The N-terminal UND motif of PUB18 is critical for the negative regulation of ABA-mediated stomatal closure and ubiquitination specificity to Exo70B1, a subunit of the exocyst complex.
Abstract
The Arabidopsis thaliana U-box E3 ligases PUB18/PUB19 and PUB22/PUB23 are negative regulators of drought stress responses. PUB18/PUB19 regulate the drought stress response in an abscisic acid (ABA)-dependent manner, whereas PUB22/PUB23 regulate this response in an ABA-independent manner. A major structural difference between PUB18/PUB19 and PUB22/PUB23 is the presence of the UND (U-box N-terminal domain). Here, we focused on elucidating the molecular mechanism that mediates the functional difference between PUB18 and PUB22 and found that the UNDPUB18 was critically involved in the negative regulation of ABA-mediated stomatal movements. Exo70B1, a subunit of the exocyst complex, was identified as a target of PUB18, whereas Exo70B2 was a substrate of PUB22. However, the ∆UND-PUB18 derivative failed to ubiquitinate Exo70B1, but ubiquitinated Exo70B2. By contrast, the UNDPUB18-PUB22 chimeric protein ubiquitinated Exo70B1 instead of Exo70B2, suggesting that the ubiquitination specificities of PUB18 and PUB22 to Exo70B1 and Exo70B2, respectively, are dependent on the presence or absence of the UNDPUB18 motif. The ABA-insensitive phenotypes of the pub18 pub19 exo70b1 triple mutant were reminiscent of those of exo70b1 rather than pub18 pub19, indicating that Exo70B1 functions downstream of PUB18. Overall, our results suggest that the UNDPUB18 motif is crucial for the negative regulation of ABA-dependent stomatal movement and for determination of its ubiquitination specificity to Exo70B1.
INTRODUCTION
As sessile organisms, terrestrial higher plants have developed intricate mechanisms to cope with adverse environmental conditions. Drought is one of the most critical factors that limit the growth, development, and productivity of agricultural crops (Chaves et al., 2002; Reynolds and Tuberosa, 2008; Osakabe et al., 2014). Under water-deficit conditions, the phytohormone abscisic acid (ABA) acts as a major internal signal that regulates stress-adaptive responses (Tuteja, 2007; Nakashima and Yamaguchi-Shinozaki, 2013; Yu et al., 2016). However, recent studies have revealed that an ABA-independent drought-tolerant pathway also plays an important role in Arabidopsis thaliana (Shinozaki and Yamaguchi-Shinozaki, 2007; Yoshida et al., 2014). Therefore, understanding the comprehensive defense mechanisms requires deciphering of the crosstalk and demarcation between ABA-dependent and ABA-independent signaling pathways against abiotic stress.
In eukaryotes, the ubiquitin (Ub)-proteasome system (UPS) plays an indispensable role in modulating various vital cellular processes, including hormone signaling, DNA repair, endocytosis, and biotic and abiotic stress responses (Welchman et al., 2005; Dreher and Callis, 2007; Vierstra, 2009; Lee and Kim, 2011; Bartel and Citovsky, 2012; Sadanandom et al., 2012; Stone, 2014; Zhang et al., 2015; Yu et al., 2016). The ubiquitination cascade requires the sequential action of three enzymes: E1 Ub-activating enzymes, E2 Ub-conjugating enzymes, and E3 Ub ligases (Guerra and Callis, 2012; Berndsen and Wolberger, 2014). The expanded diversity of E3 Ub ligases suggests that the selection of specific substrates to strictly regulate different cellular processes is determined by E3s (Mazzucotelli et al., 2006; Vierstra, 2009; Sadanandom et al., 2012).
The E3 ubiquitin ligases are classified into four different types: really interesting new gene (RING), U-box, homology to E6-AP C terminus (HECT), and Cullin (Cul)-RING ligases (CRLs) (Vierstra, 2009; Yu et al., 2016). The multisubunit E3 complexes, such as the anaphase-promoting complexes and CUL1-based SCF complexes, belong to the CRL-type E3 Ub ligases (Mazzucotelli et al., 2006; Spratt et al., 2014).
The U-box E3 ligase, which has a modified RING finger motif, was first found in yeasts. Based on sequence conservation, the U-box motif-containing proteins are widely present in eukaryotic organisms. For example, Arabidopsis, a dicot model plant, contains 64 U-box E3 Ub ligases, whereas the monocot model crop rice (Oryza sativa) contains 77 U-box containing proteins (PUBs). Recently, 125 putative PUBs were identified in soybean (Glycine max; Wang et al., 2016). By contrast, yeast and humans possess a smaller number of U-box E3 Ub ligases than higher plants do (Mudgil et al., 2004; Wiborg et al., 2008; Yee and Goring, 2009; Lyzenga and Stone, 2012). The existence of a large number of PUBs implies that the U-box E3 ligases have evolved to perform plant-specific functions, such as plant immune responses (Yang et al., 2006; Trujillo et al., 2008; Lu et al., 2011, 2012; Stegmann et al., 2012; Antignani et al., 2015; He et al., 2015), abiotic stress responses (Yan et al., 2003; Cho et al., 2006, 2008; Bergler and Hoth, 2011; Liu et al., 2011; Salt et al., 2011; Seo et al., 2012; Hwang et al., 2015), self-incompatibility (Liu et al., 2007; Samuel et al., 2008, 2009; Indriolo and Goring, 2014; Zhang et al., 2014), hormonal responses (Luo et al., 2006; Vogelmann et al., 2012; Hu et al., 2013; Kong et al., 2015), and development (Raab et al., 2009; Wang et al., 2013; Deb et al., 2014; Kinoshita et al., 2015).
Unlike other multigene families that are divided by their sequence homology, PUBs are classified based on the presence of functional domains in addition to the U-box (Azevedo et al., 2001; Wang et al., 2016). Among the 64 U-box E3 Ub ligases in Arabidopsis, 41 PUBs contain the armadillo (ARM) repeat, which is a protein-protein interacting domain, in their C-terminal regions (Azevedo et al., 2001; Mudgil et al., 2004; Zeng et al., 2008; Yee and Goring, 2009). The PUB-ARM proteins are then divided into two subgroups based on the presence or absence of the U-box N-terminal domain (UND). At least 17 members of the UND-containing PUB-ARM (UND-PUB-ARM) proteins are present in Arabidopsis. According to recent reports, the UND motif is involved in the interaction of U-box E3 ligase and its target protein (Samuel et al., 2009; Indriolo et al., 2012; Indriolo and Goring, 2014). Furthermore, UND has been reported to be not essential for E3 Ub ligase enzyme activity (Andersen et al., 2004). Nevertheless, detailed cellular functions of UND remain to be elucidated.
Our previous studies showed that four Arabidopsis U-box E3 Ub ligases, PUB18, PUB19, PUB22, and PUB23, play negative roles in response to drought stress (Cho et al., 2008; Seo et al., 2012). The PUB18 and PUB19 homologs regulate the drought stress response in an ABA-dependent manner, whereas PUB22 and PUB23 function in an ABA-independent manner. PUB18 and PUB19 possess an N-terminal UND and C-terminal ARM repeats in addition to the U-box domain in their central region. Conversely, PUB22 and PUB23 are composed of the U-box motif and the C-terminal ARM repeats. A major difference between PUB18/PUB19 and PUB22/PUB23 is the presence of the N-terminal UND. These findings raised the possibility that the N-terminal UND in PUB18/PUB19 participates in the negative regulation of an ABA-mediated drought stress response. In this study, we report that the N-terminal UND of PUB18 is indeed critical for the negative regulation of ABA-mediated stomatal closure. We identified Exo70B1, a subunit of the exocyst complex, as a target of PUB18. Our data further suggest that the N-terminal UND motif determines the binding and ubiquitination specificity of PUB18 to Exo70B1.
RESULTS
Deletion of the N-Terminal UND of PUB18 Impairs the ABA-Mediated Stomatal Movements, but Does Not Affect Drought-Induced Stomatal Closure in Arabidopsis
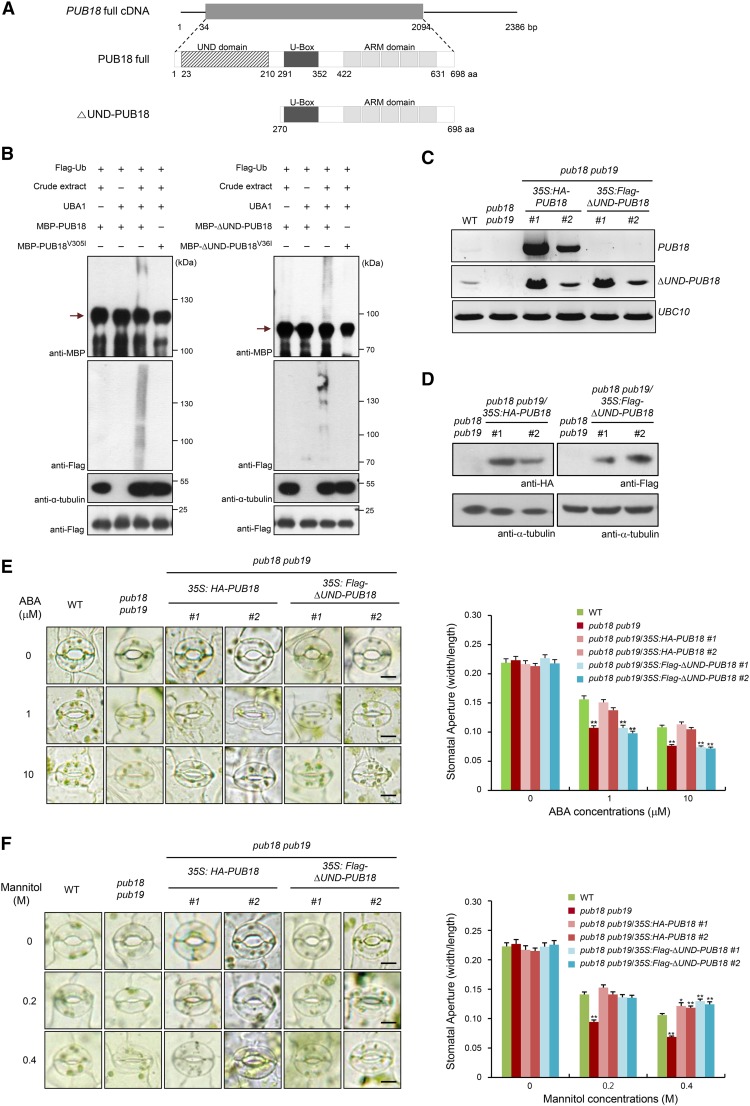
Seo et al. (2012) reported that four Arabidopsis U-box E3 Ub ligases, i.e., PUB18, PUB19, PUB22, and PUB23, play negative roles in response to drought stress. The modes of action of these negative regulators were divided into two distinct pathways: PUB18 and PUB19 regulate the drought stress response in an ABA-dependent manner, whereas PUB22 and PUB23 work in an ABA-independent manner (Cho et al., 2008; Seo et al., 2012). The homologs PUB18 and PUB19 possess N-terminal UND and C-terminal ARM repeats in addition to the U-box domain in their central region (Mudgil et al., 2004; Yee and Goring, 2009). However, PUB22 and PUB23 consist only of the U-box motif and the C-terminal ARM repeats. A major difference between PUB18/PUB19 and PUB22/PUB23 is the presence of the N-terminal UND (Supplemental Figure 1). Thus, we considered the possibility that the N-terminal UND in PUB18/PUB19 participates in the negative regulation of ABA-dependent drought stress response. To test this possibility, the ΔUND-PUB18 mutant protein, in which the N-terminal 269-amino-acid-long UND was deleted (Figure 1A), was characterized in terms of ABA-mediated stomatal movements.
Figure 1.
Deletion of the N-Terminal UND of PUB18 Impairs ABA-Mediated Stomatal Movements, but Does Not Affect the Drought-Induced Stomatal Closure in Arabidopsis.
(A) Schematic structure of full-length PUB18 cDNA and PUB18 and ∆UND-PUB18 proteins. Solid lines indicate 5ʹ- and 3ʹ-untranslated regions. The gray bar in the upper diagram represents the coding region. The N-terminal UND motif, U-box domain, and armadillo (ARM) repeats are indicated.
(B) In vitro self-ubiquitination assay of PUB18 and ∆UND-PUB18. Recombinant MBP-PUB18, MBP-PUB18V305I, MBP-∆UND-PUB18, and MBP-∆UND-PUB18V36I proteins were incubated with Flag-tagged Ub (Flag-Ub) at 30°C for 2 h in the presence or absence of E1 (UBA1) and crude extract (10 μg total proteins) prepared from ABA (100 μM)-treated pub18 pub19 pub22 pub23 quadruple mutant leaves, as a source of E2 enzyme, along with MG132 (80 μM) and protease inhibitor cocktail. Reaction products were resolved using SDS-PAGE and subjected to immunoblot analysis with anti-MBP and anti-Flag antibodies. The level of α-tubulin detected by anti-α-tubulin antibody was used as an equal loading control of input crude extract. The presence of an equal amount of Flag-Ub in each lane was confirmed by anti-Flag antibody. Arrows indicate the migration of the unmodified PUB proteins.
(C) RT-PCR analysis of wild-type, pub18 pub19 double mutant, and pub18 pub19/35S:HA-PUB18 (transgenic lines #1 and #2) and pub18 pub19/35S:Flag-∆UND-PUB18 (transgenic lines #1 and #2) complementation plants. Each experiment was performed with three independent biological replicates. The Ubiquitin Conjugating Enzyme10 (UBC10) gene was used as a loading control.
(D) Immunoblot analysis of pub18 pub19 double mutant and pub18 pub19/35S:HA-PUB18 (lines #1 and #2) and pub18 pub19/35S:Flag-∆UND-PUB18 (lines #1 and #2) complementation plants. Expression levels of HA-PUB18 and Flag-∆UND-PUB18 proteins were determined using anti-HA and anti-Flag antibodies, respectively. The level of α-tubulin was an equal loading control.
(E) Stomatal movements of wild-type, pub18 pub19 double mutant, and pub18 pub19/35S:HA-PUB18 (lines #1 and #2) and pub18 pub19/35S:Flag-∆UND-PUB18 (lines #1 and #2) complementation plants in response to ABA. Light-grown mature rosette leaves were immersed in stomatal opening solution for 2 h and transferred to the solution containing different concentrations (0, 1, and 10 μM) of ABA for 2 h. Stomata were imaged using bright-field microscopy. At least 30 stomatal apertures in each epidermal strip were measured per replicate. Three replicates were performed for each experiment. Error bars represent ±se (n = 90; **P < 0.005, one-way ANOVA). Bars = 10 μm.
(F) Stomatal movements of wild-type, pub18 pub19 double mutant, and pub18 pub19/35S:HA-PUB18 (lines #1 and #2) and pub18 pub19/35S:Flag-∆UND-PUB18 (lines #1 and #2) complementation plants in response to osmotic stress imposed by various concentrations (0, 0.2, and 0.4 M) of mannitol. Error bars represent ±se (n = 90; *P < 0.05, **P < 0.005, one-way ANOVA). Bars = 10 μm.
Liu et al. (2011) used wheat (Triticum aestivum) E1 and human E2 (UBCh5b) to measure the in vitro E3 Ub ligase activity of PUB19. We used Arabidopsis E1 (UBA1) and various E2s, including UBC7, UBC8, UBC9, UBC10, and UBC13, as well as human E2, for an in vitro self-ubiquitination assay of PUB18, but failed to show E3 Ub ligase activity of bacterially expressed MBP-PUB18. Thus, in this study, a small amount of crude extract (10 μg total proteins) prepared from ABA-treated pub18 pub19 pub22 pub23 quadruple mutant leaves was used as a source of E2 enzyme for in vitro self-ubiquitination assays of PUB18. A crude extract of pub18 pub19 pub22 pub23 mutant leaves, rather than wild-type leaves, was used as E2 to exclude a possible effect of endogenous PUB18 present in the protein crude extract on the ubiquitination assay. Recombinant full-length MBP-PUB18 and MBP-△UND-PUB18 proteins were incubated with ATP and Flag-tagged Ub (Flag-Ub) in the presence or absence of Arabidopsis UBA1 (E1) and a protein crude extract (E2) at 30°C for 2 h. The reaction mixture was analyzed by immunoblotting using anti-MBP and anti-Flag antibodies. The results indicated that MBP-△UND-PUB18 exhibited comparable E3 Ub ligase activity to the full-length MBP-PUB18, as evidenced by similar levels of high molecular mass smear ladders detected by both anti-MBP and anti-Flag antibodies (Figure 1B). Exclusion of either UBA1 (E1) or crude extract (E2) from the incubation mixture abolished these high molecular mass bands. In addition, a single amino acid substitution in both MBP-PUB18V305I and MBP-△UND-PUB18V36I abrogated E3 ligase activity even in the presence of Flag-Ub, UBA1 (E1), and crude extract (E2) (Figure 1B). When a crude protein extract was inactivated by boiling, ubiquitinated products were not detected in the presence of all reaction components (Supplemental Figure 2). These results suggest that the N-terminal UND of PUB18 is not essential for E3 Ub ligase activity, which is consistent with previous results that the UND motif in PUBs is not required for E3 Ub ligase enzyme activity (Andersen et al., 2004). For consistent experimental conditions, a crude protein extract of pub18 pub19 pub22 pub23 leaves pretreated with ABA was used as an E2 source in all other E3 Ub ligase assays in this study.
The possible role of UND in response to ABA was examined by ectopically expressing HA-PUB18 and Flag-∆UND-PUB18 in pub18 pub19 double mutant plants under the control of the 35S CaMV promoter (Figure 1C). Ectopic expression of HA-PUB18 and Flag-∆UND-PUB18 proteins was detected by anti-HA and anti-Flag antibodies, respectively, in two independent transgenic lines (#1 and #2) (Figure 1D). These complementation transgenic plants (pub18 pub19/35S:HA-PUB18 and pub18 pub19/35S:Flag-∆UND-PUB18) were subsequently used to analyze ABA-dependent stomatal movements. Consistent with previous results (Seo et al., 2012), stomatal movement of the pub18 pub19 mutant was hypersensitive compared with that in the wild-type plant in response to ABA treatments (1 and 10 μM). After 10 μM ABA treatment, the average stomatal apertures (the ratio of width to length) of the wild type and pub18 pub19 were 0.108 ± 0.003 and 0.077 ± 0.003, respectively (Figure 1E). In addition, the degree of ABA sensitivity of pub18 pub19/35S:HA-PUB18 complementation progeny was approximately the same as that of the wild-type plant, with an average stomatal aperture of 0.113 ± 0.011 (transgenic line #1) and 0.105 ± 0.003 (transgenic line #2), corroborating the role of PUB18 as a negative regulator in ABA-mediated stomatal closure. By contrast, the constitutive expression of Flag-∆UND-PUB18 in pub18 pub19 could not rescue the hypersensitive phenotype of the mutant toward ABA (Figure 1E). In the presence of 10 μM ABA, stomatal apertures of pub18 pub19/35S:Flag-∆UND-PUB18 were 0.074 ± 0.006 (transgenic line #1) and 0.072 ± 0.002 (transgenic line #2). These results suggest that the N-terminal UND in PUB18 is critical for the negative regulation of ABA-dependent stomatal behaviors in Arabidopsis.
Stomatal movements of wild-type, mutant (pub18 pub19), and complementation (pub18 pub19/35S:HA-PUB18 and pub18 pub19/35S:Flag-∆UND-PUB18) plants in response to mannitol treatments (0.2 and 0.4 M) were measured. The results revealed that the hypersensitive stomatal behavior of pub18 pub19 under mannitol treatment was efficiently offset by the ectopic expression of both HA-PUB18 and Flag-∆UND-PUB18 (Figure 1F). These results indicate that the N-terminal UND in PUB18 is not necessary for the negative regulation of mannitol-induced stomatal closure. Taken together, our data suggest that the deletion of the N-terminal UND of PUB18 impairs the ABA-mediated stomatal movements but does not affect the mannitol (drought)-induced stomatal closure in Arabidopsis.
ABA-Responsive Stomatal Movement Was Restored by Overexpression of the N-Terminal UND in pub22 pub23 Mutant Plants
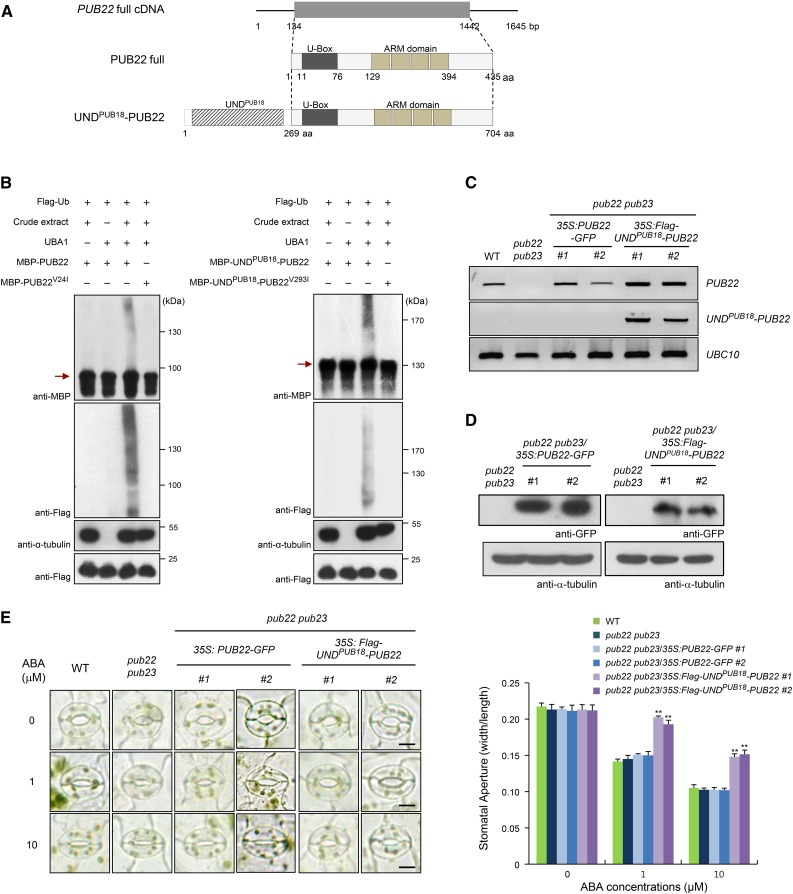
To further investigate the biological role of UND in the ABA response and unravel the possible functional relationship between PUB18 and PUB22, the UNDPUB18-PUB22 chimeric protein, in which the N-terminal 269-amino acid residues of PUB18 were fused to the N terminus of PUB22, was constructed (Figure 2A). Whether the UNDPUB18-PUB22 protein has E3 Ub ligase activity was determined by conducting an in vitro self-ubiquitination assay. The results showed that purified MBP-PUB22 and MBP-UNDPUB18-PUB22 possessed E3 Ub ligase activities detected by anti-MBP and anti-Flag antibodies, whereas MBP-PUB22V24I and MBP-UNDPUB18-PUB22V293I mutant derivatives failed to show E3 ligase activity (Figure 2B). This indicates that the attachment of UNDPUB18 to PUB22 did not affect E3 Ub ligase activity.
Figure 2.
ABA-Responsive Stomatal Movement Was Restored by the Overexpression of the N-Terminal UND in pub22 pub23 Mutant Plants.
(A) Schematic representation of full-length PUB22 cDNA and PUB22 and UNDPUB18-PUB22 proteins. Solid lines indicate 5ʹ- and 3ʹ-untranslated regions, and the gray bar represents the coding region. The UNDPUB18 motif, U-box domain, and armadillo (ARM) repeats are indicated.
(B) In vitro self-ubiquitination of PUB22 and UNDPUB18-PUB22. The E3 Ub ligase activities of bacterially expressed MBP-PUB22 and MBP-UNDPUB18-PUB22 recombinant proteins and their single amino acid substitution variants (MBP-PUB22V24I and MBP-UNDPUB18-PUB22V293I) were examined with or without E1 (UBA1) and crude extract (10 μg total proteins) of ABA (100 μM)-treated pub18 pub19 pub22 pub23 leaves, as a source of E2 enzyme, along with Flag-tagged Ub (Flag-Ub), MG132 (80 μM), and protease inhibitor cocktail. Reaction products were analyzed by SDS-PAGE, followed by immunoblotting with anti-MBP and anti-Flag antibodies. The level of α-tubulin detected by anti-α-tubulin antibody was used as an equal loading control of input crude extract. The presence of an equal amount of Flag-Ub in each lane was confirmed by anti-Flag antibody. Arrows indicate the migration of the unmodified PUB proteins.
(C) RT-PCR analysis of wild-type, pub22 pub23 double mutant, and pub22 pub23/35S:PUB22-GFP (transgenic lines #1 and #2) and pub22 pub23/35S:Flag-UNDPUB18-PUB22 (transgenic lines #1 and #2) complementation plants. Four independent biological replicate were performed. The UBC10 gene was used as a loading control.
(D) Immunoblot analysis of pub22 pub23 double mutant and pub22 pub23/35S:PUB22-GFP (lines #1 and #2) and pub22 pub23/35S:Flag-UNDPUB18-PUB22 (lines #1 and #2) complementation plants. Ectopic expression of PUB22-GFP and Flag-UNDPUB18-PUB22 proteins was detected using anti-GFP and anti-Flag antibodies, respectively. The level of α-tubulin was used as an equal loading control.
(E) Stomatal movements of wild-type, pub22 pub23 double mutant, and pub22 pub23/35S:PUB22-GFP (lines #1 and #2) and pub22 pub23/35S:Flag-UNDPUB18-PUB22 (lines #1 and #2) complementation plants in response to different concentrations (0, 1, and 10 μM) of ABA. Thirty stomatal apertures per treatment were measured, and three replicates were performed for each experiment. Error bars represent ±se (n = 90; **P < 0.005, one-way ANOVA). Bars = 10 μm.
The 35S:PUB22-GFP and 35S:Flag-UNDPUB18-PUB22 constructs were then introduced into pub22 pub23 double mutant plants. Ectopic expression of PUB22-GFP and Flag-UNDPUB18-PUB22 were detected by RT-PCR (Figure 2C) and immunoblotting using anti-GFP and anti-Flag-antibodies, respectively (Figure 2D). Subsequently, the stomatal behaviors of pub22 pub23 and complementation lines (pub22 pub23/35S:PUB22-GFP and pub22 pub23/35S:Flag-UNDPUB18-PUB22) in response to ABA were monitored. Stomatal movement of pub22 pub23 after ABA treatments (1 and 10 μM) was very similar to that of wild-type plants. The average stomatal aperture in pub22 pub23 was ∼0.103 ± 0.004 at 10 μM ABA, which was about the same as that of the wild-type plants (Figure 2E). This is consistent with the previous reports that PUB22 regulates an ABA-independent drought signaling pathway (Cho et al., 2008; Seo et al., 2012). Moreover, the overexpression of PUB22-GFP in pub22 pub23 could not change the ABA-independent phenotype. By contrast, pub22 pub23/35S:Flag-UNDPUB18-PUB22 complementation lines exhibited markedly decreased sensitivity toward ABA (stomatal apertures = 0.148 ± 0.007 for transgenic line #1 and 0.152 ± 0.01 for line #2 at 10 μM ABA), indicating that the ectopic expression of 35S:Flag-UNDPUB18-PUB22 in pub22 pub23 resulted in ABA-insensitive stomatal closure (Figure 2E). These results strongly suggest that the UNDPUB18-PUB22 chimeric protein, in contrast to PUB22, plays a negative role in ABA-dependent stomatal movement. Based on the results presented in Figures 1 and 2, we inferred that the N-terminal UND of PUB18 is negatively involved in ABA-regulated stomatal movement in Arabidopsis.
PUB18 Interacts with Exo70B1
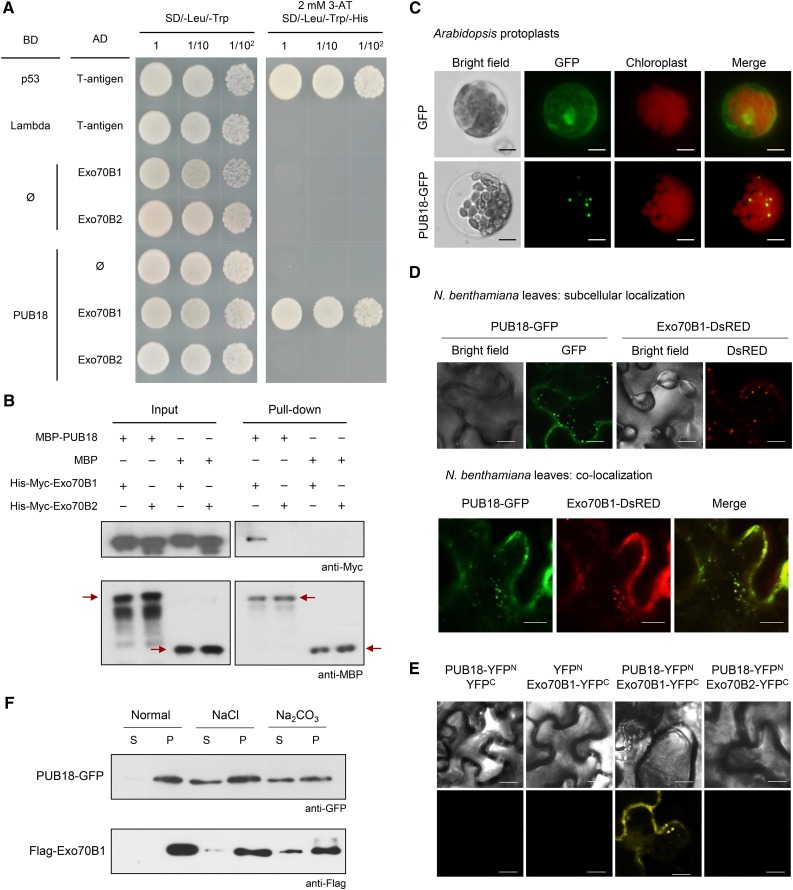
The specificity of E3 Ub ligases to their target proteins is a crucial factor for the UPS. Identification of target proteins is thus an essential step in elucidating the cellular roles of E3 ligases. Therefore, we performed a yeast two-hybrid screen to identify the target protein of PUB18. Based on this screen, we selected Exo70B1 as a putative interacting partner of PUB18. Exo70B1 is an Arabidopsis paralog of mammalian Exo70 protein, which is a subunit of the exocyst involved in vesicle tethering during exocytosis (Li et al., 2010; Wang et al., 2010; Kulich et al., 2013). Interaction of PUB18 and Exo70B1 appeared to be specific, since PUB18 was not associated with Exo70B2, a homolog of Exo70B1, in yeast cells (Figure 3A). To corroborate the interaction between PUB18 and Exo70B1, we conducted an in vitro pull-down assay. In this experiment, purified MBP-PUB18 recombinant protein was coincubated with His-Myc-Exo70B1 or His-Myc-Exo70B2 in the presence of an Excellose resin, and bound proteins were eluted with maltose (10 mM), followed by immunoblot analysis with anti-Myc and anti-MBP antibodies. His-Myc-Exo70B1 was pulled down from the Excellose affinity matrix by MBP-PUB18 (Figure 3B). This indicates a physical interaction between PUB18 and Exo70B1. By contrast, MBP-PUB18 and His-Myc-Exo70B2 did not interact with each other in vitro (Figure 3B). In addition to PUB18, PUB19 had a binding activity to Exo70B1 in yeast cells and in vitro pull-down assay (Supplemental Figures 3 and 4).
Figure 3.
PUB18 Interacts with Exo70B1.
(A) Yeast two-hybrid analysis. The full-length coding region of PUB18 was cloned into the pGBKT7 vector, and Exo70B1 and Exo70B2 were cloned into pGADT7. Different combinations of bait and prey plasmids were cotransformed as indicated into the yeast strain AH109. The protein-protein interactions were examined by plating yeast cells on SD/-Leu/-Trp/-His solid medium containing 2 mM 3-amino-1,2,4,-triazole (3-AT). p53 + T-antigen was used as a positive control. Lambda + T-antigen and empty pGADT7 (ø) plasmid were used as negative controls.
(B) In vitro pull-down assay. Purified MBP-PUB18 + His-Myc-Exo70B1, MBP-PUB18 + His-Myc-Exo70B2, MBP + His-Myc-Exo70B1, and MBP + His-Myc-Exo70B2 recombinant proteins were coincubated with an Excellose resin, and bound proteins were eluted by maltose (10 mM), followed by immunoblot analysis with anti-Myc and anti-MBP antibodies. Arrows indicate MBP-PUB18 and MBP. MBP was used as a negative control.
(C) Subcellular localization of PUB18 in Arabidopsis protoplasts. The 35S:GFP and 35S:PUB18-GFP constructs were transfected into protoplasts obtained from Arabidopsis leaf tissues using PEG-mediated transformation. The GFP signals were visualized by fluorescence microscopy under dark-field conditions. Bars = 10 μm.
(D) Subcellular localization of PUB18 and Exo70B1 in tobacco leaf epidermal cells. The 35S:PUB18-GFP and 35S:Exo70B1-DsRED constructs were introduced into tobacco leaf epidermal cells using an Agrobacterium-mediated infiltration method. The localization signals of PUB18-GFP and Exo70B1-DsRED were detected by confocal microscopy under dark-field conditions. Bars = 10 μm.
(E) BiFC analysis for in vivo interaction between PUB18 and Exo70B1. The full-length coding region of PUB18 was fused to the N-terminal region (YFPN; 1 to 155 amino acids) of YFP. Exo70B1 and Exo70B2 were fused to the C-terminal region (YFPC; 156 to 239 amino-acids) of YFP. The PUB18-YFPN + YFPC, YFPN + Exo70B1-YFPC, PUB18-YFPN + Exo70B1-YFPC, and PUB18-YFPN + Exo70B2-YFPC proteins were transiently coexpressed in tobacco leaf epidermal cells. Reconstituted fluorescent signals were detected via confocal microscopy. Bars = 20 μm.
(F) Membrane association assay of PUB18 and Exo70B1. The 35S:PUB18-GFP and 35S:Flag-Exo70B1 constructs were transiently expressed in tobacco leaves. Supernatant (S) and pellet (P) fractions of total leaf protein extracts were treated with 1 M NaCl or 0.1 M Na2CO3 and subjected to immunoblot analysis with anti-GFP and anti-Flag antibodies.
Previous subcellular localization studies showed that Arabidopsis Exo70B1 localized to small spherical bodies and/or discrete punctae (Wang et al., 2010; Kulich et al., 2013). When the 35S:PUB18-GFP construct was transiently expressed in Arabidopsis mesophyll protoplasts by means of the polyethylene glycol (PEG)-mediated transformation, the fluorescent signal for PUB18-GFP displayed a punctate pattern (Figure 3C), which was highly similar to that of Exo70B1-DsRED. These punctate localization signals for PUB18-GFP and Exo70B1-DsRED were merged in tobacco (Nicotiana benthamiana) leaf epidermal cells (Figure 3D), suggesting cosubcellular localization of PUB18 and Exo70B1.
The interaction between PUB18 and Exo70B1 was further examined by a bimolecular fluorescence complementation (BiFC) assay. The yellow fluorescent protein (YFP) was spliced into the N-terminal region (YFPN) and the C-terminal region (YFPC). YFPN and YFPC were fused to the C termini of PUB18 and Exo70B1, respectively. When PUB18-YFPN + Exo70B1-YFPC were coexpressed in tobacco leaf epidermal cells, punctate fluorescent signals were detected (Figure 3E). However, coexpression of PUB18-YFPN + Exo70B2-YFPC did not give rise to reconstituted fluorescent signal (Figure 3E; Supplemental Figure 5A). Thus, it appeared that PUB18 interacted with Exo70B1, but not with Exo70B2, in tobacco cells.
The 35S:PUB18-GFP and 35S:Flag-Exo70B1 fusion constructs were infiltrated into tobacco leaves. The total proteins were then isolated and fractionated into soluble and membrane components. As shown in Figure 3F, both PUB18-GFP and Flag-Exo70B1 were exclusively found in the membrane fraction detected by anti-GFP and anti-Flag antibodies, respectively. The PUB18-GFP and Flag-Exo70B1 proteins were partially solubilized by 1 M NaCl and 0.1 M Na2CO3. These results suggest that PUB18-GFP and Flag-Exo70B1 are membrane-associated proteins. Taken together, yeast two-hybrid, in vitro pull-down, subcellular localization, BiFC, and protein fractionation experiments indicated that PUB18 interacted with Exo70B1. This suggested that Exo70B1 might be a target protein recognized by PUB18 E3 Ub ligase.
The UNDPUB18 Motif Determines the Binding Specificities of PUB18 and PUB22 toward Exo70B1 and Exo70B2, Respectively
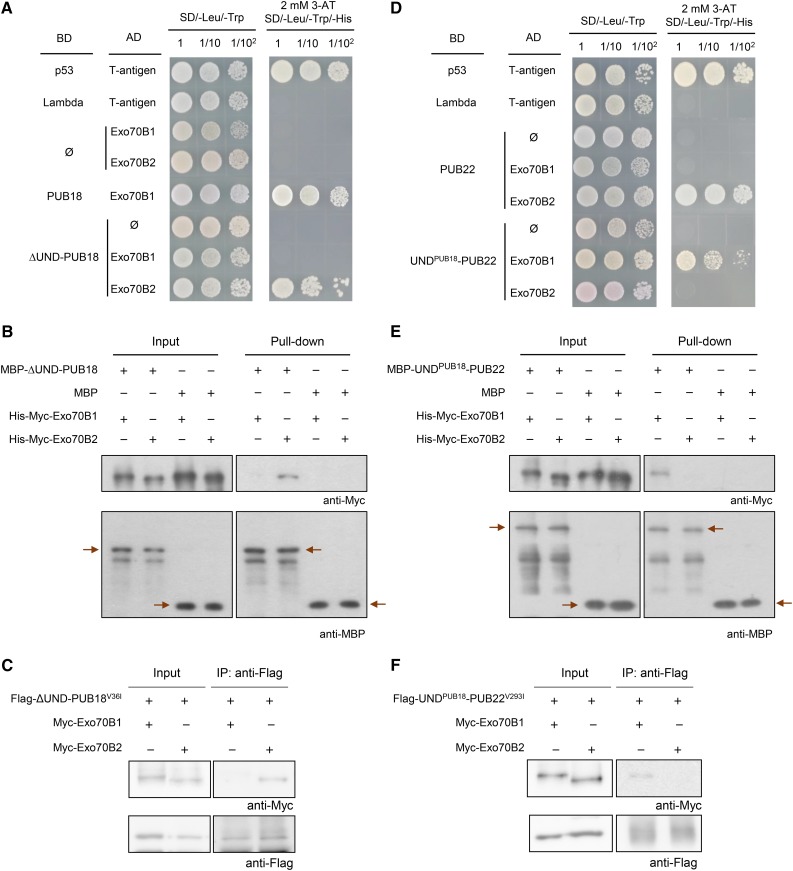
The aforementioned results provide evidence that PUB18 interacts with Exo70B1. On the other hand, Stegmann et al. (2012) reported that PUB22 binds and ubiquitinates Exo70B2, a homolog of Exo70B1, to regulate the negative response to pathogen infection in Arabidopsis. Because the presence of the N-terminal UND in PUB18 is a noteworthy distinction of PUB18 from PUB22, we speculated that the binding activities of PUB18 and PUB22 to Exo70B1 and Exo70B2, respectively, are stimulated by the presence or absence of the UNDPUB18 motif. To test this hypothesis, we estimated the binding activities of ∆UND-PUB18 to Exo70B1 and Exo70B2 by yeast two-hybrid assay. Interestingly, ∆UND-PUB18 failed to interact with Exo70B1 in yeast cells (Figure 4A). This suggests that the UNDPUB18 motif is required for the interaction of PUB18 with Exo70B1. By contrast, ∆UND-PUB18 was able to bind to Exo70B2.
Figure 4.
The UNDPUB18 Domain Determines the Binding Specificities of PUB18 and PUB22 toward Exo70B1 and Exo70B2, Respectively.
(A) ∆UND-PUB18 and Exo70B2 interact in yeast cells. The ∆UND-PUB18 coding region was cloned into the pGBKT7 vector, and Exo70B1 and Exo70B2 were cloned into pGADT7. The ∆UND-PUB18-pGBKT7 + Exo70B1-pGADT7 and ∆UND-PUB18-pGBKT7 + Exo70B2-pGADT7 plasmids were transformed into yeast AH109 cells. The yeast cells were plated on SD/-Leu/-Trp/-His solid medium in the presence of 2 mM 3-AT. p53 + T-antigen and PUB18 + Exo70B1 were used as positive controls. Lambda + T-antigen and empty pGADT7 (ø) were used as negative controls.
(B) In vitro interaction of ∆UND-PUB18 and Exo70B2. Purified MBP-∆UND-PUB18 + His-Myc-Exo70B1, MBP-∆UND-PUB18 + His-Myc-Exo70B2, MBP + His-Myc-Exo70B1, and MBP + His-Myc-Exo70B2 recombinant proteins were coincubated in the presence of an Excellose resin. After extensive washing, bound proteins were eluted with maltose (10 mM). Eluted proteins were subjected to immunoblot analysis with anti-Myc and anti-MBP antibodies. Arrows indicate MBP-∆UND-PUB18 and MBP. MBP was used as a negative control.
(C) In vivo co-IP assay of ∆UND-PUB18 and Exo70B2. The 35S:Flag-∆UND-PUB18V36I construct was transiently coexpressed with 35S:Myc-Exo70B1 or 35S:Myc-Exo70B2 in tobacco leaf epidermal cells by an Agrobacterium-mediated infiltration method. Total leaf proteins (200 μg) were extracted and immunoprecipitated with anti-Flag affinity gel matrix. The bound proteins were eluted by boiling with 4× SDS sample buffer. Eluted proteins were separated by SDS-PAGE and subjected to immunoblot analysis with anti-Flag and anti-Myc antibodies.
(D) UNDPUB18-PUB22 and Exo70B1 interact in yeast cells. The UNDPUB18-PUB22-pGBKT7 + Exo70B1-pGADT7 and UNDPUB18-PUB22-pGBKT7 + Exo70B2-pGADT7 constructs were introduced into yeast cells. The yeast cells were plated on SD/-Leu/-Trp/-His solid medium in the presence of 2 mM 3-AT. p53 + T-antigen and PUB22 + Exo70B2 and were used as positive controls. Lambda + T-antigen and empty pGADT7 (ø) plasmid were negative controls.
(E) In vitro interaction of UNDPUB18-PUB22 and Exo70B1. Purified MBP-UNDPUB18-PUB22 + His-Myc-Exo70B1, MBP-UNDPUB18-PUB22 + His-Myc-Exo70B2, MBP + His-Myc-Exo70B1, and MBP + His-Myc-Exo70B2 recombinant proteins were coincubated in the presence of an Excellose resin. After extensive washing, bound proteins were eluted with maltose (10 mM). Eluted proteins were detected by immunoblot analysis with anti-Myc and anti-MBP antibodies. Arrows indicate MBP-UNDPUB18-PUB22 and MBP. MBP was used as a negative control.
(F) In vivo co-IP assay of UNDPUB18-PUB22 and Exo70B1. The 35S:Flag-UNDPUB18-PUB22V293I construct was transiently coexpressed with 35S:Myc-Exo70B1 or 35S:Myc-Exo70B2 in tobacco leaf epidermal cells. Total leaf proteins (200 μg) were extracted and immunoprecipitated with an anti-Flag affinity gel matrix. The bound proteins were eluted by boiling with 4× SDS sample buffer and detected by immunoblot analysis with anti-Flag and anti-Myc antibodies.
Interaction of ∆UND-PUB18 to Exo70B2 was reinforced by an in vitro pull-down assay. MBP-fused ∆UND-PUB18 recombinant protein was coincubated with His-Myc-Exo70B1 or His-Myc-Exo70B2 in the presence of an Excellose resin. Bound proteins were then eluted by maltose (10 mM) and subjected to immunoblot analysis with anti-Myc and anti-MBP antibodies. As shown in Figure 4B, His-Myc-Exo70B2 was pulled down by MBP-∆UND-PUB18, indicating that ∆UND-PUB18 directly interacts with Exo70B2 in vitro. By contrast, MBP-∆UND-PUB18 did not bind to His-Myc-Exo70B1 in vitro (Figure 4B). These results were confirmed by an in vivo coimmunoprecipitation (co-IP) assay. To eliminate possible effects of self-ubiquitination activity of ∆UND-PUB18, a single amino acid substitution derivative ∆UND-PUB18V36I was used. The 35S:Flag-∆UND-PUB18V36I construct was transiently expressed with 35S:Myc-Exo70B1 or 35S:Myc-Exo70B2 in tobacco leaf cells by means of Agrobacterium tumefaciens-mediated infiltration. Total leaf proteins (200 μg) were immunoprecipitated with an anti-Flag affinity gel matrix. The bound proteins were eluted and subjected to immunoblot analysis with anti-Flag and anti-Myc antibodies. The results showed that Flag-∆UND-PUB18V36I and Myc-Exo70B2 were coimmunoprecipitated by anti-Flag antibody (Figure 4C). Thus, ∆UND-PUB18V36I interacted with Exo70B2 in tobacco cells. However, the interaction between Flag-∆UND-PUB18V36I and Myc-Exo70B1 was very weak and barely detectable in tobacco cells (Figure 4C). Therefore, the deletion of the N-terminal UND from PUB18 resulted in a change in its binding property from Exo70B1 to Exo70B2.
To further corroborate the role of UNDPUB18, we determined the binding activities of the UNDPUB18-PUB22 chimeric protein to Exo70B1 and Exo70B2 using yeast two-hybrid, in vitro pull-down analysis, and an in vivo co-IP assay. Notably, UNDPUB18-PUB22 interacted with Exo70B1 in yeast cells (Figure 4D). In addition, the interaction between UNDPUB18-PUB22 and Exo70B2 was negligible. These results were in sharp contrast to those suggesting that PUB22 does not bind to Exo70B1 (Figure 4D), but binds to Exo70B2 (Figure 4D; Stegmann et al., 2012). In vitro pull-down and in vivo co-IP experiments confirmed the interaction between UNDPUB18-PUB22 and Exo70B1 (Figures 4E and 4F). Thus, it seems highly likely that the addition of the UNDPUB18 domain to PUB22 altered its binding specificity from Exo70B2 to Exo70B1, indicating that the N-terminal UND is a critical factor for the interaction of UNDPUB18-PUB22 with Exo70B1. Collectively, the domain-swapping results presented in Figure 4 strongly suggest that the binding specificities of PUB18 and PUB22 toward Exo70B1 and Exo70B2, respectively, depend on the presence or absence of the N-terminal UNDPUB18 motif.
Turnover of Exo70B1 Is Regulated by PUB18 in a Proteasome-Dependent Manner
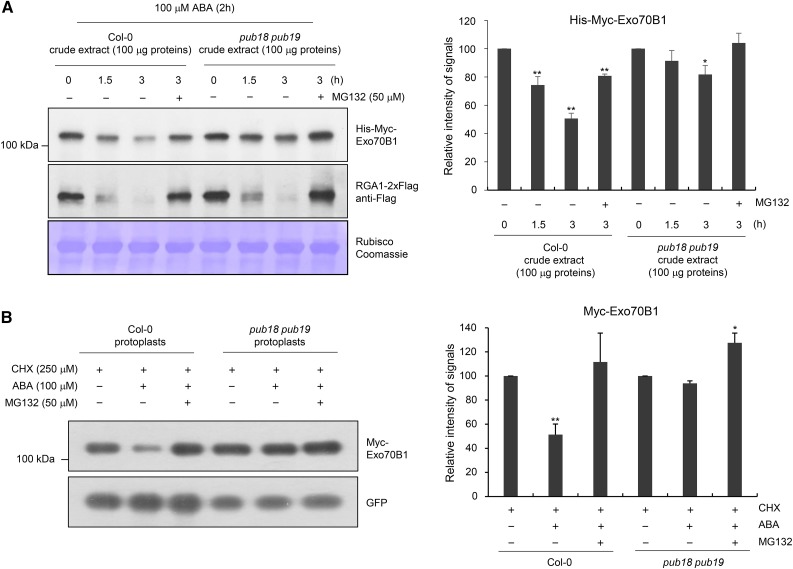
The specific interaction between PUB18 and EXO70B1 (Figure 4) suggests that the cellular level of Exo70B1 is regulated via the UPS. To investigate this possibility, we conducted a cell-free degradation assay. Bacterially expressed His-Myc-Exo70B1 recombinant protein was incubated with a crude extract (100 μg total proteins) prepared from wild-type and pub18 pub19 double mutant leaves pretreated with 100 μM ABA and subjected to immunoblot analysis with anti-Myc antibody. The level of His-Myc-Exo70B1 was rapidly reduced over time in wild-type cell-free extracts. As shown in Figure 5A, 49.3% ± 3.7% of His-Myc-Exo70B1 was degraded after 3.0 h of incubation. By contrast, the His-Myc-Exo70B1 protein remained stable in the presence of MG132, an inhibitor of the proteasome complex. After 3 h of incubation with 50 μM MG132, 19.2% ± 1.2% of His-Myc-Exo70B1 was degraded (Figure 5A), suggesting that Exo70B1 is degraded in a proteasome-dependent manner with wild-type cell-free extracts. The results further show that His-Myc-Exo70B1 was degraded more slowly in pub18 pub19 cell-free extracts than in the wild-type extracts. After a 3.0-h incubation, 81.7% ± 6.4% of His-Myc-Exo70B1 was still detected in the pub18 pub19 extracts, and this slower degradation was almost completely impeded by MG132 (Figure 5A). The degradation of REPRESSOR OF GA1-3, which is regulated by the UPS (Dill et al., 2004; Lee et al., 2010), exhibited similar degradation patterns in wild-type and pub18 pub19 crude extracts, confirming the specific role of PUB18 in Exo70B1 degradation (Figure 5A). The amount of Rubisco large subunit (rbcL) remained unchanged in both wild-type and pub18 pub19 extracts.
Figure 5.
In Vitro and in Vivo Degradation Assays of Exo70B1.
(A) Cell-free degradation assay of Exo70B1. Bacterially expressed His-Myc-Exo70B1 was incubated with a cell-free crude extract (100 μg total proteins) prepared from ABA (100 μM)-treated mature rosette leaves of wild-type or pub18 pub19 mutant plants in the presence or absence of 50 μM MG132. The time-dependent changes of protein level were monitored by immunoblotting with anti-Myc antibody. The RGA1-2xFlag protein was used as a positive control for the 26S proteasome-dependent degradation. Equal loading of cell-free extracts was confirmed with Rubisco and visualized by Coomassie staining. The protein levels were quantified using ImageJ software. Results are presented as an average value of five independent biological replicates. Error bars represent ±se (n = 5; *P < 0.05, **P < 0.005, one-way ANOVA).
(B) In vivo degradation assay of Exo70B1 in wild-type and pub18 pub19 protoplasts. The 35S:Myc-Exo70B1 and 35S:GFP constructs were transfected into protoplasts freshly isolated from wild-type and pub18 pub19 leaf tissues using PEG-mediated transformation. After overnight transfection, protoplasts were incubated with CHX (250 μM) for 2 h with or without MG132 (50 μM) and ABA (100 μM). Total proteins were extracted and examined by immunoblot analysis with anti-Myc and anti-GFP antibodies. The levels of GFP are shown as transfection efficiency of protoplast and equal loading control. Results are presented as an average value of three independent biological replicates. Error bars represent ±se (n = 6; *P < 0.05, **P < 0.005, one-way ANOVA).
The PUB18-dependent turnover of Exo70B1 was further investigated using a protoplast transient expression system. The 35S:Myc-Exo70B1 and 35S:sGFP constructs were transfected into protoplasts prepared from wild-type and pub18 pub19 double mutant leaves using PEG-mediated transformation (Kim et al., 2016). After overnight transfection, protoplasts were incubated with cycloheximide (CHX; 250 μM), an inhibitor of protein synthesis, for 2 h with or without MG132 (50 μM) and ABA (100 μM). Total proteins were extracted and examined by immunoblot analysis with anti-Myc and anti-GFP antibodies. The results revealed that the Myc-Exo70B1 level was rapidly decreased up to 51.3% ± 8.8% in the presence of ABA in wild-type protoplasts (Figure 5B). However, His-Myc-Exo70B1 was more stable in pub18 pub19 protoplasts and 93.8% ± 2.1% of the protein was detected in the presence of ABA. Degradation of Myc-Exo70B1 was inhibited by MG132 in both wild-type and mutant protoplasts (Figure 5B). The amount of Myc-Exo70B1 was slightly increased in the presence of MG132, suggesting that degradation of Myc-Exo70B1 is subject to control of 26S proteasome. The level of GFP, which was used to gauge the protoplast transfection efficiency and as an equal loading control, remained unchanged regardless of the presence of ABA and MG132. Overall, the results of in vitro and in vivo protein degradation assays suggest that the turnover of Exo70B1 is regulated, at least in part, by PUB18 in a proteasome-dependent fashion.
The UNDPUB18 Motif Is Critical for the Ubiquitination of Exo70B1 and Exo70B2 by PUB18 and PUB22, Respectively
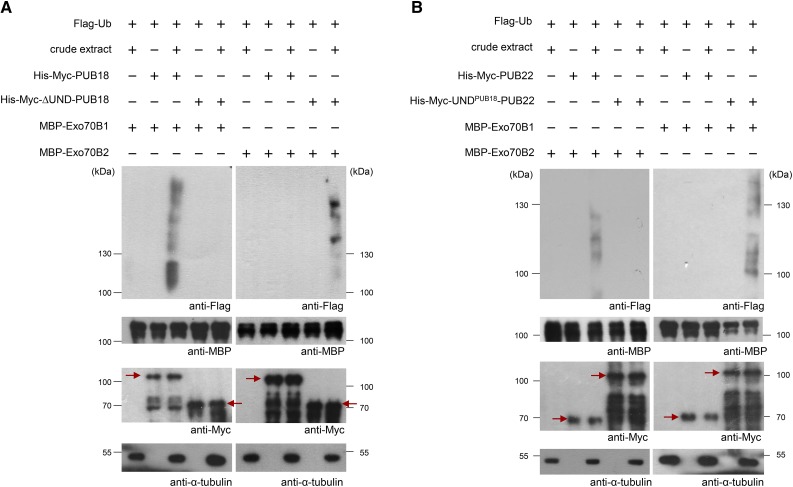
Next, an in vitro ubiquitination assay was performed (Supplemental Figure 6; Figure 6). His-Myc-PUB18 and His-Myc-∆UND-PUB18 were coincubated with MBP-Exo70B1 or MBP-Exo70B2, along with Flag-Ub, E1, and ATP, in the presence or absence of a protein crude extract (as a source of E2) prepared from ABA-treated pub18 pub19 pub22 pub23 mutant leaves. The reaction mixture was then subjected to immunoblot analysis with anti-Flag, anti-MBP, anti-Myc, and anti-α-tubulin antibodies. Ubiquitinated bands were detected by anti-Flag antibody. Equal loading of each lane was confirmed by anti-MBP and anti-Myc antibodies. The level of α-tubulin was used as a loading control of crude extract. The results showed that coincubation of His-Myc-PUB18 and MBP-Exo70B1 yielded high molecular mass smear bands detected by anti-Flag antibody (left panel in Figure 6A). The produced smear bands were not because of the endogenous PUB18 present in the protein crude extract, since this was clearly detected with a crude extract of ABA-pretreated pub18 pub19 pub22 pub23 mutant leaves. This result in conjunction with those of yeast two-hybrid and pull-down assays (Figure 4) indicates that Exo70B1 is a substrate of PUB18. However, coincubation of His-Myc-∆UND-PUB18 with MBP-Exo70B1 failed to produce any detectable high molecular mass smear ladders (left panel in Figure 6A), suggesting that Exo70B1 was ubiquitinated by PUB18, but not by ∆UND-PUB18. Conversely, His-Myc-∆UND-PUB18 gave rise to high molecular mass smear bands when it was coincubated with MBP-Exo70B2 (right panel in Figure 6A). These results indicate that deletion of the N-terminal UND of PUB18 resulted in the alteration of ubiquitination specificity of PUB18 from Exo70B1 to Exo70B2.
Figure 6.
The UNDPUB18 Domain Is Critical for the Ubiquitination of Exo70B1 and Exo70B2 by PUB18 and PUB22, Respectively.
(A) In vitro ubiquitination assays of Exo70B1 and Exo70B2 by PUB18 and ∆UND-PUB18, respectively. Recombinant His-Myc-PUB18 and His-Myc-∆UND-PUB18 E3 Ub ligases were incubated with MBP-Exo70B1 (left panel) or MBP-Exo70B2 (right panel) with or without crude extract (10 μg total proteins) of ABA (100 μM)-treated pub18 pub19 pub22 pub23 quadruple mutant leaves, along with Flag-tagged Ub (Flag-Ub), E1 (UBA1), MG132 (80 μM), and protease inhibitor cocktail. The reaction mixtures were subjected to immunoblot analysis with anti-Flag, anti-MBP, anti-Myc, and anti-α-tubulin antibodies. Ubiquitinated bands were detected by anti-Flag antibody. Equal loading of each lane was confirmed by anti-MBP and anti-Myc antibodies. The level of α-tubulin was used as a loading control of crude extract. Arrows indicate the migration of the unmodified PUB proteins.
(B) In vitro ubiquitination assays of Exo70B2 and Exo70B1 by PUB22 and UNDPUB18-PUB22, respectively. His-Myc-PUB22 and His-Myc-UNDPUB18-PUB22 E3 Ub ligases were incubated with MBP-Exo70B2 (left panel) or MBP-Exo70B1 (right panel) with or without crude extract (10 μg total proteins) of ABA (100 μM)-treated pub18 pub19 pub22 pub23 quadruple mutant leaves, along with Flag-Ub, E1 (UBA1), MG132 (80 μM), and protease inhibitor cocktail. The samples were resolved by SDS-PAGE and immunoblotted with anti-Flag, anti-MBP, anti-Myc, and anti-α-tubulin antibodies. Ubiquitinated bands were detected by anti-Flag antibody. Equal loading of each lane was confirmed by anti-MBP and anti-Myc antibodies. The level of α-tubulin was used as a loading control of crude extract. Arrows indicate the migration of the unmodified PUB proteins.
An identical set of in vitro ubiquitination experiments was performed with His-Myc-PUB22 and His-Myc-UNDPUB18-PUB22 E3 ligases. Consistent with previous findings (Stegmann et al., 2012), His-Myc-PUB22 effectively ubiquitinated MBP-Exo70B2 (left panel in Figure 6B). By contrast, His-Myc-UNDPUB18-PUB22 was unable to ubiquitinate MBP-Exo70B2, suggesting that the addition of UNDPUB18 to PUB22 hampered its ubiquitination activity toward Exo70B2 (left panel in Figure 6B). Although His-Myc-PUB22 failed to ubiquitinate MBP-Exo70B1, His-Myc-UNDPUB18-PUB22 was able to ubiquitinate MBP-Myc-Exo70B1, as evidenced by the formation of high molecular weight ladders detected by anti-Flag antibody (right panel in Figure 6B). Taken together, the results of in vitro ubiquitination assays indicate that the deletion of the N-terminal UND motif from PUB18 changes the target specificity from Exo70B1 to Exo70B2. By contrast, addition of UNDPUB18 to PUB22 resulted in the change in its ubiquitination activity from Exo70B2 to Exo70B1. Therefore, the ubiquitination specificities of PUB18 and PUB22 to Exo70B1 and Exo70B2, respectively, are most likely dependent on the presence or absence of the N-terminal UNDPUB18 motif.
Exo70B1 and Exo70B2 Are Positive Regulators of the Response to Mannitol (Drought) Treatments in ABA-Dependent and ABA-Independent Manners, Respectively
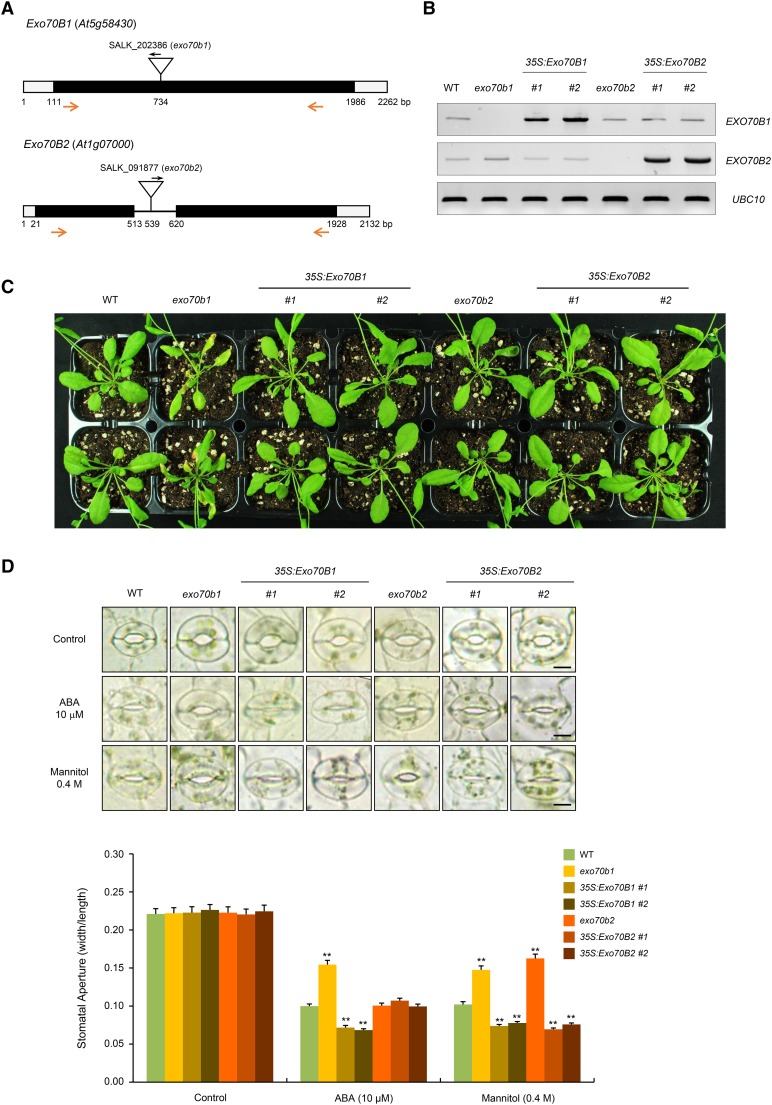
Our results indicate that ABA responsiveness and target specificities of PUB18 and PUB22 were dependent on the presence or absence of the N-terminal UNDPUB18 motif (Figures 1 to 4). Exo70B1 is a substrate of PUB18 that contains the N-terminal UND, whereas Exo70B2 is a target protein of PUB22, in which the UNDPUB18 motif is absent (Figures 5 and 6; Stegmann et al., 2012). Based on these results, we presumed that Exo70B1 plays a positive role in the ABA response and, by contrast, the cellular role of Exo70B2 is ABA independent. To elucidate this possibility, we obtained the loss-of-function T-DNA inserted knockout mutants of Exo70B1 and Exo70B2. The exo70b1 (SALK_202386) and exo70b2 (SALK_091877) mutant lines contained a single T-DNA insertion in the exon at nucleotide 734 and in the intron at nucleotide 539, respectively (Figure 7A). In addition, transgenic Arabidopsis plants that constitutively expressed Exo70B1 and Exo70B2 under the control of the 35S CaMV promoter were constructed. Disruption and ectopic expression of Exo70B1 and Exo70B2 were verified by genotyping PCR (Supplemental Figure 7) and RT-PCR (Figure 7B), respectively. As shown in Figure 7C, the exo70b1 mutant line displayed retarded growth with wrinkled rosette leaves under long-day growth conditions (16 h light/8 h dark) when compared with growth of wild-type plants. On the other hand, 35S:Exo70B1, exo70b2, and 35S:Exo70B2 progeny were morphologically normal under the same growth conditions.
Figure 7.
Exo70B1 and Exo70B2 Are Positive Regulators of the Response to Mannitol Treatments in ABA-Dependent and ABA-Independent Manners, Respectively
(A) Schematic structure of exo70b1 and exo70b2 T-DNA inserted loss-of-function mutant alleles. Inverted triangles indicate T-DNA insertion sites. Black bars, open bars, and solid line represent coding regions, untranslated regions, and intron, respectively. Gene-specific and T-DNA-specific primers used in RT-PCR are shown with arrows.
(B) RT-PCR analysis to detect the transcript levels of Exo70B1 and Exo70B2 in wild-type, exo70b1, 35S:Exo70B1 (transgenic lines #1 and #2), exo70b2, and 35S:Exo70B2 (transgenic lines #1 and #2) plants. Each experiment was performed with three independent biological replicates. UBC10 was used as a loading control.
(C) Phenotypes of wild-type, exo70b1, 35S:Exo70B1, exo70b2, and 35S:Exo70B2 plants under normal growth conditions. Seedlings were grown in half-strength Murashige and Skoog medium containing 1% sucrose and 0.7% phytoagar (pH 5.7) for 9 d, transferred to soil, and grown for 4 weeks in a growth chamber at 22°C under long-day conditions (16 h light/8 h darkness).
(D) ABA- and mannitol-induced stomatal closure in wild-type, exo70b1, 35S:Exo70B1 (transgenic lines #1 and #2), exo70b2, and 35S:Exo70B2 (transgenic lines #1 and #2) plants. Light-grown mature rosette leaves were immersed in stomatal opening solution for 2 h and transferred to solution that contained 10 μM ABA or 0.4 M mannitol for 2 h. Stomata were captured using bright-field microscopy. At least 30 stomatal apertures in each epidermal strip were measured per replicate. Three replicates were performed for each experiment. Error bars represent ±se (n = 90, **P < 0.005, one-way ANOVA). Bars = 10 μm.
Next, ABA-mediated stomatal behaviors of these mutant and overexpressing lines were monitored. After 10 μM ABA treatment, the average stomatal apertures of wild-type, exo70b1, and 35S:Exo70B1 were 0.100 ± 0.004, 0.154 ± 0.010, and 0.072 ± 0.003 (line #1) to 0.068 ± 0.005 (line #2), respectively (Figure 7D). Thus, ABA-mediated stomatal closure was inhibited in exo70b1 mutant plants and, by contrast, highly enhanced in Exo70B1-overexpressing plants, implying that Exo70B1 plays a positive role in ABA-promoted stomatal closure. To further examine the role of Exo70B1, the second mutant allele (exo70b1-1, GK-114C03; Hong et al., 2016) of Exo70B1 was obtained and its response to ABA was tested (Supplemental Figure 8). Recently, Hong et al. (2016) reported that exo70b1-1 showed similar stomatal movements to wild-type plants in response to ABA treatment (1 μM for 0.5 to 1 h). Although phenotypes of exo70b1-1 were less severe than exo70b1, the exo70b1-1 progeny exhibited retarded growth under long-day conditions and were hyposensitive to ABA (10 μM for 2 h) in stomatal closure compared with wild-type plants (Supplemental Figure 8). By contrast, stomatal apertures of wild-type, exo70b2 mutant, and 35S:Exo70B2 transgenic plants were indistinguishable in response to ABA treatment, indicating that Exo70B2 is not involved in the ABA-induced stomatal movements (Figure 7D).
However, in response to mannitol treatment (0.4 M), both exo70b1 and exo70b2 mutants displayed insensitive phenotypes in stomatal closure (Figure 7D). Furthermore, stomatal movements in Exo70B1 and Exo70B2 overexpressors showed similar levels of hypersensitivity in response to mannitol-derived water deficit. These results suggest that Exo70B1, a target protein of PUB18, is a positive regulator of both ABA-promoted and mannitol (drought)-promoted stomatal closure. Conversely, Exo70B2, a substrate of PUB22, is positively involved in mannitol-induced stomatal closure, but not in the ABA-mediated stomatal movement.
PUB18 Is Epistatic to Exo70B1 in the ABA-Mediated Drought Stress Responses
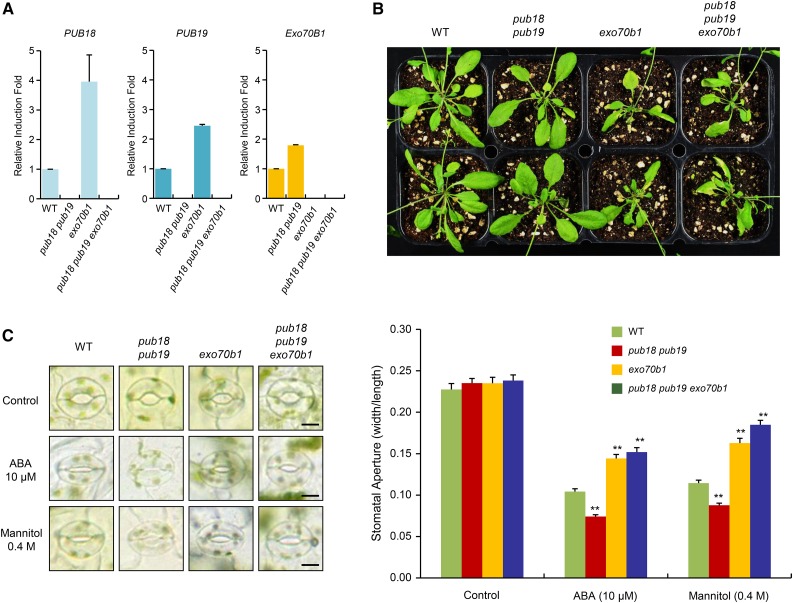
Exo70B1 is a target protein of PUB18 and plays a positive role in the mannitol (drought)-promoted stomatal closure in an ABA-dependent manner (Figure 7). To define whether Exo70B1 acts downstream of PUB18, we generated the pub18 pub19 exo70b1 triple knockout mutant line (Figure 8A), and its phenotypic properties were compared with those of wild-type, pub18 pub19, and exo70b1 plants. Real-time RT-qPCR revealed that the expressions of PUB18 and PUB19 were markedly higher in exo70b1 knockout mutant than in wild-type plants (Figure 8A). In addition, the expression of EXO70B1 was higher in the pub18 pub19 double mutant line than in wild-type plants. The reason for these higher expression levels of PUB18/PUB19 and EXO70B1 in exo70b1 and pub18 pub19 progeny, respectively, is unknown.
Figure 8.
PUB18 Is Epistatic to Exo70B1 in the ABA-Mediated Drought Stress Responses.
(A) Real-time qRT-PCR analysis to detect the transcripts levels of PUB18, PUB19, and Exo70B1 in wild-type and pub18 pub19 double, exo70b1 single, and pub18 pub19 exo70b1 triple mutant plants. ACT8 was used as an endogenous control gene to normalize the expression fold of PUB18, PUB19, and Exo70B1. Error bars represent ±se from three independent experiments.
(B) Growth morphologies of wild-type and pub18 pub19, exo70b1, and pub18 pub19 exo70b1 mutant plants under normal conditions. Seedlings were grown on half-strength Murashige and Skoog agar plates for 9 d, transplanted to individual pots, and grown for 4 weeks in a growth chamber at 22°C under long-day conditions (16 h light/8 h darkness).
(C) Stomatal movements of wild-type and pub18 pub19, exo70b1, and pub18 pub19 exo70b1 mutant plants in response to ABA (10 μM) and mannitol (0.4 M). ABA- and mannitol-mediated stomatal behaviors were monitored as described in Figure 7D. Images of stomata were captured using bright-field microscopy. At least 30 stomatal apertures in each epidermal strip were measured per replicate. Three replicates were performed for each experiment. Error bars represent ±se (n = 90, **P < 0.005, one-way ANOVA). Bars = 10 μm.
As indicated in Figures 7B and 8B, the exo70b1 single mutant showed smaller leaves with partial lesions and a twisted morphology compared with the leaves of wild-type and pub18 pub19 double mutant plants under long-day growth conditions. These morphological abnormalities were also evident in pub18 pub19 exo70b1 triple knockout mutant plants (Figure 8B), suggesting that Exo70B1 acts downstream of PUB18/PUB19. To further examine the epistatic interaction of Exo70B1 and PUB18, the ABA- and mannitol-mediated stomatal behaviors of wild-type, exo70b1 single, pub18 pub19 double, and pub18 pub19 exo70b1 triple mutant plants were compared. In response to 10 μM ABA and 0.4 M mannitol treatments, stomatal movements of the pub18 pub19 exo70b1 triple mutant exhibited insensitive phenotypes, which were reminiscent of those of the exo70b1 single mutant rather than the pub18 pub19 double mutant (Figure 8C). These morphological and stomatal movement studies suggest that PUB18 is epistatic to Exo70B1 in the ABA-mediated drought stress responses. Taken together, we concluded that Exo70B1 is a target protein of the U-box E3 ligase PUB18 and exerts its positive effects on the ABA signaling pathway downstream of PUB18.
DISCUSSION
A large number of plant PUBs, arising from extensive gene proliferation and diversification, compared with those in yeasts and mammals, provides the possibility that U-box E3 Ub ligases play crucial roles in various cellular processes in higher plants. Of the 64 U-box E3 ligases in Arabidopsis, the largest group of PUBs is composed of 41 PUB-ARM proteins, among which 17 proteins (UND-PUB-ARM) possess the UND motif in their N termini. Although the UND motifs were first identified based on their N-terminal positional conservation in a subset of PUBs (Mudgil et al., 2004), a high level of amino acid sequence diversity of UND implies that functional specificities of UND-PUB-ARM proteins are dependent, at least in part, on the UND motifs (Supplemental Figure 9; Samuel et al., 2009; Antignani et al., 2015). However, the detailed cellular functions of UND are largely unknown in higher plants.
Arabidopsis U-box E3 Ub ligases PUB18, PUB19, PUB22, and PUB23 act as negative regulators of drought stress responses, but they exhibit distinct modes of action: PUB18/PUB19 regulate the drought stress response in an ABA-dependent manner, whereas the role of PUB22/PUB23 was ABA-independent (Cho et al., 2008; Seo et al., 2012). In addition, PUB22/PUB23 along with PUB24 regulate the negative response to pathogen infection (Trujillo et al., 2008). In this study, we focused on elucidating the molecular mechanism that mediates this functional separation of PUB18 and PUB22 in relation to ABA responses. We found an apparent difference between PUB18/PUB19 and PUB22/PUB23: the presence of the N-terminal UND motif in the former. According to the phylogenetic tree of 41 PUB-ARM proteins constructed on the basis of the alignment of U-box sequences, UND-PUB-ARM proteins were divided into three distinct clusters, indicating that the UND motifs were independently obtained multiple times for specialized functions during evolution (Mudgil et al., 2004; Ishizaki et al., 2013; Supplemental Figure 10 and Supplemental Data Set 1). Interestingly, PUB18/PUB19 and PUB22/PUB23 fall into the same cluster.
Our results indicate that the UNDPUB18 motif is critically involved in the negative regulation of ABA-dependent stomatal behaviors (Figures 1E and 2E). Because the mannitol-induced drought response was not affected by UNDPUB18 (Figure 1F), the UNDPUB18 motif is not likely to be a general negative factor for stress responses, but is specific to the ABA-mediated drought response. Thus, the functional differentiation of PUB18 and PUB22 in response to ABA might result from the presence or absence of UND. Liu et al. (2016) recently reported that the N-terminal domain of Brassica oleracea ARC1, the closest homolog of Arabidopsis PUB17, confers a binding specificity of ARC1 toward Exo70A1.
Exo70B1 was identified as an interaction partner of PUB18 (Figures 3A and 3B). The Exo70 (exocyst component of 70 kD) protein is a core component of an evolutionarily conserved exocyst vesicle-tethering complex, which consisted of eight subunits (Novick et al., 1980). Among these, Exo70 has undergone the most remarkable expansion in plants. Therefore, there are 23 paralogs of Exo70, including Exo70B1, in Arabidopsis. This expansion implies that plants have developed a functionally differentiated Exo70 gene family to mediate plant-specific processes at the evolutionary point of view (Chong et al., 2010; Li et al., 2010; Cvrčková et al., 2012; Zárský et al., 2013; Indriolo et al., 2014; Lin et al., 2015). Punctate subcellular localization and membrane association patterns of PUB18 were highly similar to those of Exo70B1, further supporting their physical interactions as binding partners (Figures 3C to 3F). Furthermore, Exo70B1 is degraded by a 26S proteasome complex in a PUB18-depenent fashion (Figure 5). Exo70B2 was shown to be a target substrate of PUB22 E3 Ub ligase (Stegmann et al., 2012). Among the 23 Exo70 paralogous proteins in Arabidopsis, Exo70B2 is the closest homolog of Exo70B1 (54% identity) (Li et al., 2010; Stegmann et al., 2012; Supplemental Figure 11). With this in mind, we speculated that the UNDPUB18 motif affects the binding activities of PUB18 and PUB22 to Exo70B1 and Exo70B2, respectively.
It was previously shown that the GFP-PUB18 fusion protein was localized to the cytosol and nucleus, rather than to punctate bodies, in Arabidopsis protoplasts (Supplemental Figure 1 in Drechsel et al., 2011). The reason for this discrepancy between those and our results is currently unknown. It is possible that the N-terminally fused GFP to PUB18 may cause a subtle conformational change in the UND domain, which resulted in different subcellular localization of GFP-PUB18. However, this possibility is unlikely because ΔUND-PUB18-GFP also displayed a punctate subcellular localization (Supplemental Figure 5B). Alternatively, proper trafficking of transiently expressed proteins to different subcellular organelles (e.g., cytosol and punctate exocyst bodies) in the protoplasts may need differential temporal processes; thus, more detailed time-course localization studies of GFP-PUB18 after protoplast transformation appear to be necessary to answer these questions.
The domain-exchange analysis indicated that the ubiquitination specificities, as well as binding activities, of PUB18 and PUB22 to Exo70B1 and Exo70B2, respectively, are dependent on the presence or absence of the UNDPUB18 motif (Figures 4 and 6). Because ∆UND-PUB18 colocalized to punctate bodies with Exo70B1 (Supplemental Figure 5B), the UNDPUB18 motif may not be involved in the subcellular localization of PUB18. Consistently, UNDPUB18 was able to interact with Exo70B1 in yeast cells (Supplemental Figure 12). Exo70B1 plays a positive role in both ABA- and mannitol-induced stomatal closure downstream of PUB18, whereas Exo70B2 positively regulated mannitol-induced stomatal closure, but in an ABA-independent manner (Figures 7D and 8C). Taken together, these results strongly suggest that Exo70B1 is regulated by PUB18 in ABA-mediated stomatal movement and that the N-terminal UNDPUB18 is critically involved in this negative regulation of ABA-dependent drought responses.
Although PUB18 was identified as a functional U-box E3 Ub ligase (Mudgil et al., 2004; Bergler and Hoth, 2011; Seo et al., 2012), we failed to detect in vitro E3 self-ubiquitination activity of bacterially expressed MBP-PUB18 using Arabidopsis E1 (UBA1) and various E2 enzymes. This was in sharp contrast to the results that in vitro E3 Ub ligase activities of recombinant PUB19, PUB22, UNDPUB18-PUB22, and PUB23 proteins were routinely detectable (Cho et al., 2008; Trujillo et al., 2008; Liu et al., 2011; Stegmann et al., 2012; Supplemental Figure 13). Thus, the E3 ligase activity of PUB18 was speculated to be mediated by another specific E2 protein or to require an as yet unidentified cellular factor. The in vitro E3 activity of PUB18 was clearly detected when a small amount of crude extract (10 μg total proteins) prepared from ABA-treated leaves was included as a source of E2 in the reaction mixture (Figures 1B and 6A). Because an ABA-treated crude extract was essential for the detection of E3 activity of PUB18, we are tempted to propose that the activation of E3 Ub ligase activity of PUB18 is subject to control by ABA-mediated cellular processes such as protein modifications. This hypothesis is supported by recent results that an Ub ligase enzyme activity of RING-type E3 RZFP34/CHYR1 was enhanced by SnRK2.6-mediated phosphorylation, a key regulator of ABA signal transduction in Arabidopsis (Ding et al., 2015). In addition, the phosphorylation of the C-terminal ARM repeats of OsPUB15, a rice UND-PUB-ARM protein, triggered its E3 Ub ligase activity (Wang et al., 2015).
Exocytosis is a fundamental cellular mechanism that regulates the transport of various compounds to the plasma membrane and extracellular matrix (TerBush et al., 1996; Hsu et al., 2004; Orlando and Guo, 2009; Liu and Guo, 2012). In higher plants, exocytosis plays various roles in cell growth, pollen incompatibility, and the response to pathogen invasion (Wen et al., 2005; Samuel et al., 2009; Sup Yun et al., 2013; Kissoudis et al., 2014). The Exo70B1 subunit contributes to the regulation of autophagy-related transport, vesicle trafficking, and pathogen-induced immune responses (Kulich et al., 2013; Stegmann et al., 2013; Teh and Hofius, 2014; Zhao et al., 2015), whereas Exo70B2 participates in the process of pathogen-associated molecular pattern (PAMP)-triggered defense responses (Pecenková et al., 2011; Stegmann et al., 2012). Stegmann et al. (2012) proposed that the detailed mechanisms by which Exo70B1 and Exo70B2 regulate PAMP-induced immune signaling are different. The exo70b1 knockout mutant plants displayed morphological abnormalities, such as smaller and twisted leaves with partial lesions, whereas the exo70b2 progeny were phenotypically normal (Figure 7C). These results further indicate that Exo70B1 and Exo70B2 paralogs perform distinct cellular roles.
Under adverse growth conditions, many stress-related genes are activated systematically and simultaneously (Cramer et al., 2011; Lv et al., 2014). Because stress-responsive proteins occasionally resulted in multiple destructive traits, they function transiently and then should be subsequently degraded (Gilmour et al., 2000; Xiong et al., 2007; Flick and Kaiser, 2012). However, how these transiently induced proteins are effectively removed has not yet been determined. Recently, physiological relevance between autophagy and ABA and abiotic stress responses has attracted considerable interest (Xiong et al., 2007; Slavikova et al., 2008; Liu et al., 2009; Han et al., 2011). Notably, Vanhee and Batoko (2011) proposed the existence of a selective autophagy system to remove the ABA-induced At-TSPO. Furthermore, NBR1 was reported to be an important regulator of selective autophagy-mediated antiproteotoxic pathways in abiotic stress responses (Zhou et al., 2014). Tzfadia and Galili (2013) suggested that 21 paralogs of Arabidopsis EXO70 exocyst subunits, including Exo70B1 and Exo70B2, contain an ATG8-interacting motif (strict consensus W/YxxL/V/I), implying that the functions of Exo70 paralogs could be related to autophagy regulation. Moreover, Exo70B1 was found to participate in the autophagy-related transport of exocysts to the vacuole (Kulich et al., 2013). Interestingly, PUB22 ubiquitinates 26S proteasome subunits RPN6 and RPN12 (Cho et al., 2008, 2015). Ubiquitination of RPN12 by PUB22 resulted in the partial dissociation of the 26S proteasome complex, whereas the ubiquitinated RPN6 subunit was rapidly degraded, both of which might decrease the proteasomal protein degradation activity. The changes in the proteasome activity by drought-induced PUB22 might affect cellular autophagy activity to maintain continuous elimination of toxic or unnecessary proteins to fine-tune the plant responses to drought stress. Thus, further studies are warranted to elucidate the possible regulatory links between PUB18 and PUB22 U-box E3 Ub ligases and Exo70-related autophagy in ABA-mediated as well as ABA-independent drought stress responses.
In conclusion, our results suggest that the N-terminal UND motif of the U-box E3 ubiquitin ligase PUB18 is critical for the negative regulation of ABA-mediated stomatal movements and determines its binding and ubiquitination specificity to Exo70B1, a subunit of the exocyst complex in Arabidopsis.
METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Columbia (Col-0) was used as the wild type in this study. Mutant alleles used to produce the pub18 pub19 and pub22 pub23 double mutants have been previously described (Cho et al., 2008; Seo et al., 2012). The T-DNA insertion exo70b1 (SALK_202386) and exo70b2 (SALK_091877) mutants were obtained from the ABRC at the Ohio State University (http://www.arabidopsis.org). The pub18 pub19 exo70b1 triple knockout mutant line was generated through a genetic cross between pub18 pub19 and exo70b1 homozygous mutant plants.
Chimeric transgenes were constructed by obtaining the N-terminal UND region of PUB18 by using PCR and ligating it to the N terminus of the full-length coding sequence of PUB22. The UNDPUB18-PUB22 and ∆UND-PUB18 constructs, as well as a full-length PUB18, were inserted into the modified pENTR SD/D topo vector (Invitrogen) and transferred to the binary vector pEarleyGate202 (ABRC stock no. CD3-688) using a Gateway cloning kit (Invitrogen). The full-length coding sequence of PUB22 was introduced into a GFP-fused pENTR SD/D topo vector and transferred to the pEarlyGate100 destination vector (ABRC stock no. CD3-724). The Exo70B2 cDNA clone (ABRC stock no. CD4-36) was acquired from the ABRC. The full-length Exo70B1 and Exo70B2 coding sequences were amplified using PrimeSTAR polymerase (Takara) and incorporated downstream of the CaMV 35S promoter in the pEarleyGate202 vector containing the Flag-tag sequence.
All transgenic plants used in this study were generated via floral-dip transformation. Methods for seed surface sterilization and growth conditions of wild-type and transgenic Arabidopsis plants were described by Ryu et al. (2010) and were applied with slight modifications. All plants were grown at 22°C under continuous spectrum light. Primer sequences used in RT-qPCR, genotyping PCR, and plasmid constructions are listed in Supplemental Tables 1 and 2.
Stomatal Aperture Measurements
Light-grown fully expanded rosette leaves of 4- to 6-week-old plants were detached and submerged in stomatal opening solution (30 mM KCl, 100 mM CaCl2, and 10 mM MES, pH 6.15) for 2 h at 25°C as described previously (Kwak et al., 2001). The adaxial surface of the leaves was applied to label tape (Bel-Art Products) to peel off the abaxial epidermal layers. Epidermal strips were floated on stomatal opening solution containing various concentrations of mannitol (0, 0.2, and 0.4 M) or ABA (0, 1, and 10 μM) for 2 h in white light (100 μmol m−2 s−1). After incubation, leaf strips were mounted on glass slides. Stomata images were observed and photographed using an Olympus CX41 microscope equipped with a JUJAK560 CCD camera (Dixi Optics). At least 30 stomatal apertures in each epidermal peel were measured and analyzed using Photoshop CS6. Excel data files of stomatal apertures are provided in Supplemental Data Set 2.
In Vitro Self-Ubiquitination and Substrate Ubiquitination Analyses
Recombinant MBP-PUB18, MBP-∆UND-PUB18, MBP-PUB22, MBP-UNDPUB18-PUB22, and their single amino acid substitution mutant variant proteins (MBP-PUB18V305I, MBP-∆UND-PUB18V36I, MBP-PUB22V24I, and MBP-UNDPUB18-PUB22V293I) were expressed in Escherichia coli and purified by affinity chromatography using MBP Excellose resin (Takara). Purified MBP-tagged proteins were immobilized on the resin without elution and applied for in vitro self-ubiquitination analyses. Each reaction of 60 μL final volume contained ubiquitination buffer (50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 2 mM DTT, 4 mM ATP, 15 μg ubiquitin, 400 μM PMSF, 80 μM MG132 [AG Scientific], and 1× protease inhibitor cocktail) and 1 μg of bacterially expressed E3s. The reaction was conducted at 30°C for 2 h in the presence or absence of E1 (Arabidopsis UBA1) (50 ng) and a crude extract (10 μg total proteins), as a source of E2 enzyme, prepared from ABA (100 μM)-pretreated pub18 pub19 pub22 pub23 quadruple mutant leaves. The pub18 pub19 pub22 pub23 quadruple mutant leaves, instead of wild-type leaves, were used as E2 to avoid the possible effects of endogenous PUB18 and PUB22 present in the protein crude extract on the ubiquitination assays. The resin-bound proteins were washed five times with MBP column buffer (20 mM Tris-HCl, pH 7.4, 200 mM NaCl, and 1 mM EDTA) and eluted with 10 mM maltose. The eluted samples were mixed with 4× SDS sample buffer and heated at 90°C for 5 min. The reaction products were separated on a 6% (w/v) SDS-PAGE gel, followed by immunoblot analysis with anti-MBP antibody (rabbit, 1:5000; Applied Biological Materials; cat no. G079; lot no. AP4779).
For in vitro substrate ubiquitination assays, Ni-NTA resin (Qiagen)-bound His-Myc-Exo70B1 and His-Myc-Exo70B2 were used as target substrates. The reaction was performed in 80 μL of total mixture containing ubiquitination buffer, 50 ng of E1, and 1 μg of target protein in the presence or absence of an ABA-pretreated pub18 pub19 pub22 pub23 crude extract (10 μg total proteins) and 200 ng of E3s at 30°C for 3 h. After incubation, the resin-bound substrate proteins were washed five times with Ni-NTA washing buffer (1× PBS, 300 mM NaCl, and 20 mM imidazole) and eluted using 4× SDS sample buffer with boiling for 5 min. The eluted proteins were subjected to immunoblot analysis with anti-Myc antibody (rabbit, 1:5000; Applied Biological Materials; cat no. G077; lot no. 0512)
A protein crude extract was prepared from the pub18 pub19 pub22 pub23 quadruple mutant plants. Fully expanded mutant rosette leaves pretreated with 100 μM ABA for 3 h were ground under liquid nitrogen and suspended in an extraction buffer containing 50 mM Tris-HCl, pH 7.4, 10 mM MgCl2, 1 mM EDTA, 1 M NaCl, 80 μM MG132, 1 mM PMSF, and 1× protease inhibitor cocktail. The suspensions were vortexed gently at 4°C for 30 min. After centrifugation (13,200 rpm) at 4°C for 10 min, the supernatant was used as a source of E2 for ubiquitination assays.
Yeast Two-Hybrid Assays
The PUB18, ∆UND-PUB18, PUB22, and UNDPUB18-PUB22 constructs were cloned in the pGBKT7 vector (Clontech) as described previously (Lee et al., 2009). The full-length coding sequences of Exo70B1 and Exo70B2 were amplified and introduced into the pGADT7 vector (Clontech). Various combinations of bait and prey plasmids were cotransformed into the yeast strain AH109. Yeast two-hybrid analyses were performed according to the manufacturer’s instructions of the Matchmaker Yeast Two-Hybrid System (Clontech). Interactions between two proteins were confirmed by growth on SD/-Leu/-Trp/-His media plates, including 2 mM 3-amino1,2,4,-triazole.
In Vitro Pull-Down Assay
The MBP-PUB18 and MBP-∆UND-PUB18 recombinant proteins were immobilized on the MBP Excellose resin (Takara) and incubated with His-Myc-Exo70B1 and His-Myc-Exo70B2, respectively, in immunoprecipitation buffer at 30°C for 1 h. In addition, the resin-bound MBP-UNDPUB18-PUB22 chimeric protein was coincubated with His-Myc-Exo70B1 under the same conditions. The affinity beads were extensively washed five times with MBP column buffer, and bound proteins were eluted with 10 mM maltose. The eluted proteins were separated by SDS-PAGE and analyzed by immunoblotting using anti-MBP (rabbit, 1:5000; Applied Biological Materials; cat no. G079; lot no. AP4779) and anti-Myc (rabbit, 1:5000; Applied Biological Materials; cat no. G077; lot no. 0512) antibodies.
Subcellular Localization
The full-length PUB18 coding sequence was inserted into the 326-GFP expression vector provided by Inhwan Hwang at POSTECH (Pohang, Korea). The PUB18-GFP plasmid was transformed into protoplasts prepared from wild-type Arabidopsis rosette leaves using the PEG method (Ryu et al., 2010). For an Agrobacterium tumefaciens-mediated transient expression assay, the 35S:PUB18-GFP, 35S:GFP, 35S:Exo70B1-DsRED, and 34S:DsRED constructs were introduced into the binary vector pEarlyGate100 and transformed into Agrobacterium strain GV3101 (Kim and Kim, 2013). Agrobacterium cells harboring each plasmid were incubated in the infiltration medium (10 mM MgCl2, 10 mM MES, pH 5.7, and 500 μM acetosyringone) containing 40 μM MG132 for 30 min and infiltrated into the abaxial side of Nicotiana benthamiana leaves. The expression of PUB18-GFP and Exo70B1-DsRED was enhanced by coexpressing a viral-encoded silencing suppressor P19 that enables high levels of transient expression (Hellens et al., 2005). Transiently expressed GFP signals were obtained using a fluorescence microscope (BX51; Olympus) and a cooled CCD camera (PCO) or by confocal microscopy (LSM510 META; Carl Zeiss). The laser signals were measured using a sliding 0.313-μm detection window in a confocal microscope. GFP and YFP were excited by a 488-nm laser, and DsRED was excited by 550-nm laser.
BiFC Assay
PUB18 and ∆UND-PUB18 were cloned in the pSPYNE-35S vector that contained the N-terminal region (YFPN; 1 to 155 amino acids) of YFP (Walter et al., 2004). The full-length coding sequences of Exo70B1 and Exo70B2 were amplified and introduced into the pSPYCE-35S vector containing the C-terminal region (YFPC; 156 to 239 amino acids) of YFP (Walter et al., 2004). Various combinations of Agrobacterium strain GV3101 harboring the YFPN- and YFPC-fused constructs were coinfiltrated into the abaxial side of tobacco leaves. After 3 d, reconstituted fluorescent signals in epidermal cells of infiltrated leaves were examined by confocal microscopy.
Protein Membrane Association Analysis
The 35S:PUB18-GFP and 35S:Flag-Exo70B1 fusion genes were transiently coexpressed with 35S:P19 using Agrobacterium-mediated infiltration in N. benthamiana as described by Kim et al. (2016). Tobacco leaves were ground in liquid nitrogen, suspended in extraction buffer (50 mM Tris-HCl, pH 7.4, 10 mM MgCl2, 1 mM EDTA, and 1 M NaCl), and centrifuged at 28,000g for 10 min at 4°C. The pellet was resuspended in extraction buffer with or without NaCl (1 M) and Na2CO3 (100 mM) and vigorously vortexed at 4°C for 15 min. Protein samples were then centrifuged at 28,000g for 30 min at 4°C. The supernatant and pellet fractions were analyzed using immunoblotting with anti-GFP (mouse, 1:5000; Clontech; cat no. 632381; lot no. A5033481) and anti-Flag (mouse, 1:5000; Sigma-Aldrich; cat no. A8592; batch no. SLBD9930) antibodies.
In Vivo Co-IP Assay
The 35S:Flag-∆UND-PUB18V36I and 35S:Flag-UNDPUB18-PUB22V293I constructs were introduced into the binary vector pEarlyGate202. The 35S:Myc-Exo70B1 and 35S:Myc-Exo70B2 constructs were inserted into the modified pENTR SD/D topo vector (Invitrogen) and transferred to the binary vector pEarleyGate100. 35S:Flag-∆UND-PUB18V36I and 35S:Flag-UNDPUB18-PUB22V293I were transiently coexpressed with 35S:Myc-Exo70B1 or 35S:Myc-Exo70B2 in the presence of 35S:P19 in N. benthamiana leaves using Agrobacterium-mediated infiltration as described by Kim et al. (2016). After 2.5 d, harvested tobacco leaves were ground in liquid nitrogen, suspended in the extraction buffer (50 mM Tris-HCl, pH 7.4, 10 mM MgCl2, 1 mM EDTA, and 1 M NaCl), and vigorously vortexed at 4°C for 15 min. Total leaf proteins (200 μg) were incubated for 1 h at 4°C with anti-Flag affinity gel matrix. The precipitated samples were extensively washed three times with TBS buffer and eluted by boiling with 4× SDS sample buffer. Each sample was separated by SDS-PAGE and analyzed by immunoblotting with anti-Flag and anti-Myc antibodies.
In Vitro and in Vivo Protein Degradation Assay
Light-grown mature rosette leaves of wild-type and pub18 pub19 mutant plants were treated with ABA (100 μM) for 2 h and rapidly frozen with liquid nitrogen. Frozen powder of sample leaves was suspended in extraction buffer and vigorously vortexed at 4°C for 1 h. After the mixture was centrifuged at 28,000g at 4°C for 10 min, the supernatant was collected. The His-Myc-Exo70B1 and RGA1-2xFlag recombinant proteins were incubated with a cell-free crude extract (100 μg total proteins) for 1.5 and 3 h in the presence or absence of 50 μM MG132 (AG Scientific). The reactions were terminated by adding 2× SDS sample buffer. The protein degradation patterns were analyzed by immunoblotting with anti-Myc (Applied Biological Materials) and anti-Flag (Sigma-Aldrich) antibodies.
For the in vivo protein degradation assay, the full-length Exo70B1 coding sequence was inserted into the 326-GFP expression vector that was provided by Inhwan Hwang. The Exo70B1-GFP and GFP only plasmids were transfected into protoplasts prepared from Arabidopsis rosette leaves of wild-type and pub18 pub19 double mutant plants using the PEG method (Ryu et al., 2010). After incubation for 12 h, the protoplasts were treated with MG132 (50 μM) or DMSO for 3 h. Subsequently, protoplasts were incubated with CHX (250 μM) for 2 h with or without ABA (100 μM). The reactions were terminated by adding 4× SDS sample buffer. The patterns of protein degradation were analyzed by immunoblot analysis with anti-Myc and anti-GFP antibodies. The levels of GFP and α-tubulin were shown as transfection efficiency control of protoplasts and equal loading control, respectively. The protein levels were quantified using ImageJ software (NIH; http://imagej.nih.gov/ij/).
Sequence Alignment and Phylogenetic Analysis
The U-box domain sequences of Arabidopsis PUB-ARM proteins were aligned using ClustalW2 with default parameters. The multiple sequence alignment was generated and the phylogenetic tree was constructed. The evolutionary history was inferred using the maximum likelihood method based on the Poisson correction model. The bootstrap consensus tree inferred from 5000 replicates is taken to represent the evolutionary history of the taxa analyzed (Felsenstein, 1985). The initial tree(s) for the heuristic search was obtained automatically by applying neighbor-joining and BioNJ algorithms to a matrix of pairwise distances estimated using a JTT model and then selecting the topology with superior log likelihood value. The phylogenetic dendrogram was constructed in MEGA5 software (Tamura et al., 2011).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: PUB18 (At1g10560), PUB19 (At1g60190), PUB22 (At3g52450), PUB23 (At2g35930), Exo70B1 (At5g58430), Exo70B2 (At1g07000), UBA1 (At2g30110), UBC7 (At5g59300), UBC8 (At5g41700), UBC9 (At4g27960), UBC10 (At5g53300), and UBC13 (At3g46460).
Supplemental Data
Supplemental Figure 1. Multiple Alignment of Amino Acid Sequences of PUB18, PUB19, PUB22, and PUB23 Arabidopsis U-box E3 Ub Ligases.
Supplemental Figure 2. In Vitro Self-Ubiquitination Assay of PUB18, PUB18V305I, PUB22, and PUB22V24I with or without Heat-Inactivated Leaf Crude Protein Extract.
Supplemental Figure 3. Arabidopsis PUB19 Interacts with Exo70B1 in Yeast Cells.
Supplemental Figure 4. The in Vitro Pull-Down Assay between MBP-PUB19 and His-Myc-Exo70B1.
Supplemental Figure 5. BiFC Assay and Subcellular Localization of GFP, DsRED, ∆UND-PUB18-GFP, and Exo70B1-DsRED.
Supplemental Figure 6. In Vitro Ubiquitination Assays of Exo70B1 and Exo70B2 by PUB18, ∆UND-PUB18, PUB22, and UNDPUB18-PUB22.
Supplemental Figure 7. Molecular Characterization of exo70b1 and exo70b2 T-DNA-Inserted Homozygous Knockout Mutant Plants.
Supplemental Figure 8. Characterization of the Second Mutant Allele (exo70b1-1) of Exo70B1.
Supplemental Figure 9. Multiple Alignments of Amino Acid Sequences of Arabidopsis UND Motifs.
Supplemental Figure 10. Molecular Phylogenetic Analysis of Arabidopsis PUB-ARM Proteins Using the Maximum Likelihood Method.
Supplemental Figure 11. Amino Acid Sequence Alignment of Arabidopsis Exo70B1 and Exo70B2.
Supplemental Figure 12. Yeast Two-hybrid Assay of the UNDPUB18 Motif and Exo70B1.
Supplemental Figure 13. In Vitro Self-Ubiquitination Assay of UNDPUB18-PUB22.
Supplemental Table 1. Primer Sequences Used for Genotyping PCR, qRT-PCR, and RT-PCR.
Supplemental Table 2. Primer Sequences Used for Gene Constructions and Mutagenesis.
Supplemental Data Set 1. Alignment used to generate the phylogeny presented in Supplemental Figure 10.
Supplemental Data Set 2. Excel Data Files of Stomatal Aperture Analyses.
Supplementary Material
Acknowledgments
This work was supported by grants from the Woo Jang Chun Special Project (PJ009106) funded by the Rural Development Administration and from the National Research Foundation, Project No. 2014R1A2A2A01003891, Republic of Korea, to W.T.K.
AUTHOR CONTRIBUTIONS
D.H.S., M.Y.A., K.Y.P., and E.Y.K performed the experiments. D.H.S., M.Y.A., and W.T.K. analyzed the data. D.H.S. and W.T.K. planned the project and drafted the manuscript. W.T.K. supervised the project and complemented the writing.
Glossary
- ABA
abscisic acid
- UPS
ubiquitin-proteasome system
- PEG
polyethylene glycol
- BiFC
bimolecular fluorescence complementation
- co-IP
coimmunoprecipitation
- CHX
cycloheximide
References
- Andersen P., Kragelund B.B., Olsen A.N., Larsen F.H., Chua N.H., Poulsen F.M., Skriver K. (2004). Structure and biochemical function of a prototypical Arabidopsis U-box domain. J. Biol. Chem. 279: 40053–40061. [DOI] [PubMed] [Google Scholar]
- Antignani V., Klocko A.L., Bak G., Chandrasekaran S.D., Dunivin T., Nielsen E. (2015). Recruitment of PLANT U-BOX13 and the PI4Kβ1/β2 phosphatidylinositol-4 kinases by the small GTPase RabA4B plays important roles during salicylic acid-mediated plant defense signaling in Arabidopsis. Plant Cell 27: 243–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo C., Santos-Rosa M.J., Shirasu K. (2001). The U-box protein family in plants. Trends Plant Sci. 6: 354–358. [DOI] [PubMed] [Google Scholar]
- Bartel B., Citovsky V. (2012). Focus on ubiquitin in plant biology. Plant Physiol. 160: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergler J., Hoth S. (2011). Plant U-box armadillo repeat proteins AtPUB18 and AtPUB19 are involved in salt inhibition of germination in Arabidopsis. Plant Biol. (Stuttg.) 13: 725–730. [DOI] [PubMed] [Google Scholar]
- Berndsen C.E., Wolberger C. (2014). New insights into ubiquitin E3 ligase mechanism. Nat. Struct. Mol. Biol. 21: 301–307. [DOI] [PubMed] [Google Scholar]
- Chaves M.M., Pereira J.S., Maroco J., Rodrigues M.L., Ricardo C.P., Osório M.L., Carvalho I., Faria T., Pinheiro C. (2002). How plants cope with water stress in the field. Photosynthesis and growth. Ann. Bot. (Lond.) 89: 907–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S.K., Bae H., Ryu M.Y., Wook Yang S., Kim W.T. (2015). PUB22 and PUB23 U-BOX E3 ligases directly ubiquitinate RPN6, a 26S proteasome lid subunit, for subsequent degradation in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 464: 994–999. [DOI] [PubMed] [Google Scholar]
- Cho S.K., Chung H.S., Ryu M.Y., Park M.J., Lee M.M., Bahk Y.-Y., Kim J., Pai H.S., Kim W.T. (2006). Heterologous expression and molecular and cellular characterization of CaPUB1 encoding a hot pepper U-Box E3 ubiquitin ligase homolog. Plant Physiol. 142: 1664–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S.K., Ryu M.Y., Song C., Kwak J.M., Kim W.T. (2008). Arabidopsis PUB22 and PUB23 are homologous U-Box E3 ubiquitin ligases that play combinatory roles in response to drought stress. Plant Cell 20: 1899–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong Y.T., Gidda S.K., Sanford C., Parkinson J., Mullen R.T., Goring D.R. (2010). Characterization of the Arabidopsis thaliana exocyst complex gene families by phylogenetic, expression profiling, and subcellular localization studies. New Phytol. 185: 401–419. [DOI] [PubMed] [Google Scholar]
- Cramer G.R., Urano K., Delrot S., Pezzotti M., Shinozaki K. (2011). Effects of abiotic stress on plants: a systems biology perspective. BMC Plant Biol. 11: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvrčková F., Grunt M., Bezvoda R., Hála M., Kulich I., Rawat A., Zárský V. (2012). Evolution of the land plant exocyst complexes. Front. Plant Sci. 3: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb S., Sankaranarayanan S., Wewala G., Widdup E., Samuel M.A. (2014). The S-domain receptor kinase Arabidopsis receptor kinase2 and the U Box/armadillo repeat-containing E3 ubiquitin ligase9 module mediates lateral root development under phosphate starvation in Arabidopsis. Plant Physiol. 165: 1647–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill A., Thomas S.G., Hu J., Steber C.M., Sun T.P. (2004). The Arabidopsis F-box protein SLEEPY1 targets gibberellin signaling repressors for gibberellin-induced degradation. Plant Cell 16: 1392–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S., Zhang B., Qin F. (2015). Arabidopsis RZFP34/CHYR1, a ubiquitin E3 ligase, regulates stomatal movement and drought tolerance via SnRK2.6-mediated phosphorylation. Plant Cell 27: 3228–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsel G., Bergler J., Wippel K., Sauer N., Vogelmann K., Hoth S. (2011). C-terminal armadillo repeats are essential and sufficient for association of the plant U-box armadillo E3 ubiquitin ligase SAUL1 with the plasma membrane. J. Exp. Bot. 62: 775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher K., Callis J. (2007). Ubiquitin, hormones and biotic stress in plants. Ann. Bot. (Lond.) 99: 787–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. (1985). Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39: 783–791. [DOI] [PubMed] [Google Scholar]
- Flick K., Kaiser P. (2012). Protein degradation and the stress response. Semin. Cell Dev. Biol. 23: 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour S.J., Sebolt A.M., Salazar M.P., Everard J.D., Thomashow M.F. (2000). Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol. 124: 1854–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra D.D., Callis J. (2012). Ubiquitin on the move: the ubiquitin modification system plays diverse roles in the regulation of endoplasmic reticulum- and plasma membrane-localized proteins. Plant Physiol. 160: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Yu B., Wang Y., Liu Y. (2011). Role of plant autophagy in stress response. Protein Cell 2: 784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q., McLellan H., Boevink P.C., Sadanandom A., Xie C., Birch P.R., Tian Z. (2015). U-box E3 ubiquitin ligase PUB17 acts in the nucleus to promote specific immune pathways triggered by Phytophthora infestans. J. Exp. Bot. 66: 3189–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens R.P., Allan A.C., Friel E.N., Bolitho K., Grafton K., Templeton M.D., Karunairetnam S., Gleave A.P., Laing W.A. (2005). Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong D., Jeon B.W., Kim S.Y., Hwang J.-U., Lee Y. (2016). The ROP2-RIC7 pathway negatively regulates light-induced stomatal opening by inhibiting exocyst subunit Exo70B1 in Arabidopsis. New Phytol. 209: 624–635. [DOI] [PubMed] [Google Scholar]
- Hsu S.C., TerBush D., Abraham M., Guo W. (2004). The exocyst complex in polarized exocytosis. Int. Rev. Cytol. 233: 243–265. [DOI] [PubMed] [Google Scholar]
- Hu X., Qian Q., Xu T., Zhang Y., Dong G., Gao T., Xie Q., Xue Y. (2013). The U-box E3 ubiquitin ligase TUD1 functions with a heterotrimeric G α subunit to regulate brassinosteroid-mediated growth in rice. PLoS Genet. 9: e1003391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J.H., Seo D.H., Kang B.G., Kwak J.M., Kim W.T. (2015). Suppression of Arabidopsis AtPUB30 resulted in increased tolerance to salt stress during germination. Plant Cell Rep. 34: 277–289. [DOI] [PubMed] [Google Scholar]
- Indriolo E., Goring D.R. (2014). A conserved role for the ARC1 E3 ligase in Brassicaceae self-incompatibility. Front. Plant Sci. 5: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indriolo E., Safavian D., Goring D.R. (2014). The ARC1 E3 ligase promotes two different self-pollen avoidance traits in Arabidopsis. Plant Cell 26: 1525–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indriolo E., Tharmapalan P., Wright S.I., Goring D.R. (2012). The ARC1 E3 ligase gene is frequently deleted in self-compatible Brassicaceae species and has a conserved role in Arabidopsis lyrata self-pollen rejection. Plant Cell 24: 4607–4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki K., Mizutani M., Shimamura M., Masuda A., Nishihama R., Kohchi T. (2013). Essential role of the E3 ubiquitin ligase nopperabo1 in schizogenous intercellular space formation in the liverwort Marchantia polymorpha. Plant Cell 25: 4075–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.Y., Park K.Y., Seo Y.S., Kim W.T. (2016). Arabidopsis small rubber particle protein homolog SRPs play dual roles as positive factors for tissue growth and development and in drought stress responses. Plant Physiol. 170: 2494–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Kim W.T. (2013). The Arabidopsis RING E3 ubiquitin ligase AtAIRP3/LOG2 participates in positive regulation of high-salt and drought stress responses. Plant Physiol. 162: 1733–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita A., et al. (2015). A plant U-box protein, PUB4, regulates asymmetric cell division and cell proliferation in the root meristem. Development 142: 444–453. [DOI] [PubMed] [Google Scholar]
- Kissoudis C., van de Wiel C., Visser R.G., van der Linden G. (2014). Enhancing crop resilience to combined abiotic and biotic stress through the dissection of physiological and molecular crosstalk. Front. Plant Sci. 5: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L., Cheng J., Zhu Y., Ding Y., Meng J., Chen Z., Xie Q., Guo Y., Li J., Yang S., Gong Z. (2015). Degradation of the ABA co-receptor ABI1 by PUB12/13 U-box E3 ligases. Nat. Commun. 6: 8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulich I., Pečenková T., Sekereš J., Smetana O., Fendrych M., Foissner I., Höftberger M., Zárský V. (2013). Arabidopsis exocyst subcomplex containing subunit EXO70B1 is involved in autophagy-related transport to the vacuole. Traffic 14: 1155–1165. [DOI] [PubMed] [Google Scholar]
- Kwak J.M., Murata Y., Baizabal-Aguirre V.M., Merrill J., Wang M., Kemper A., Hawke S.D., Tallman G., Schroeder J.I. (2001). Dominant negative guard cell K+ channel mutants reduce inward-rectifying K+ currents and light-induced stomatal opening in arabidopsis. Plant Physiol. 127: 473–485. [PMC free article] [PubMed] [Google Scholar]
- Lee H.K., Cho S.K., Son O., Xu Z., Hwang I., Kim W.T. (2009). Drought stress-induced Rma1H1, a RING membrane-anchor E3 ubiquitin ligase homolog, regulates aquaporin levels via ubiquitination in transgenic Arabidopsis plants. Plant Cell 21: 622–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-H., Kim W.T. (2011). Regulation of abiotic stress signal transduction by E3 ubiquitin ligases in Arabidopsis. Mol. Cells 31: 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Yoon H.J., Terzaghi W., Martinez C., Dai M., Li J., Byun M.O., Deng X.W. (2010). DWA1 and DWA2, two Arabidopsis DWD protein components of CUL4-based E3 ligases, act together as negative regulators in ABA signal transduction. Plant Cell 22: 1716–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., van Os G.M., Ren S., Yu D., Ketelaar T., Emons A.M., Liu C.M. (2010). Expression and functional analyses of EXO70 genes in Arabidopsis implicate their roles in regulating cell type-specific exocytosis. Plant Physiol. 154: 1819–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Ding Y., Wang J., Shen J., Kung C.H., Zhuang X., Cui Y., Yin Z., Xia Y., Lin H., Robinson D.G., Jiang L. (2015). Exocyst-positive organelles and autophagosomes are distinct organelles in plants. Plant Physiol. 169: 1917–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Guo W. (2012). The exocyst complex in exocytosis and cell migration. Protoplasma 249: 587–597. [DOI] [PubMed] [Google Scholar]
- Liu J., Li W., Ning Y., Shirsekar G., Cai Y., Wang X., Dai L., Wang Z., Liu W., Wang G.L. (2012). The U-Box E3 ligase SPL11/PUB13 is a convergence point of defense and flowering signaling in plants. Plant Physiol. 160: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Zhang H., Lian X., Converse R., Zhu L. (2016). Identification of interacting motifs between Armadillo repeat containing 1 (ARC1) and exocyst 70 A1 (Exo70A1) proteins in Brassica oleracea. Protein J. 35: 34–43. [DOI] [PubMed] [Google Scholar]
- Liu P., Sherman-Broyles S., Nasrallah M.E., Nasrallah J.B. (2007). A cryptic modifier causing transient self-incompatibility in Arabidopsis thaliana. Curr. Biol. 17: 734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Xiong Y., Bassham D.C. (2009). Autophagy is required for tolerance of drought and salt stress in plants. Autophagy 5: 954–963. [DOI] [PubMed] [Google Scholar]
- Liu Y.C., Wu Y.R., Huang X.H., Sun J., Xie Q. (2011). AtPUB19, a U-box E3 ubiquitin ligase, negatively regulates abscisic acid and drought responses in Arabidopsis thaliana. Mol. Plant 4: 938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Lin W., Gao X., Wu S., Cheng C., Avila J., Heese A., Devarenne T.P., He P., Shan L. (2011). Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science 332: 1439–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Shen G., Yan J., He C., Zhang H. (2006). AtCHIP functions as an E3 ubiquitin ligase of protein phosphatase 2A subunits and alters plant response to abscisic acid treatment. Plant J. 46: 649–657. [DOI] [PubMed] [Google Scholar]
- Lv Q., Cheng R., Shi T. (2014). Regulatory network rewiring for secondary metabolism in Arabidopsis thaliana under various conditions. BMC Plant Biol. 14: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyzenga W.J., Stone S.L. (2012). Abiotic stress tolerance mediated by protein ubiquitination. J. Exp. Bot. 63: 599–616. [DOI] [PubMed] [Google Scholar]
- Mazzucotelli E., Belloni S., Marone D., De Leonardis A., Guerra D., Di Fonzo N., Cattivelli L., Mastrangelo A. (2006). The e3 ubiquitin ligase gene family in plants: regulation by degradation. Curr. Genomics 7: 509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgil Y., Shiu S.H., Stone S.L., Salt J.N., Goring D.R. (2004). A large complement of the predicted Arabidopsis ARM repeat proteins are members of the U-box E3 ubiquitin ligase family. Plant Physiol. 134: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K., Yamaguchi-Shinozaki K. (2013). ABA signaling in stress-response and seed development. Plant Cell Rep. 32: 959–970. [DOI] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. (1980). Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 21: 205–215. [DOI] [PubMed] [Google Scholar]
- Orlando K., Guo W. (2009). Membrane organization and dynamics in cell polarity. Cold Spring Harb. Perspect. Biol. 1: a001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakabe Y., Osakabe K., Shinozaki K., Tran L.S. (2014). Response of plants to water stress. Front. Plant Sci. 5: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecenková T., Hála M., Kulich I., Kocourková D., Drdová E., Fendrych M., Toupalová H., Zársky V. (2011). The role for the exocyst complex subunits Exo70B2 and Exo70H1 in the plant-pathogen interaction. J. Exp. Bot. 62: 2107–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab S., Drechsel G., Zarepour M., Hartung W., Koshiba T., Bittner F., Hoth S. (2009). Identification of a novel E3 ubiquitin ligase that is required for suppression of premature senescence in Arabidopsis. Plant J. 59: 39–51. [DOI] [PubMed] [Google Scholar]
- Reynolds M., Tuberosa R. (2008). Translational research impacting on crop productivity in drought-prone environments. Curr. Opin. Plant Biol. 11: 171–179. [DOI] [PubMed] [Google Scholar]
- Ryu M.Y., Cho S.K., Kim W.T. (2010). The Arabidopsis C3H2C3-type RING E3 ubiquitin ligase AtAIRP1 is a positive regulator of an abscisic acid-dependent response to drought stress. Plant Physiol. 154: 1983–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadanandom A., Bailey M., Ewan R., Lee J., Nelis S. (2012). The ubiquitin-proteasome system: central modifier of plant signalling. New Phytol. 196: 13–28. [DOI] [PubMed] [Google Scholar]
- Salt J.N., Yoshioka K., Moeder W., Goring D.R. (2011). Altered germination and subcellular localization patterns for PUB44/SAUL1 in response to stress and phytohormone treatments. PLoS One 6: e21321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel M.A., Chong Y.T., Haasen K.E., Aldea-Brydges M.G., Stone S.L., Goring D.R. (2009). Cellular pathways regulating responses to compatible and self-incompatible pollen in Brassica and Arabidopsis stigmas intersect at Exo70A1, a putative component of the exocyst complex. Plant Cell 21: 2655–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel M.A., Mudgil Y., Salt J.N., Delmas F., Ramachandran S., Chilelli A., Goring D.R. (2008). Interactions between the S-domain receptor kinases and AtPUB-ARM E3 ubiquitin ligases suggest a conserved signaling pathway in Arabidopsis. Plant Physiol. 147: 2084–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D.H., Ryu M.Y., Jammes F., Hwang J.H., Turek M., Kang B.G., Kwak J.M., Kim W.T. (2012). Roles of four Arabidopsis U-box E3 ubiquitin ligases in negative regulation of abscisic acid-mediated drought stress responses. Plant Physiol. 160: 556–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Yamaguchi-Shinozaki K. (2007). Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 58: 221–227. [DOI] [PubMed] [Google Scholar]
- Slavikova S., Ufaz S., Avin-Wittenberg T., Levanony H., Galili G. (2008). An autophagy-associated Atg8 protein is involved in the responses of Arabidopsis seedlings to hormonal controls and abiotic stresses. J. Exp. Bot. 59: 4029–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt D.E., Walden H., Shaw G.S. (2014). RBR E3 ubiquitin ligases: new structures, new insights, new questions. Biochem. J. 458: 421–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmann M., Anderson R.G., Ichimura K., Pecenkova T., Reuter P., Žársky V., McDowell J.M., Shirasu K., Trujillo M. (2012). The ubiquitin ligase PUB22 targets a subunit of the exocyst complex required for PAMP-triggered responses in Arabidopsis. Plant Cell 24: 4703–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmann M., Anderson R.G., Westphal L., Rosahl S., McDowell J.M., Trujillo M. (2013). The exocyst subunit Exo70B1 is involved in the immune response of Arabidopsis thaliana to different pathogens and cell death. Plant Signal. Behav. 8: e27421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone S.L. (2014). The role of ubiquitin and the 26S proteasome in plant abiotic stress signaling. Front. Plant Sci. 5: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sup Yun H., Yi C., Kwon H., Kwon C. (2013). Model for regulation of VAMP721/722-mediated secretion: growth vs. stress responses. Plant Signal. Behav. 8: e27116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh O.K., Hofius D. (2014). Membrane trafficking and autophagy in pathogen-triggered cell death and immunity. J. Exp. Bot. 65: 1297–1312. [DOI] [PubMed] [Google Scholar]
- TerBush D.R., Maurice T., Roth D., Novick P. (1996). The exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 15: 6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Trujillo M., Ichimura K., Casais C., Shirasu K. (2008). Negative regulation of PAMP-triggered immunity by an E3 ubiquitin ligase triplet in Arabidopsis. Curr. Biol. 18: 1396–1401. [DOI] [PubMed] [Google Scholar]
- Tuteja N. (2007). Abscisic acid and abiotic stress signaling. Plant Signal. Behav. 2: 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzfadia O., Galili G. (2013). The Arabidopsis exocyst subcomplex subunits involved in a Golgi-independent transport into the vacuole possess consensus autophagy-associated atg8 interacting motifs. Plant Signal. Behav. 8: 4161–, 26732.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhee C., Batoko H. (2011). Autophagy involvement in responses to abscisic acid by plant cells. Autophagy 7: 655–656. [DOI] [PubMed] [Google Scholar]
- Vierstra R.D. (2009). The ubiquitin-26S proteasome system at the nexus of plant biology. Nat. Rev. Mol. Cell Biol. 10: 385–397. [DOI] [PubMed] [Google Scholar]
- Vogelmann K., Drechsel G., Bergler J., Subert C., Philippar K., Soll J., Engelmann J.C., Engelsdorf T., Voll L.M., Hoth S. (2012). Early senescence and cell death in Arabidopsis saul1 mutants involves the PAD4-dependent salicylic acid pathway. Plant Physiol. 159: 1477–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M., Chaban C., Schütze K., Batistic O., Weckermann K., Näke C., Blazevic D., Grefen C., Schumacher K., Oecking C., Harter K., Kudla J. (2004). Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40: 428–438. [DOI] [PubMed] [Google Scholar]
- Wang H., Lu Y., Jiang T., Berg H., Li C., Xia Y. (2013). The Arabidopsis U-box/ARM repeat E3 ligase AtPUB4 influences growth and degeneration of tapetal cells, and its mutation leads to conditional male sterility. Plant J. 74: 511–523. [DOI] [PubMed] [Google Scholar]
- Wang J., Ding Y., Wang J., Hillmer S., Miao Y., Lo S.W., Wang X., Robinson D.G., Jiang L. (2010). EXPO, an exocyst-positive organelle distinct from multivesicular endosomes and autophagosomes, mediates cytosol to cell wall exocytosis in Arabidopsis and tobacco cells. Plant Cell 22: 4009–4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., et al. (2015). The E3 ligase OsPUB15 interacts with the receptor-like kinase PID2 and regulates plant cell death and innate immunity. BMC Plant Biol. 15: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Liu Y., Cong Y., Wang T., Zhong X., Yang S., Li Y., Gai J. (2016). Genome-wide identification of soybean U-box E3 ubiquitin ligases and roles of GmPUB88 in negative regulation of drought stress response in Arabidopsis. Plant Cell Physiol. 57: 1189–1209. [DOI] [PubMed] [Google Scholar]
- Welchman R.L., Gordon C., Mayer R.J. (2005). Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat. Rev. Mol. Cell Biol. 6: 599–609. [DOI] [PubMed] [Google Scholar]
- Wen T.J., Hochholdinger F., Sauer M., Bruce W., Schnable P.S. (2005). The roothairless1 gene of maize encodes a homolog of sec3, which is involved in polar exocytosis. Plant Physiol. 138: 1637–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiborg J., O’Shea C., Skriver K. (2008). Biochemical function of typical and variant Arabidopsis thaliana U-box E3 ubiquitin-protein ligases. Biochem. J. 413: 447–457. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Contento A.L., Nguyen P.Q., Bassham D.C. (2007). Degradation of oxidized proteins by autophagy during oxidative stress in Arabidopsis. Plant Physiol. 143: 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Wang J., Li Q., Hwang J.R., Patterson C., Zhang H. (2003). AtCHIP, a U-box-containing E3 ubiquitin ligase, plays a critical role in temperature stress tolerance in Arabidopsis. Plant Physiol. 132: 861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.W., González-Lamothe R., Ewan R.A., Rowland O., Yoshioka H., Shenton M., Ye H., O’Donnell E., Jones J.D., Sadanandom A. (2006). The E3 ubiquitin ligase activity of arabidopsis PLANT U-BOX17 and its functional tobacco homolog ACRE276 are required for cell death and defense. Plant Cell 18: 1084–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee D., Goring D.R. (2009). The diversity of plant U-box E3 ubiquitin ligases: from upstream activators to downstream target substrates. J. Exp. Bot. 60: 1109–1121. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Mogami J., Yamaguchi-Shinozaki K. (2014). ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant Biol. 21: 133–139. [DOI] [PubMed] [Google Scholar]
- Yu F., Wu Y., Xie Q. (2016). Ubiquitin-proteasome system in ABA signaling: from perception to action. Mol. Plant 9: 21–33. [DOI] [PubMed] [Google Scholar]
- Zárský V., Kulich I., Fendrych M., Pečenková T. (2013). Exocyst complexes multiple functions in plant cells secretory pathways. Curr. Opin. Plant Biol. 16: 726–733. [DOI] [PubMed] [Google Scholar]
- Zeng L.R., Park C.H., Venu R.C., Gough J., Wang G.L. (2008). Classification, expression pattern, and E3 ligase activity assay of rice U-box-containing proteins. Mol. Plant 1: 800–815. [DOI] [PubMed] [Google Scholar]
- Zhang J., Rea A.C., Fu T., Ma C., Nasrallah J.B. (2014). Exploring the role of a stigma-expressed plant U-box gene in the pollination responses of transgenic self-incompatible Arabidopsis thaliana. Plant Reprod. 27: 59–68. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Li J., Liu H., Chong K., Xua Y. (2015). Roles of ubiquitination-mediated protein degradation in plant responses to abiotic stresses. Environ. Exp. Bot. 114: 92–103. [Google Scholar]
- Zhao T., Rui L., Li J., Nishimura M.T., Vogel J.P., Liu N., Liu S., Zhao Y., Dangl J.L., Tang D. (2015). A truncated NLR protein, TIR-NBS2, is required for activated defense responses in the exo70B1 mutant. PLoS Genet. 11: e1004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Zhang Y., Qi J., Chi Y., Fan B., Yu J.Q., Chen Z. (2014). E3 ubiquitin ligase CHIP and NBR1-mediated selective autophagy protect additively against proteotoxicity in plant stress responses. PLoS Genet. 10: e1004116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.