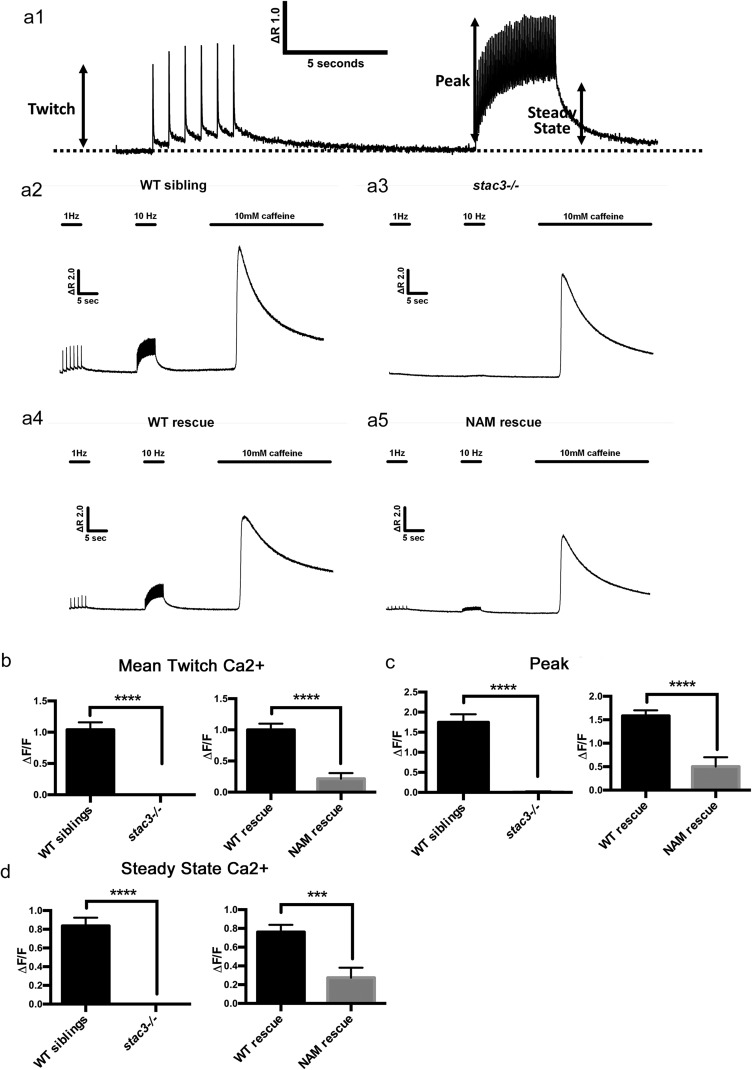
Ca2+ transients are reduced in dissociated muscle fibers from stac3 mutants in response to electrically evoked twitch and 10-Hz trains of stimulation. (A1) Representative Fluo-4 fluorescence trace in a WT fiber during six successive single electrically evoked (twitch) stimuli, a 5-s 10-Hz stimulation train showing both measurement of peak and steady-state levels during the train. (A2–A5) Representative Fluo-4 fluorescence traces from WT sibling, stac3−/−, WT rescue (muscle actin:stac3WT-EGFP; stac3−/−), and NAM rescue (muscle actin:stac3NAM-EGFP; stac3−/−) following stimulation. The stimulation protocol consisted of six successive single-voltage pulses (1-Hz) followed by a 5-s 10-Hz stimulation, and finally addition of 10 mM caffeine. (B) Histograms showing that average (±SE) peak electrically evoked change in relative Fluo-4 fluorescence (ΔF/F) during twitch stimulation is dramatically reduced in stac3−/− and NAM rescue fibers compared with WT sibling and WT rescue fibers (t test, WT sibling versus stac3−/−, P < 0.0001 and WT rescue versus NAM rescue, P < 0.0001). (C) Histograms showing that average (±SE) peak change in relative Fluo-4 fluorescence during twitch stimulation is reduced in stac3−/− and NAM rescue fibers compared with WT sibling and WT rescue fibers (t test, WT sibling versus stac3−/−, P < 0.0001 and WT rescue versus NAM rescue, P < 0.0001). (D) Histograms showing that average (±SE) steady-state change in relative Fluo-4 fluorescence during 10-Hz stimulation is reduced in stac3−/− and NAM rescue fibers compared with WT sibling and WT rescue fibers (t test, WT sibling versus stac3−/−, P < 0.0001 and WT rescue versus NAM rescue, P < 0.001). WT sibling, n = 42; stac3−/−, n = 16; WT rescue, n = 27; NAM rescue, n = 14. SEMs are indicated.
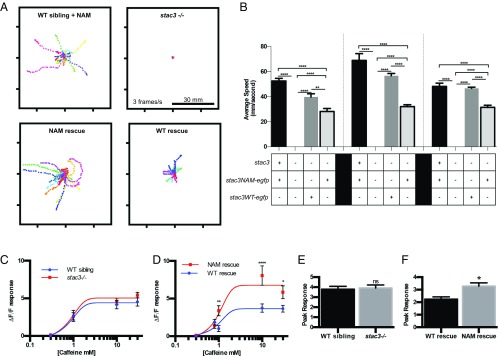
Embryos Expressing Stac3NAM Exhibit Decreased Swimming and Increased Caffeine Responsiveness.
Stac3NAM-expressing myofibers are able to release a low level of Ca2+ in response to voltage-clamp depolarization (Fig. 4D) and electrical stimulation (Fig. S5). This predicts that muscle actin:stac3NAM-EGFP; stac3−/− transgenic embryos should exhibit more mobility than stac3−/− embryos. Indeed, muscle actin:stac3NAM transgenic embryos were significantly more motile than stac3−/− embryos but less motile than stac3−/− embryos expressing muscle actin:stac3WT (Fig. 5 A and B). Presumably, the lack of swimming in embryos injected with stac3NAM expression constructs previously reported (9) was due to mosaic and inconsistent expression of Stac3NAM. Thus, the stable and consistent expression of Stac3NAM within transgenic fish used here is likely sufficient for the low-level swimming observed in these fish despite low-level Ca2+ transients observed in Stac3NAM muscle fibers (Fig. 4 and Fig. S5).
Fig. 5.
Stac3NAM transgenic zebrafish have reduced motility and hallmarks of malignant hyperthermia. (A) Overlaid traces of touch-evoked swimming by transgenic 72-hpf WT siblings expressing stac3NAM-EGFP (WT sibling + NAM), stac3−/−, transgenic stac3−/−;stac3WT-EGFP (WT rescue), transgenic stac3−/−; stac3NAM-EGFP (NAM rescue), and stac3−/− showing that whereas stac3−/− embryos do not swim, NAM rescue embryos do. (B) Histograms of the speed of swimming by WT sibling + NAM (n = 55, 11, and 32 at 56, 72, and 96 hpf, respectively), stac3−/− (n = 15, 15, and 15), WT rescue (n = 8, 20, and 91), and NAM rescue (n = 18, 55, and 58) show that NAM rescue zebrafish exhibit partial rescue of swimming compared with WT rescue (ANOVA Tukey multiple comparisons, ****P < 0.0001, **P < 0.001). (C) Dose–response plots of Ca2+ release as a function of caffeine concentration indicate that stac3−/− muscles do not show increased Ca2+ release in response to caffeine compared with WT sibling muscles (0.3 mM, n = 30, 30; 1.0 mM, n = 19, 14; 10.0 mM, n = 16, 20; 30.0 mM, n = 18, 29). The data were fit with a sigmoidal with Hill slope of 1. (D) Dose–response plots of Ca2+ release as a function of caffeine concentration show that NAM rescue transgenic muscles release more Ca2+ compared with WT rescue transgenic muscles. Each point represents the average maximal caffeine response relative to the baseline immediately before caffeine application (0.3 mM, n = 171, 192; 1.0 mM, n = 24, 20; 10.0 mM, n = 16, 16; 30.0 mM, n = 22, 23) (t-test comparisons, ****P < 0.0001, **P < 0.01, *P < 0.05). (E) Histogram showing that mean peaks of Ca2+ released from internal stores induced by application of the ICE release mixture are comparable between WT sibling (n = 27) and stac3−/− (n = 35) myofibers (t test, P < 0.7). ns, not significant. (F) Histogram showing that the mean peak of Ca2+ released from NAM rescue fibers (n = 45) is higher than in WT rescue fibers (n = 30, t test, *P = 0.01). SEMs are indicated.
Individuals homozygous for the Stac3NAM mutation are susceptible to malignant hyperthermia, a pharmacogenic disease characterized by Ca2+ dysregulation in skeletal muscle. Because NAM is found in a relatively homogeneous population, the Lumbee Native Americans, other genetic factors within the population could contribute to the MH susceptibility seen in NAM patients. To determine whether stac3−/− and/or NAM muscle fibers might exhibit characteristics of MH, the caffeine responsiveness of fibers of stac3−/− and muscle actin:stac3NAM-EGFP; stac3−/− embryos was examined, because an increased sensitivity to caffeine is observed in muscle fibers exhibiting MH. Ca2+ release in stac3−/− fibers in response to increasing concentrations of caffeine was not different from that observed for WT siblings (Fig. 5C). However, myofibers from muscle actin:stac3NAM-EGFP embryos exhibited significantly higher Ca2+ release than fibers from muscle actin:stac3WT-EGFP; stac3−/− embryos (Fig. 5D) at all concentrations of caffeine tested. Thus, doses of caffeine above saturation (>10 mM) induced significantly greater Ca2+ release in muscle actin:stac3NAM-EGFP; stac3−/− fibers, consistent with higher luminal Ca2+ levels in the SR of myofibers from muscle actin:stac3NAM-EGFP; stac3−/− embryos. This idea was also tested by application of a rapid Ca2+ release mixture (ICE; 10 μM ionomycin, 30 μM cyclopiazonic acid, and 100 μM EGTA/0 Ca2+) to deplete intracellular Ca2+ stores as an index of total Ca2+ store content (22). Using this assay, total releasable Ca2+ store content was not different between myofibers from WT and stac3−/− embryos (Fig. 5E) but was significantly increased in myofibers from muscle actin:stac3NAM embryos compared with myofibers from muscle actin:stac3WT transgenics (Fig. 5F). These results are consistent with the idea that luminal SR Ca2+ is increased in stac3NAM embryos.
Discussion
Our results indicate that a relatively modest reduction (∼24 to 34%) in DHPRα1S expression within the triad junction of stac3−/− and stac3NAM muscle is not sufficient to explain the near-complete loss of depolarization-induced Ca2+ release. In addition, our freeze-fracture results support previous work indicating that complete tetrads represent the critical DHPR structural unit required for functional EC coupling (17, 23–25). Indeed, the number of complete tetrads and amount of DHPR charge movement are tightly correlated in each genotype studied (Table 1). Additionally, knockdown of maternal stac3 message in stac3−/− embryos resulted in few to no complete tetrads (Fig. 2 and Table 1) and complete immotility (10). These data demonstrate the importance of defects in tetrads as a fundamental mechanism for altered EC coupling in human myopathies.
As expected, the number of complete tetrads varies as the fourth power of DHPR expression in all genotypes examined, suggesting that the decrease in complete tetrads is due to a decrease in triadic DHPRs in stac3 mutants. Interestingly, DHPR charge movement and SR Ca2+ release are decreased disproportionately to that of complete tetrads and DHPR particles in stac3−/− and stac3NAM fibers (Table 1). This suggests that Stac3 regulates charge movement and EC coupling in ways beyond simply regulating the amount of complete DHPRs and tetrads at triads. One possibility is that Stac3 may be required for the proper conformation of DHPRs in T tubules required for voltage-dependent charge movement. Consistent with this, charge movement and voltage-gated Ca2+ release are also reduced in myotubes from Stac3-null mice (26), although the magnitude of charge movement reduction in Stac3-null and Stac3NAM-expressing zebrafish fibers was greater than that observed in mouse myotubes. In addition, the lack of proper folding of the DHPRα in the T-tubule membrane has been proposed as the basis for a similar near-complete absence of DHPR charge movement in the DHPRβ1a-null zebrafish (17, 24).
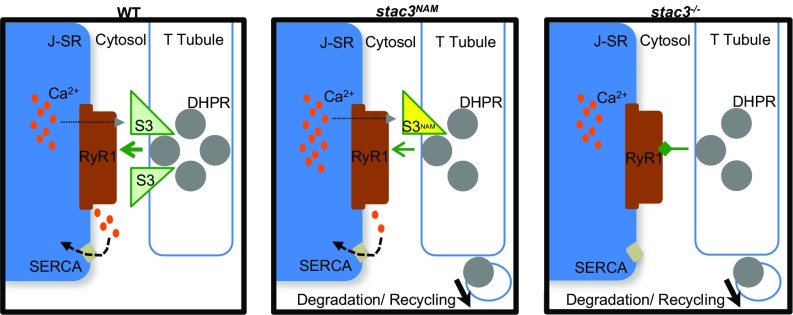
DHPRα is less stable at triads in stac3−/− and stac3NAM fibers. The reduced stability of DHPRα is presumably responsible for the decrease in triadic DHPRs observed in these fibers. How Stac3 regulates the stability of DHPRs is unknown. Stability of DHPRs could be regulated by Stac3 once DHPRs are in the triadic T-tubule membrane. Another possibility is that Stac3 may act as a triad-localized chaperone responsible for proper insertion of DHPRs into the T-tubule membrane. The absence of Stac3 to properly fold/insert DHPRα1S proteins into the T-tubule membrane would increase the fraction of DHPRα1S proteins available for degradation, perhaps explaining the increased DHPR mobility and instability in stac3−/− fibers (Fig. 6).
Fig. 6.
Model for the role of Stac3 in DHPR trafficking and maintenance of functional tetrads. In WT fibers (Left), Stac3 facilitates direct EC coupling between DHPR tetrads and RyR1, allowing normal Ca2+ release (gray arrowhead) and subsequent refilling of Ca2+ SR stores by SERCA. In stac3NAM fibers (Middle), DHPRα and Stac3NAM are unstable, causing DHPRα to enter degradation and recycling pathways and leaving triadic DHPRα reduced and in incomplete tetrads. EC coupling is less efficient, reducing Ca2+ release, but resulting in excessive SR Ca2+ buildup. In stac3−/− fibers (Right), DHPRα are unstable, reduced, and in incomplete tetrads, and EC coupling is inhibited.
DHPRβ1a-null zebrafish also display reduced DHPR triadic levels, charge movements, tetrad formation, and voltage-gated Ca2+ release. However, unlike DHPRβ1a, a chaperone that is cotransported with DHPRα1S to the triadic junction, Stac3 is required instead for stability of DHPRα1S within the triadic junction (17, 23, 24). Interestingly, whereas both Stac3-null and DHPRβ1a-null fibers show a 90% reduction in Qmax, Stac3-null shows a rightward shift in voltage dependence whereas DHPRβ1a-null exhibits a leftward shift. Additional work is needed to further elucidate how DHPRβ1a and Stac3 cooperate to facilitate the formation of DHPR into functional tetrads.
Although NAM patients are susceptible to MH, a causal role of Stac3NAM in MH had not been previously established. As a result, STAC3 is not routinely examined as a candidate gene in screens for MH in the general population (27, 28). The data presented here strengthen the link between Stac3 and MH susceptibility and potentially provide a novel animal model for the study of MH susceptibility. Furthermore, because zebrafish are amenable to high-throughput drug screens, stac3NAM zebrafish have the potential to become a powerful tool for new drug discovery in the treatment of NAM and, potentially, MH susceptibility.
Analysis of stac3NAM zebrafish may also contribute to a greater understanding of the pathogenesis and mechanism of MH. Intriguingly, the mechanics of Ca2+ dysregulation in Stac3NAM-expressing myofibers appears to be unique. Previous studies have shown that mutations in RyR1(29–31) or DHPRα1S (32, 33) linked to increased MH susceptibility result in an increase in the sensitivity of caffeine-induced Ca2+ release that occurs in the absence of a change in maximal caffeine-induced Ca2+ release. In contrast, we found that whereas caffeine-induced Ca2+ release was unaltered in stac3−/− fibers, stac3NAM fibers exhibited increased Ca2+ release in response to all concentrations of caffeine, suggesting that SR luminal Ca2+ may be higher in stac3NAM fibers and that this may contribute to higher MH susceptibility. The increased Ca2+ release in response to caffeine observed in stac3NAM fibers could in principle be caused by increases in several factors, including the number of activated RyR1 channels, RyR1 open-channel probability or single-channel conductance, and/or the chemical driving force Ca2+ release. However, because triadic RyR1 levels were not increased in stac3NAM myofibers, an increase in RyR1s appears not to underlie the increase in caffeine-induced Ca2+ release. Although a potential effect of stac3NAM on RyR1 open-channel probability and single-channel conductance is unclear, we found that total releasable Ca2+ store content was increased in rescued stac3NAM fibers compared with rescued stac3WT fibers. This observation raises the possibility that increased SR Ca2+ content, and possibly luminal Ca2+ regulation of RyR1 activity (34), contributes to the increased caffeine responsiveness observed in rescued stac3NAM fibers.
Increased luminal Ca2+ levels in rescued Stac3NAM fibers could be explained by either increased ability to sequester Ca2+ and store Ca2+ within the SR such as through increased sarco/endoplasmic reticulum calcium ATPase (SERCA) activity or calsequestrin expression, or through a reduction of steady-state SR Ca2+ leak. Interestingly, a significant component of myoplasmic Ca2+ homeostasis depends on SR Ca2+ leak via RyR1, which is tightly controlled through the orthograde interaction between DHPRα1S and RyR1 (35, 36). DHPRα1S normally suppresses RyR1 Ca2+ leak, and a mutation in DHPRα1S linked to MH (R174W) has been proposed to promote RyR1 in the leak state (37). The continued presence of DHPRα1S and its ability to suppress RyR1 opening could also explain how elevated SR Ca2+ in Stac3NAM fibers does not induce RyR1 to open at resting state, as has been reported when RyR1 is expressed in HEK293 cells without DHPRα1S (34). Our data indicate that although inefficient, DHPRα1S structural (Fig. 2) and functional (Fig. 4) coupling to RyR1 is maintained in rescued Stac3NAM fibers. Thus, the observed increase in Ca2+ store content and caffeine responsiveness in rescued Stac3NAM fibers could result in part from increased DHPRα1S-mediated suppression of RyR1 Ca2+ leak that facilitates accumulation of SR luminal Ca2+ levels. Additionally, the marked reduction in voltage-gated Ca2+ release observed in rescued Stac3NAM fibers would further promote an increase in luminal SR Ca2+ content. The consequences of excessive SR Ca2+ levels and its contribution to the pathology and MH susceptibility of NAM are areas that require further investigation.
Materials and Methods
Animal Behavioral Analysis.
Zebrafish were bred and maintained according to approved guidelines of the University Committee on Use and Care of Animals at the University of Michigan. stac3mi34 (stac3−/−) carriers were raised to 48 hpf and stac3−/− mutant embryos were behaviorally identified as previously described (10).
Disassociation of Zebrafish Myotubes and Quantification of Triadic Fluorescence.
Zebrafish myofibers were dissociated with collagenase, and fluorescence was quantified as previously described (17). Embryos were identified by behavior and fluorescence, and the heads of embryos were removed for genotyping before disassociation of tails. Photoactivation of mEos3.2 and quantification of fluorescence are described in SI Materials and Methods.
Measurement of Cav1.1 Gating Charge Movement and Depolarization-Induced Ca2+ Transients.
The whole-cell patch-clamp technique was used to quantify the magnitude and voltage dependence of CaV1.1 channel Q movement in isolated zebrafish fibers as described previously (24). Ca2+ transients were measured by loading fibers with Fluo-4 AM from the patch pipette. Detailed methods are in SI Materials and Methods.
Intracellular Ca2+ Measurements in Intact Muscle Fibers.
Fibers were loaded with 5 µM Fluo-4 AM and excited at 480 ± 15 nm, and fluorescence emission detected at 535 ± 20 nm was collected at 10 kHz using a photomultiplier system. Electrically evoked Ca2+ transients were measured in dissociated fibers and elicited by electrical field stimulation using a glass electrode filled with 200 mM NaCl placed adjacent to the cell of interest. Caffeine concentration–response curves were obtained by sequential exposure of fibers to various concentrations of caffeine applied through a rapid (response time <5 s) local perfusion system (Warner Instruments).
Freeze-Fracture Electron Microscopy.
Four-day-old zebrafish larvae were fixed at room temperature with 6% (vol/vol) glutaraldehyde in 0.1 M cacodylate buffer at neutral pH after removal of tail skin, stored at 4 °C, and shipped at room temperature to C.F.-A.’s laboratory while in the fixative. Thin-section EM and freeze-fracture analysis were performed as previously described (24, 38).
SI Materials and Methods
Zebrafish Behavior.
Embryos for behavioral analysis were enzymatically dechorionated using 2 mg/mL Pronase (protease, type XIV; Sigma) for 20 min. Embryos were placed individually in a dish and touched lightly on the tail with no. 2 forceps while recording a 30-s movie. Movies were imported into ImageJ (NIH) and tracked manually using a manual tracking plugin. Using a custom script in R with the ggplot package (39), each track was calibrated to the middle of the plate and the path of swimming from each embryo was traced and overlaid into a figure. Tg:muscle actin:Stac3NAM-EGFP+/−; stac3−/− and Tg:muscle actin:Stac3NAM-EGFP+/−; stac3+ embryos were genotyped after behavioral recordings.
Antibody Conjugation and Immunolabeling.
Anti-DHPRα1S (mAb1) MA3-920 (Thermo) and anti-pan RyR 34c (DSHB) were each purified using a NAb Protein A/G Spin Kit (Thermo). Purified flowthrough was buffer-exchanged with PBS (pH 7.0) and concentrated to 1 mg/mL with Pierce Protein Concentrators PES, 10-kDa molecular-weight cutoff. Antibodies were conjugated with Alexa Fluor 488 or 568 protein labeling kits. Dye/IgG ratio was estimated by spectrophotometry with a NanoDrop (Invitrogen). Anti–DHPRα1S-Alexa488 was found to give ideal labeling at 2.1 dye per IgG and anti–panRyR-Alexa568 at 5.5 dye per IgG. Anti–panRyR-Alexa568 was used at 1:500, anti–DHPRα1S-Alexa488 at 1:100 unless otherwise noted, anti-DHPRα1S ACC-314 (Alomone) at 1:500. Whole-mount immunolabeling was performed after fixation with 4% (vol/vol) paraformaldehyde (PFA) at 4 °C overnight using a previously described method (40).
Electrophysiology.
To test the effect of the stac3NAM mutation on the DHPR charge movement, stable muscle actin:stac3NAM-EGFP; stac3−/− transgenic lines were generated and compared with muscle actin:stacWT-EGFP; stac3−/− transgenic embryos. Isolated single fibers cultured for 24 h were bathed in an external recording solution containing 10 mM Ca(OH)2, 100 mM l-aspartate, 10 mM Hepes [pH 7.4 with tetramethylammonium hydroxide (TEA-OH)], and 0.1 mM N-benzyl-p-toluenesulfonamide (pH 7.4 adjusted with TEA-OH). The patch pipette internal solution contained 100 mM Cs-aspartate, 10 mM Hepes, 0.5 mM CsEGTA, 3 mM MgATP, and 0.2 mM Fluo-4 AM (pH 7.4 adjusted with CsOH). Patch pipette resistance when placed in the external solution was 3 to 6 MΩ. Fibers were voltage-clamped at a holding potential of −80 mV. Series resistance was compensated up to 80%. Data were sampled every 90 µs and filtered using a low-pass Bessel filter with 2-kHz cutoff frequency. QON, observed as positive gating current, was generated by 20-ms depolarizing pulses to potentials ranging from −60 mV to +70 mV in 10-mV increments delivered every 2 s. To improve signal-to-noise ratio, three runs of the stimulation protocol were used to obtain averaged gating current records for each depolarizing voltage. A prepulse step for 1 s to −50 mV was used to inactivate endogenous T-type channels. Gating charge was calculated by integrating the outward gating current transient normalized to fiber capacitance (nC/µF) and plotted against the testing depolarizing voltage (V test). The voltage dependence of charge movement was fitted with the following Boltzmann equation: Q = Qmax/(1 + exp[(VQ1/2 − Vm)/kQ]), where Qmax is the maximum charge movement, Vm is the membrane test potential, VQ1/2 is the voltage for half-maximal activation of Qmax, and kQ is a slope factor.
Quantification of Triadic Fluorescence.
Tg:muscle actin:Stac3WT-EGFP+/−; stac3−/− adults were bred and embryos were identified as Tg:muscle actin:Stac3WT-EGFP+; stac3−/− by behavior and fluorescence. The parental stac3−/− carrier fish (Tg:muscle actin:Stac3NAM-EGFP+/−; stac3+/−) were individually identified by genotyping heads of progeny embryos. For examining dissociated muscle fibers, tails from single 48-hpf embryos were incubated in collagenase type II (3.125 mg/mL in CO2-independent medium) at 20 °C for ∼1 h. Fibers were spun at 380 × g for 5 min, the supernatant was removed, and the pellet was resuspended and allowed to settle on polylysine-coated coverslips. Fibers were cultured in Leibovitz’s L-15 medium supplemented with 5 mM l-glutamine, 10% (vol/vol) FBS, and penicillin/streptomycin/amphotericin B and incubated at 28.5 °C overnight before being fixed in 4% (vol/vol) PFA. Quantification of immunolabeled triadic DHPRα1S or RyR was determined by measuring the average fluorescence along a line across a row of triadic junctions. Cultures were immunostained simultaneously and images were obtained using identical exposure times, gain, and excitation. Average fluorescence was obtained across five triadic junctions from each myotube and at least seven myotubes per genotype or antibody titration. For immunolabeling and physiological analysis of dissociated muscle fibers, heads were removed for genotyping before dissociation of trunks and used for genotyping.
Caffeine Dose–Response Experiments.
For caffeine concentration–response experiments, fibers were first exposed for 30 s to 0.3 mM caffeine, followed by a 30-s wash with control Ringer’s solution and then a final 30-s exposure to either 1.0, 10, or 30 mM caffeine. The magnitude of total Ca2+ store content was assessed from a 30-s application of a release mixture designed to rapidly dump intracellular Ca2+ sores (ICE; 10 µM ionomycin, 30 µM cyclopiazonic acid, and 100 µM EGTA in a Ca2+-free Ringer’s solution). For all intact fiber Ca2+ measurements, peak changes in relative Fluo-4 fluorescence were measured as ΔF/F, where F is the baseline fluorescence immediately before depolarization and ΔF is the fluorescence change from baseline.
Electron Microscopy.
For thin-section EM, the tails were postfixed in 2% (vol/vol) OsO4 in 0.1 M cacodylate buffer for 1 h at 4 °C, en bloc stained with a saturated solution of uranyl acetate in H2O, and embedded in Epon (41, 42). Thin sections were stained with lead acetate for contrast. For freeze-fracture EM, tails were infiltrated in 30% (vol/vol) glycerol in H2O and cut into two or three segments, and segments from five or six larvae were mounted vertically into the central hole of a small gold holder, allowing adhesion to each other to hold them upright. The mounted tails were frozen in liquid nitrogen-cooled propane, and then mounted on the cold freeze-fracture table, brought under vacuum, fractured, shadowed with platinum at 45°, and replicated with carbon in a Balzers BAF 400. Each freeze-fracture replica contained information from four or five fish. Thin sections and fracture replicas were examined in a Philips EM 410 (Philips Electron Optics). The images were digitally recorded with a Hamamatsu C4742-95 digital camera (Advanced Microscopy Techniques).
Cloning of cacna1sa.
Pooled mRNA from 50 WT 48-hpf embryos was extracted using TRIzol (Invitrogen) reagent, and SuperScript II and Oligo dT (Invitrogen) were used for reverse transcription to create a cDNA library. cDNA was amplified by PCR and subcloned into pGemT-easy using Max Efficiency Stbl2 competent cells (Invitrogen). Kozak sequences containing EGFP and mEos3.2 were amplified using SacII-tagged primers, digested with FastDigest SacII (Thermo), and ligated in-frame to the N terminus of cacna1sa, which has been shown to produce a fully functional Cav1.1 protein (41). gfp-cacna1sa and mEos3.2-cacna1sa were digested out of the pGEM-T Easy backbone, gel-purified using a SNAP UV-Free Gel Purification Kit (Invitrogen), and ligated into the pα-actin:SV40 backbone for skeletal muscle expression. Sanger sequencing using 17 primers spaced throughout the length of cacna1sa confirmed 100% sequence identity with the amino acid prediction from the Zv9 genome assembly (ftp://ftp.sanger.ac.uk/pub/zfish/assembly/Zv9/).
In Vivo Live-Cell Imaging and Quantification.
Progeny from stac3mi34 carriers were injected at the one-cell stage with either muscle actin:EGFP-cacna1sa or muscle actin:mEos3.2-cacna1sa and in combination with muscle actin:mKate2-stac3NAM, muscle actin:mKate2-stac3WT, muscle actin:mCherry-stac3NAM, or muscle actin:mCherry-stac3WT. Embryos were raised to 24 hpf at 28.5 °C, enzymatically dechorionated, and put into 200 μM 1-phenyl 2-thiourea (PTU) E2 medium at 28.5 °C to inhibit melanogenesis (42, 43). At 48 hpf, embryos were identified behaviorally and left at room temperature (22 °C). All imaging experiments were conducted on embryos from 2 to 4 d postfertilization at room temperature unless otherwise noted. For temperature-controlled experiments, embryos were imaged on a TS-4MP thermal microscope stage (Physitemp). EGFP- or mEos3.2-expressing embryos were identified using a fluorescence dissecting microscope, and anesthetized in 0.02% Tricaine in 200 μM PTU (Sigma) for at least 2 h. For mounting, embryos were embedded in 1% low-melting point agarose on a plastic Petri dish, covered with 0.02% Tricaine and 200 μM PTU, and imaged using a 40× objective on a Leica SP5 upright confocal microscope using a 10× digital zoom. FRAP within the triad was quantified and the data were fit to a nonlinear regression to obtain best-fit values for mobile fraction and diffusion rates. For each individual FRAP trace used in the analysis, the mean fluorescence of all postbleach triads was significantly higher than the mean fluorescence of all triads at T 0 (ANOVA, P < 0.05). In mEos3.2 imaging, photomultiplier tube (PMT) settings were set so that no bleedthrough of preactivated green fluorescence was seen in the red channel (band pass 575 to 730 nm). For mEos3.2 and mKate2 imaging, PMT settings were set so that minimal mKate2 could be seen in the red channel (red channel 575 to 600, mKate2 635 to 688). Low laser intensities were used to minimize bleaching (8% 488-nm and 10% 568-nm excitation laser intensities). FRAP bleaching was achieved with 10 scans of 100% 488-nm laser intensity over the course of 1 min and subsequent imaging with 8% 488-nm excitation laser (band pass 493 to 555 nM).
Statistical Analyses.
For particles per T-tubule length, data for the number of particles per tetrad site were converted to a z score and multiplied by z score-converted values of T-tubule length with groups of particles per total length; error bars represent SD. Similarly, for complete tetrads per tetrad site, data for the number of complete tetrads per tetrad site were converted to a z score and multiplied by z score-converted values of T-tubule length with groups of particles per total length; error bars represent SD. For optical pulse-labeling experiments, a series of randomization tests implemented in R (https://www.r-project.org/) captured the statistical significance of the observed differences in fluorescence decay between the wild-type group and each other group. This procedure was executed in three steps. First, exponential decay traces were natural log-transformed, and linear regressions were fit to the time course of fluorescence. Second, a mean difference score between β-coefficients was calculated for each comparison of interest (e.g., wild type versus mutant). Third, for each comparison of interest, the group membership of the β-coefficients was randomized before recalculating a mean difference score. This randomization procedure was repeated 10,000 times for the comparisons of interest, yielding distributions of mean difference scores to be expected under the null hypothesis of each. Because there was a clear hypothesis about the sign of the mean difference score for each comparison, P values were determined from the positive tail null permutation distributions. Permutation testing was used to avoid distributional assumptions during statistical testing. Nevertheless, parametric tests (e.g., independent sample t tests) provided similar results. At probabilities P < 0.05, the null hypothesis was rejected and the results were judged as statistically significant. Data are shown as mean ± SEM. Statistical analysis was performed using two-sample t test, or one-way ANOVA with Tukey multiple comparison correction.
Acknowledgments
We thank Alex Migda, Matthew Lacey, Sean Lowe, Hoaxing Xu, and Richard Hume for technical assistance and discussions. Research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health Grant R01-AR-063056 (to J.Y.K.); Grants NIAMS AR059646 and AR053349 (to R.T.D.); Grant NIAMS AR060831 (to V.Y.); and Grant NIAMS 2P01 AR 052354-06A1, PI: P. D. Allen (to C.F.-A.). J.W.L. was supported in part by a Rackham Merit Fellowship (University of Michigan) and NIGMS (Grant T32 GM007315).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1619238114/-/DCSupplemental.
References
- 1.Flucher BE, Franzini-Armstrong C. Formation of junctions involved in excitation-contraction coupling in skeletal and cardiac muscle. Proc Natl Acad Sci USA. 1996;93(15):8101–8106. doi: 10.1073/pnas.93.15.8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dowling JJ, Lawlor MW, Dirksen RT. Triadopathies: An emerging class of skeletal muscle diseases. Neurotherapeutics. 2014;11(4):773–785. doi: 10.1007/s13311-014-0300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanabe T, Beam KG, Powell JA, Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988;336(6195):134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- 4.Lai FA, Erickson HP, Rousseau E, Liu QY, Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- 5.Paolini C, Fessenden JD, Pessah IN, Franzini-Armstrong C. Evidence for conformational coupling between two calcium channels. Proc Natl Acad Sci USA. 2004;101(34):12748–12752. doi: 10.1073/pnas.0404836101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grabner M, Dirksen RT, Suda N, Beam KG. The II-III loop of the skeletal muscle dihydropyridine receptor is responsible for the bi-directional coupling with the ryanodine receptor. J Biol Chem. 1999;274(31):21913–21919. doi: 10.1074/jbc.274.31.21913. [DOI] [PubMed] [Google Scholar]
- 7.Nakai J, et al. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature. 1996;380(6569):72–75. doi: 10.1038/380072a0. [DOI] [PubMed] [Google Scholar]
- 8.Bannister RA. Bridging the myoplasmic gap II: More recent advances in skeletal muscle excitation-contraction coupling. J Exp Biol. 2016;219(Pt 2):175–182. doi: 10.1242/jeb.124123. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg H, Davis M, James D, Pollock N, Stowell K. Malignant hyperthermia. Orphanet J Rare Dis. 2007;2:21. doi: 10.1186/1750-1172-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horstick EJ, et al. Stac3 is a component of the excitation-contraction coupling machinery and mutated in Native American myopathy. Nat Commun. 2013;4:1952. doi: 10.1038/ncomms2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson BR, et al. Skeletal muscle-specific T-tubule protein STAC3 mediates voltage-induced Ca2+ release and contractility. Proc Natl Acad Sci USA. 2013;110(29):11881–11886. doi: 10.1073/pnas.1310571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cong X, et al. The SH3 and cysteine-rich domain 3 (Stac3) gene is important to growth, fiber composition, and calcium release from the sarcoplasmic reticulum in postnatal skeletal muscle. Skelet Muscle. 2016;6:17. doi: 10.1186/s13395-016-0088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polster A, Perni S, Bichraoui H, Beam KG. Stac adaptor proteins regulate trafficking and function of muscle and neuronal L-type Ca2+ channels. Proc Natl Acad Sci USA. 2015;112(2):602–606. doi: 10.1073/pnas.1423113112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stamm DS, et al. Native American myopathy: Congenital myopathy with cleft palate, skeletal anomalies, and susceptibility to malignant hyperthermia. Am J Med Genet A. 2008;146A(14):1832–1841. doi: 10.1002/ajmg.a.32370. [DOI] [PubMed] [Google Scholar]
- 15.Kugler G, Grabner M, Platzer J, Striessnig J, Flucher BE. The monoclonal antibody mAB 1A binds to the excitation-contraction coupling domain in the II-III loop of the skeletal muscle calcium channel alpha(1S) subunit. Arch Biochem Biophys. 2004;427(1):91–100. doi: 10.1016/j.abb.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Schredelseker J, Shrivastav M, Dayal A, Grabner M. Non-Ca2+-conducting Ca2+ channels in fish skeletal muscle excitation-contraction coupling. Proc Natl Acad Sci USA. 2010;107(12):5658–5663. doi: 10.1073/pnas.0912153107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schredelseker J, et al. The beta 1a subunit is essential for the assembly of dihydropyridine-receptor arrays in skeletal muscle. Proc Natl Acad Sci USA. 2005;102(47):17219–17224. doi: 10.1073/pnas.0508710102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Block BA, Imagawa T, Campbell KP, Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol. 1988;107(6 Pt 2):2587–2600. doi: 10.1083/jcb.107.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang M, et al. Rational design of true monomeric and bright photoactivatable fluorescent proteins. Nat Methods. 2012;9(7):727–729. doi: 10.1038/nmeth.2021. [DOI] [PubMed] [Google Scholar]
- 20.Gupta V, et al. The zebrafish dag1 mutant: A novel genetic model for dystroglycanopathies. Hum Mol Genet. 2011;20(9):1712–1725. doi: 10.1093/hmg/ddr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buss RR, Drapeau P. Activation of embryonic red and white muscle fibers during fictive swimming in the developing zebrafish. J Neurophysiol. 2002;87(3):1244–1251. doi: 10.1152/jn.00659.2001. [DOI] [PubMed] [Google Scholar]
- 22.Zvaritch E, et al. An Ryr1I4895T mutation abolishes Ca2+ release channel function and delays development in homozygous offspring of a mutant mouse line. Proc Natl Acad Sci USA. 2007;104(47):18537–18542. doi: 10.1073/pnas.0709312104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dayal A, Bhat V, Franzini-Armstrong C, Grabner M. Domain cooperativity in the β1a subunit is essential for dihydropyridine receptor voltage sensing in skeletal muscle. Proc Natl Acad Sci USA. 2013;110(18):7488–7493. doi: 10.1073/pnas.1301087110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schredelseker J, Dayal A, Schwerte T, Franzini-Armstrong C, Grabner M. Proper restoration of excitation-contraction coupling in the dihydropyridine receptor beta1-null zebrafish relaxed is an exclusive function of the beta1a subunit. J Biol Chem. 2009;284(2):1242–1251. doi: 10.1074/jbc.M807767200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takekura H, et al. Differential contribution of skeletal and cardiac II-III loop sequences to the assembly of dihydropyridine-receptor arrays in skeletal muscle. Mol Biol Cell. 2004;15(12):5408–5419. doi: 10.1091/mbc.E04-05-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polster A, Nelson BR, Olson EN, Beam KG. Stac3 has a direct role in skeletal muscle-type excitation-contraction coupling that is disrupted by a myopathy-causing mutation. Proc Natl Acad Sci USA. 2016;113(39):10986–10991. doi: 10.1073/pnas.1612441113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monnier N, et al. Presence of two different genetic traits in malignant hyperthermia families: Implication for genetic analysis, diagnosis, and incidence of malignant hyperthermia susceptibility. Anesthesiology. 2002;97(5):1067–1074. doi: 10.1097/00000542-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg H, Pollock N, Schiemann A, Bulger T, Stowell K. Malignant hyperthermia: A review. Orphanet J Rare Dis. 2015;10:93. doi: 10.1186/s13023-015-0310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang T, Ta TA, Pessah IN, Allen PD. Functional defects in six ryanodine receptor isoform-1 (RyR1) mutations associated with malignant hyperthermia and their impact on skeletal excitation-contraction coupling. J Biol Chem. 2003;278(28):25722–25730. doi: 10.1074/jbc.M302165200. [DOI] [PubMed] [Google Scholar]
- 30.Estève E, et al. A malignant hyperthermia-inducing mutation in RYR1 (R163C): Alterations in Ca2+ entry, release, and retrograde signaling to the DHPR. J Gen Physiol. 2010;135(6):619–628. doi: 10.1085/jgp.200910328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chelu MG, et al. Heat- and anesthesia-induced malignant hyperthermia in an RyR1 knock-in mouse. FASEB J. 2006;20(2):329–330. doi: 10.1096/fj.05-4497fje. [DOI] [PubMed] [Google Scholar]
- 32.Weiss RG, et al. Functional analysis of the R1086H malignant hyperthermia mutation in the DHPR reveals an unexpected influence of the III-IV loop on skeletal muscle EC coupling. Am J Physiol Cell Physiol. 2004;287(4):C1094–C1102. doi: 10.1152/ajpcell.00173.2004. [DOI] [PubMed] [Google Scholar]
- 33.Pirone A, et al. Identification and functional characterization of malignant hyperthermia mutation T1354S in the outer pore of the Cavalpha1S-subunit. Am J Physiol Cell Physiol. 2010;299(6):C1345–C1354. doi: 10.1152/ajpcell.00008.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang D, et al. Reduced threshold for luminal Ca2+ activation of RyR1 underlies a causal mechanism of porcine malignant hyperthermia. J Biol Chem. 2008;283(30):20813–20820. doi: 10.1074/jbc.M801944200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eltit JM, et al. Orthograde dihydropyridine receptor signal regulates ryanodine receptor passive leak. Proc Natl Acad Sci USA. 2011;108(17):7046–7051. doi: 10.1073/pnas.1018380108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eltit JM, et al. RyR1-mediated Ca2+ leak and Ca2+ entry determine resting intracellular Ca2+ in skeletal myotubes. J Biol Chem. 2010;285(18):13781–13787. doi: 10.1074/jbc.M110.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eltit JM, et al. Malignant hyperthermia susceptibility arising from altered resting coupling between the skeletal muscle L-type Ca2+ channel and the type 1 ryanodine receptor. Proc Natl Acad Sci USA. 2012;109(20):7923–7928. doi: 10.1073/pnas.1119207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arikkath J, Campbell KP. Auxiliary subunits: Essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol. 2003;13(3):298–307. doi: 10.1016/s0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 39.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer; New York: 2009. [Google Scholar]
- 40.Hirata H, et al. Zebrafish bandoneon mutants display behavioral defects due to a mutation in the glycine receptor beta-subunit. Proc Natl Acad Sci USA. 2005;102(23):8345–8350. doi: 10.1073/pnas.0500862102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grabner M, Dirksen RT, Beam KG. Tagging with green fluorescent protein reveals a distinct subcellular distribution of L-type and non-L-type Ca2+ channels expressed in dysgenic myotubes. Proc Natl Acad Sci USA. 1998;95(4):1903–1908. doi: 10.1073/pnas.95.4.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nüsslein-Volhard C, Dahm R, editors. Zebrafish: A Practical Approach. Oxford Univ Press; New York: 2002. [Google Scholar]
- 43.Karlsson J, von Hofsten J, Olsson PE. Generating transparent zebrafish: A refined method to improve detection of gene expression during embryonic development. Mar Biotechnol (NY) 2001;3(6):522–527. doi: 10.1007/s1012601-0053-4. [DOI] [PubMed] [Google Scholar]