Abstract
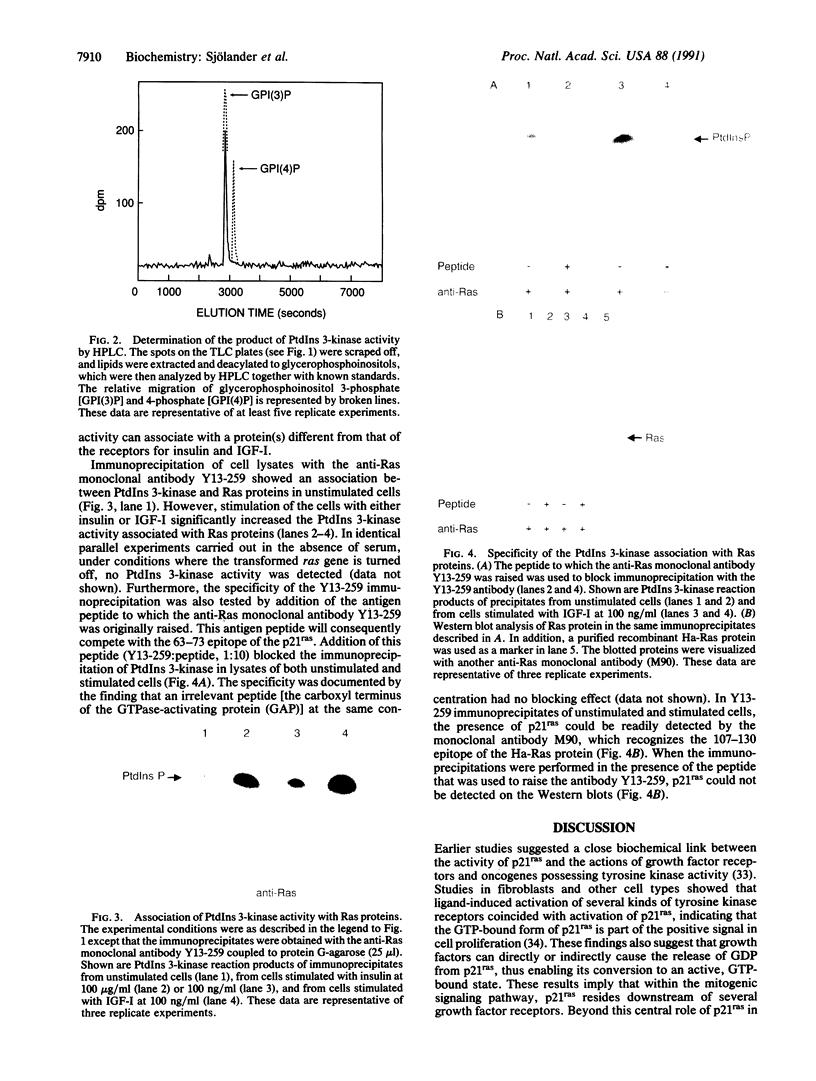
In mammalian cells, ras genes code for 21-kDa GTP-binding proteins. Increased expression and mutations in specific amino acids have been closely linked to alterations of normal cell morphology, growth, and differentiation and, in particular, to neoplastic transformation. The signal transduction induced by these p21ras proteins is largely unknown; however, the signaling pathways of several growth factors have been reported to involve phosphatidylinositol (PtdIns) 3-kinase. In the present study of a Ha-ras-transformed epithelial cell line, we demonstrated increased PtdIns 3-kinase activity in anti-phosphotyrosine and anti-receptor (insulin and hybrid insulin-like growth factor I) immunoprecipitates of cells that had been stimulated with insulin or insulin-like growth factor I. The PtdIns 3-kinase activity was also immunoprecipitated in these experiments by the anti-Ras monoclonal antibody Y13-259. The specificity of this association with p21ras was ascertained by the neutralizing effect of the antigen peptide and the absence of PtdIns 3-kinase activity in Y13-259 immunoprecipitates from cells in which the ras gene was turned off. These data indicate that PtdIns 3-kinase activity is an important step in the cascade of reactions in p21ras signal transduction, suggesting that the alterations of the cytoskeleton and growth in ras-transformed cells could be mediated by PtdIns 3-kinase activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres A. C., van der Valk M. A., Schönenberger C. A., Flückiger F., LeMeur M., Gerlinger P., Groner B. Ha-ras and c-myc oncogene expression interferes with morphological and functional differentiation of mammary epithelial cells in single and double transgenic mice. Genes Dev. 1988 Nov;2(11):1486–1495. doi: 10.1101/gad.2.11.1486. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bengtsson T., Rundquist I., Stendahl O., Wymann M. P., Andersson T. Increased breakdown of phosphatidylinositol 4,5-bisphosphate is not an initiating factor for actin assembly in human neutrophils. J Biol Chem. 1988 Nov 25;263(33):17385–17389. [PubMed] [Google Scholar]
- Bos J. L. ras oncogenes in human cancer: a review. Cancer Res. 1989 Sep 1;49(17):4682–4689. [PubMed] [Google Scholar]
- Bouton A. H., Kanner S. B., Vines R. R., Wang H. C., Gibbs J. B., Parsons J. T. Transformation by pp60src or stimulation of cells with epidermal growth factor induces the stable association of tyrosine-phosphorylated cellular proteins with GTPase-activating protein. Mol Cell Biol. 1991 Feb;11(2):945–953. doi: 10.1128/mcb.11.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Carpenter C. L., Cantley L. C. Phosphoinositide kinases. Biochemistry. 1990 Dec 25;29(51):11147–11156. doi: 10.1021/bi00503a001. [DOI] [PubMed] [Google Scholar]
- Clarke N. G., Dawson R. M. Alkaline O leads to N-transacylation. A new method for the quantitative deacylation of phospholipids. Biochem J. 1981 Apr 1;195(1):301–306. doi: 10.1042/bj1950301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condorelli G., Formisano P., Villone G., Smith R. J., Beguinot F. Insulin and insulin-like growth factor I (IGF I) stimulate phosphorylation of a Mr 175,000 cytoskeleton-associated protein in intact FRTL5 cells. J Biol Chem. 1989 Jul 25;264(21):12633–12638. [PubMed] [Google Scholar]
- Coughlin S. R., Escobedo J. A., Williams L. T. Role of phosphatidylinositol kinase in PDGF receptor signal transduction. Science. 1989 Mar 3;243(4895):1191–1194. doi: 10.1126/science.2466336. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A., Heber A. An 81 kd protein complexed with middle T antigen and pp60c-src: a possible phosphatidylinositol kinase. Cell. 1987 Sep 25;50(7):1031–1037. doi: 10.1016/0092-8674(87)90169-3. [DOI] [PubMed] [Google Scholar]
- Dadabay C. Y., Patton E., Cooper J. A., Pike L. J. Lack of correlation between changes in polyphosphoinositide levels and actin/gelsolin complexes in A431 cells treated with epidermal growth factor. J Cell Biol. 1991 Mar;112(6):1151–1156. doi: 10.1083/jcb.112.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle M., Traynor-Kaplan A. E., Sklar L. A., Norgauer J. Is there a relationship between phosphatidylinositol trisphosphate and F-actin polymerization in human neutrophils? J Biol Chem. 1990 Oct 5;265(28):16725–16728. [PubMed] [Google Scholar]
- Ellis C., Moran M., McCormick F., Pawson T. Phosphorylation of GAP and GAP-associated proteins by transforming and mitogenic tyrosine kinases. Nature. 1990 Jan 25;343(6256):377–381. doi: 10.1038/343377a0. [DOI] [PubMed] [Google Scholar]
- Endemann G., Yonezawa K., Roth R. A. Phosphatidylinositol kinase or an associated protein is a substrate for the insulin receptor tyrosine kinase. J Biol Chem. 1990 Jan 5;265(1):396–400. [PubMed] [Google Scholar]
- Escobedo J. A., Navankasattusas S., Kavanaugh W. M., Milfay D., Fried V. A., Williams L. T. cDNA cloning of a novel 85 kd protein that has SH2 domains and regulates binding of PI3-kinase to the PDGF beta-receptor. Cell. 1991 Apr 5;65(1):75–82. doi: 10.1016/0092-8674(91)90409-r. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Hanafusa H. Requirement of phosphatidylinositol-3 kinase modification for its association with p60src. Mol Cell Biol. 1991 Apr;11(4):1972–1979. doi: 10.1128/mcb.11.4.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager G. L. Expression of a viral oncogene under control of the mouse mammary tumor virus promoter: a new system for the study of glucocorticoid regulation. Prog Nucleic Acid Res Mol Biol. 1983;29:193–203. doi: 10.1016/s0079-6603(08)60447-x. [DOI] [PubMed] [Google Scholar]
- Huber B. E., Cordingley M. G. Expression and phenotypic alterations caused by an inducible transforming ras oncogene introduced into rat liver epithelial cells. Oncogene. 1988 Sep;3(3):245–256. [PubMed] [Google Scholar]
- Kaplan D. R., Whitman M., Schaffhausen B., Pallas D. C., White M., Cantley L., Roberts T. M. Common elements in growth factor stimulation and oncogenic transformation: 85 kd phosphoprotein and phosphatidylinositol kinase activity. Cell. 1987 Sep 25;50(7):1021–1029. doi: 10.1016/0092-8674(87)90168-1. [DOI] [PubMed] [Google Scholar]
- Lacal J. C., Aaronson S. A. ras p21 deletion mutants and monoclonal antibodies as tools for localization of regions relevant to p21 function. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5400–5404. doi: 10.1073/pnas.83.15.5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassing I., Lindberg U. Specific interaction between phosphatidylinositol 4,5-bisphosphate and profilactin. Nature. 1985 Apr 4;314(6010):472–474. doi: 10.1038/314472a0. [DOI] [PubMed] [Google Scholar]
- Majerus P. W., Ross T. S., Cunningham T. W., Caldwell K. K., Jefferson A. B., Bansal V. S. Recent insights in phosphatidylinositol signaling. Cell. 1990 Nov 2;63(3):459–465. doi: 10.1016/0092-8674(90)90442-h. [DOI] [PubMed] [Google Scholar]
- McCormick F. ras GTPase activating protein: signal transmitter and signal terminator. Cell. 1989 Jan 13;56(1):5–8. doi: 10.1016/0092-8674(89)90976-8. [DOI] [PubMed] [Google Scholar]
- Moxham C. P., Duronio V., Jacobs S. Insulin-like growth factor I receptor beta-subunit heterogeneity. Evidence for hybrid tetramers composed of insulin-like growth factor I and insulin receptor heterodimers. J Biol Chem. 1989 Aug 5;264(22):13238–13244. [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- Nolan R. D., Lapetina E. G. Thrombin stimulates the production of a novel polyphosphoinositide in human platelets. J Biol Chem. 1990 Feb 15;265(5):2441–2445. [PubMed] [Google Scholar]
- Ostrowski M. C., Huang A. L., Kessel M., Wolford R. G., Hager G. L. Modulation of enhancer activity by the hormone responsive regulatory element from mouse mammary tumor virus. EMBO J. 1984 Aug;3(8):1891–1899. doi: 10.1002/j.1460-2075.1984.tb02064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu M., Hiles I., Gout I., Fry M. J., Ruiz-Larrea F., Panayotou G., Thompson A., Dhand R., Hsuan J., Totty N. Characterization of two 85 kd proteins that associate with receptor tyrosine kinases, middle-T/pp60c-src complexes, and PI3-kinase. Cell. 1991 Apr 5;65(1):91–104. doi: 10.1016/0092-8674(91)90411-q. [DOI] [PubMed] [Google Scholar]
- Price B. D., Morris J. D., Marshall C. J., Hall A. Scrape-loaded p21ras down-regulates agonist-stimulated inositol phosphate production by a mechanism involving protein kinase C. Biochem J. 1989 May 15;260(1):157–161. doi: 10.1042/bj2600157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechler M. M., Nissley S. P. The nature and regulation of the receptors for insulin-like growth factors. Annu Rev Physiol. 1985;47:425–442. doi: 10.1146/annurev.ph.47.030185.002233. [DOI] [PubMed] [Google Scholar]
- Santos E., Nebreda A. R. Structural and functional properties of ras proteins. FASEB J. 1989 Aug;3(10):2151–2163. doi: 10.1096/fasebj.3.10.2666231. [DOI] [PubMed] [Google Scholar]
- Satoh T., Endo M., Nakafuku M., Akiyama T., Yamamoto T., Kaziro Y. Accumulation of p21ras.GTP in response to stimulation with epidermal growth factor and oncogene products with tyrosine kinase activity. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7926–7929. doi: 10.1073/pnas.87.20.7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjölander A., Grönroos E., Hammarström S., Andersson T. Leukotriene D4 and E4 induce transmembrane signaling in human epithelial cells. Single cell analysis reveals diverse pathways at the G-protein level for the influx and the intracellular mobilization of Ca2+. J Biol Chem. 1990 Dec 5;265(34):20976–20981. [PubMed] [Google Scholar]
- Stacey D. W., Kung H. F. Transformation of NIH 3T3 cells by microinjection of Ha-ras p21 protein. Nature. 1984 Aug 9;310(5977):508–511. doi: 10.1038/310508a0. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. From signal to pseudopod. How cells control cytoplasmic actin assembly. J Biol Chem. 1989 Nov 5;264(31):18261–18264. [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Varticovski L., Druker B., Morrison D., Cantley L., Roberts T. The colony stimulating factor-1 receptor associates with and activates phosphatidylinositol-3 kinase. Nature. 1989 Dec 7;342(6250):699–702. doi: 10.1038/342699a0. [DOI] [PubMed] [Google Scholar]
- Whitman M., Downes C. P., Keeler M., Keller T., Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 1988 Apr 14;332(6165):644–646. doi: 10.1038/332644a0. [DOI] [PubMed] [Google Scholar]
- Whitman M., Kaplan D. R., Schaffhausen B., Cantley L., Roberts T. M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985 May 16;315(6016):239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Graziani A., Carpenter C., Cantley L. C., Lapetina E. G. A novel pathway for the formation of phosphatidylinositol 3,4-bisphosphate. Phosphorylation of phosphatidylinositol 3-monophosphate by phosphatidylinositol-3-monophosphate 4-kinase. J Biol Chem. 1990 Dec 25;265(36):22086–22089. [PubMed] [Google Scholar]
- Zick Y., Sasaki N., Rees-Jones R. W., Grunberger G., Nissley S. P., Rechler M. M. Insulin-like growth factor-I (IGF-I) stimulates tyrosine kinase activity in purified receptors from a rat liver cell line. Biochem Biophys Res Commun. 1984 Feb 29;119(1):6–13. doi: 10.1016/0006-291x(84)91610-3. [DOI] [PubMed] [Google Scholar]