Abstract
Background
Early life stress is thought to contribute to psychiatric disorders, but the precise mechanisms underlying this link are poorly understood. As neonatal stress decreases adult hippocampal neurogenesis, which, in turn, functionally contributes to many behavioral phenotypes relevant to psychiatric disorders, we examined how in vivo neonatal maternal separation (NMS) impacts the capacity of adult hippocampal neural precursor cells via epigenetic alterations in vitro.
Methods
Rat pups were separated from their dams for 3 hours daily from postnatal day (PND) 2 to PND 14 or were never separated from the dam (as control animals). We isolated adult neural precursor cells from the hippocampal dentate gyrus at PND 56 and assessed rates of proliferation, apoptosis, and differentiation in cell culture. We also evaluated the effect of DNA methylation at the retinoic acid receptor (RAR) promoter stemming from NMS on adult neural precursor cells.
Results
NMS attenuated neural differentiation of adult neural precursor cells but had no detectible effect on proliferation, apoptosis, or astroglial differentiation. The DNA methyltransferase (DNMT) inhibitor, 5-aza-dC, reversed a reduction by NMS of neural differentiation of adult neural precursor cells. NMS increased DNMT1 expression and decreased expression of RARα. An RARα agonist increased neural differentiation and an antagonist reduced retinoic acid-induced neural differentiation. NMS increased the methylated portion of RARα promoter, and the DNMT inhibitor reversed a reduction by NMS of RARα messenger RNA expression.
Conclusions
NMS attenuates the capacity of adult hippocampal neural precursor cells to differentiate into neurons by decreasing expression of RARα through DNMT1-mediated methylation of its promoter.
Keywords: Adult neurogenesis, dentate gyrus, DNA methylation, DNA methyltransferase, maternal separation, retinoic acid receptor
One of the fundamental issues in neurobiology is how environmental factors alter molecular states in the brain, ultimately leading to behavioral phenotypes. Neonatal and postnatal stress are thought to have long-lasting effects on individuals, resulting in heightened risk for many psychiatric disorders, including schizophrenia, substance abuse disorders, personality disorders, and mood and anxiety disorders (1). The precise mechanisms of this process are still poorly understood in humans.
In rodents, neonatal maternal separation (NMS) alters behavioral phenotypes related to neuropsychiatric disorders later in life. Defective prepulse inhibition (PPI) is nonselectively associated with many neuropsychiatric disorders, including schizophrenia, bipolar disorder, schizotypal personality disorder, obsessive-compulsive disorder, and panic disorder in humans (2). NMS reduces PPI from adolescence to adulthood but not before puberty in rats (3–7). Moreover, NMS exacerbates stress responses and anxiety-like behaviors (8–10), heightens preference for ethanol (8,11), and induces cognitive impairments (5,12) in rats by the time they reach adulthood.
NMS induces a host of neuronal phenotypes in many rodent brain regions (13), but neuronal alterations in the hippocampus are likely to mediate some of the long-lasting effects of NMS on behaviors (14). Indirect evidence suggests that adult neurogenesis in the hippocampus contributes to the behavioral effects of NMS. First, NMS reduces adult neurogenesis in the rat hippocampal dentate gyrus in vivo (15). Second, direct alterations in adult neurogenesis in the hippocampus affect PPI (16), mood-related behaviors (17), and fear-related memory (18–20).
Epigenetic alterations in hippocampal neural precursor cells are increasingly appreciated as contributors to many aspects of adult neurogenesis (21,22). Methyl-CpG binding domain protein 1, a member of the methylated DNA-binding protein family, binds methylated gene promoters and facilitates transcriptional repression. Loss of this gene reduces neural differentiation in vivo and in vitro (23) through a basic fibroblast growth factor 2 promoter in vitro (24) and induces PPI deficits and defective fear conditioning and heightens anxiety- and depression-related behaviors in vivo (25).
We hypothesized that NMS alters the rate of adult neurogenesis in the hippocampal dentate gyrus via methylation of a neurogenesis-related gene. Because adult neural precursor cells represent a small fraction of the total hippocampal cell population, in vivo analysis cannot identify an epigenetic modification for this specific cell population. To circumvent this technical obstacle, we evaluated the impact of in vivo environmental stress on adult neural precursor cells in the hippocampal dentate gyrus, using our in vitro cell culture system. Our cell culture system uses adult dentate gyrus-derived neural precursor cells (ADP) and does not include ependymal cells (26). Pups were separated from their dams on postnatal days (PNDs) 2 to 14, and we evaluated how this environmental stress altered the capacity of in vitro adult neural precursor cells and DNA methylation at PND 56. Rats become sexually mature by 6 weeks of age (i.e., enter adolescence). They are considered to be young adult from PND 63, reaching socially mature adulthood around 6 months of age (27). We focused on young adulthood, because onset of many neuropsychiatric disorders occurs during the period from late adolescence through young adulthood.
Methods and Materials
Animals
Pregnant Sprague-Dawley rats (Shizuoka Laboratory Animal Center, Shizuoka, Japan) were delivered on gestation day 14 and singly housed. All rats were housed in standard animal cages with ad libitum access to food and water in a temperature-controlled environment (22°C ± 1°C) on a 12-hour light/dark cycle (light phase: 6:00 AM–6:00 PM). All procedures were approved by the Hokkaido University School of Medicine Animal Care and Use Committee and complied with the Guide for the Care and Use of Laboratory Animals, Hokkaido University School of Medicine.
Neonatal Maternal Separation
We used a brief maternal separation procedure previously reported by Plotsky and Meaney (28). Pups were cross-fostered on PND2 to minimize litter differences; eight male and two female pups were placed in each litter. Ten pups per litter were assigned to neonatal maternal separation or typical animal facility rearing (AFR) groups. Because cross-fostering could have long-lasting effects on emotional behaviors (29), this factor was held constant for both groups. Under the cross-fostering condition, NMS, but not AFR, results in reduced adult neurogenesis (15). Maternal separation took place for 3 hours per day (9:30 AM to 12:30 PM each day) from PND 2 to PND 14. Dams were removed from the cage and placed in a separate cage; pups were also removed from the cage, placed in a clean plastic cage with wood-chip bedding in an incubator to maintain an ambient temperature at 27°C to 30°C in another room, and returned 3 hours later to the original cage with the dams. Pups in the NMS group were permitted to position themselves, which included huddling with littermates, during the separation period. Pups in the AFR group were not disturbed and were maintained with dams. Bedding for both AFR and NMS groups was changed once a week by an animal care technician.
The same pool of animals that simultaneously underwent NMS was randomly divided into two subgroups. One subgroup was tested for fear conditioning and the other for the present cell culture analysis. The efficacy of our NMS procedure was validated as NMS-treated rats showed fear-related phenotypes (30). We removed all pups from the dam for 3 hours each day. Other published procedures keep two to three pups with the dam to minimize her stress and subsequent maternal abuse. The precise environmental factor in the NMS procedure that causes behavioral phenotypes cannot be easily or definitively isolated. Nonetheless, the version we employed has been demonstrated to cause robust behavioral phenotypes (30) and alteration in adult neurogenesis (15). In the literature, control for NMS is our AFR, brief handling, or both. A brief-handing group is handled for 30 seconds to 15 minutes; however, this also inevitably results in maternal separation during handling. Thus, this control does not isolate the impact of handling alone. As pointed out by Matthews and Robbins (31), it is not realistic to apply a pure experimental condition that would permit definitive descriptions of the effects of handling or maternal separation. In reality, the AFR and brief isolation with handing do not result in consistently different behavioral phenotypes (8,31–35). We conducted a pilot study to compare the impact of the AFR and 15-minute handling but did not find phenotypic differences in anxiety-related behaviors between these two groups and thus did not include the handling control.
Isolation and Culture of ADP Cells
At weaning, male and female rats were separated and group-housed. At PND 56, all eight male rats from each of the NMS groups and AFR groups were used to dissect the dentate gyrus. We used four NMS groups and four AFR groups as one set. Tissues from 32 rats of each treatment group (NMS or AFR) were pooled and digested using proteases and DNase (Worthington Biochemical Corp., Lakewood, New Jersey). ADP cells were isolated using Percoll-gradient centrifugation and then prepared in monolayer culture in nonserum medium with basic fibroblast growth factor (bFGF) (Invitrogen, Carlsbad, California), using our standard procedure (26). Each cell culture was derived from 32 rats (8 rats per foster mother and 4 foster mother lines per treatment group). As each assay was repeated in three to six cell cultures, the sample size ranged from three to six.
Drugs
We used retinoic acid (Invitrogen), Ro 41-5213 (Enzo Life Sciences, Farmingdale, New York), and 5-aza-dC (Sigma, St. Louis, Missouri). Staurosporine was kindly donated by Asahi-Kasei Corporation (Tokyo, Japan) and CD1556 was kindly donated by Garderma (Sophia-Antipolis, France).
Proliferation Assay
ADP cells (1 × 104 per well) were placed on laminin-ornithine coated Lab-Tek П eight-chamber slides (Nalge Nunc International, Naperville, Illinois) with .5% fetal bovine serum medium (Invitrogen). After 24 hours, cells were treated with 5 ng/mL bFGF, a major stimulator of proliferation in neural precursor cells (36). Bromodeoxyuridine (BrdU) (Sigma) was added 24 hours later (10 nmol/L). Immunocytochemistry assays were conducted with anti-BrdU antibody 24 hours later as described in our previous study (37). Fluorescent signals were detected using an IX-71 fluorescence microscope (Olympus, Tokyo, Japan). We evaluated BrdU and 4′,6-diamidino-2-phenylindole (DAPI) signal in four randomly selected fields per well and then calculated the ratio of BrdU-derived signals to DAPI signals.
Apoptosis Assay
ADP cells (2 × 104 per well) were placed on laminin-ornithine coated Lab-Tek П eight-chamber slides with medium including 20 ng/mL bFGF, and 24 hours later, apoptosis was induced using staurosporine (300 nmol/L) (38). While apoptosis could be mediated by tumor necrosis factor alpha (TNF-α)- and staurosporine-dependent pathways, we previously demonstrated that in adult neural progenitor cells, TNF-α does not induce apoptosis but staurosporine does at this concentration (38). Two days later, we performed a terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling (TUNEL) assay using the DeadEnd Fluorometric TUNEL System (Promega, Madison, Wisconsin), as described in our previous study (38). We detected fluorescent signals using an IX-71 fluorescence microscope (Olympus); we then counted the number of TUNEL and DAPI-positive cells in four randomly selected fields per well and calculated the signal ratio of TUNEL to DAPI.
Differentiation Assay
ADP cells (2 × 104 per well) were placed on laminin-ornithine coated Lab-Tek П eight-chamber slides with medium including .5% fetal bovine serum. Following overnight incubation, differentiation was induced using 1 μmol/L retinoic acid. Little is known about potential multiple pathways for differentiation of adult neural progenitor cells. However, we previously demonstrated that retinoic acid is one of the most likely endogenous factors that induces differentiation of adult neural progenitor cells (38). Seven days later, we performed immunocytochemistry assays using anti-Tuj1 antibody (Covance Inc., Princeton, New Jersey), a marker of immature neurons, and anti-glial fibrillary acidic protein (GFAP) antibody (Dako, Glostrup, Denmark), a marker of glial cells, as described in our previous study (38). It is difficult to induce complete differentiation of adult neural progenitor cells to the extent that they assume features of genuinely mature neurons. Microtubule-associated protein 2 is a marker of mature neurons and use of this marker would not identify all differentiating cells, including immature neurons. Because Tuj1 is a marker of immature neurons, we used it as a marker to evaluate the rate of differentiation. We counted the numbers of cells positive for markers in four randomly selected fields per well and calculated the ratio of each cell marker to DAPI.
To evaluate the role of retinoic acid receptor (RAR)α and DNA methyltransferase (DNMT) in differentiation of ADP cells, we used CD1556, a selective RARα agonist; Ro 41-5213, a selective RARα antagonist; and 5-aza-dC, a DNMT inhibitor. In a pilot study, we used a wide range of concentrations based on published studies and chose 2 μmol/L for CD1556, 1 μmol/L for Ro 41-5213, and 10 μmol/L for 5-aza-dC, because they induced the expected effect without toxicity. To evaluate the effect of 5-aza-dC on RARα messenger RNA (mRNA) expression, we used the same concentration (10 μmol/L).
Total RNA Isolation and Quantitative Real Time Polymerase Chain Reaction
ADP cells (2 × 105) were placed on laminin-ornithine coated 35-mm dishes or six-well plates in medium 20 ng/mL bFGF. For 35-mm dishes, after 24 hours, we isolated total RNA using All Prep DNA/RNAMini (Qiagen, Hilden, Germany). For six-well plates, drugs were added 24 hours later, and we isolated total RNA 3 days later. We performed RNA isolation and quantitative real time polymerase chain reaction (RT-PCR) 24 hours after cell seeding and quantitative RT-PCR using our standard procedure (26), using a glyceraldehyde 3-phosphate dehydrogenase as a control. The results were analyzed by using SDS 2.0 software (Applied Biosystems, Foster, California).
Western Blotting
ADP cells (2 × 105) were placed on laminin-ornithine coated 35-mm dishes or six-well plates in medium with 20 ng/mL bFGF. We prepared cells 24 hours after cell seeding. Total protein was prepared using the Mammalian Cell Lysis Kit (Sigma). We performed western blotting using anti-DNMT1 antibody (1:1000) (Active Motif, Carlsbad, California) and anti-RARα antibody (1:1000) (Cell Signaling, Danvers, Massachusetts) as described in our previous study (26). Protein expression was detected using the Amersham ECL Plus Western Blotting Detection System (GE Healthcare, Milwaukee, Wisconsin) and Amersham Hyperfilm ECL (GE Healthcare). Images were converted to digital files and the intensity of bands was analyzed using ImageJ software (National Institutes of Health, Bethesda, Maryland).
Methylation Analysis of RARα Promoter
ADP cells (2 × 105) were placed on laminin-ornithine coated 35-mm dishes in medium with 20 ng/mL bFGF. Twenty-four hours later, we isolated genome DNA of ADP cells using Allprep DNA/RNA Mini (Qiagen) and digested with Mse 1 (New England Biolabs, Ipswich, Massachusetts). We enriched CpG-methylated DNA using Mse 1-digested DNA fragments with MethylCollector Ultra (Active Motif) and performed PCR with AmpliTaq Gold 360 Master Mix (Applied Biosystems). PCR conditions were 95°C for 10 minutes, followed by 35 cycles of 95°C for 30 seconds, 56°C for 30 seconds, and 72°C for 1 minute. Primers for semi-quantitative PCR were designed to cover 340 base pair (bp) of the CpG island of RARα promoter (Figure S1 in Supplement 1). We used the following sequences of forward and reverse primers: TAGGGGCTGGAATCCCAGAG and AAGTTGTGCAGGTTGGAGGAAG. PCR products were electrophoresed with 2% agarose gel. Digital images of this gel were acquired and we analyzed the intensity of each band using ImageJ (National Institute of Health).
Statistical Analysis
We conducted statistical analyses using unpaired t test or analysis of variance followed by Bonferroni post hoc comparisons. Significance was set as p < .05. Data are expressed as the means ± SEM.
Results
Neonatal Maternal Separation Attenuates Neural Differentiation of ADP Cells
To identify if neonatal maternal separation, from neonatal days 2 to 14, has a long-lasting effect on ADP cells at PND 56, we evaluated the rates of proliferation, apoptosis, and differentiation into neurons and astrocytes. We found no difference in the numbers of BrdU-positive cells in AFR group compared with the NMS group (Figure 1B). Using staurosporine-induced apoptosis, cells were examined with TUNEL staining. We found no difference in the numbers of TUNEL-positive cells between the AFR and NMS groups (Figure 1C). We next examined the rates of differentiation of ADP cells. Because ADP cells lose their capacity to spontaneously differentiate as rats age, we used retinoic acid to induce differentiation (38). We evaluated neural and astroglial differentiation using immunocytochemistry assays with anti-Tuj1 antibody and anti-GFAP antibody, respectively (38). While the number of Tuj1-positive cells was significantly reduced in the NMS group compared with the AFR group, there was no difference between groups for the number of GFAP-positive cells (Figure 1D). Here, we showed that the impact of neonatal stress applied in vivo can be evaluated using in vitro experimental methods. Taken together, these data indicate that neonatal maternal separation has a long-lasting (>42 days) effect on the capacity of ADP cells to differentiate into neurons but has no detectable effect on proliferation, apoptosis, or differentiation into astrocytes.
Figure 1.
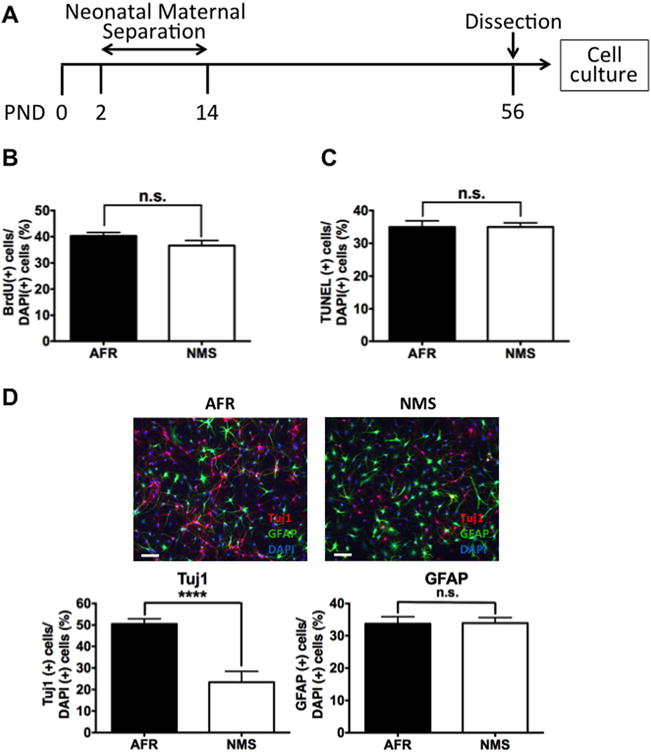
Effects of neonatal maternal separation (NMS) on proliferation, apoptosis, and differentiation of adult dentate gyrus-derived precursor cells in vitro. (A) Rats underwent NMS between postnatal days (PND) 2 and 14. Adult dentate gyrus-derived precursor cells were collected from the hippocampal dentate gyrus at PND 56. (B) NMS had no effect on proliferation, as assessed by bromodeoxyuridine (BrdU)-positive cells (t10 = .149, n.s.) or (C) staurosporine-induced apoptosis, as assessed by terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling (TUNEL) assay (t10 = .9954, n.s.). (D) NMS reduced retinoic acid-induced neural differentiation, as assessed by Tuj1-positive cells (t10 = 11.96, p < .0001) but had no effect on astroglial differentiation, as assessed by glial fibrillary acidic protein (GFAP)-positive cells (t10 = .062, n.s.). Data are shown as the means ± SEM. ****Statistically significant difference at p < .0001. AFR, animal facility reared; DAPI, 4′,6-diamidino-2-phenylindole; n.s., no statistically significant difference.
Neonatal Maternal Separation Attenuates Neural Differentiation of ADP Cells via DNA Methylation
We hypothesized that the long-lasting effect might be mediated by epigenetic alterations. Histone acetylation acts as an epigenetic modification to mediate neural differentiation of neural progenitor cells (39). However, histone acetylation might not account for a reduction in neural differentiation of ADP cells. Valproate, a histone deacetylase inhibitor, promotes neural differentiation of cells derived from embryonic rat hippocampus (39) and facilitates astroglial differentiation and attenuates neural differentiation of ADP cells (38). In the current study, we thus examined the role of DNA methylation as an epigenetic alteration, because previous reports show that DNA methylation at a CpG island decreases expression of a globin gene (40,41).
We examined the effect of 5-aza-dC, a common inhibitor of DNA methyltransferases (DNMTs), on the diminished rate of neural differentiation of ADP cells. If DNA methylation was involved, then its inhibition by 5-aza-dC would restore neural differentiation that was decreased due to neonatal maternal separation. The number of Tuj1-positive cells was equally increased by 5-aza-dC in the AFR and NMS groups; the number of Tuj1-positive cells in the NMS group increased to normal levels of the AFR group (Figure 2A). We observed a small variance (~10%) in differentiation rates, as judged byTuj1, among different cell lines (Figures 1D and 2A). However, NMS consistently decreased the rate of neural differentiation. These results suggest that increased DNA methylation by DNMTs was involved in the diminished neural differentiation of ADP cells. Given that there are three DNMT subtypes, namely DNMT1, 3a, and 3b (42), using quantitative RT-PCR, we next examined whether neonatal maternal separation induces long-lasting effects on expression of the three DNMT subtypes in ADP cells. DNMT1 mRNA, but not DNMT 3a or 3b, was selectively increased in ADP cells from the NMS group (Figure 2B). We additionally confirmed that DNMT1 protein was also increased in ADP cells from the NMS animals (Figure 2C), suggesting that neonatal maternal separation has a long-lasting effect on DNMT1 expression in ADP cells.
Figure 2.
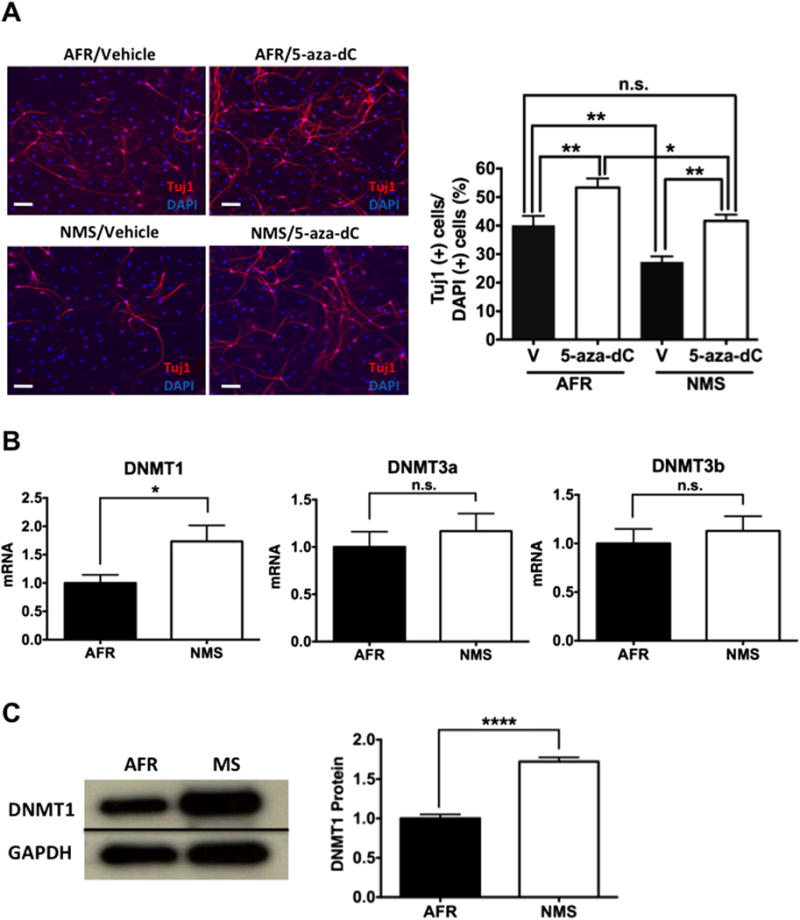
Effects of neonatal maternal separation (NMS) on neural differentiation of adult dentate gyrus-derived neural precursor cells via DNA methyltransferase (DNMT) and on regulation of DNMT subtypes in vitro. (A) A reduction in neural differentiation following NMS was reversed by 5-aza-dC (10 μmol/L), a DNMT inhibitor. Immunocytochemistry assays were performed 7 days after drug treatment. Two-way analysis of variance showed that the main group (animal facility reared [AFR] vs. NMS) effect (F1,60 = 17.95, p < .0001) and the drug (vehicle [V] vs. 5-aza-dC) effect (F1,60 = 22.76, p < .0001) were significant without an interaction effect (F1,60 = .101, n.s.). Post hoc comparison of the vehicle-treated AFR and 5-aza-dC-treated NMS groups showed that the groups did differ. (B) NMS increased messenger RNA (mRNA) levels of the DNMT1 subtype (t10 = 2.306, p < .05) but had no effect on DNMT3a (t10 = .678, n.s.) or DNMT3b (t10 = .609, n.s.). (C) NMS increased protein levels of DNMT1 (t6 = 9.801, p < .0001). Data are shown as the means ± SEM. A statistically significant difference is indicated at *p < .05, **p < .01, and ****p < .0001. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; n.s., no statistically significant difference.
Neonatal Maternal Separation Selectively Reduces Retinoic Acid Receptor α Subtype Expression in ADP Cells
Because endogenous retinoic acid is involved in neural differentiation in adult hippocampus in vivo (43,44) and we used retinoic acid to induce differentiation, we hypothesized that the receptor for this ligand might mediate the impact of neonatal maternal separation. Among the three known subtypes of retinoic acid receptors, RARα, RARβ, and RARγ (45), RARα and RARβ, but not RARγ, mediates neural differentiation of embryonic neural progenitor cells (46). RARα mRNA, but not that of RARβ, was decreased in ADP cells of the NMS group (Figure 3A); RARγ mRNA was not detectable in this cell population (data not shown). Moreover, immunoblotting analysis confirmed that RARα protein was similarly reduced in ADP cells of the NMS group (Figure 3B), indicating that neonatal maternal separation selectively reduces RARα mRNA and protein in ADP cells.
Figure 3.
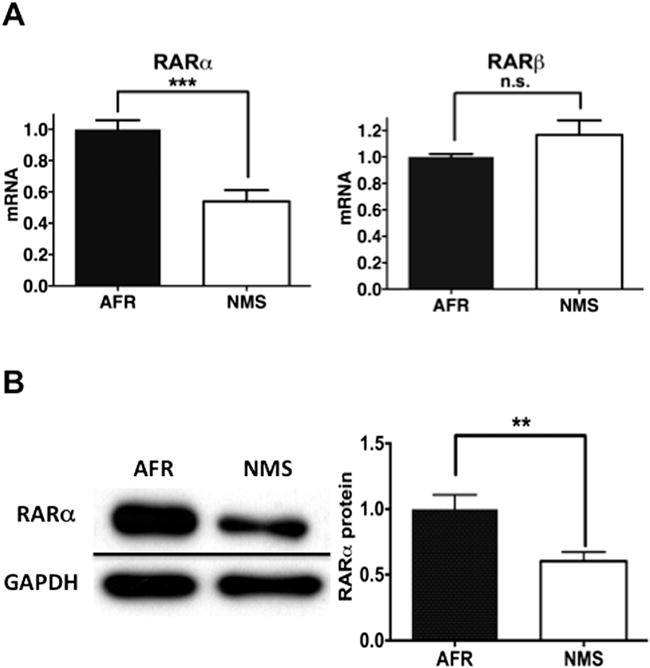
Effects of neonatal maternal separation (NMS) on expression of retinoic acid receptor (RAR)α and RARβ subtypes in adult dentate gyrus-derived neural precursor cells in vitro. (A) NMS decreased messenger RNA (mRNA) expression of RARα (t10 = 4.991, p < .001) but not RARβ (t10 = 1.513, n.s.). (B) NMS decreased expression of RARα protein (t14 = 3.088, p <.01). Data are shown as the means ± SEM. A statistically significant difference is indicated at **p < .01 or ***p < .001. AFR, animal facility reared; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; n.s., no statistically significant difference.
RARα Expression Is a Determinant of the Balance of Differentiation of ADP Cells into Neurons and Astrocytes
To more directly evaluate if RARα is functionally involved in neural differentiation of ADP cells, we examined the effect of the RARα-selective agonist CD1556 and the RARα-selective antagonist Ro 41-5253 on neural differentiation using ADP cells from the AFR group. CD1556 more robustly increased Tuj1-positive cells than retinoic acid (Figure 4A). Conversely, Ro 41-5253 reduced retinoic acid-induced differentiation into Tuj1-positive neurons (Figure 4B). These data suggest that RARα activation is a determinant for differentiation of ADP cells into neurons.
Figure 4.
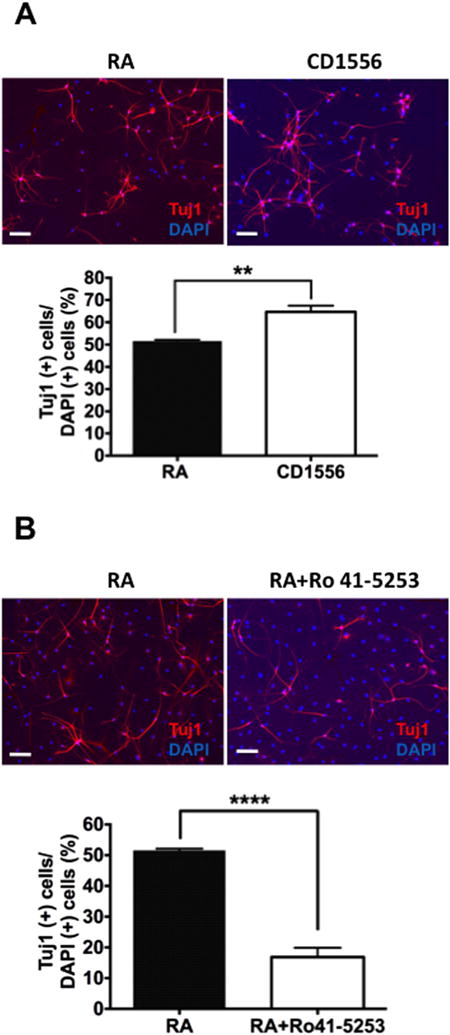
Effects of a retinoic acid receptor (RAR)α agonist (CD1556) and antagonist (Ro 41-5253) on neural differentiation of adult dentate gyrus-derived neural precursor (ADP) cells in vitro. (A) Retinoic acid (RA) and CD1556 (2 μmol/L), a selective RARα agonist, induced neural differentiation but the latter had a more robust effect (t6 = 4.651, p < .01) in ADP cells from the animal facility reared group. (B) Ro 41-5253 (1 μmol/L), a selective RARα antagonist, attenuated neural differentiation induced by RA of ADP cells in the animal facility reared group (t6 = 11.370, p < .0001). Data are shown as the means ± SEM. Statistical significance is shown at **p < .01 or ****p < .0001. DAPI, 4′,6-diamidino-2-phenylindole.
Neonatal Maternal Separation Increases Methylated RARα Promoter Levels and Reduces RARα mRNA Expression in ADP Cells
We next examined the level of DNA methylation in a RARα gene promoter in the AFR and NMS groups. RARα gene promoter was more highly methylated in the NMS group than the AFR group (Figure 5A). If DNA methylation is causally involved in diminished expression of RARα in ADP cells of the NMS group, inhibition of DNA methylation would be expected to increase RARα expression. To test this hypothesis, we added 5-aza-dC, an inhibitor of DNA methylation, to ADP cells. This treatment increased expression of RARα mRNA in ADP cells of both the AFR and NMS groups, thereby normalizing the diminished level of RARα mRNA in the NMS group (Figure 5B). These data suggest that maternal separation reduces RARα expression by increasing methylation of its promoter.
Figure 5.
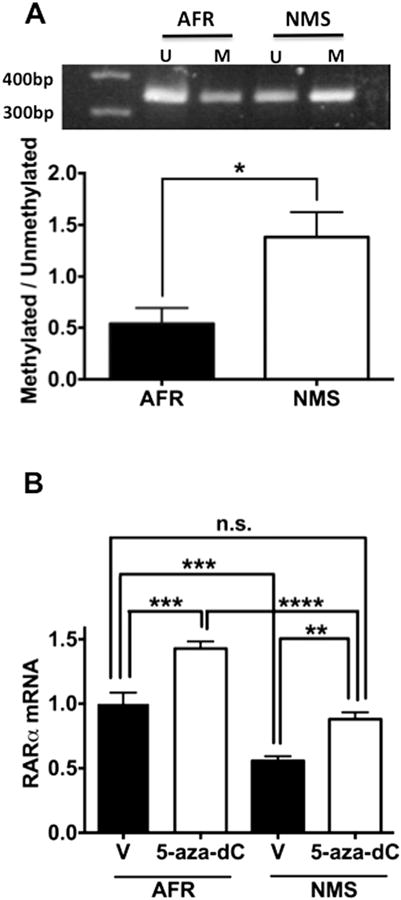
Effects of neonatal maternal separation (NMS) on methylation and methylation-dependent messenger RNA (mRNA) expression of retinoic acid receptor (RAR)α in adult dentate gyrus-derived neural precursor cells in vitro. (A) NMS increased the ratio of methylated (M) fraction to unmethylated fraction (U) of RARα promoter (t4 = 2.956, p < .05). (B) The DNA methyltransferase inhibitor 5-aza-dC (10 μmol/L) increased expression of RARα mRNA in adult dentate gyrus-derived neural precursor cells from the animal facility reared (AFR) and NMS groups (AFR vs. NMS, F1,20 = 67.160, p < .0001; vehicle [V] vs. 5-aza-dC, F1,20 = 39.090, p < .0001; interaction, F1,20 = .780, n.s.). Post hoc comparison of vehicle-treated AFR and 5-aza-dC-treated NMS groups showed that the groups did differ. Data are shown as the means ± SEM. Statistical significance is shown at *p < .05, **p < .01, ***p < .001, or ****p < .0001. n.s., no statistically significant difference.
Discussion
Our in vitro analyses showed that neonatal maternal separation diminishes the capacity of adult neural precursor cells to differentiate into neurons; increases expression of DNMT1, but not 3a or 3b; reduces expression of RARα, but not β subtype; and increases methylation of RARα promoter. Functional analysis showed that direct activation of RARα increased neural differentiation and blockade of RARα reduced neural differentiation induced by retinoic acid. Finally, inhibition of DNMT methylation reversed the reduction of neural differentiation and RARα expression seen following neonatal maternal separation (Figure 6). Taken together, our data suggest that neonatal maternal separation reduces the capacity of adult hippocampal neural precursor cells to differentiate into neurons and this effect is dependent on a reduction in RARα expression through methylation of its promoter.
Figure 6.
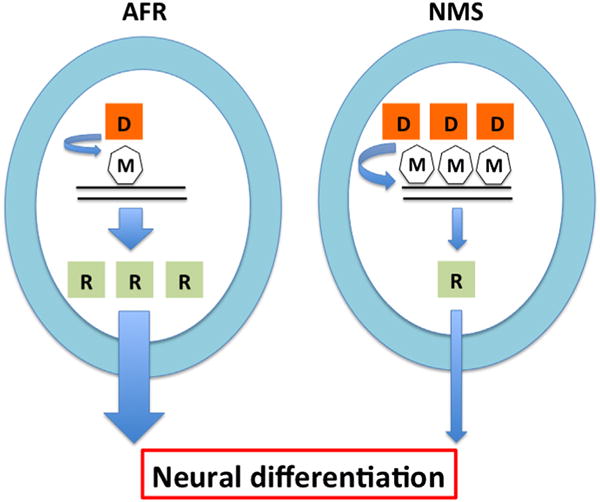
Hypothetical epigenetic mechanisms underlying the effects of neonatal maternal separation (NMS) on neural differentiation of adult dentate gyrus-derived neural precursor cells. NMS reduces neural differentiation by decreasing retinoic acid receptor α (R) expression via increased DNA methyltransferase 1 (D) expression and DNA methylation (M) at a retinoic acid receptor α promoter. AFR, animal facility reared.
Among the three DNMT subtypes, neonatal maternal separation selectively decreased DNMT1 expression, but not DNMT3a or DNMT3b. DNMT1 is highly expressed in the central nervous system of adult rodents (47), specifically in the hippocampus (48), and especially in the hippocampal dentate gyrus (49). However, DNMT3a is also highly expressed in adult hippocampal dentate gyrus (49) and mediates neural differentiation in embryonic neural stem cells (50) and in vivo adult dentate gyrus (51). DNMT3b also is present in the rat hippocampal dentate gyrus (52) and thought to contribute to embryonic neurogenesis (53). As DNMT inhibitor 5-aza-dC does not differentiate among the three DNMT subtypes, we cannot rule out the possibility that other DNMT subtypes functionally contribute to the impact of neonatal maternal separation on adult hippocampal neurogenesis.
It remains unclear exactly how NMS increases DNMT1 expression. While it is possible that NMS increased DNMT1 gene expression by decreasing methylation of the DNMT1 promoter, this is highly unlikely because there is no CpG island up to 1 kilobase of the transcription start site of DNMT1. Indirect evidence suggests that glucocorticoids might mediate this link. We recently reported that NMS resulted in increased basal and inducible corticosterone (30). More work is needed to further elucidate the upstream mechanisms of DNMT1 regulation.
We showed that neonatal maternal separation selectively decreased RARα, but not RARβ, in ADP cells. Further, an RARα agonist and antagonist facilitated and decreased, respectively, neural differentiation of this cell population. Neonatal maternal separation increased methylation at a promoter region of this gene and 5-aza-dC, a DNA methylation inhibitor, diminished methylation of this gene. Together with our observation that the methylation inhibitor also increased neural differentiation of adult hippocampal neural precursor cells, we submit that neonatal maternal separation increases methylation of RARα promoter, resulting in reduced levels of RARα expression and neural differentiation.
Given that neonatal maternal separation increased DNMT1 mRNA and protein levels and a common inhibitor of DNMTs reversed the reduction by NMS of RARα expression, the activity of DNMT1 is likely to be increased. However, there is no currently available reliable method to differentially detect activities of the three DNMT subtypes. Analysis of total DNMT activities is not suitable for validation of DNMT1-specific mRNA and protein regulation at the activity level. We also caution that because many genes are likely to contribute to neural differentiation (54), neonatal maternal separation might additionally affect differentiation of adult neural precursor cells of the hippocampal dentate gyrus through DNA methylation of other genes.
While the in vitro molecular and cellular events observed in this study could in theory be examined in vivo by knocking down genes by a viral vector, promoters designed to affect a specific cell population often do not confer intended cell specificity (55,56). Such preparation would include effects of gene knockdown in the target cells and other cell types. Moreover, in vivo isolation of a small fraction of cells (i.e., adult neural progenitor cells) and detection of epigenetic alterations in that cell population alone from tissue pose another technical challenge. A future challenge involves development of a reliable technique to validate in vitro mechanisms under in vivo conditions. Notwithstanding this challenge, our work provides an alternative to circumvent these technical difficulties in vivo and has an innovative translational value in psychiatry. Our in vitro cellular model makes it possible to delve into precise molecular mechanisms underlying neonatal stress in a select population of cells, thereby providing an assay system for development of novel therapeutic options. Drugs and other therapeutic options developed using this assay could then be directly tested, as a means of validation, for their effects on behavioral abnormalities caused by neonatal stress in rodents and ultimately in humans. Moreover, the molecular and cellular outcomes of such therapeutic options applied to rodents in vivo could be validated using our in vitro assay.
The recent discovery of many copy number variants (e.g., 22q11.2), which are robustly associated with schizophrenia and autism, as well as mood and anxiety disorders, has made it possible to establish reliable genetic mouse models of these variants (57,58). Given that these genetic variants do not show complete penetrance, genetic and environmental modifiers are likely to contribute to variability. Adult neurogenesis in the hippocampus is a potential intermediate substrate, which is altered by many environmental factors (e.g., environmental enrichment) (59,60), in addition to stress. Our in vitro assay protocol provides novel technical methods to elucidate epigenetic and molecular mechanisms underlying the impact of environmental factors on adult neurogenesis and behavioral phenotypes relevant to neuropsychiatric disorders.
Supplementary Material
Acknowledgments
This work was supported in part by a grant-in-aid No. 18591269 for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and by a grant from SENSHIN Medical Research Foundation, Japan, to SB and a National Institutes of Health grant (MH099660) to NH.
We thank Drs. T. Masuda, H. Nishikawa, Y. Omiya, N. Song, and N. Takamura for their helpful critiques.
TI has received honoraria from GlaxoSmithKline, Pfizer, Astellas, Eli Lilly, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Otsuka Pharmaceutical, Meiji Seika Pharma, Asahi Kasei Pharma, Shionogi, Janssen Pharmaceutical, Takeda Pharmaceutical, and Yoshitomi Pharmaceutical and has received grant support from Otsuka Pharmaceutical. He is a member of the advisory boards of GlaxoSmithKline, Eli Lilly, Mochida Pharmaceutical, and Mitsubishi Tanabe Pharma. SN has received honoraria from GlaxoSmithKline, Eisai, Pfizer, Daiichi-Sankyo, Meiji Seika Pharma, Ono Pharmaceutical, and Eli Lilly and has received grant support from Pfizer, Eli Lilly, Eisai, and Ono Pharmaceutical. IK has received honoraria from Eli Lilly and grant support from Takeda Pharmaceutical, Astellas, and Dainippon Sumitomo Pharma; he is a member of the advisory board of Dainippon Sumitomo Pharma and Tanabe Mitsubishi Pharma. TK has received honoraria from GlaxoSmithKline, Astellas, and Eli Lilly and grant support from Astellas and GlaxoSmithKline; he is a member of the advisory boards of GlaxoSmithKline and Mitsubishi Tanabe Pharma. NH has received honoraria from Dainippon Sumitomo Pharma. Inc., Japan.
Footnotes
All other authors declare no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2014.07.008.
References
- 1.Carr CP, Martins CM, Stingel AM, Lemgruber VB, Juruena MF. The role of early life stress in adult psychiatric disorders: A systematic review according to childhood trauma subtypes. J Nerv Ment Dis. 2013;201:1007–1020. doi: 10.1097/NMD.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 2.Geyer MA. The family of sensorimotor gating disorders: Comorbidities or diagnostic overlaps? Neurotox Res. 2006;10:211–220. doi: 10.1007/BF03033358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellenbroek BA, van den Kroonenberg PT, Cools AR. The effects of an early stressful life event on sensorimotor gating in adult rats. Schizophr Res. 1998;30:251–260. doi: 10.1016/s0920-9964(97)00149-7. [DOI] [PubMed] [Google Scholar]
- 4.Ellenbroek BA, de Bruin NM, van Den Kroonenburg PT, van Luijtelaar EL, Cools AR. The effects of early maternal deprivation on auditory information processing in adult Wistar rats. Biol Psychiatry. 2004;55:701–707. doi: 10.1016/j.biopsych.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Garner B, Wood SJ, Pantelis C, van den BM. Early maternal deprivation reduces prepulse inhibition and impairs spatial learning ability in adulthood: No further effect of post-pubertal chronic corticosterone treatment. Behav Brain Res. 2007;176:323–332. doi: 10.1016/j.bbr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 6.Husum H, Termeer E, Mathe AA, Bolwig TG, Ellenbroek BA. Early maternal deprivation alters hippocampal levels of neuropeptide Y and calcitonin-gene related peptide in adult rats. Neuropharmacology. 2002;42:798–806. doi: 10.1016/s0028-3908(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 7.Lovic V, Fleming AS. Artificially-reared female rats show reduced prepulse inhibition and deficits in the attentional set shifting task–reversal of effects with maternal-like licking stimulation. Behav Brain Res. 2004;148:209–219. doi: 10.1016/s0166-4328(03)00206-7. [DOI] [PubMed] [Google Scholar]
- 8.Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 2001;158:366–373. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- 9.Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol Biochem Behav. 2002;73:131–140. doi: 10.1016/s0091-3057(02)00781-5. [DOI] [PubMed] [Google Scholar]
- 10.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 11.Moffett MC, Vicentic A, Kozel M, Plotsky P, Francis DD, Kuhar MJ. Maternal separation alters drug intake patterns in adulthood in rats. Biochem Pharmacol. 2007;73:321–330. doi: 10.1016/j.bcp.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aisa B, Tordera R, Lasheras B, Del RJ, Ramirez MJ. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology. 2007;32:256–266. doi: 10.1016/j.psyneuen.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: Consistent or confusing? Rev Neurosci. 2000;11:383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- 14.Roceri M, Hendriks W, Racagni G, Ellenbroek BA, Riva MA. Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus. Mol Psychiatry. 2002;7:609–616. doi: 10.1038/sj.mp.4001036. [DOI] [PubMed] [Google Scholar]
- 15.Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- 16.Maekawa M, Takashima N, Matsumata M, Ikegami S, Kontani M, Hara Y, et al. Arachidonic acid drives postnatal neurogenesis and elicits a beneficial effect on prepulse inhibition, a biological trait of psychiatric illnesses. PLoS One. 2009;4:e5085. doi: 10.1371/journal.pone.0005085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 18.Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh J, Eisch AJ. Epigenetics, hippocampal neurogenesis, and neuropsychiatric disorders: Unraveling the genome to understand the mind. Neurobiol Dis. 2010;39:73–84. doi: 10.1016/j.nbd.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu Rev Cell Dev Biol. 2009;25:253–275. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- 23.Zhao X, Ueba T, Christie BR, Barkho B, McConnell MJ, Nakashima K, et al. Mice lacking methyl-CpG binding protein 1 have deficits in adult neurogenesis and hippocampal function. Proc Natl Acad Sci U S A. 2003;100:6777–6782. doi: 10.1073/pnas.1131928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Barkho BZ, Luo Y, Smrt RD, Santistevan NJ, Liu C, et al. Epigenetic regulation of the stem cell mitogen Fgf-2 by Mbd1 in adult neural stem/progenitor cells. J Biol Chem. 2008;283:27644–27652. doi: 10.1074/jbc.M804899200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allan AM, Liang X, Luo Y, Pak C, Li X, Szulwach KE, et al. The loss of methyl-CpG binding protein 1 leads to autism-like behavioral deficits. Hum Mol Genet. 2008;17:2047–2057. doi: 10.1093/hmg/ddn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boku S, Nakagawa S, Masuda T, Nishikawa H, Kato A, Kitaichi Y, et al. Glucocorticoids and lithium reciprocally regulate the proliferation of adult dentate gyrus-derived neural precursor cells through GSK-3beta and beta-catenin/TCF pathway. Neuropsychopharmacology. 2009;34:805–815. doi: 10.1038/npp.2008.198. [DOI] [PubMed] [Google Scholar]
- 27.Sengupta P. The laboratory rat: Relating its age with human’s. Int J Prev Med. 2013;4:624–630. [PMC free article] [PubMed] [Google Scholar]
- 28.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 29.Lu L, Mamiya T, Lu P, Niwa M, Mouri A, Zou LB, et al. The long-lasting effects of cross-fostering on the emotional behavior in ICR mice. Behav Brain Res. 2009;198:172–178. doi: 10.1016/j.bbr.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 30.Toda H, Boku S, Nakagawa S, Inoue T, Kato A, Takamura N, et al. Maternal separation enhances conditioned fear and decreases the mRNA levels of the neurotensin receptor 1 gene with hypermethylation of this gene in the rat amygdala. PLoS One. 2014;9:e97421. doi: 10.1371/journal.pone.0097421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews K, Robbins TW. Early experience as a determinant of adult behavioural responses to reward: The effects of repeated maternal separation in the rat. Neurosci Biobehav Rev. 2003;27:45–55. doi: 10.1016/s0149-7634(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 32.Kosten TA, Kehoe P. Immediate and enduring effects of neonatal isolation on maternal behavior in rats. Int J Dev Neurosci. 2010;28:53–61. doi: 10.1016/j.ijdevneu.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci. 2004;19:1863–1874. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- 34.Kalinichev M, Easterling KW, Holtzman SG. Early neonatal experience of Long-Evans rats results in long-lasting changes in reactivity to a novel environment and morphine-induced sensitization and tolerance. Neuropsychopharmacology. 2002;27:518–533. doi: 10.1016/S0893-133X(02)00326-3. [DOI] [PubMed] [Google Scholar]
- 35.Kosten TA, Kehoe P. Neonatal isolation is a relevant model for studying the contributions of early life stress to vulnerability to drug abuse: Response to Marmendal et al. (2004) Dev Psychobiol. 2005;47:108–110. doi: 10.1002/dev.20083. [DOI] [PubMed] [Google Scholar]
- 36.Palmer TD, Ray J, Gage FH. FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci. 1995;6:474–486. doi: 10.1006/mcne.1995.1035. [DOI] [PubMed] [Google Scholar]
- 37.Boku S, Hisaoka-Nakashima K, Nakagawa S, Kato A, Kajitani N, Inoue T, et al. Tricyclic antidepressant amitriptyline indirectly increases the proliferation of adult dentate gyrus-derived neural precursors: An involvement of astrocytes. PLoS One. 2013;8:e79371. doi: 10.1371/journal.pone.0079371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boku S, Nakagawa S, Masuda T, Nishikawa H, Kato A, Toda H, et al. Effects of mood stabilizers on adult dentate gyrus-derived neural precursor cells. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:111–117. doi: 10.1016/j.pnpbp.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 39.Yu IT, Park JY, Kim SH, Lee JS, Kim YS, Son H. Valproic acid promotes neuronal differentiation by induction of proneural factors in association with H4 acetylation. Neuropharmacology. 2009;56:473–480. doi: 10.1016/j.neuropharm.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 40.Busslinger M, Hurst J, Flavell RA. DNA methylation and the regulation of globin gene expression. Cell. 1983;34:197–206. doi: 10.1016/0092-8674(83)90150-2. [DOI] [PubMed] [Google Scholar]
- 41.Murray EJ, Grosveld F. Site specific demethylation in the promoter of human gamma-globin gene does not alleviate methylation mediated suppression. EMBO J. 1987;6:2329–2335. doi: 10.1002/j.1460-2075.1987.tb02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robertson KD, Uzvolgyi E, Liang G, Talmadge C, Sumegi J, Gonzales FA, Jones PA. The human DNA methyltransferases (DNMTs) 1, 3a and 3b: Coordinate mRNA expression in normal tissues and overexpression in tumors. Nucleic Acids Res. 1999;27:2291–2298. doi: 10.1093/nar/27.11.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacobs S, Lie DC, DeCicco KL, Shi Y, DeLuca LM, Gage FH, Evans RM. Retinoic acid is required early during adult neurogenesis in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:3902–3907. doi: 10.1073/pnas.0511294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCaffery P, Zhang J, Crandall JE. Retinoic acid signaling and function in the adult hippocampus. J Neurobiol. 2006;66:780–791. doi: 10.1002/neu.20237. [DOI] [PubMed] [Google Scholar]
- 45.Leid M, Kastner P, Chambon P. Multiplicity generates diversity in the retinoic acid signalling pathways. Trends Biochem Sci. 1992;17:427–433. doi: 10.1016/0968-0004(92)90014-z. [DOI] [PubMed] [Google Scholar]
- 46.Goncalves MB, Boyle J, Webber DJ, Hall S, Minger SL, Corcoran JP. Timing of the retinoid-signalling pathway determines the expression of neuronal markers in neural progenitor cells. Dev Biol. 2005;278:60–70. doi: 10.1016/j.ydbio.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 47.Goto K, Numata M, Komura JI, Ono T, Bestor TH, Kondo H. Expression of DNA methyltransferase gene in mature and immature neurons as well as proliferating cells in mice. Differentiation. 1994;56:39–44. doi: 10.1046/j.1432-0436.1994.56120039.x. [DOI] [PubMed] [Google Scholar]
- 48.Simmons RK, Stringfellow SA, Glover ME, Wagle AA, Clinton SM. DNA methylation markers in the postnatal developing rat brain. Brain Res. 2013;1533:26–36. doi: 10.1016/j.brainres.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown SE, Weaver IC, Meaney MJ, Szyf M. Regional-specific global cytosine methylation and DNA methyltransferase expression in the adult rat hippocampus. Neurosci Lett. 2008;440:49–53. doi: 10.1016/j.neulet.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 50.Wu Z, Huang K, Yu J, Le T, Namihira M, Liu Y, et al. Dnmt3a regulates both proliferation and differentiation of mouse neural stem cells. J Neurosci Res. 2012;90:1883–1891. doi: 10.1002/jnr.23077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu H, Coskun V, Tao J, Xie W, Ge W, Yoshikawa K, et al. Dnmt3a-dependent nonpromoter DNA methylation facilitates transcription of neurogenic genes. Science. 2010;329:444–448. doi: 10.1126/science.1190485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia-Fuster MJ, Flagel SB, Mahmood ST, Mayo LM, Thompson RC, Watson SJ, Akil H. Decreased proliferation of adult hippocampal stem cells during cocaine withdrawal: Possible role of the cell fate regulator FADD. Neuropsychopharmacology. 2011;36:2303–2317. doi: 10.1038/npp.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feng J, Chang H, Li E, Fan G. Dynamic expression of de novo DNA methyltransferases Dnmt3a and Dnmt3b in the central nervous system. J Neurosci Res. 2005;79:734–746. doi: 10.1002/jnr.20404. [DOI] [PubMed] [Google Scholar]
- 54.Castro DS, Guillemot F. Old and new functions of proneural factors revealed by the genome-wide characterization of their transcriptional targets. Cell Cycle. 2011;10:4026–4031. doi: 10.4161/cc.10.23.18578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nathanson JL, Yanagawa Y, Obata K, Callaway EM. Preferential labeling of inhibitory and excitatory cortical neurons by endogenous tropism of adeno-associated virus and lentivirus vectors. Neuroscience. 2009;161:441–450. doi: 10.1016/j.neuroscience.2009.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nathanson JL, Jappelli R, Scheeff ED, Manning G, Obata K, Brenner S, Callaway EM. Short promoters in viral vectors drive selective expression in mammalian inhibitory neurons, but do not restrict activity to specific inhibitory cell-types. Front Neural Circuits. 2009;3:19. doi: 10.3389/neuro.04.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hiroi N, Takahashi T, Hishimoto A, Izumi T, Boku S, Hiramoto T. Copy number variation at 22q11.2: From rare variants to common mechanisms of developmental neuropsychiatric disorders. Mol Psychiatry. 2013;18:1153–1165. doi: 10.1038/mp.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hiroi N. Small cracks in the dam: Rare genetic variants provide opportunities to delve into mechanisms of neuropsychiatric disorders. Biol Psychiatry. 2014;76:91–92. doi: 10.1016/j.biopsych.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 60.Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol. 1999;39:569–578. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


