Abstract
Mechanisms of opioid tolerance have focused on adaptive modifications within cells containing opioid receptors, defined here as cellular allostasis, emphasizing regulation of the opioid receptor signalosome. We review additional regulatory and opponent processes involved in behavioral tolerance, and include mechanistic differences both between agonists (agonist bias), and between μ- and δ-opioid receptors. In a process we will refer to as pass-forward allostasis, cells modified directly by opioid drugs impute allostatic changes to downstream circuitry. Because of the broad distribution of opioid systems, every brain cell may be touched by pass-forward allostasis in the opioid-dependent/tolerant state. We will implicate neurons and microglia as interactive contributors to the cumulative allostatic processes creating analgesic and hedonic tolerance to opioid drugs.
What is Tolerance?
Tolerance (see Glossary) is defined as a reduction in effect following prolonged drug administration that results in a loss of drug potency indicated by a pharmacological shift to the right in the dose–response curve. The development and extent of tolerance are dependent on the drug interactions with the opioid receptor(s), dose, and frequency of administration. There are many mechanisms that can contribute to opioid tolerance at a behavioral level, including upregulation of drug metabolism (metabolic tolerance), desensitization of receptor signaling, and downregulation of receptors, as well as the initiation of compensatory/opponent processes. Analgesic tolerance is not always noteworthy, and many physicians argue that analgesic tolerance can simply be overcome by increasing the opioid therapeutic dose to maintain patient satisfaction or by implementing opioid rotation. However, this is not always feasible because other pharmacological effects, such as constipation, can limit patient compliance/satisfaction – opioid-induced gut motility exhibits minimal tolerance compared to other effects such as sedation and nausea. Moreover, the management of opioid-tolerant patients during acute episodes of care is also a challenge among healthcare providers because these patients have a significantly longer length of hospital stay and a greater 30 day readmission rate, after adjusting for risk assessment including risk of mortality (APR-DRG 3 M model) and comorbid conditions [1]. Furthermore, increasing the dose of opioids can precipitate or exacerbate opioid-induced hyperalgesia, which in turn contributes to behavioral analgesic-tolerance and may be misdiagnosed as disease progression-induced pain. In addiction medicine, tolerance is a key component of dependence and addiction liability. The USA FDA defines tolerance as 60 mg morphine-equivalent daily, and prescriptions of over 100 mg morphine-equivalent daily are often subject to scrutiny for opioid abuse [2]. Importantly, tolerance to opioid-induced respiratory depression may be seen as beneficial for pain patients, but can cause an increase mortality when opioid addicts take the same dose of opioid in a different environment/context [3] or relapse following a period of abstinence. For this latter population, tolerance has abated during this drug-free period, and relapse with a pre-abstinence opioid dose triggers life-threatening consequences. Another reason tolerance is central in addiction medicine is that treating pain in opioid addicts is exceedingly challenging, where methadone-maintained patients are often refractory to the analgesic effects of opioid therapies as a result of excessive tolerance [4]. In this review we will consider behavioral tolerance mechanisms to include drug-induced adaptations or allostatic changes at the cellular, circuitry, and system levels that require increased opioid drug to achieve the same effect. We argue that opioids generate opponent processes not only in nociceptive circuits but also in circuits modulating mood, and these contribute to the genesis of an addicted state via tolerance to the hedonic aspects of drug taking. The purpose of this review is to take a neurobiological journey, beginning with agonist-dependent regulation of the opioid receptor signalosome mediating allostatic changes within cells expressing opioid receptors, and which leads to pass-forward allostasis – thereby modifying circuits and networks to create behavioral tolerance.
Opioid drugs such as morphine and fentanyl activate opioid receptors, a family of G protein-coupled receptors that primarily signal through heterotrimeric G protein Gi/o subunits. On agonist binding, opioid receptors activate a signalosome to inhibit adenylate cyclase and calcium channels, while activating potassium channels and several kinase cascades such as extracellular signal-regulated kinase (ERK1/2), c-Jun N-terminal kinases (JNKs), and AKT/protein kinase B. The hallmark outcome of acute opioid agonist treatment is that neurons containing opioid receptors are less excitable, thereby influencing circuit dynamics. There are four members of the opioid receptor family in vertebrates (μ, δ, κ and nociceptin/orphanin FQ), and activation of all four can produce analgesia, whereas substance abuse is primarily associated with activation of μ-opioid receptors (MOPs) [5]. Because of their differential cellular and regional distributions, agonists to the different opioid receptors elicit disparate pharmacological effects. The MOPs mediate the canonical effects of clinically used opioids, namely analgesia, euphoria, respiratory depression, and constipation. Activation of the δ-opioid receptor (DOP) also produces analgesia in chronic pain states, and agonists are being developed by industry for the treatment of anxiety and migraine [6,7]. κ-Opioid receptor (KOP) agonists elicit anxiogenic and dysphoric effects, and are notable for exhibiting opposing effects to MOP agonists on emotional regulation [8]. Industry development of KOP antagonists is being vigorously pursued for their ability to alleviate negative affect in addiction and substance-abuse disorders, whereas there is much interest in developing a peripherally acting KOP agonist for pain relief [9,10]. The nociceptin/orphanin FQ receptor (NOP) is the most abundant member of the opioid receptor family in brain and affects opioid analgesia, tolerance development, and reward via modulating MOP effects [11]. In addition to different receptor types, different drugs acting at the same receptor can cause different conformational changes, resulting in the coupling of the receptor to different intracellular signaling cascades that produce distinct pharmacological effects. This phenomenon, referred to as biased agonism, is well known to occur for various opioid receptor types (for review see Pradhan et al. [12,13]).
Cellular Allostasis and the Opioid Receptor Signalosome (MOP and DOP)
Mechanisms of opioid receptor desensitization and resensitization have been thoroughly reviewed for different agonists at the MOP [13]. The ability of different agonists to induce receptor internalization has been shown to affect divergent downstream regulatory processes contributing to the activity of the opioid receptor signalosome and cellular tolerance (Figure 1). Many other processes contribute to cellular allostasis. MOP signaling initiates a cascade of cellular adaptations involving perturbation of kinase pathways and protein complexes (as reviewed in depth [14]). In turn, these perturbations mediate changes in transcriptome and proteome profiles, as well as morphological changes, for instance in spine remodeling [15]. Cellular allostasis from continued opioid treatment is substantial, although not all adaptations triggered by MOP activation will contribute to tolerance. One well-studied opponent system that undoubtedly contributes to cellular tolerance is adenylate cyclase supersensitivity. Both DOP and MOP agonists inhibit adenylate cyclase, thereby decreasing cellular cAMP levels. Sustained agonist stimulation is accompanied by compensatory upregulation of both basal and stimulated cAMP levels. Mechanisms that account for this adaptation include regulation of the levels of different adenylate cyclase isoforms and protein kinase A (PKA) [16]. In addition, a noncanonical pathway involving a switch from the classical Gi/Go-coupled signaling to one involving Src has been proposed. This leads to Raf1 recruitment and phosphorylation of specific cyclase isoforms to increase cAMP levels [17]. Many cellular adaptations occur as a result of protracted signaling via opioid receptors, modifying not only the signalosome but also the transcriptome and proteome to affect the structural integrity, connectivity, and activity of opioid receptor-containing neurons [16].
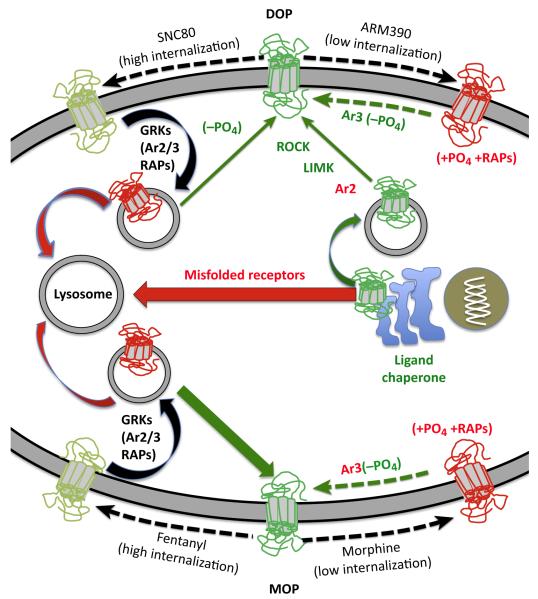
Figure 1. Differences in Opioid Receptor Regulation by Low- and High-Internalizing Agonists.
The cartoon depicts the DOP (δ-opioid receptor) and MOP (μ-opioid receptor) (receptors in red have lost membrane signaling ability) after activation by a high-internalizing agonist; fentanyl (for MOP), or SNC80 (for DOP), and a low-internalizing agonist, morphine (for MOP), or ARM390 (for DOP). Red arrows represent processes that downregulate opioid receptors (lysosomal degradation of agonist-internalized receptors or lysosome-targeted misfolded receptors following de novo synthesis). Green arrows represent mechanisms of resensitization or enhancement of receptor activity. Several membrane-permeable DOP agonists and antagonists act as chaperones for DOP resulting in increased levels of surface receptors [108]. Externalization of vesicle-stored receptors can be enabled by a ROCK/LIMK mechanism, which is held in check by arrestins (Ar). High-internalizing agonists of DOP and MOP recruit G protein receptor kinases (GRKs), arrestins 2 or 3, and other receptor-associated proteins or RAPs (Ras-related proteins) (left side of the figure) to promote internalization as well as arrestin-dependent and vesicle-mediated signaling [109]. Once internalized the receptors are targeted either to lysosomes or recycled back to the cell surface in a resensitized, dephosphorylated (−PO4) state. MOPs effectively recycle back to the membrane (robust green arrow) whereas DOPs are predominantly targeted to lysosomes for degradation (robust red arrow)[110] although, unlike many other receptors, this appears not to require receptor ubiquitination [111]. By contrast, activation by low-internalizing agonists of MOP and DOP only weakly recruits arrestin 3, and desensitization is reported to be dependent upon other kinases such as JNK and PKC (+PO4). Resensitization of DOP and MOPs, presumably by dephosphorylation, following low-internalizing agonist desensitization is differentially regulated by arrestin 3. In the case of MOP, arrestin 3 (Ar3 in red) attenuates resensitization [112] whereas for DOP arrestin 3 (Ar3 in green) facilitates resensitization [19].
The Complex Role of Arrestins in Behavioral Tolerance to Opioids
Following the initial report that mutant mice lacking arrestin 3 (β-arrestin 2) exhibit attenuated tolerance to morphine [18], there is now a plethora of studies demonstrating the involvement of arrestins in opioid receptor desensitization and tolerance. Both high- and low-internalizing MOP and DOP agonists induce analgesic tolerance [19–22], albeit via different mechanisms. Moreover, MOP receptor agonists, independently of their internalization efficacy, exhibit comparable symptoms of physical withdrawal [20]. That behavioral tolerance and withdrawal are comparable between arrestin and internalization-biased agonists suggests that MOP agonists regardless of mechanisms of cellular tolerance trigger overlapping allostatic circuitry regulating behavior. There are several explanations for the modulation of tolerance by arrestins. At face value, the classical mechanism would be that arrestin binding, as the name would imply, blocks G-protein signaling and promotes clathrin-mediated endocytosis. However, attenuated signaling or cellular tolerance is the sum of multiple processes including: receptor desensitization, receptor resensitization, receptor internalization (recycling or downregulation), de novo receptor synthesis, and Golgi stability, as well as receptor trafficking to the cell membrane (externalization). Modification by arrestins at any juncture in the receptor signalosome regulatory machinery could influence cellular and behavioral opioid tolerance. Opioid agonists can differentially recruit arrestin-dependent signaling cascades to regulate opioid tolerance in a ligand- and tissue-biased manner. For example, morphine analgesic tolerance is attenuated in the absence of arrestin 3, whereas that of fentanyl is not, despite both agonists being able to recruit arrestin 3 (Figure 1). Arrestin 3 in the colon appears to be necessary for tolerance to the MOP agonist DAMGO, but not fentanyl or etorphine, despite all three agonists effectively inducing MOP internalization [23]. It was also found that JNK2 can regulate MOP tolerance, and recruitment of this molecule can be arrestin-dependent or -independent, depending on the MOP agonist used [24].
As illustrated in Table 1 and Figure 1, arrestin isoforms are a major modifier of DOP tolerance. Furthermore, arrestins are key to ligand bias of DOP agonists to trigger different intracellular signaling cascades and functional outcomes [25]. In its resting state DOP appears to form a complex with arrestin 3, and high-internalizing DOP agonists preferentially recruit arrestin 2 (β-arrestin 1), resulting in DOP desensitization and acute behavioral tolerance. Thus, knockout of arrestin 2 results in dramatically increased potency and decreased acute behavioral tolerance to high-internalizing DOP agonists, such as SNC80 [19,26] (Figure 1). By contrast, low-internalizing DOP agonists preferentially recruit arrestin 3 and show no phenotype in the arrestin 2 knockout mice. In the arrestin 3 knockout, there is no phenotype for high-internalizing agonists. However, for low-internalizing agonists, such as ARM390 and JNJ20788560, arrestin 3 facilitates the rate of receptor resensitization. Hence, in complete contrast to the MOP, knockout of arrestin 3 resulted in increased tolerance to low-internalizing DOP agonists and reduced the rates of DOP resensitization [19]. These data for MOP and DOP reveal a role of arrestins in regulating receptor resensitization, although they are traditionally considered for their role in mediating desensitization (Figure 1). The mechanisms of desensitization and resensitization by low-internalizing opioid agonists are currently unknown, although kinase/phosphatase activity is a likely component and PKC and JNK isoforms are implicated for MOP [24,27]. We speculate that arrestin 3-containing complexes attenuate the recruitment of relevant phosphatases for MOP resensitization, but facilitate the recruitment of relevant phosphatases for DOP resensitization.
Table 1.
The Effects of Arrestin Deletion on Ligand-Induced MOP and DOP Desensitization, Resensitization, and Tolerance
| MOP | DOP | |||
|---|---|---|---|---|
| High-Internalizing | Low-Internalizing | High-Internalizing | Low-Internalizing | |
| Arrestin 2 (β-arrestin 1) deletion |
No reported effects on desensitization, resensitization, or tolerance |
No observed effect on tolerance, or reported effects on desensitization or resensitization |
High desensitization [26] High externalization [26] High efficacy and potency [19,26] Reduced acute tolerance [19] |
No effect on efficacy/potency, or acute tolerance [19] |
| Arrestin 3 (β-arrestin 2) deletion |
No observed effects on tolerance or desensitization, but resensitization was affected [20,31,112,113] |
Low desensitization [27] High resensitization [112] Decreased tolerance [18,113] |
No effect on desensitization or acute tolerance [19] |
Low resensitization [19] Increased tolerance [19] |
Agonists used. DOP, high-internalizing, SNC80; low-internalizing, ARM390, JNJ20788560; MOP, high-internalizing, fentanyl, DAMGO; low-internalizing, morphine. Note that tolerance mechanisms and thus arrestin dependence may differ with measured behaviors, and the table above should not be generalized to behaviors not assessed in the referenced papers.
As a part of multifaceted protein complexes, the arrestins play important roles in opioid receptor signaling independently of ligand-induced receptor desensitization, resensitization, and internalization. For example, both arrestin 2 and 3 control the cytoskeletal protein actin by binding to cofilin and its inactivating kinase, Lim domain kinase, and activating the phosphatases slingshot or chronophin [28–30]. Relevant to DOP and nociceptin/orphanin FQ receptor function is that arrestin 2 acts as a chaperone controlling the rate of ligand-dependent export of these opioid receptors to the cell membrane [27]. Furthermore, a c-Src/arrestin 3 complex regulates MOP to promote ligand-independent or constitutively active MOP signaling [31]. In this complex, arrestin constrains c-Src activity by keeping this tyrosine kinase in an inactive state. Together these interactions demonstrate that the arrestins regulate opioid receptor function as part of catalytically active scaffolding complexes.
Given that arrestins are key components of opioid receptor signaling and trafficking, they are well situated to contribute to the changes that initiate cellular allostasis, and subsequent pass-forward system-wide allostasis. Because of the arrestin-dependent processes involved in MOP signaling and tolerance there has been a drive to develop drugs that activate MOP without recruiting either arrestin 2 or 3. Such drugs would be anticipated to have increased analgesia (possibly either promoting recycling or inducing constitutive activity) and reduced respiratory depression [32]. However, on the negative side, arrestin deletion increased constipation, a major issue with opioid drugs. Recently, clinical trials have begun for TRV130, a MOP agonist that elicits very low arrestin recruitment. There are some promising results with this drug. TRV130 has increased analgesic potency compared with morphine (1.5 mg TRV130 given intravenously had an equivalent analgesic effect to 10 mg morphine). At some timepoints, the ratio of respiratory depression to analgesic efficacy is less than morphine, implying better safety (therapeutic index) profile [33]. However, the pharmacokinetic differences confound therapeutic advantage over morphine because TRV130 appears to have a shorter duration of action in both respiratory depression and analgesia. This is clearly an area of agonist bias drug development that could have positive outcome, although at this point it is unclear if MOP agonists that do not recruit arrestins at all will elicit increased gut immobility and/or altered addiction liability. The assessment of such drugs in self-administration paradigms and relapse models will provide valuable insight for whether biased ligands produce less addictive-like behaviors compared to current opioid therapeutics.
Opponent Processes Contribute to a Tolerant State
Behavioral opioid tolerance is most often studied in the context of analgesia and cellular signaling mechanisms, as reviewed above. However, opponent processes are also evident at a circuit-level in the regulation of mood and affect. In this section we will focus on how chronic opioid exposure causes adaptations of the mesocorticolimbic circuitry as a result of the development of opponent processes in the striatum and ventral tegmental area (VTA). The striatum is an important hub of the opioid reward pathway and receives dense dopaminergic innervation from the midbrain (such as the VTA), as well as excitatory input from the basolateral amygdala, prefrontal cortex, ventral hippocampus, and paraventricular thalamic nucleus that drive opioid reward-seeking behaviors [34–36]. The striatum also has high intrinsic expression and projection input of endogenous opioids, and expresses opioid receptors in various cell types including dopamine D1 receptor (prodynorphin)- and D2 receptor (proenkephalin)-expressing striatal medium spiny projection neurons (Figure 2). Importantly, the VTA and striatum undergo synaptic adaptations that initiate opponent processes in response to chronic opioid use, with implications for substance abuse and comorbid affective disorders.
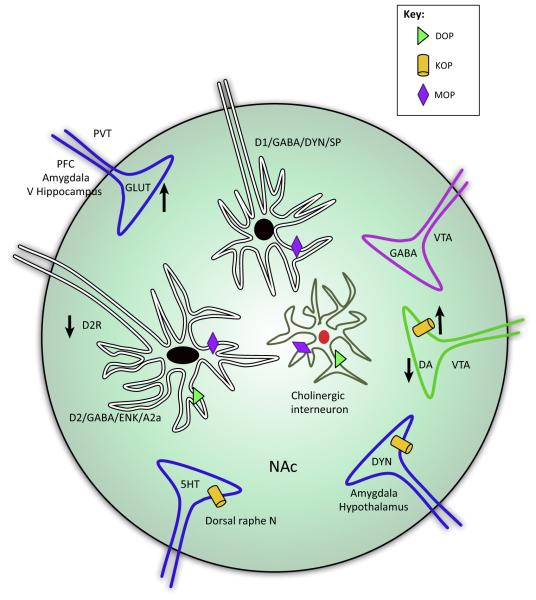
Figure 2. Schematic Cartoon of How Inputs to the Nucleus Accumbens (NAc) Are Modified by Chronic Opioids and their Contribution to Negative Affect.
This cartoon depicts sites of neuronal allostasis in the NAc, potentially contributing to negative affect following chronic opioid treatments. Dopamine neurons from the ventral tegmental area (VTA) project onto two types of GABAergic medium spiny neurons (MSNs) defined by the ability of dopamine to inhibit or excite these neurons within the NAc. Excitatory MSNs are characterized by expression of dopamine D1 receptors, GABA, dynorphin, and substance P, whereas inhibitory MSNs are characterized by the expression of dopamine D2, GABA, enkephalin, and adenosine A2a receptors. The NAc also receives excitatory (glutamatergic) input from the basolateral amygdala, ventral hippocampus, and prefrontal cortex that drives drug reinforcement, whereas input from the paraventricular nucleus of the thalamus can drive reward or aversion associated with withdrawal [78,101]. Chronic morphine is known to increase or decrease long-term potentiation (LTP) and long-term depression (LTD) of some of these glutamatergic inputs, and this may depend on the method of morphine administration (contingent versus non-contingent) [75]. Other inputs originate from the dorsal raphe nucleus, hypothalamus, and GABAergic projection neurons from the VTA. κ-Opioid receptors (KOPs) are expressed on the axon terminals of dopamine neurons, where they can inhibit dopamine release. Chronic opioid treatment increases the expression of KOPs and likely contributes to dopamine hypofunction in this circuitry. Dynorphin, the endogenous agonist at KOP, is released by excitatory MSNs; however, projection neurons from the amygdala and hypothalamus are also sources of striatal dynorphin. KOPs are also present on the axon terminals of these dynorphin projection neurons. δ-Opioid receptors (DOPs) and μ-opioid receptors (MOPs) are expressed on cholinergic interneurons and are involved in modulating reward and motivation, but it is unknown to what extent they contribute to opioid tolerance and opponent processes. MOPs are also present on both excitatory, D1, and inhibitory, D2, MSNs, and activation of these receptors on excitatory D1 neurons is sufficient to drive reward-like behavior [39]. By contrast, DOPs appear to be expressed in D2-, but not D1-, enriched MSNs [114]. Synaptic insertion of GluA2-lacking AMPA receptors in D2 MSNs is implicated in mediating the aversion as a result of activation of the paraventricular-accumbens circuitry during withdrawal [78]. Abbreviations: α2a, A2a cholinergic receptor; DA, dopamine; D1/2R, dopamine receptor 1/2; DYN, dynorphin; ENK, enkephalin; GLUT, glutamate; 5HT, serotonin; PFC, prefrontal cortex; PVT, paraventricular thalamic nucleus; SP, substance P; V Hippocampus, ventral hippocampus.
Allostatic Processes in the VTA
Dopamine release from VTA dopaminergic neurons reinforces natural rewarding behavior and attributes motivational salience to otherwise neutral environmental stimuli or unanticipated salient stimuli [34,37,38]. Dopaminergic neurons from the VTA exhibit a rich and complex organization, with widespread projections to forebrain targets [39–43]. The rewarding effects of morphine are mediated via activation of MOP because mice lacking this receptor do not express conditioned place preference and do not self-administer opioid drugs [44]. MOP in the VTA elicits rewarding effects via disinhibition of GABAergic neurons to stimulate dopamine release [45]. Disinhibition of VTA dopaminergic neurons is primarily driven by effects of MOP on GABA input from the rostral tegmental nucleus (RMTg), rather than from VTA GABAergic interneurons or GABA input from the nucleus accumbens (NAc) [46]. However, expression of MOP on D1/prodynorphin medium spiny projection neurons is sufficient to produce opioid reward [47]. Following chronic morphine, MOP desensitization on VTA input from the RMTg, rather than from NAc or VTA GABAergic interneurons, is responsible for the loss of MOP-induced disinhibition of dopamine neurons [46]. Interestingly, opioid reward in naïve animals (non-opioid dependent) does not rely on dopamine [48,49], whereas dopamine signaling is required for expression of reward in opioid-dependent animals, as evidenced by systemic blockade using a non-specific dopamine antagonist [50–53]. It should be emphasized that opioid-induced reward is dependent on dopamine in opioid-dependent states, despite chronic morphine causing hypofunction of the dopaminergic mesolimbic circuitry [54,55]. This dopaminergic hypofunction is thought to contribute to the negative affect associated with opioid dependence, possibly a result of increased KOP function.
KOPs are present on dopaminergic terminals in the NAc and inhibit dopamine release (Figure 2). They are activated by the endogenous release of neuropeptides derived from prodynorphin, and produce behavioral phenotypes indicative of negative emotional states such as place aversion and depressive-like affective behaviors [56]. The prominent neuromodulatory effect of KOP on the dopaminergic system is now the subject of clinical development, with the goal of removing opioid-induced protracted abstinence syndrome characterized by negative affect that can drive relapse. There is convincing evidence that KOP in the NAc and extended amygdala drive stress-induced dysphoria and vulnerability to drug relapse. Indeed, elimination of KOP in amygdala neurons projecting to the bed nucleus of the stria terminalis [57] or within dopamine neurons [58] produced an anxiolytic phenotype. This suggests that KOP expression can contribute to negative affect, and identifies a novel treatment strategy for mood disorders such as anxiety and depression. In addition, KOPs in the VTA also drive aversive behavior because conditional knockout of KOP from dopaminergic neurons blocked KOP agonist-induced place aversion [59]. Further research is necessary to identify the circuitry important for the genesis of a protracted abstinence syndrome following chronic opioid treatment and determine whether KOP modulation can eliminate this negative affect [60].
Brain-derived neurotrophic factor (BDNF) is a key modulator of VTA dopamine neuronal activity in opioid-dependent animals [61–63], and impacts on VTA neuronal activity by shifting the GABAA receptor reversal potential in VTA GABAergic interneurons [64]. Evidence from other systems have shown that BDNF decreases the GABAA receptor reversal potential via downregulation of the K+/Cl− cotransporter KCC2 [65–68], which depletes the Cl− electrochemical gradient, effectively reducing GABAA-mediated hyperpolarization ([69] for review). Because GABAergic, but not dopaminergic, neurons express KCC2 [70], this population is particularly affected by the BDNF-driven hyperexcitability in opioid dependence. We hypothesize that this increased excitability of GABAergic neurons impairs the ability of opioid agonists to inhibit GABAergic neurons. Although neurons release BDNF, we have proposed that activated microglia are the source of BDNF during abstinence following prolonged morphine administration (Box 1). Interestingly, BDNF injections reverse changes in VTA dopamine soma size that are observed following chronic opioids, and suggests multifaceted effects of BDNF on allostatic processes triggered by chronic opioids [71].
Box 1. Neuroinflammation and Opioid Tolerance.
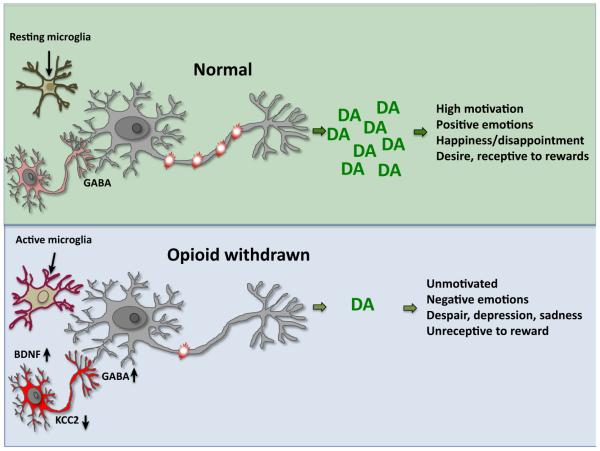
Chronic exposure to opioids has been shown to activate microglia and astrocytes in the spinal cord ([115] for review). BDNF levels are increased in the spinal cord 24 h after morphine abstinence, and the increase can be blocked by in vivo treatment with microglial inhibitors [65]. Inhibition of glia, and of many of the downstream signaling components (including chemokines, cytokines, fractalkine, nitric oxide, and connexin 43) is effective at restoring morphine analgesic efficacy after chronic administration [116–120]. While initial reports have implicated activated astrocytes and microglia in the development of behavioral tolerance and opioid-induced hyperalgesia, careful analysis of spinal behavioral effects of opioids identified that microglia contribute specifically to the development of the opioid-induced hyperalgesic component of behavioral tolerance, but not to drug analgesic potency [65]. This study identified a P2X4–BDNF signaling pathway from microglia to neurons that resulted in a shift in inhibitory potential in the GABAA-expressing nociceptive specific lamina I spinal cord neurons. Importantly, targeted genetic deletion of BDNF from microglia was sufficient to abrogate the development of morphine-induced hyperalgesia. Thus, opponent processes can also be initiated by non-neuronal mechanisms and contribute to analgesic behavioral tolerance. Microglial activation following chronic morphine treatment has also been reported in several brain regions, including the VTA, NAc, frontal cortex, and periaqueductal grey [63,121–123]. While initial studies implicated activated microglia as modulators of centrally mediated acute morphine actions [124], subsequent studies emphasized modulation only in opioid-dependent states [63,125]. Blocking glial activation in opioid-dependent states restores mesolimbic dopamine function, alleviates withdrawal, and prevents the relapse of drug-seeking behavior after prolonged drug abstinence [63,121,123,126,127]. Given that microglial inhibitors also reverse the modulation of KCC2 in brain, neuroinflammation appears to be a key process in generating opioid-induced allostatic changes. In Figure I we propose a model whereby microglia are activated during opioid withdrawal and shift the electrochemical gradient of VTA GABAergic neurons via a BDNF–KCC2 mechanism. This leads to an increased inhibitory tone on dopaminergic neurons, resulting in blunted VTA-dependent reward behaviors [63].
Figure I.
Neuroimmune Contributions to Opioid Withdrawal.
Allostatic Processes in Striatal Cells, Signaling, and Circuits
Similarly to other drugs of abuse, chronic morphine induces extensive and region-specific synaptic plasticity within the striatum. Glutamate homeostasis is affected by changes in glutamate release and reuptake, and in the composition, number, and role of ionotropic and metabotropic receptors ([72] for review). At the level of the synapse, a 10 day period of withdrawal from chronic non-contingent (i.e., experimenter-delivered) morphine increased synaptic strength, as assessed by the ratio of AMPA:NMDA currents in the NAc shell [73,74]. Conversely, 14 days of withdrawal from contingent (i.e., subject-initiated) heroin impaired long-term potentiation (LTP) and long-term depression (LTD) in the NAc core [75]. These differences could be region-specific or a result of experimenter- versus self-administered drug delivery. Opioid-induced synaptic plasticity is closely related to changes in dendritic spine density that show similar regional and drug-regimen effects. For example, spine density in the orbitofrontal cortex showed a greater increase following self-administered than experimenter-delivered morphine. In this same study, both contingent and non-contingent opioid administration decreased spine density in the NAc shell [76]. Heroin self-administration has also been shown to reduce spine density in the NAc core, but this recovered following heroin reinstatement and was accompanied by increased GluN2B NMDARs. Interestingly, blocking these receptors prevented heroin-seeking behaviors [77]. Although experimenter-delivered morphine also increased GluN2B-NMDARs, the effect of this opiate differed from that of heroin, and resulted in an increased spine density in the same region [78]. Together these findings suggest that the effect of opioids on spine morphology parallels changes in synaptic plasticity and possibly GluN2B NMDARs. These alterations in glutamatergic input will undoubtedly affect signaling within the striatum and striatal output. Indeed, striatal neurons show increased intrinsic excitability [74], which would induce neuron-specific adaptations in regions such as the pallidum that receive striatal input. However, it is important to note that the role of drug-seeking versus drug exposure per se and region-specificity must be considered when identifying opioid-induced allostatic changes in circuitry.
Studies of immediate-early gene expression further demonstrate how administration of morphine not only affects MOP-expressing cells but also the circuits that interact with these neurons. Thus, the effect of morphine can spread across the striatum and associated regions. A single morphine injection initially activates neurons in the dorsomedial striatum and NAc shell, but after 4 h activity is observed across the dorsal striatum and neocortex [79]. Colabeling with markers of D1 and D2 medium spiny neurons (MSNs) shows that a single morphine injection increases c-Fos expression in D1 MSNs within the NAc, whereas naloxone precipitated withdrawal activates c-Fos in D2 MSNs [80]. Optogenetic experiments further define the role of these neuronal subtypes, with D1, but not D2, MSNs mediating tolerance and reward [81,82]. Transcriptional and proteomic profiles outline morphine-induced changes in phosphorylation cascades, energy status, and cell morphology [83,84]. It is unclear if the cellular adaptations observed in the striatum are restricted to opioid receptor-expressing cells or are a result of pass-forward allostasis (Box 2).
Box 2. Pass-Forward Allostasis.
It would difficult to envisage that allostatic mechanisms accompanying opioid tolerance would be restricted to neurons containing opioid receptors, given the extensive perturbation of circuits and systems following opioid drug treatment. In the case of VTA dopaminergic cells, major changes in morphology and excitability occur after chronic morphine administration, despite these neurons not expressing MOPs [71]. Changes in dopaminergic neurons are likely initiated by altered GABAergic inhibitory function within the VTA or RMTg, which in turn are modulated by neuroinflammation (Box 1). Unclear at this juncture is whether opioid drugs directly activate microglia or if the activation occurs as a result of MOP-regulated circuitry during withdrawal. Evidence for pass-forward allostasis can also be observed in patterns of kinase activation following opioid treatment. Morphine activates ERK1/2 in many areas of the nervous system, which, as described earlier in the review, is involved in opioid tolerance in several different systems. Interestingly, in many different areas of the cortex (anterior cingulate and somatosensory), opioid-induced activation of ERK1/2 is prominent only in cells adjacent to MOP-expressing cells [128]. The inference is that modifications in neuronal populations not containing opioid receptors can have perhaps greater allostatic changes than opioid receptor-containing neurons initiating opioid behaviors.
Opioid-Mediated Aversion Circuitry
The negative affect and ‘anti-reward’ that is created by an opioid-dependent state is now recognized as a driving contributor to drug-seeking behaviors [85–87], and the neurocircuitry of the learned association between drug-induced relief of these aversive states may be particularly important for opioid substance-abuse disorder. Repeated or chronic use of opioids induces adaptive or allostatic changes that modify neuronal circuitry and create an altered normality – the ‘drug-dependent’ state [88]. The striatum, and in particular the shell of the NAc, is often associated with reward-processing in the acute and subsequently opioid-dependent, or reward-deficient, aspects of drug addiction. However, there are several projections to the striatum that have been implicated in aversive responses during withdrawal, revealing opponent processes developed during chronic drug treatments. Striatal expression of dynorphin and corticotropin-releasing factor (CRF), the prototypical stress-associated signaling molecules, are increased by morphine withdrawal [89], and inhibition of either system reverses chronic drug-seeking behavior [89,90]. The modulatory role of dynorphin and KOPs in the VTA–NAc pathway has been discussed in the section on allosteric modulation of the VTA. The recruitment of the central and basolateral amygdala, the bed nucleus of the stria terminalis, the hippocampus, and the hypothalamus have all been implicated in producing an anti-reward state, with direct or indirect consequences on the functioning of NAc circuitry. Noradrenergic inputs from the caudal medulla into the bed nucleus of the stria terminalis are also crucial for opioid withdrawal-induced aversion [91].
Figure 2 outlines how chronic or prolonged exposure to opioids modifies inputs to the NAc that contribute to negative affect and the anti-reward state. Several different areas of the brain are involved in mediating aversion associated with opioid withdrawal. For example, withdrawal-induced aversion has been shown to involve a thalamic–NAc pathway from the paraventricular nucleus to D2-expressing MSNs of the NAc [92]. In this study, chronic morphine increased the excitatory input to NAc D2 MSNs via insertion of GluA1-containing AMPA receptors. Inhibition of this activity reversed the somatic symptoms of morphine withdrawal, and naloxone precipitated withdrawal place aversion. The contribution of glutamatergic inputs to the NAc is also evidenced by the ability of microinjections of a GluA1 antagonist in the NAc shell to inhibit naloxone conditioned place aversion [93]. Inputs from the basolateral amygdala to the NAc appear to control opioid reward by activating D1 MSNs in the opioid-naïve state, followed by D2 MSNs in the opioid-dependent state [94]. These basolateral amygdala inputs to the NAc are modulated by cannabinoid 1 (CB1) receptors which can modulate the activity of either fast-spiking interneurons or MSNs within the NAc shell to potentiate reward or induce aversion [95,96]. Another glutamatergic innervation to the NAc that plays a role in opioid withdrawal and aversion are those form the infralimbic cortex. Activating the infralimbic cortex projections to the NAc shell blocks morphine reinstatement and reverses the effects of morphine abstinence [97]. There is also evidence that glutamatergic projections from the VTA to the NAc may drive aversion by activating parvalbumin GABAergic interneurons, which in turn inhibit MSNs [98]. However, the role of this projection in opioid aversion has also not been defined. The lateral habenula, an important site of aversion, reduces striatal DA levels by inhibiting VTA dopamine neurons via projections to GABAergic neurons in the RMTg [99–101]. While this lateral habenula circuitry is an important component of the negative prediction error and negative affect [43], the role of this pathway in the negative affect following chronic opioids is unknown. Within the striatum, optogenetic activation of dorsal or ventral KOP-expressing neurons has recently been shown to drive reward or aversion, respectively, suggesting that, similarly to MOPs and DOPs, KOPs show specific hedonic hotspots in the NAc shell [102,103]. Finally, orexin neurons from the lateral region of the hypothalamus that project to the NAc, implicated in the brain stress system, block both the somatic physical symptoms and increased corticosterone levels associated with opioid withdrawal [104].
Network and fMRI analyses have also identified widespread changes during opioid withdrawal. Adaptations in local field potentials in regions including the basolateral amygdala, NAc, and prefrontal cortex reach a new allostatic set-point 2 days following continuous morphine treatment. This widespread allostatic state was disrupted by opioid receptor blockade and precipitation of withdrawal [105]. Studies in humans show that withdrawal from chronic morphine alters fMRI signals in many of the limbic nuclei associated with reward, including the striatum [106]. Together, this research implicates the extended amygdala and striatum as playing a central role in the allostatic changes contributing to affective opponent processes that may drive addictive behaviors.
Concluding Remarks and Future Directions
Tolerance to opioids per se is not a reliable predictor of abuse liability but presents a challenge for treating both acute and chronic pain, and becomes a liability for addicts that relapse. Opioid tolerance is also a predictor of significantly longer length of hospital stay and readmission rates. Tolerance often masks opioid-induced hyperalgesia and contributes to the development of affective disorders such as anxiety and depression, where the emergence of anxiety during withdrawal can be triggered from a single acute opioid exposure [107], but is most prominent following protracted abstinence. Opponent processes in the mesocorticolimbic circuitry, especially the striatum and the VTA, contribute to the neuro-adaptations that lead to comorbid conditions including affective disorders. In this review we have taken a holistic view of opioid tolerance, beginning with adaptations of the opioid receptor signalosome leading on to cellular allostasis and progressing to pass-forward system-wide allostasis resulting in opponent processes that counter opioid behaviors. We highlight biased agonism as a promising avenue for developing novel therapeutics that evade selective allostasis involved in some of the negative sequelae of acute and chronic opioid use. Furthermore, microglia are identified as a mediator of the development of opponent processes (negative affect and hyperalgesia) that contribute to behavioral tolerance. Future research will determine if biased agonism or blockade of neuroinflammation will have clinical relevance in the treatment of pain or opioid addiction (see Outstanding Questions).
Outstanding Questions.
Are different populations of dopamine neurons activated following chronic versus acute opioid treatment?
Do KOPs preferentially modulate excitatory or dopaminergic input to the ventral striatum during chronic opioid exposure or during abstinence?
Will biased agonism identify novel treatment strategies to obviate the allostatic changes within opioid receptor-containing cells and mitigate the pass-forward allostasis that modifies circuits and networks, perhaps targeting specific arrestin-dependent signaling pathways?
What is the significance of pass-forward allostasis in neurons not containing opioid receptors for the generation of behavioral tolerance and opponent processes?
What is the mechanism by which opioids activate microglia, and can co-therapeutics be developed to effectively block activation induced by opioid treatment paradigms?
Trends.
Behavioral tolerance to opioids results from many interacting processes including desensitization, resensitization, and cellular and pass-forward allostasis.
Opioid-biased agonism presents new pharmacological approaches to avoid detrimental side effects and negate aspects of opioid tolerance and dependence.
Negative affect is an opponent process that creates behavioral tolerance to the rewarding effects of opioid drugs, and constitutes a likely driver of relapse following drug cessation.
Microglia activation following chronic opioids is a potential therapeutic target contributing to behavioral tolerance and dependence mechanisms.
Acknowledgments
Financial support was provided by the Shirley and Stefan Hatos Foundation (C.J.E., A.M.W.T., W.W)., Shirley Hatos (C.M.C.), Cousins Center for Psychoneuroimmunology (AMWT), National Institutes of Health (NIH) K99DA040016 (A.M.W.T.), NIH DA005010 (C.J.E., A.M.W.T., W.W.), NIH DA031243 (A.A.A.P.), Department of Defense MR141282 (C.M.C.), and the University of Illinois at Chicago Department of Psychiatry (A.A.A.P.).
Glossary
- Allostasis
adaptive modifications of the nervous system following chronic opioid use that create a new stable state dependent on presence of the drug.
- Brain-derived neurotrophic factor (BDNF)
factor released from neurons and/or microglia that has been shown to contribute to neuronal plasticity during development and pathology, including chronic opioid exposure.
- Long-term depression (LTD)
an activity-dependent decrease in the strength (or efficacy) of a neuronal synapse that can last for hours or longer.
- Long-term potentiation (LTP)
the persistent strengthening of synapses between neurons that can last hours or longer.
- Mesocorticolimbic circuit
the major dopaminergic pathway originating from the midbrain and projecting to the striatum and prefrontal cortical structures. It is primarily involved in mediating motivation, and adaptations in this circuit are often observed in addicted states.
- Nucleus accumbens (NAc)
region of the striatum composed of heterogeneous cell populations that are a major target of dopamine projections from the VTA.
- δ-Opioid receptor (DOP)
member of the opioid receptor family associated with agonist-induced antidepressant and anxiolytic effects.
- κ-Opioid receptor (KOP)
member of the opioid receptor family, noted for its agonist-induced dysphoria and opposite effects to both MOP and DOP activation with regards to affect.
- μ-Opioid receptor (MOP)
member of the opioid receptor family eliciting the hallmark effects of opioid drugs, including euphoria, analgesia, and respiratory depression.
- Rostral tegmental nucleus (RMTg)
region immediately rostral to the ventral tegmental area rich in GABAergic neurons that project to the VTA to control dopaminergic output; highly implicated in opioid control of VTA dopaminergic function.
- Striatum
forebrain region involved in movement and both goal-directed and habit behaviors. Receives dense glutamatergic and dopaminergic inputs from several brain areas including the amygdala, frontal cortex, and VTA, and projects to the basal ganglia and midbrain nuclei.
- Tolerance
reduction in drug effect following prolonged administration resulting in a loss of drug potency indicated by a pharmacological shift to the right in the dose–response curve.
- Ventral tegmental area (VTA)
component of the mesolimbic reward system that is rich in dopamine projection neurons to the striatum and frontal cortical areas.
- Withdrawal
the manifestation of allostatic processes developed during drug dependence that emerge following drug cessation.
References
- 1.Gulur P, et al. Opioid tolerance – a predictor of increased length of stay and higher readmission rates. Pain Physician. 2014;17:E503–E507. [PubMed] [Google Scholar]
- 2.Volkow ND, McLellan AT. Opioid abuse in chronic pain – misconceptions and mitigation strategies. N. Engl. J. Med. 2016;374:1253–1263. doi: 10.1056/NEJMra1507771. [DOI] [PubMed] [Google Scholar]
- 3.Siegel S, et al. Heroin ‘overdose’ death: contribution of drug-associated environmental cues. Science. 1982;216:436–437. doi: 10.1126/science.7200260. [DOI] [PubMed] [Google Scholar]
- 4.Voon P, et al. Pain among high-risk patients on methadone maintenance treatment. J. Pain. 2015;16:887–894. doi: 10.1016/j.jpain.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanderah TW. Delta and kappa opioid receptors as suitable drug targets for pain. Clin. J. Pain. 2010;26(Suppl 10):S10–S15. doi: 10.1097/AJP.0b013e3181c49e3a. [DOI] [PubMed] [Google Scholar]
- 6.Gendron L, et al. Molecular pharmacology of delta-opioid receptors. Pharmacol. Rev. 2016;68:631–700. doi: 10.1124/pr.114.008979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charles A, Pradhan AA. Delta-opioid receptors as targets for migraine therapy. Curr. Opin. Neurol. 2016;29:314–319. doi: 10.1097/WCO.0000000000000311. [DOI] [PubMed] [Google Scholar]
- 8.Bruchas MR, et al. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chavkin C, Martinez D. Kappa antagonist JDTic in Phase 1 clinical trial. Neuropsychopharmacology. 2015;40:2057–2058. doi: 10.1038/npp.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chavkin C, Ehrich JM. How does stress-induced activation of the kappa opioid system increase addiction risk? Biol. Psychiatry. 2014;76:760–762. doi: 10.1016/j.biopsych.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toll L, et al. Nociceptin/orphanin FQ receptor structure, signaling, ligands, functions, and interactions with opioid systems. Pharmacol. Rev. 2016;68:419–457. doi: 10.1124/pr.114.009209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pradhan AA, et al. Ligand-directed signalling within the opioid receptor family. Br. J. Pharmacol. 2012;167:960–969. doi: 10.1111/j.1476-5381.2012.02075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams JT, et al. Regulation of mu-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol. Rev. 2013;65:223–254. doi: 10.1124/pr.112.005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology. 2011;115:1363–1381. doi: 10.1097/ALN.0b013e318238bba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugiura H, et al. Transducing neuronal activity into dendritic spine morphology: new roles for p38 MAP kinase and N-cadherin. Neuroscientist. 2009;15:90–104. doi: 10.1177/1073858408324024. [DOI] [PubMed] [Google Scholar]
- 16.Mazei-Robison MS, Nestler EJ. Opiate-induced molecular and cellular plasticity of ventral tegmental area and locus coeruleus catecholamine neurons. Cold Spring Harb. Perspect. Med. 2012;2:a012070. doi: 10.1101/cshperspect.a012070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, et al. A novel noncanonical signaling pathway for the mu-opioid receptor. Mol. Pharmacol. 2013;84:844–853. doi: 10.1124/mol.113.088278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bohn LM, et al. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- 19.Pradhan AA, et al. Agonist-specific recruitment of arrestin isoforms differentially modify delta opioid receptor function. J. Neurosci. 2016;36:3541–3551. doi: 10.1523/JNEUROSCI.4124-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raehal KM, Bohn LM. The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology. 2011;60:58–65. doi: 10.1016/j.neuropharm.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker EA, Young AM. Differential tolerance to antinociceptive effects of mu opioids during repeated treatment with etonitazene, morphine, or buprenorphine in rats. Psychopharmacology. 2001;154:131–142. doi: 10.1007/s002130000620. [DOI] [PubMed] [Google Scholar]
- 22.Pradhan AA, et al. Ligand-directed trafficking of the delta-opioid receptor in vivo: two paths toward analgesic tolerance. J. Neurosci. 2010;30:16459–16468. doi: 10.1523/JNEUROSCI.3748-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maguma HT, et al. Differences in the characteristics of tolerance to mu-opioid receptor agonists in the colon from wild type and beta-arrestin2 knockout mice. Eur. J. Pharmacol. 2012;685:133–140. doi: 10.1016/j.ejphar.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuhar JR, et al. Mu opioid receptor stimulation activates c-Jun N-terminal kinase 2 by distinct arrestin-dependent and independent mechanisms. Cell Signal. 2015;27:1799–1806. doi: 10.1016/j.cellsig.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu C, et al. Serine 363 of the δ-opioid receptor is crucial for adopting distinct pathways to activate ERK1/2 in response to stimulation with different ligands. J. Cell Sci. 2010;123:4259–4270. doi: 10.1242/jcs.073742. [DOI] [PubMed] [Google Scholar]
- 26.Mittal N, et al. Select G protein-coupled receptors modulate agonist-induced signaling via a ROCK, LIMK, and betaarrestin 1 pathway. Cell Rep. 2013;5:1010–1021. doi: 10.1016/j.celrep.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mittal N, et al. Evidence that behavioral phenotypes of morphine in beta-arr2−/− mice are due to the unmasking of JNK signaling. Neuropsychopharmacology. 2012;37:1953–1962. doi: 10.1038/npp.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zoudilova M, et al. Beta-arrestin-dependent regulation of the cofilin pathway downstream of protease-activated receptor-2. J. Biol. Chem. 2007;282:20634–20646. doi: 10.1074/jbc.M701391200. [DOI] [PubMed] [Google Scholar]
- 29.Pontrello CG, et al. Cofilin under control of beta-arrestin-2 in NMDA-dependent dendritic spine plasticity, long-term depression (LTD), and learning. Proc. Natl. Acad. Sci. U.S.A. 2012;109:E442–E451. doi: 10.1073/pnas.1118803109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zoudilova M, et al. Beta-arrestins scaffold cofilin with chronophin to direct localized actin filament severing and membrane protrusions downstream of protease-activated receptor-2. J. Biol. Chem. 2010;285:14318–14329. doi: 10.1074/jbc.M109.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walwyn W, et al. Beta-arrestin2 and c-Src regulate the constitutive activity and recycling of mu opioid receptors in dorsal root ganglion neurons. J. Neurosci. 2007;27:5092–5104. doi: 10.1523/JNEUROSCI.1157-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raehal KM, et al. Morphine side effects in beta-arrestin 2 knockout mice. J. Pharmacol. Exp. Ther. 2005;314:1195–1201. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- 33.Soergel DG, et al. Biased agonism of the mu-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: A randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain. 2014;155:1829–1835. doi: 10.1016/j.pain.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Smith KS, et al. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc. Natl. Acad. Sci. U.S.A. 2011;108:E255–E264. doi: 10.1073/pnas.1101920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Britt JP, et al. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stuber GD, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Brain Res. Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 38.Pignatelli M, Bonci A. Role of dopamine neurons in reward and aversion: a synaptic plasticity perspective. Neuron. 2015;86:1145–1157. doi: 10.1016/j.neuron.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Hitchcott PK, et al. Enhanced stimulus-reward learning by intra-amygdala administration of a D3 dopamine receptor agonist. Psychopharmacology. 1997;133:240–248. doi: 10.1007/s002130050397. [DOI] [PubMed] [Google Scholar]
- 40.Ikemoto S. Involvement of the olfactory tubercle in cocaine reward: intracranial self-administration studies. J. Neurosci. 2003;23:9305–9311. doi: 10.1523/JNEUROSCI.23-28-09305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Renard GM, et al. Withdrawal from chronic amphetamine reduces dopamine transmission in the rat lateral septum. J. Neurosci. Res. 2014;92:937–943. doi: 10.1002/jnr.23369. [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat. Neurosci. 2009;12:77–84. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 44.Matthes HW, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the muopioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 45.Fields HL, Margolis EB. Understanding opioid reward. Trends Neurosci. 2015;38:217–225. doi: 10.1016/j.tins.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsui A, et al. Separate GABA afferents to dopamine neurons mediate acute action of opioids, development of tolerance, and expression of withdrawal. Neuron. 2014;82:1346–1356. doi: 10.1016/j.neuron.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cui Y, et al. Targeted expression of mu-opioid receptors in a subset of striatal direct-pathway neurons restores opiate reward. Nat. Neurosci. 2014;17:254–261. doi: 10.1038/nn.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olson VG, et al. Role of noradrenergic signaling by the nucleus tractus solitarius in mediating opiate reward. Science. 2006;311:1017–1020. doi: 10.1126/science.1119311. [DOI] [PubMed] [Google Scholar]
- 49.Pettit HO, et al. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology. 1984;84:167–173. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- 50.Bechara A, et al. A two-separate-motivational-systems hypothesis of opioid addiction. Pharmacol. Biochem. Behav. 1998;59:1–17. doi: 10.1016/s0091-3057(97)00047-6. [DOI] [PubMed] [Google Scholar]
- 51.Laviolette SR, et al. Opiate state controls bi-directional reward signaling via GABAA receptors in the ventral tegmental area. Nature neuroscience. 2004;7:160–169. doi: 10.1038/nn1182. [DOI] [PubMed] [Google Scholar]
- 52.Laviolette SR, et al. Motivational state determines the functional role of the mesolimbic dopamine system in the mediation of opiate reward processes. Behav. Brain Res. 2002;129:17–29. doi: 10.1016/s0166-4328(01)00327-8. [DOI] [PubMed] [Google Scholar]
- 53.Laviolette SR, van der Kooy D. GABAA receptors in the ventral tegmental area control bidirectional reward signalling between dopaminergic and non-dopaminergic neural motivational systems. Eur. J. Neurosci. 2001;13:1009–1015. doi: 10.1046/j.1460-9568.2001.01458.x. [DOI] [PubMed] [Google Scholar]
- 54.Taylor AM, et al. Mesolimbic dopamine signaling in acute and chronic pain: implications for motivation, analgesia, and addiction. Pain. 2016;157:1194–1198. doi: 10.1097/j.pain.0000000000000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor AM, et al. Microglia disrupt mesolimbic reward circuitry in chronic pain. J. Neurosci. 2015;35:8442–8450. doi: 10.1523/JNEUROSCI.4036-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chavkin C, Koob GF. Dynorphin, dysphoria, and dependence: the stress of addiction. Neuropsychopharmacology. 2016;41:373–374. doi: 10.1038/npp.2015.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crowley NA, et al. Dynorphin controls the gain of an amygdalar anxiety circuit. Cell Rep. 2016;14:2774–2783. doi: 10.1016/j.celrep.2016.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van’t Veer A, et al. Ablation of kappa-opioid receptors from brain dopamine neurons has anxiolytic-like effects and enhances cocaine-induced plasticity. Neuropsychopharmacology. 2013;38:1585–1597. doi: 10.1038/npp.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ehrich JM, et al. Kappa opioid receptor-induced aversion requires p38 MAPK activation in VTA dopamine neurons. J. Neurosci. 2015;35:12917–12931. doi: 10.1523/JNEUROSCI.2444-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cahill CM, et al. Does the kappa opioid receptor system contribute to pain aversion? Front Pharmacol. 2014;5:253. doi: 10.3389/fphar.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koo JW, et al. BDNF is a negative modulator of morphine action. Science. 2012;338:124–128. doi: 10.1126/science.1222265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vargas-Perez H, et al. BDNF signaling in the VTA links the drug-dependent state to drug withdrawal aversions. J. Neurosci. 2014;34:7899–7909. doi: 10.1523/JNEUROSCI.3776-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taylor AM, et al. Neuroimmune regulation of GABAergic neurons within the ventral tegmental area during withdrawal from chronic morphine. Neuropsychopharmacology. 2016;41:949–959. doi: 10.1038/npp.2015.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vargas-Perez H, et al. Ventral tegmental area BDNF induces an opiate-dependent-like reward state in naive rats. Science. 2009;324:1732–1734. doi: 10.1126/science.1168501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferrini F, et al. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl− homeostasis. Nature neuroscience. 2013;16:183–192. doi: 10.1038/nn.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gagnon M, et al. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat. Med. 2013;19:1524–1528. doi: 10.1038/nm.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ting AKR, et al. Infusion of brain-derived neurotrophic factor into the ventral tegmental area switches the substrates mediating ethanol motivation. Eur. J. Neurosci. 2013;37:996–1003. doi: 10.1111/ejn.12105. [DOI] [PubMed] [Google Scholar]
- 68.Coull JA, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 69.Doyon N, et al. Chloride regulation: a dynamic equilibrium crucial for synaptic inhibition. Neuron. 2016;89:1157–1172. doi: 10.1016/j.neuron.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 70.Gulacsi A, et al. Cell type-specific differences in chloride-regulatory mechanisms and GABAA receptor-mediated inhibition in rat substantia nigra. J. Neurosci. 2003;23:8237–8246. doi: 10.1523/JNEUROSCI.23-23-08237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sklair-Tavron L, et al. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc. Natl. Acad. Sci. U.S.A. 1996;93:11202–11207. doi: 10.1073/pnas.93.20.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 73.Wu X, et al. Effects of morphine withdrawal on the membrane properties of medium spiny neurons in the nucleus accumbens shell. Brain Res. Bull. 2013;90:92–99. doi: 10.1016/j.brainresbull.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 74.Wu X, et al. Potentiation of synaptic strength and intrinsic excitability in the nucleus accumbens after 10 days of morphine withdrawal. J. Neurosci. Res. 2012;90:1270–1283. doi: 10.1002/jnr.23025. [DOI] [PubMed] [Google Scholar]
- 75.Shen H, Kalivas PW. Reduced LTP and LTD in prefrontal cortex synapses in the nucleus accumbens after heroin self-administration. Int. J. Neuropsychopharmacol. 2013;16:1165–1167. doi: 10.1017/S1461145712001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Robinson TE, et al. Widespread but regionally specific effects of experimenter- versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse. 2002;46:271–279. doi: 10.1002/syn.10146. [DOI] [PubMed] [Google Scholar]
- 77.Shen H, et al. Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proc. Natl. Acad. Sci. U.S.A. 2011;108:19407–19412. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kobrin KL, et al. Acquisition of morphine conditioned place preference increases the dendritic complexity of nucleus accumbens core neurons. Addict. Biol. 2015 doi: 10.1111/adb.12273. Published online June 19, 2015. http://dx.doi.org/10.1111/adb.12273. [DOI] [PubMed]
- 79.Ziolkowska B, et al. Temporal and anatomic patterns of immediate-early gene expression in the forebrain of C57BL/6 and DBA/2 mice after morphine administration. Neuroscience. 2015;284:107–124. doi: 10.1016/j.neuroscience.2014.09.069. [DOI] [PubMed] [Google Scholar]
- 80.Enoksson T, et al. Nucleus accumbens D2- and D1-receptor expressing medium spiny neurons are selectively activated by morphine withdrawal and acute morphine, respectively. Neuropharmacology. 2012;62:2463–2471. doi: 10.1016/j.neuropharm.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 81.Gaspari S, et al. Nucleus accumbens-specific interventions in RGS9-2 activity modulate responses to morphine. Neuropsychopharmacology. 2014;39:1968–1977. doi: 10.1038/npp.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koo JW, et al. Loss of BDNF signaling in D1R-expressing NAc neurons enhances morphine reward by reducing GABA inhibition. Neuropsychopharmacology. 2014;39:2646–2653. doi: 10.1038/npp.2014.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feng K, et al. Comparative proteomic analysis of the nucleus accumbens during extinction and reinstatement of morphine dependence. West Indian Med. J. 2013;62:210–215. [PubMed] [Google Scholar]
- 84.Tapocik JD, et al. Neuroplasticity, axonal guidance and micro-RNA genes are associated with morphine self-administration behavior. Addict. Biol. 2013;18:480–495. doi: 10.1111/j.1369-1600.2012.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu. Rev. Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 86.Koob GF, et al. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76:370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koob GF. Negative reinforcement in drug addiction: the darkness within. Curr. Opin. Neurobiol. 2013;23:559–563. doi: 10.1016/j.conb.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 88.Evans CJ, Cahill CM. Neurobiology of opioid dependence in creating addiction vulnerability. F1000Res. 2016;5:1748. doi: 10.12688/f1000research.8369.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Papaleo F, et al. Disruption of the CRF/CRF1 receptor stress system exacerbates the somatic signs of opiate withdrawal. Neuron. 2007;53:577–589. doi: 10.1016/j.neuron.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 90.Park PE, et al. Chronic CRF1 receptor blockade reduces heroin intake escalation and dependence-induced hyperalgesia. Addict. Biol. 2015;20:275–284. doi: 10.1111/adb.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Delfs JM, et al. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- 92.Zhu Y, et al. A thalamic input to the nucleus accumbens mediates opiate dependence. Nature. 2016;530:219–222. doi: 10.1038/nature16954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Russell SE, et al. Nucleus accumbens AMPA receptors are necessary for morphine-withdrawal-induced negative-affective states in rats. J. Neurosci. 2016;36:5748–5762. doi: 10.1523/JNEUROSCI.2875-12.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lintas A, et al. Inputs from the basolateral amygdala to the nucleus accumbens shell control opiate reward magnitude via differential dopamine D1 or D2 receptor transmission. Eur. J. Neurosci. 2012;35:279–290. doi: 10.1111/j.1460-9568.2011.07943.x. [DOI] [PubMed] [Google Scholar]
- 95.Baharlouei N, et al. Blockage of acquisition and expression of morphine-induced conditioned place preference in rats due to activation of glutamate receptors type II/III in nucleus accumbens. Pharmacol. Biochem. Behav. 2015;135:192–198. doi: 10.1016/j.pbb.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 96.Haghparast A, et al. Cannabinoid receptors in the basolateral amygdala are involved in the potentiation of morphine rewarding properties in the acquisition, but not expression of conditioned place preference in rats. Brain Res. 2014;1565:28–36. doi: 10.1016/j.brainres.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 97.Hearing MC, et al. Reversal of morphine-induced cell-type-specific synaptic plasticity in the nucleus accumbens shell blocks reinstatement. Proc. Natl. Acad. Sci. U.S.A. 2016;113:757–762. doi: 10.1073/pnas.1519248113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qi J, et al. VTA glutamatergic inputs to nucleus accumbens drive aversion by acting on GABAergic interneurons. Nat. Neurosci. 2016;19:725–733. doi: 10.1038/nn.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Root DH, et al. Role of glutamatergic projections from ventral tegmental area to lateral habenula in aversive conditioning. J. Neurosci. 2014;34:13906–13910. doi: 10.1523/JNEUROSCI.2029-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Velasquez KM, et al. The role of the habenula in drug addiction. Front Hum. Neurosci. 2014;8:174. doi: 10.3389/fnhum.2014.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gardon O, et al. Expression of mu opioid receptor in dorsal diencephalic conduction system: new insights for the medial habenula. Neuroscience. 2014;277:595–609. doi: 10.1016/j.neuroscience.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Al-Hasani R, et al. Distinct subpopulations of nucleus accumbens dynorphin neurons drive aversion and reward. Neuron. 2015;87:1063–1077. doi: 10.1016/j.neuron.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Castro DC, Berridge KC. Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness ‘liking’ and ‘wanting’. J. Neurosci. 2014;34:4239–4250. doi: 10.1523/JNEUROSCI.4458-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Laorden ML, et al. Hypothalamic orexin – a neurons are involved in the response of the brain stress system to morphine withdrawal. PLoS ONE. 2012;7:e36871. doi: 10.1371/journal.pone.0036871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dejean C, et al. Opiate dependence induces network state shifts in the limbic system. Neurobiol. Dis. 2013;59:220–229. doi: 10.1016/j.nbd.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 106.Chu LF, et al. Acute opioid withdrawal is associated with increased neural activity in reward-processing centers in healthy men: a functional magnetic resonance imaging study. Drug Alcohol. Depend. 2015;153:314–322. doi: 10.1016/j.drugalcdep.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 107.Radke AK, et al. An anatomical basis for opponent process mechanisms of opiate withdrawal. J. Neurosci. 2011;31:7533–7539. doi: 10.1523/JNEUROSCI.0172-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Petaja-Repo UE, et al. Ligands act as pharmacological chaperones and increase the efficiency of delta opioid receptor maturation. EMBO J. 2002;21:1628–1637. doi: 10.1093/emboj/21.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Irannejad R, von Zastrow M. GPCR signaling along the endocytic pathway. Curr. Opin. Cell Biol. 2014;27:109–116. doi: 10.1016/j.ceb.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eisinger DA, Schulz R. Mechanism and consequences of delta-opioid receptor internalization. Crit. Rev. Neurobiol. 2005;17:1–26. doi: 10.1615/critrevneurobiol.v17.i1.10. [DOI] [PubMed] [Google Scholar]
- 111.Henry AG, et al. The role of ubiquitination in lysosomal trafficking of delta-opioid receptors. Traffic. 2011;12:170–184. doi: 10.1111/j.1600-0854.2010.01145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dang VC, et al. Cellular morphine tolerance produced by betaarrestin-2-dependent impairment of mu-opioid receptor resensitization. J. Neurosci. 2011;31:7122–7130. doi: 10.1523/JNEUROSCI.5999-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Connor M, et al. Beta-arrestin-2 knockout prevents development of cellular mu-opioid receptor tolerance but does not affect opioid-withdrawal-related adaptations in single PAG neurons. Br. J. Pharmacol. 2015;172:492–500. doi: 10.1111/bph.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Banghart MR, et al. Enkephalin disinhibits mu opioid receptor-rich striatal patches via delta opioid receptors. Neuron. 2015;88:1227–1239. doi: 10.1016/j.neuron.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Watkins LR, et al. The ‘toll’ of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol. Sci. 2009;30:581–591. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Horvath RJ, et al. Inhibition of microglial P2X4 receptors attenuates morphine tolerance, Iba1, GFAP and mu opioid receptor protein expression while enhancing perivascular microglial ED2. Pain. 2010;150:401–413. doi: 10.1016/j.pain.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Johnston IN, et al. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J. Neurosci. 2004;24:7353–7365. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Muscoli C, et al. Counter-regulation of opioid analgesia by glial-derived bioactive sphingolipids. J. Neurosci. 2010;30:15400–15408. doi: 10.1523/JNEUROSCI.2391-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stefano GB. Autoimmunovascular regulation: morphine and anandamide and ancondamide stimulated nitric oxide release. J. Neuroimmunol. 1998;83:70–76. doi: 10.1016/s0165-5728(97)00223-3. [DOI] [PubMed] [Google Scholar]
- 120.Shen N, et al. A novel role of spinal astrocytic connexin 43: mediating morphine antinociceptive tolerance by activation of NMDA receptors and inhibition of glutamate transporter-1 in rats. CNS Neurosci. Ther. 2014;20:728–736. doi: 10.1111/cns.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schwarz JM, et al. Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression. J. Neurosci. 2011;31:17835–17847. doi: 10.1523/JNEUROSCI.3297-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schwarz JM, Bilbo SD. Adolescent morphine exposure affects long-term microglial function and later-life relapse liability in a model of addiction. J. Neurosci. 2013;33:961–971. doi: 10.1523/JNEUROSCI.2516-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hao S, et al. The role of TNFalpha in the periaqueductal gray during naloxone-precipitated morphine withdrawal in rats. Neuropsychopharmacology. 2011;36:664–676. doi: 10.1038/npp.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hutchinson MR, et al. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J. Neurosci. 2012;32:11187–11200. doi: 10.1523/JNEUROSCI.0684-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zwicker JD, et al. Glial TLR4 signaling does not contribute to opioid-induced depression of respiration. J. Appl. Physiol. 2014;117:857–868. doi: 10.1152/japplphysiol.00534.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang XQ, et al. Activation of p38 signaling in the microglia in the nucleus accumbens contributes to the acquisition and maintenance of morphine-induced conditioned place preference. Brain Behav. Immun. 2012;26:318–325. doi: 10.1016/j.bbi.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 127.Theberge FR, et al. Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biol. Psychiatry. 2013;73:729–737. doi: 10.1016/j.biopsych.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Eitan S, et al. Brain region-specific mechanisms for acute morphine-induced mitogen-activated protein kinase modulation and distinct patterns of activation during analgesic tolerance and locomotor sensitization. J. Neurosci. 2003;23:8360–8369. doi: 10.1523/JNEUROSCI.23-23-08360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]