Abstract
Objectives
Effects of daily caffeine consumption on open-field behaviours, serum corticosterone and brain antioxidant levels were investigated after six hours of total sleep-deprivation in prepubertal mice. We tested the hypothesis that daily caffeine consumption may significantly alter behaviour, stress and antioxidative response of prepubertal mice to an acute episode of total sleep-deprivation.
Methods
Prepubertal Swiss mice of both sexes were assigned to two main groups of 120 each (subdivided into 6 groups of 10 each, based on sex), and administered vehicle or graded oral doses of caffeine (10, 20, 40, 80 and 120 mg/kg/day) for 14 days. On day 14, a main group was subjected to 6 h of total sleep-deprivation by ‘gentle-handling’. Open-field behaviours were then assessed in both groups, after which animals were euthanized, and levels of corticosterone, superoxide dismutase and glutathione peroxidase assayed.
Results
Horizontal locomotion, rearing and grooming increased significantly, compared to control, with sleep-deprived (SD) mice showing stronger caffeine-driven responses at higher doses; and SD female mice showing sustained response to caffeine, compared to respective males. Plasma corticosterone increased with increasing doses of caffeine in both non sleep-deprived (NSD) and SD mice; although SD mice had higher corticosterone levels. Sleep-deprivation and/or higher doses of caffeine were associated with derangements in brain antioxidant levels.
Conclusion
Repeated caffeine consumption and/or acute sleep-deprivation led to significant changes in pattern of open-field behaviour and stress/antioxidant response in mice. Responses seen in the study are probably due to modulatory effects of caffeine on the total body response to stressful stimuli.
Keywords: Sleep-deprivation, Caffeine, Sex, Open-field arena, Stress hormones, Antioxidant
1. Introduction
Caffeine is a central nervous system stimulator that belongs to the class of molecules known as xanthines [1], [2]. Caffeine is found in tea, coffee, mate, guarana paste and kola nuts, and it is consumed globally, irrespective of age or social status [3]. In humans, studies continue to shed light on the behavioural effects of caffeine [4], [5], especially in adults; however, there is also a noticeable increase in the consumption of caffeine and caffeine-containing products by children and adolescents [6], [7], [8], [9]. Studies have also been conducted to evaluate caffeine safety in the young [6], [7], and to determine dose/consumer-dependent influences of caffeine use on the heart, blood-pressure and general body physiology in children [8], [9]; however, in comparison to adults, data on caffeine research in the young is still less available.
A major reason for deliberate caffeine consumption is to combat sleepiness. Sleepiness is defined as difficulty in maintaining alertness during the major wake period of the day, resulting in unintended lapses into drowsiness or sleep [10]. Sleepiness is a known consequence of sleep-deprivation in healthy humans; however, the quantity of sleep needed by children, adolescents and young adults is still debated [11], [12]. Recent literature reveal that about 7–9 h of sleep is adequate in young adults (18−25), 8–10 h in teenagers and 9–11 h in school aged children (6−13) [13]. It is also reported that sleep-deficits are higher in adolescents compared to other age-groups, with females showing greater need for sleep than males [14]. A number of human studies have reported no difference in total sleep time all through the adolescence period on non-school days, and significant loss of total sleep time only during school days [15], [16], suggesting psychosocial factors as a sleep-time determinant in adolescents [17]. Sleep loss, and poor quality of sleep have been associated with alterations in emotional behaviour and decreased quality of life in young adults [18], and now studies are beginning to show evidence of increasing caffeine consumption in adolescents, to help cope with sleep loss [19]. Therefore, in this group, cycles of caffeine consumption and insufficient sleep tend to succeed each other.
The existence of sex differences in the response to caffeine in young subjects have been studied in humans [8], [20] and rodents [21], [22], [23], with different conclusions. Soellner et al. [24] reported improved object-discrimination in female rats, following caffeine ingestion. Elkins et al. [20], looking at effects of caffeine in prepubertal boys, concluded that memory-impairment associated with caffeine in males was due to increased vigilance and decreased reaction-time with caffeine at lower doses. Fischer and Guillet [25] reported sex-differences in caffeine effect on memory retention in neonatal rats; however Temple et al., [8] reported no sex-differences in cardiovascular response to caffeine in adolescent boys or girls. The differences in the results of these studies would suggest that in the outcomes of tasks involving caffeine administration, the influences of sex are probably task-specific. Sex-differences in drug self-administration was ruled out in the present study, by giving caffeine through gavage; since studies have reported that female rodents are more vulnerable to stimulant self administration [26], [27] or drug seeking behaviour [28].
There is a dearth of information on the influence of sex on effects of caffeine on open- field behaviours in rodents or motor activity in humans; and where available, results of studies vary. Uzbay et al. [23] reported the absence of sex-differences with caffeine administration in mice, although a sex-differential response was seen with agmatine on caffeine-induced locomotor response. In an earlier study [21], we reported that male mice (more than females) showed an early locomotor response, although females showed higher basal levels of locomotion. However, the present study differs from our previous study in a few areas: age of animals used, doses of caffeine used and presence of sleep-deprivation.
Sleep-deprivation by gentle-handling is a stressor, and has been shown to be associated with elevated corticosterone [29] and alterations in antioxidant activity [30], [31]. Oxidative stress occurs due to an imbalance between the production of oxidants and the strength of antioxidant defences. These imbalances can cause structural changes due to oxidation of proteins, lipids, and nucleic acids [32]. A number of studies have reported evidence of oxidative stress following acute or chronic sleep-deprivation [31], [32], [33], [34]. An overall effect of sleep-deprivation on the body is a general sympathetic activation.
The rationale for this study was the need to answer the question: can a history of caffeine consumption affect behavioural phenotypes, stress and antioxidant response, after a single episode of total sleep deprivation? Therefore, we tested the hypothesis that background caffeine consumption may significantly alter novelty-induced behaviours, plasma corticosterone levels, superoxide dismutase and glutathione peroxidase activity in prepubertal mice (with or without sleep deprivation); and that these parameters could be influenced by sex.
2. Methods
2.1. Drugs
99.9% anhydrous caffeine (E. Merck, Darmstadt, Germany) was weighed and dissolved in distilled water to get the desired concentrations. Caffeine (10, 20, 40, 80 and 120 mg/kg) was administered orally by using a cannula. The selection of these doses was based on a previous study [22], [35], although cognizance was also taken of the LD50 (oral) of caffeine in mice which is 127 mg/kg (male) and 137 mg/kg (female) [36].
2.2. Animals
Five-week old Swiss mice (Empire Breeders, Osogbo, Osun State, Nigeria) weighing 10–15 g at the commencement of study were used. Male and female mice were housed separately in plastic cages measuring 16×12×10 in. (10 mice in each cage). General housing is a temperature-controlled (22.5 °C±2.5 °C) quarters with 12 h of light. Mice had free access to food and water except during the behavioural test. All animals were fed commercial standard chow (Calories: 29% protein, 13% fat, 58% carbohydrate) from weaning. All procedures were conducted in accordance with the approved institutional protocols and within the provisions for animal care and use prescribed in the scientific procedures on living animals, European Council Directive (EU2010/63).
2.3. Experimental methods
Two main groups comprising one hundred and twenty mice each were used (i.e. 120 males and 120 females). Each main group was further subdivided into: Sleep deprived (SD) or non sleep-deprived (NSD) of 60 each. Each subdivision has six sub-groups of 10 animals each (Table 1); which received either vehicle (distilled water) or one of five doses of caffeine (10, 20, 40, 80 and 120 mg/kg/day) for a period of 14 days. Tests were carried out after the last dose of caffeine or vehicle in the NSD groups. Mice in the sleep-deprived groups (SD) were subjected to six hours of gentle-handling after the last dose of vehicle or caffeine, and then exposed to the behavioural tests. Immediately after the behavioural tests, animals were euthanized and blood taken via cardiac puncture for estimation of plasma corticosterone, while whole brain homogenates were used for estimation of antioxidant levels.
Table 1.
Experimental groups, time-line and treatments administered.
| Day 1–14 | Day 14 | ||
|---|---|---|---|
| Group/Treatment | DW or Caffeine | SD(6 h) or NSD | Open-field (30 min) |
| Vehicle | + | + | + |
| 10 mg/kg | + | + | + |
| 20 mg/kg | + | + | + |
| 40 mg/kg | + | + | + |
| 80 mg/kg | + | + | + |
| 120 mg/kg | + | + | + |
Number of animals/group=10, Total number of animals/main group=120 (60 NSD, 60 SD), NSD: non sleep-deprived, SD: sleep-deprived, DW: distilled water
2.3.1. Sleep-deprivation
Gentle-handling as a method of sleep-deprivation was first used in feline and rodents [37]. It has also been validated for use as a method of total sleep-deprivation in mice [38]. It is a widely accepted as a way to keep mice awake for periods of hours, while minimally disturbing ongoing activity. Gentle-handling was the method of total sleep- deprivation employed in this study. The gentle-handling protocol used lasted for 6 h, starting at 7 a.m. It involved cage-shaking, touching the animals with the hand or a soft brush, introducing novel objects into the cage and cage-tapping.
2.3.2. Behavioural test
Behavioural tests were conducted in a quiet room between the hours of 1 p.m and 3 p.m on day 14 of administration (5 animals/day). On the test days, mice were transported in their home cages to the testing room, and allowed to acclimatise for 30 min before testing. At the beginning of the test, each mouse was placed in the open-field box and its behaviour videotaped for subsequent analysis. After testing, the mouse was removed from the maze and all interior surfaces of the open-field box were cleaned thoroughly with 70% ethanol, and then wiped dry to remove any trace of odour.
2.3.2.1. Open field novelty-induced behaviours
The open-field test is a complex behavioural paradigm whose components have been used and accepted as measures of exploration and general activity or locomotion [21], [39], [40] in rodents. The open-field used in this study is a wooden rectangular arena composed of a floor measuring 36×36×26 cm. The floor was divided by permanent red markings into 16 equal squares at the bottom. It was placed in a sound-isolated room with dim lighting. Locomotion (number of floor units entered with all paws), rearing frequency (number of times the animal stood on its hind legs or with its forearms against the walls of the observation cage or free in the air) and frequency of grooming (number of body-cleaning with paws, picking of the body and pubis with the mouth, and face-washing actions) were recorded for thirty minutes, and scored. An increase in locomotion, rearing and grooming connotes a central excitatory response, while a decrease connotes central inhibition.
2.3.3. Biochemical assay
2.3.3.1. Corticosterone assay
Plasma corticosterone (CORT) levels in NSD/SD mice were analysed using commercially-available corticosterone ELISA assay kit, which is a competitive enzyme immunoassay for the quantitative measurement of corticosterone. Standard or samples were added in duplicate to wells of the micro-titre plate. Corticosterone conjugate and corticosterone antibody were added to each well and allowed to incubate for 2 h at room temperature. Unbound enzyme was washed. Streptavidin-peroxidase conjugate was added to the wells and incubated for 30 min, following which plates were washed again and chromogen substrate was added to each well and allowed to incubate for about 20 min. Stop solution was then added and absorbance read immediately at 450 nm. The concentration of corticosterone was calculated according to the standard curves.
2.3.3.2. Brain antioxidant assay
The whole brain was removed and homogenized (1:10, w/v) in ice-cold phosphate-buffered saline using Teflon-glass homogenizer. Homogenate was centrifuged at 10,000 rpm at 4 °C for 15 min to remove cell debris. Superoxide dismutase level was assayed (using a UV–vis spectrophotometer) based on the enzyme's ability to inhibit phenazine methosulphate-mediated reduction of nitro-blue tetrazolium dye; the change in absorbance at 560 nm over 5 min was measured. Glutathione peroxidase (GPX) catalyzes the reduction of hydroperoxides (like hydrogen peroxide), by reduced glutathione (GSH) and functions to protect the cell from oxidative damage. GPX levels measure the change in absorbance at 340 nm that follow NADPH consumption in the presence of H2O2, according to the manufacturer's instruction.
2.4. Statistical analysis
Data were analysed using Chris Rorden's ezANOVA for windows, version 0.98. We tested the hypothesis that background caffeine consumption may significantly alter novelty-induced behaviours, plasma corticosterone levels, superoxide dismutase and glutathione peroxidase activity in prepubertal mice (with or without sleep deprivation); and that the responses may be modulated by sex, using analysis of variance (ANOVA). Multi-factorial ANOVA was used to test effects of 3 main factors (dose of caffeine, sex and sleep-deprivation) on locomotion, rearing, grooming, plasma corticosterone, and brain antioxidant levels. Tukey HSD test was used for within and between-group comparisons. Results are expressed as mean±(S.E.M), p values less than 0.05 were considered statistically significant.
3. Results
3.1. Horizontal locomotion
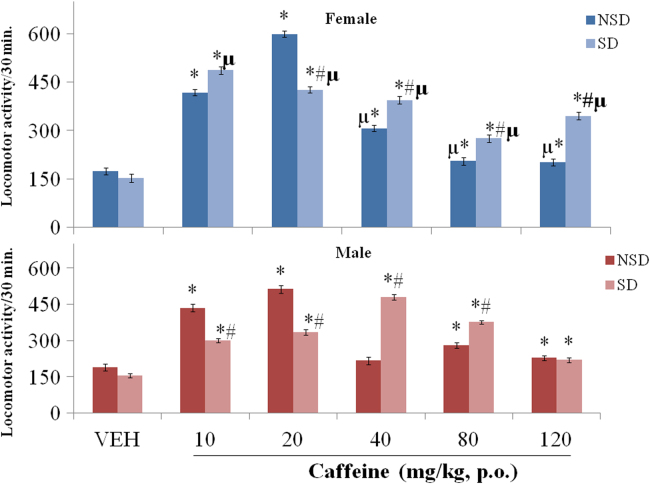
Fig. 1 shows effect of increasing doses of caffeine on horizontal locomotion in sleep-deprived (SD) and non sleep-deprived (NSD) male/female mice. MANOVA with drug dose, sex and sleep deprivation as main factors revealed a significant effect of drug dose {F (5,120) = 119, p<0.001} and sex {F (1,120) = 6.94, p<0.011}, with no significant effect of sleep-deprivation {F (1,120) = 1.68, p<0.421). There was however significant interactions between drug dosage×sex (F (5,120) = 9.32 p<0.001), drug dosage×sleep- deprivation {F(5,120) = 27.0, p<0.001}, sex×sleep-deprivation {F(1,120) = 8.82 p<0.003} and drug dosage×sex ×sleep-deprivation {F(5,120) = 14.2 p<0.001}.
Fig. 1.
Effect of Caffeine on horizontal locomotion in NSD/SD female and male mice. Each bar represents mean±SE.M. Comparisons are: *p<0.05 versus VEH, #p<0.05 SD versus NSD mice, µp<0.05 female versus male. VEH: Vehicle, SD: sleep deprived, NSD: non sleep deprived, mg/kg, p.o: milligram/kilogram per oral, number of animals per group-10.
Pairwise comparisons of the effect of caffeine and vehicle revealed a significant (p<0.001) increase in locomotor activity with caffeine at 10 (p<0.001, p<0.001), 20 (p<0.001, p<0.001), 40 (p<0.001, p<0.001), 80 (p<0.001, p<0.001) and 120 (p<0.001, p<0.001) in NSD and SD female mice respectively, compared to either NSD or SD vehicle. In male mice, there was a significant increase in locomotor activity with caffeine at 10 (p<0.002), 20 (p<0.001), 80 (p<0.001) and 120 mg/kg (p<0.001) in NSD male mice compared to NSD vehicle, and a significant increase with caffeine at 10 (p<0.001), 20 (p<0.001), 40 (p<0.001), 80 (p<0.001) and 120 mg/kg (p<0.001) in SD male mice compared to SD vehicle.
Comparisons between NSD and SD (female/male mice) revealed no significant difference in locomotor activity between SD and NSD female/male mice administered vehicle. With caffeine administration, there was a significant decrease in locomotor activity at 20 mg/kg (p<0.030), and a significant increase at 40 (p<0.025), 80 (p<0.001) and 120 mg/kg (p<0.016) in SD compared to NSD female mice; while in male mice, locomotor activity decreased significantly at 10 (p<0.001) and 20 mg/kg (p<0.041), and increased at 40 (p<0.001) and 80 mg/kg (p<0.024) in SD compared to NSD male mice.
Comparisons between female and male (NSD/SD) mice revealed no significant difference in locomotor activity between NSD/SD female and male mice administered vehicle. Caffeine administration resulted in a significant increase in locomotor activity at 40 (p<0.009), and a significant decrease at 80 (p<0.001) and 120 mg/kg (p<0.001) in NSD female compared to NSD male mice. With sleep-deprivation, locomotor activity increased significantly at 10 (p<0.001), 20 (p<0.001) and 120 mg/kg (p<0.011), and decreased at 40 (p<0.001) and 80 mg/kg (p<0.046) in SD female compared to SD male mice.
3.2. Rearing activity
Fig. 2 shows the effect of increasing doses of caffeine on rearing in NSD/SD male and female mice. MANOVA with drug dose, sex and sleep-deprivation as main factors revealed a significant effect of drug dose {F (5,120) = 27.8 p<0.001} and sex {F(1,120) = 5.90, p<0.017}, but no significant effect of sleep-deprivation {F(1,120) = 1.82, p<0.254}. There was however significant interactions between drug dose×sleep-deprivation {F (5,120) = 4.23, p<0.001} and sex ×sleep-deprivation {F(1,120) = 5.31, p<0.022}; with no significant interactions between drug dose×sex {F(5,120) = 1.30, p<0.185} or drug dose×sex ×sleep-deprivation {F(5,120) = 1.96, p<0.324}.
Fig. 2.
Effect of Caffeine on rearing in NSD/SD female and male mice. Each bar represents mean±SE.M. Comparisons are: *p<0.05 versus VEH, #p<0.05 SD versus NSD mice, µp<0.05 female versus male. VEH: Vehicle, SD: sleep deprived, NSD: non sleep deprived, mg/kg, p.o: milligram/kilogram per oral, number of animals per group-10.
Pairwise comparisons of the effect of caffeine and vehicle, revealed a significant increase in rearing at 10 (p<0.001), 20 (p<0.001) and 40 mg/kg (p<0.001); with a significant decrease at 80 (p<0.029) and 120 mg/kg (p<0.016) in NSD female mice compared to NSD vehicle. In NSD males, rearing activity increased with caffeine at 10 (p<0.011), 20 (p<0.033), 40 (p<0.027) and 80 mg/kg (p<0.011), and decreased at 120 mg/kg (p<0.020) compared to NSD vehicle. In both SD females and SD males, rearing activity increased significantly at 10 (p<0.001, p<0.001), 20 (p<0.001, p<0.001), 40 (p<0.001, p<0.001) and 80 mg/kg (p<0.005, p<0.001) respectively compared to respective SD female/male mice administered vehicle.
Comparisons between NSD and SD (female/male) mice revealed no significant difference in rearing activity between SD and NSD mice administered vehicle. Caffeine administration resulted in significant increase in rearing activity at 40 (p<0.012), 80 (p<0.001) and 120 mg/kg (p<0.011) in SD females compared to NSD females, and a significant decrease at 10 (p<0.037) and 20 mg/kg (p<0.020) in SD males compared to NSD male mice.
Comparisons between female and male (NSD/SD) mice revealed no significant difference in rearing activity between NSD/SD female and male mice administered vehicle. Caffeine administration resulted in a significant increase in rearing at 20 mg/kg (p<0.019), and a decrease at 80 (p<0.001) and 120 mg/kg (p<0.001) in NSD females compared to NSD male mice; while a significant increase in rearing was seen at 10 (p<0.001), 20 (p<0.001) and 80 mg/kg (p<0.001) in SD female compared to SD male mice.
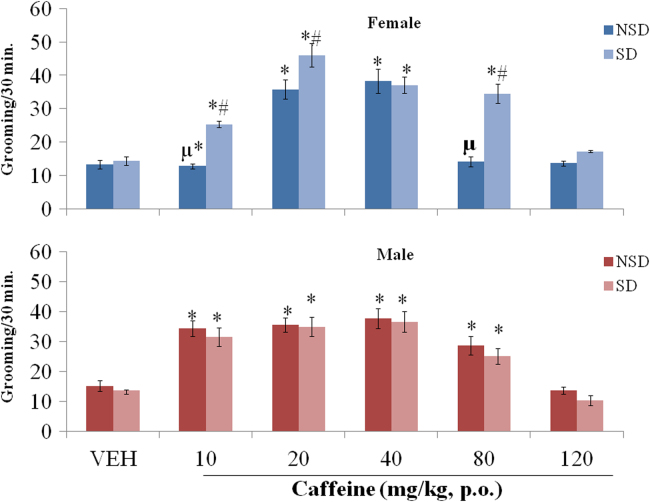
3.3. Grooming Behaviour
Fig. 3 shows the effect of increasing doses of caffeine on grooming in NSD/SD male and female mice. MANOVA with drug dose, sex and sleep-deprivation as main factors revealed a significant effect of sleep-deprivation {F (1,120) = 8.26, p<0.005} but no significant effect of drug dose {F (5,120) = 1.12, p<0.556} or sex {F(1,120) = 0.070, p<0.315}. There were no significant interactions between drug dose×sleep-deprivation {F (5,120) = 0.427, p<0.153}, drug dose×sex {F (5,120) = 0.083, p<0.151}, sex×sleep-deprivation {F (1,120) = 0.127 p<0.355} drug dose×sex ×sleep-deprivation {F(5,120) = 0.081, p<0.180}.
Fig. 3.
Effect of Caffeine on rearing in NSD/SD female and male mice. Each bar represents mean±S.E.M. Comparisons are: *p<0.05 versus VEH, #p<0.05 SD versus NSD mice, µp<0.05 female versus male. VEH: Vehicle, SD: sleep deprived, NSD: non sleep deprived, mg/kg, p.o: milligram/kilogram per oral, number of animals per group-10.
Pairwise comparisons of the effect of caffeine and vehicle revealed a significant decrease in grooming at 10 mg/kg (p<0.023), and an increase at 20 (p<0.001), 40 (p<0.021) and 80 mg/kg (p<0.001) in NSD females compared to NSD vehicle. In SD female mice, grooming increased at 10 (p<0.034), 20(p<0.011), 40 (p<0.024) and 80 mg/kg (p<0.042), compared to SD vehicle. Grooming increased significantly at 10 (p<0.040, p<0.032), 20 (p<0.001, p<0.025), 40 (p<0.011, p<0.01) and 80 mg/kg (p<0.021, p<0.011) in NSD and SD male mice respectively, compared to NSD/SD vehicle.
Comparisons between NSD and SD (female/male) mice revealed no significant difference in grooming between SD and NSD female/male mice administered vehicle. Caffeine administration resulted in a significant increase in grooming in SD female mice at 10 (p<0.001), 20 (p<0.001) and 80 mg/kg (p<0.001) compared to NSD females. In male mice, there was no significant difference in grooming in SD compared to NSD groups.
Comparisons between female and male (NSD/SD) mice revealed no significant difference in grooming behaviour between NSD/SD female and male mice administered vehicle. Caffeine administration resulted in a significant decrease in grooming at 10 (p<0.011) and 80 mg/kg (p<0.041) in NSD females compared to NSD males, and no significant difference between SD female and SD male mice.
3.4. Plasma corticosterone assay
Table 2 shows the effect of caffeine on plasma corticosterone levels in NSD/SD male and female mice. MANOVA with drug dose, sex and sleep-deprivation as main factors revealed a significant effect of drug dose {F (5,120) = 21.5 p<0.001}, sex {F(1,120) = 99.3, p<0.002} and sleep-deprivation {F(1,120) = 141 p<0.001}. There were also significant interactions between drug dose×sex {F(5,120) = 3.40, p<0.007} and drug dose×sleep-deprivation {F(5,120) = 3.55,p<0.005}; and no significant interactions between sex×sleep-deprivation {F(1,120) = 0.06, p<0.804} and drug dose×sex×sleep-deprivation {F(5,120) = 1.82, p<0.113}.
Table 2.
Corticosterone (CORT) levels (ng/ml).
| Female NSD | Male NSD | Female SD | Male SD | |
|---|---|---|---|---|
| VEH | 116.5±2.95 | 95.67±5.69 | 128.5±7.31 | 122.83±2.78 |
| 10 | 105.5±2.6* | 93.67±6.08 | 121.67±2.99µ# | 115.50±2.54# |
| 20 | 117.17±1.95µ | 97.00±6.57 | 125.00±2.59 | 114.17±3.63# |
| 40 | 118.17±2.54µ | 97.88±8.25 | 147.50±6.48µ# | 120.83±2.38# |
| 80 | 122.17±3.11µ | 102.33±3.31 | 160.17±9.55*µ# | 127.17±2.98# |
| 120 | 138.00±5.13*µ | 114.17±3.63* | 177.67±5.30*µ# | 140.33±4.83*# |
Mean±S.E.M. Comparisons are: *p<0.05 versus VEH, #p<0.05 SD versus NSD mice, µp<0.05 male versus female. VEH: Vehicle, SD: sleep-deprived, NSD: non sleep-deprived, number of animals per group-7.
Pairwise comparisons of the effect of caffeine and vehicle revealed a significant decrease in corticosterone levels at 10 mg/kg (p<0.019), and a significant increase at 120 mg/kg (p<0.005) in NSD females; while in SD females, corticosterone levels increased at 80 (p<0.032) and 120 mg/kg (p<0.005). In males, corticosterone levels increased significantly at 120 mg/kg (p<0.011, p<0.001) in both NSD and SD groups compared to vehicle.
Comparisons between NSD and SD (female/male) mice revealed no significant difference in corticosterone levels between SD and NSD female/male mice administered vehicle. Caffeine administration resulted in a significant increase in corticosterone levels at 10 (p<0.001), 40 (p<0.001), 80 (p<0.005) and 120 mg/kg (p<0.001) in SD females compared to NSD female mice. A significant increase is seen at 10 (p<0.013), 20 (p<0.002), 40 (p<0.045), 80 (p<0.006) and 120 mg/kg (p<0.001) in SD males compared to NSD male mice.
Comparisons between female and male (NSD/SD) mice revealed no significant difference in corticosterone levels between NSD/SD female and male mice administered vehicle. Caffeine administration results in a significant increase in corticosterone levels in female NSD mice at 20 (p<0.001), 40 (p<0.013), 80 (p<0.030) and 120 mg/kg (p<0.001) compared to male NSD mice. With sleep-deprivation, corticosterone levels increased significantly in SD females at 10 (p<0.035), 40 (p<0.001), 80 (p<0.019) and 120 mg/kg (p<0.045) compared to SD male mice.
3.5. Brain antioxidant levels
Table 3 shows the effect of increasing doses of caffeine on super-oxide dismutase activity in NSD/SD male and female mice. MANOVA with drug dose, sex and sleep-deprivation as main factors revealed a significant effect of drug dose {F (5,120) = 26.6, p<0.001}, sex {F(1,120) = 4.52, p<0.036}, and sleep-deprivation {F(1,120) = 7.64, p<0.007}, with no significant interactions between drug dose ×sex {F(5,120) = 0.596, p<0.703}, drug dose×sleep-deprivation {F(5,120) = 0.411, p<0.841}, sex×sleep-deprivation {F(1,120) = 0.152, p<0.697} and drug dose×sex×sleep-deprivation {F(5,120) = 0.199, p<0.902}.
Table 3.
Superoxide dismutase and glutathione activity.
| Female NSD | Male NSD | Female SD | Male SD | ||
|---|---|---|---|---|---|
| SOD/U/g | VEH | 31.33±1.71 | 29.83±1.74 | 34.33±1.76 | 33.50±1.78 |
| 10 | 31.17±1.30 | 33.17±1.74 | 35.00±2.13 | 34.83±2.14 | |
| 20 | 34.83±0.79 | 34.50±2.15 | 38.67±1.65 | 37.00±1.57 | |
| 40 | 37.67±2.33 | 37.33±2.30* | 42.17±2.01* | 38.50±1.18* | |
| 80 | 43.00±2.79* | 39.33±1.30* | 41.33±1.20* | 44.33±1.94* | |
| 120 | 46.67±2.17* | 46.33±1.82* | 43.67±2.04* | 43.83±1.28* | |
| GPX U/g | VEH | 5.50±0.34 | 5.59±0.26 | 4.80±0.31 | 4.75±0.49 |
| 10 | 4.67±0.48 | 4.73±0.59 | 4.22±0.99 | 4.20±0.35 | |
| 20 | 4.39±0.37 | 4.46±0.38 | 4.14±0.33 | 4.10±0.25 | |
| 40 | 4.27±0.21 | 4.28±0.42 | 4.08±0.39 | 4.00±0.57 | |
| 80 | 2.67±0.43* | 3.07±0.44* | 2.00±0.28* | 2.13±0.28* | |
| 120 | 1.93±0.22* | 2.01±0.53* | 1.81±0.19* | 1.84±0.55* | |
Mean±S.E.M. Comparisons are: *p<0.05 versus VEH, VEH: Vehicle, SD: sleep-deprived, NSD: non sleep-deprived, number of animals per group-7.
Pairwise comparisons of the effect of caffeine and vehicle revealed a significant increase in super-oxide dismutase (SOD) activity at 80 (p<0.005) and 120 mg/kg (p<0.002) in NSD females, and an increase at 40 (p<0.015), 80 (p<0.003) and 120 mg/kg (p<0.001) in SD female mice compared to vehicle. SOD activity increased significantly at 40 (p<0.027, p<0.041), 80 (p<0.008, p<0.05) and 120 mg/kg (p<0.001, p<0.001) in NSD and SD males respectively compared to vehicle. Comparisons between NSD and SD (female/male) mice revealed no significant difference in SOD activity between SD and NSD female/male mice administered vehicle, or at any of the doses of caffeine. Comparisons between female and male (NSD/SD) mice revealed no significant difference in SOD activity between NSD/SD female and male mice administered vehicle or at any of the doses of caffeine.
Table 3 also shows the effect of increasing doses of caffeine on glutathione peroxidase activity in NSD/SD male and female mice. MANOVA with drug dose, sex and sleep-deprivation as main factors revealed a significant effect of drug dose {F (5,120) = 24.8, p<0.001}, sex {F(1,120) = 3.38, p<0.043}, and sleep-deprivation {F(1,120) = 8.63, p<0.010}. There was no significant interactions between drug dose×sex {F(5,120) = 0.857, p<0.512}, drug dose×sleep-deprivation {F(5,120) = 0.784, p<0.563}, sex×sleep-deprivation {F(1,120) = 0.760, p<0.385} and drug dose×sex ×sleep-deprivation {F(5,120) = 2.22, p<0.056}.
Pairwise comparisons of the effect of caffeine and vehicle revealed a significant decrease in glutathione peroxidase (GPX) activity at 80 (p<0.001, p<0.021) and 120 mg/kg (p<0.023, p<0.011) in NSD and SD females respectively compared to NSD/SD vehicle; in males, GPX activity also decreased significantly at 80 (p<0.013, p<0.001) and 120 mg/kg (p<0.002, p<0.011) in NSD and SD males respectively compared to NSD/SD vehicle.
Comparisons between NSD and SD (female/male) mice revealed no significant difference in GPX activity between SD and NSD female/male mice administered vehicle, or at any of the doses of caffeine. Comparisons between female and male (NSD/SD) mice revealed no significant difference in GPX activity between NSD/SD female and male mice administered vehicle or at any of the doses of caffeine.
4. Discussion
This study set out to assess the interactions that exist among three main factors (graded doses of caffeine, sex and sleep-deprivation) in relation to novelty-induced behaviours, biochemical markers of stress and antioxidant status in prepubertal mice. The overall finding was that in the determination of open-field behavioural response, strong associations exist among these factors; while with respect to plasma corticosterone levels and brain antioxidant activities, the associations were not as strong. Sex and caffeine dose independently altered locomotor activity and rearing. Sleep-deprivation did not significantly affect locomotion, although it potentiated the effects of caffeine dose and sex. Assessment of grooming however showed that total sleep-deprivation independently altered outcome, while sex and caffeine dose did not exert any significant main effect. With the biochemical and antioxidant tests, caffeine dose, sex and sleep-deprivation influenced outcomes, each independent of the other; with no significant interactions among them.
Novelty-induced horizontal locomotion (Fig. 1) and rearing (Fig. 2) showed a general locomotor-stimulating effect of caffeine in both females and males (with or without sleep-deprivation), when compared to their respective vehicle groups. Horizontal locomotion and rearing are well-documented measures of explorative ability; and as seen in our study, caffeine administration (within the range of doses used) resulted in a biphasic effect on locomotion, with enhanced locomotor activity at low doses and suppression at high doses; a result consistent with several studies in adult rodents [41], [42]. El Yacoubi et al. [41] also reported a biphasic effect of caffeine on locomotion of wild-type mice not habituated to the open-field, which is similar to the response seen in NSD prepubertal mice in this study. The stimulatory effect of caffeine has been attributed to its effects on adenosine receptors [41], [42]. Specifically, the stimulant effect of low doses of caffeine is known to be mediated by A2A receptor blockade [41]. Effects of caffeine on both horizontal locomotion and rearing in the present study, supports the results of a number of other studies [41], [43], and the suggestions that these effects are direct consequences of caffeine antagonism at ventral and dorsal striatum adenosine A2A receptors respectively [41]. However, the biphasic effect of caffeine dose on locomotion and rearing seen in NSD groups in this study is blunted by acute sleep-deprivation (regardless of sex); we observed that in the SD mice, excitatory effects seem to persist to the higher doses.
In mice, spontaneous resting behaviour, humoral constitution and synaptic function may all be affected by sleep-deprivation. It is known that repeated brief handling provokes a number of changes in both mouse behaviour and physiological constitution and these changes are known to persist for a number of days. There have been reports on the effects of sleep-deprivation on brain function [35], [44], [45], [46] (with varying results). Berro et al., [45] and Saito et al. [46] reported a potentiation of the effects of psycho-stimulants (cocaine and amphetamine) on locomotion in sleep-deprived mice, possibly through a synergism at dopamine receptors. Onaolapo et al., [35], while studying the effect of sleep-deprivation and caffeine administration on memory, concluded that caffeine/sleep-deprivation interactions resulted in varying effects on memory in adult mice. Sleep-deprivation influences the dopaminergic system; causing increased density of both D1 and D2 dopaminergic receptors [47], [48]. Studies have also shown that sleep-deprivation induced alteration of locomotion and rearing are related extensively to an increase in dopaminergic neurotransmission [49]. In this study, following sleep-deprivation, we saw a blunting of the expected biphasic effect of caffeine on both locomotion and rearing, with a potentiation of locomotion and rearing at higher doses of caffeine in both sexes, although more consistently in females.
Grooming behaviour (Fig. 3) increased significantly in both NSD and SD mice across all doses relative to vehicle; although at some doses (especially in females), SD mice showed a higher tendency to groom compared to NSD. Grooming is an important aspect of the behavioural repertoire in rodents, and it is exhibited as a complex hierarchy of patterns that are sensitive to stress and drugs. Increase in grooming seen in NSD animals could be due to increased anxiety that has been associated with caffeine administration at doses similar to those used in this study [50], [51] while the significant increase in grooming in SD mice (compared to NSD) could be due to summation of both anxiety from caffeine and stress response of sleep-deprivation. Studies have shown that stress [52] and elevated stress hormones [53] induce grooming. Activation of central dopaminergic (D1) [54] and/or inhibition of γ-amino-butyric acid (GABA A and B) ergic receptors are also known to induce intense grooming [55].
In this study, peak behavioural response following caffeine administration was higher in females compared to males (with both locomotion and rearing), although no difference was seen with grooming. Generally, the differences in response is more dose-related than sex-specific; with significant increase seen in females compared to males at certain doses and decrease at other doses. This supports our observation from another study [22] in which we concluded that with respect to prepubertal mice, caffeine's influence on another behavioural response (memory) was both dose-specific and sex-related. Also, in the present study, an increase in corticosteroid level was seen in females compared to males, and this could be responsible for the higher response to caffeine in SD female mice.
Studies have reported sleep-deprivation-dependent alterations in glucocorticoid levels in rodents and humans [56], [57]. In this study, we saw a non-significant increase in plasma corticosterone levels (Table 2) with increasing doses of caffeine in the NSD groups, and a significant increase at the higher doses of caffeine in the SD groups. In a study assessing the effects of sleep-deprivation in neonatal rats, Hairston et al., [57] reported an increase in corticosterone levels; a result similar to what was seen in our study. NSD mice only showed minimal increase in corticosterone levels, which suggest that sleep-deprivation in itself elicits a stress response, the severity of which, is related to the length or mode of sleep-deprivation. Sex differences in stress response have been reported in a number of studies; in a study by Romeo et al., [58], prepubertal male mice had higher levels of glucocorticoids compared to adult males, although the female response was not age-dependent. In this study, levels of corticosterone in females were higher than males in both SD and NSD mice; the reasons for these differences in stress response are still being examined.
In the present study, brain glutathione activity decreased, while brain superoxide dismutase activity (Table 3) increased with increasing doses of caffeine in both SD and NSD. However, no significant effect of sleep-deprivation or sex influence was seen. Sleep-deprivation studies are based on the premise that wakefulness represents an oxidative challenge for the brain, which is controlled by sleep [59], [60]. There are also suggestions that during sleep, glutathione and uridine may aid brain oxidative detoxification by facilitating γ-amino butyric acid-related transmission and inhibiting glutamatergic transmission [61]. However, in our study, the results of comparisons between SD and NSD mice revealed no significant effect of stress on antioxidant status. This supports the results of a study by Noschang et al. [62] in which no difference in antioxidant enzyme activity between stressed and non-stressed rats was observed. In both SD and NSD mice, glutathione peroxidase activity decreased and superoxide dismutase activity increased with increasing doses of caffeine suggesting an imbalance in brain antioxidant status in prepubertal mice. Studies have shown that caffeine has antioxidant properties [62], [63], [64]; linked to its ability to regulate reactive oxygen species via adenosine receptors activation [65], [66]. Also caffeine's ability to mop up peroxide radicals [63] may be responsible for the slight increase in SOD activity seen at low doses of caffeine in this study. However, studies have also shown that the effects of caffeine on antioxidant status are dose related; with reports of increased oxidative stress with high doses of caffeine [67], [68]. The significant increase in SOD activity and decrease in GPX activity seen in this study (with increasing doses of caffeine) is suggestive of oxidative stress.
5. Conclusion
A combination of caffeine and sleep-deprivation influences open-field behaviours, stress response and brain antioxidant status in prepubertal mice; leading to the exhibition of a pattern of response that is modulated by sex.
Conflict of interest
None.
Funding
None.
Footnotes
Peer review under responsibility of Brazilian Association of Sleep.
References
- 1.Coffin V.L., Spealman R.D. Psychomotor-stimulant effects of 3-isobutyl-1-methylxanthine: comparison with caffeine and 7-(2-chloroethyl) theophyllline. Eur J Pharm. 1989;170(1–2):35–40. doi: 10.1016/0014-2999(89)90130-1. [DOI] [PubMed] [Google Scholar]
- 2.Svenningsson P., Nomicos G., Fredholm B. The stimulatory action and the development of tolerance to caffeine is associated with alterations in gene expression in specific brain regions. J Neurosci. 1999;19(10):4011–4022. doi: 10.1523/JNEUROSCI.19-10-04011.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guedes R.C.A., Lima De-Aguiar M.R.J., Alves De-Aguiar C.R.R. Caffeine and nutrition: an overview. Caffeine Chem. 2012:3–21. [Google Scholar]
- 4.Smith A. Effects of caffeine on human behavior. Food Chem Toxicol. 2002;40(9):1243–1255. doi: 10.1016/s0278-6915(02)00096-0. [DOI] [PubMed] [Google Scholar]
- 5.Rogers P.J., Heatherley S.V., Hayward R.C., Seers H.E., Hill J., Kane M. Effects of caffeine and caffeine withdrawal on mood and cognitive performance degraded by sleep restriction. Psychopharmacol (Berl) 2005;179:742–752. doi: 10.1007/s00213-004-2097-y. [DOI] [PubMed] [Google Scholar]
- 6.Castellanos F.X., Rapoport T.L. Effects of caffeine on development and behaviour in infancy and childhood: a review of the published literature. Food Chem Tox. 2002;40:1235–1242. doi: 10.1016/s0278-6915(02)00097-2. [DOI] [PubMed] [Google Scholar]
- 7.Knight C.A., Knight I., Mitchell D.C. Beverage caffeine intakes in young children in Canada and the US. Can J Diet Pract Res. 2006;67:96–99. doi: 10.3148/67.2.2006.96. [DOI] [PubMed] [Google Scholar]
- 8.Temple J.L., Bulkley A.M., Briatico L., Dewey A.M. Sex differences in reinforcing value of caffeinated beverages in adolescents. Behav Pharm. 2009;20:731–741. doi: 10.1097/FBP.0b013e328333b27c. [DOI] [PubMed] [Google Scholar]
- 9.Temple J.L., Dewey A.M., Briatico L.N. Effects of acute caffeine administration on adolescents. Exp Clin Psychopharmacol. 2010;18:510–520. doi: 10.1037/a0021651. [DOI] [PubMed] [Google Scholar]
- 10.Fisone G., Borgkvist A.U. Caffeine as a psychomotor stimulant: mechanism of action. Cell Mol Life Sci. 2004;6 doi: 10.1007/s00018-003-3269-3. [857] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Millman R.P. Working group on sleepiness in adolescents/young adults, AAP committee on adolescence. Excessive sleepiness in adolescents and young adults: causes, consequences, and treatment strategies. Pediatrics. 2005;115:1774–1786. doi: 10.1542/peds.2005-0772. [pmid:15930245] [DOI] [PubMed] [Google Scholar]
- 12.Owens Judith. Adolescent sleep working group, committee on adolescence. Insufficient sleep in adolescents and young adults: an update on causes and consequences. Paediatric. 2014:134. doi: 10.1542/peds.2014-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirshkowitz M., Whiton K., Albert S.M., Alessi C., Bruni O., DonCarlos L., Hazen N., Herman J., Katz E.S., Kheirandish-Gozal L., Neubauer D.N., O’Donnell A.E., Ohayon M., Peever J., Rawding R., Sachdeva R.C., Setters B., Vitiello M.V., Ware J.C., Hillard P.J.A. National sleep foundation's sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1:40–43. doi: 10.1016/j.sleh.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Oginska H., Pokorski J. Fatigue and mood correlates of sleep length in three age-social groups: School children, students, and employees. Chrono- Int. 2006;23(6):1317–1328. doi: 10.1080/07420520601089349. [DOI] [PubMed] [Google Scholar]
- 15.Carskadon M.A. Sleep in adolescents: the perfect storm. Pediatr Clin North Am. 2011;58:637–647. doi: 10.1016/j.pcl.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feinberg I., Davis N.M., de Bie E., Grimm K.J., Campbell I.G. The maturational trajectories of NREM and REM sleep durations differ across adolescence on both school-night and extended sleep. Am J Physiol Regul Integr Comp Physiol. 2012;302:R533–R540. doi: 10.1152/ajpregu.00532.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson A.B., Faraguna U., Zoltan J.T., Tononi G., Cirelli C. Sleep patterns and homeostatic mechanisms in adolescent mice. Brain Sci. 2013;3:318–343. doi: 10.3390/brainsci3010318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilcher J.J., Ginter D.R., Sadowsky B. Sleep quality versus Sleep quantity: relationships between Sleep and measures of health, well-being and sleepiness in college students. J Psychosom Res. 1997;42(6):583–596. doi: 10.1016/s0022-3999(97)00004-4. [DOI] [PubMed] [Google Scholar]
- 19.Calamaro C.J., Mason T.B.A., Ratcliffe S.J. Adolescents living the 24/7 lifestyle: effects of caffeine and technology on sleep duration and daytime functioning. Pediatric. 2009;123(6) doi: 10.1542/peds.2008-3641. [DOI] [PubMed] [Google Scholar]
- 20.Elkins R.N., Rapoport J.L., Zahn T.P., Buchsbaum M.S., Weingartner H., Kopin I.J., Langer D., Johnson C. Acute effects of caffeine in normal prepubertal boys. Am J Psychiatry. 1981;138:178–183. doi: 10.1176/ajp.138.2.178. [DOI] [PubMed] [Google Scholar]
- 21.Onaolapo O.J., Onaolapo A.Y. Sex differential effects of acute caffeine administration on open field novelty induced behaviour in Swiss albino mice J. Neurosc Behav Health. 2011;3:99–106. [Google Scholar]
- 22.Onaolapo A.Y., Onaolapo O.J. Caffeine's influence on object recognition and working-memory in prepubertal mice and its modulation by gender. Pathophysiol. 2015;22:223–230. doi: 10.1016/j.pathophys.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Uzbay T., Kose A., Kayir H., Ulusoy G., Celik T. Sex-related effects of agmatine on caffeine-induced locomotor activity in Swiss Webster mice. Eur J Pharm. 2009;25(630):69–73. doi: 10.1016/j.ejphar.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 24.Soellner D.E., Grandys T., Nuñez J.L. Chronic prenatal caffeine exposure impairs novel object recognition and radial arm maze behaviors in adult rats. Behav Brain Res. 2009;205:191–199. doi: 10.1016/j.bbr.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher S., Guillet R. Neonatal caffeine alters passive avoidance retention in rats in an age- and gender-related manner Developmental. Brain Res. 1997;98:145–149. doi: 10.1016/s0165-3806(96)00158-7. [DOI] [PubMed] [Google Scholar]
- 26.Lynch W.J., Carroll M.E. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacol. 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- 27.Donny E.C., Caggiula A.R., Rowell P.P., Gharib M.A., Maldovan V., Booth S. Nicotine self-administration in rats: oestrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacol. 2000;151:392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- 28.Campbell U.C., Morgan A.D., Carroll M.E. Sex differences in the effects of baclofen on the acquisition of intravenous cocaine self administration in rats. Drug Alcohol Depend. 2002;(1):66. doi: 10.1016/s0376-8716(01)00185-5. [DOI] [PubMed] [Google Scholar]
- 29.Cohen S., Kozlovsky N., Matar M.A., Kaplan Z., Zohar J., Cohen H. Post-exposure sleep deprivation facilitates correctly timed interactions between glucocorticoid and adrenergic systems, which attenuate traumatic stress responses. Neuropsychopharmacol. 2012;37:2388–2404. doi: 10.1038/npp.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Almeida V., Lobo L.L., Hipolide D.C., De Oliveira A.C., Nobrega J.N., Tufik S. Sleep deprivation induces brain region-specific decreases in glutathione levels. Neuroreport. 1998;9:2853–2856. doi: 10.1097/00001756-199808240-00031. [DOI] [PubMed] [Google Scholar]
- 31.Ramanathan L., Gulyani S., Nienhuis R., Siegel J.M. Sleep deprivation decreases superoxide dismutase activity in rat hippocampus and brainstem. Neuroreport. 2002;13:1387–1390. doi: 10.1097/00001756-200208070-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gopalakrishnan A., Ji L.L., Cirelli C. Sleep deprivation and cellular responses to oxidative stress. Sleep. 2004;27(1):27–35. doi: 10.1093/sleep/27.1.27. [DOI] [PubMed] [Google Scholar]
- 33.Kalinchuk A.V., McCarley R.W., Porkka-Heiskanen T., Basheer R. Sleep deprivation triggers inducible nitric oxide-dependent nitric oxide production in wake-active basal forebrain neurons. J Neurosci. 2010;30(40):13254–13264. doi: 10.1523/JNEUROSCI.0014-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alzoubi K.H., Khabour O.F., Rashid B.A., Damaj I.M., Salah H.A. The neuroprotective effect of vitamin E on chronic sleep deprivation-induced memory impairment: the role of oxidative stress. Behav Brain Res. 2012;226(1):205–210. doi: 10.1016/j.bbr.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 35.Onaolapo O.J., Onaolapo A.Y., Akanmu M.A., Olayiwola G. Caffeine/sleep-deprivation interaction in mice produces complex memory effects. Ann Neurosci. 2015;22:139–149. doi: 10.5214/ans.0972.7531.220304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The Registry of Toxic Effects of Chemical Substances (RTECS) 〈www.cdc.gov/niosh/rtecs/yq1d694c〉. 2004
- 37.Franken P., Dijk D.J., Tobler I., Borbély A.A. Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol. 1991;261:R198–R208. doi: 10.1152/ajpregu.1991.261.1.R198. [DOI] [PubMed] [Google Scholar]
- 38.Longordo F., Fan J., Steimer T., Kopp C., Lüthi A. Do mice habituate to “gentle handling?” A comparison of resting behavior, corticosterone levels and synaptic function in handled and undisturbed C57BL/6J mice. Sleep. 2011;34:679–681. doi: 10.1093/sleep/34.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Onaolapo O.J., Onaolapo A.Y. Acute low dose monosodium glutamate retards novelty induced behaviours in male Swiss albino mice. J Neurosc Behav Health. 2011;3:51–56. [Google Scholar]
- 40.Onaolapo O.J., Onaolapo A.Y., Akanmu M.A., Olayiwola G. Foraging enrichment modulates open field response to monosodium glutamate in mice. Ann Neurosci. 2015;22:162–170. doi: 10.5214/ans.0972.7531.220306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Yacoubi M., Ledent C., Ménard J.-F., Parmentier M., Costentin J., Vaugeois J.M. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A2A receptors. Br J Pharm. 2000;129:1465–1473. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Svenningsson P., Nomikos G.G., Ongini E., Fredholm B.B. Antagonism of adenosine A2A receptors underlies the behavioural activating effect of caffeine and is associated with reduced expression of messenger RNA for NGFI-A and NGFI-B in caudate-putamen and nucleus accumbens. Nucl Accumbens Neurosci. 1997;79:753–764. doi: 10.1016/s0306-4522(97)00046-8. [DOI] [PubMed] [Google Scholar]
- 43.Svenningsson P., Nomikos G.G., Fredholm B.B. Biphasic changes in locomotor behavior and in expression of mRNA for NGFI-A and NGFI-B in rat striatum following acute caffeine administration. J Neurosci. 1995;75(11):7612–7624. doi: 10.1523/JNEUROSCI.15-11-07612.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alhaider I.A., Aleisa A.M., Tran T.T., Alzoubi K.H., Alkadhi K.A. Chronic caffeine treatment prevents sleep deprivation-induced impairment of cognitive function and synaptic plasticity. Sleep. 2010;33(4):437–444. doi: 10.1093/sleep/33.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berro L.F., Santos R., Hollais A.W., Wuo-Silva R., Fukushiro D.F., Mári-Kawamoto E., Costa J.M., Trombin T.F., Patti C.L., Grapiglia S.B., Tufik S., Andersen M.L., Frussa-Filho Acute total sleep deprivation potentiates cocaine-induced hyperlocomotion in mice. Neurosci Lett. 2014;579:130–133. doi: 10.1016/j.neulet.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 46.Saito L.P., Fukushiro D.F., Hollais A.W., Mári-Kawamoto E., Costa J.M., Berro L.F., Aramini T.C., Wuo-Silva R., Andersen M.L., Tufik S., Frussa-Filho R. Acute total sleep deprivation potentiates amphetamine-induced locomotor-stimulant effects and behavioral sensitization in mice. Pharm Biochem Behav. 2014;117:7–16. doi: 10.1016/j.pbb.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 47.Demontis M.G., Fadda P., Devoto P., Martellota M.C., Fratta W. Sleep deprivation increases dopamine D1 receptor agonist [3H]SCH 23390 binding and dopamine-stimulated adenylate cyclase in the rat limbic system. Neurosci Lett. 1990;117:224–227. doi: 10.1016/0304-3940(90)90148-3. [DOI] [PubMed] [Google Scholar]
- 48.Nunes G.P., Tufik S., Nobrega J.N. Autoradiographic analysis of D1 and D2 dopaminergic receptors in rat brain after paradoxical sleep deprivation. Brain Res Bull. 1994;34:453–456. doi: 10.1016/0361-9230(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 49.Ebert D., Berger M. Neurobiol similar antidepressant sleep deprivation psychostimulant use: a psychostimulant theory antidepressant Sleep deprivation. Psychopharmacology. 1998;140:1–10. doi: 10.1007/s002130050732. [DOI] [PubMed] [Google Scholar]
- 50.Jain N.S., Hirani K., Chopde C.F. Reversal of caffeine-induced anxiety by neurosteroid 3-alpha-hydroxy-5-alpha-pregnane-20-one in rats. Neuropharmacol. 2005;48:627–638. doi: 10.1016/j.neuropharm.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 51.El Yacoubi M., Ledent C., Parmentier M., Costentin J., Vaugeois J.M. The anxiogenic-like effect of caffeine in two experimental procedures measuring anxiety in the mouse is not shared by selective A (2A) adenosine receptor antagonists. Psychopharmacol. 2000;148:153–163. doi: 10.1007/s002130050037. [DOI] [PubMed] [Google Scholar]
- 52.Moyaho A., Valencia J. Grooming and yawning trace adjustment to unfamiliar environments in laboratory Sprague–Dawley rats (Rattus norvegicus) J Comp Psychol. 2002;116 doi: 10.1037/0735-7036.116.3.263. [263–26] [DOI] [PubMed] [Google Scholar]
- 53.Dunn A.J., Berridge C.W., Lai Y.I., Yachabach T.L., File S.E. Excessive grooming behavior in rats and mice induced by corticotropin-releasing factor. Ann N Y Acad Sci. 1988;525:391–393. [Google Scholar]
- 54.Stoessl A.J. Dopamine D1 receptor agonist induced grooming is blocked by the Opioid receptor antagonist maloxone. Eur J Pharm. 1996;259:301–303. doi: 10.1016/0014-2999(94)90657-2. [DOI] [PubMed] [Google Scholar]
- 55.Spruijt B.M., Van–Hoff J., Grispn W.H. Aetiology and neurobiology of grooming behaviour. Physiol Rev. 1992;72:825–852. doi: 10.1152/physrev.1992.72.3.825. [DOI] [PubMed] [Google Scholar]
- 56.Spiegel K., Leproult R., Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 57.Hairston I.S., Ruby N.F., Brooke S., Peyron C., Denning D.P., Heller H.C., Sapolsky R.M. Sleep deprivation elevates plasma corticosterone levels in neonatal rats. Neurosc Lett. 2001;315:29–32. doi: 10.1016/s0304-3940(01)02309-6. [DOI] [PubMed] [Google Scholar]
- 58.Romeo R.D., Kaplowitz E.T., Ho A., Franco D. The influence of puberty on stress reactivity and forebrain glucocorticoid receptor levels in inbred and outbred strains of male and female mice. Psychoneuroendocrinology. 2012;38:592–596. doi: 10.1016/j.psyneuen.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 59.Gopalakrishnan A., Ji L.L., Cirelli C. Sleep deprivation cell responses oxid stress. Sleep. 2004;27(1):27–35. doi: 10.1093/sleep/27.1.27. [DOI] [PubMed] [Google Scholar]
- 60.Reimund E. The free radical flux theory of sleep. Med Hypotheses. 1994;43:231–233. doi: 10.1016/0306-9877(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 61.Inoué S., Honda K., Komoda Y. Sleep as neuronal detoxification and restitution. Behav Brain Res. 1995;69(91):96. doi: 10.1016/0166-4328(95)00014-k. [DOI] [PubMed] [Google Scholar]
- 62.Noschang C.G., Krolow R., Pettenuzzo L.F., Ávila M.C., Fachin A., Arcego D., Toigo E.V., Crema L.M., Diehl L.A., Vendite D., Dalmaz C. Interactions between chronic stress and chronic consumption of caffeine on the enzymatic antioxidant system. Neurochem Res. 2009;34:1568–1574. doi: 10.1007/s11064-009-9945-4. [DOI] [PubMed] [Google Scholar]
- 63.Lee C. Antioxidant ability of caffeine and its metabolites based on the study of oxygen radical absorbing capacity and inhibition of LDL peroxidation. Clin Chim Acta. 2000;295:141–154. doi: 10.1016/s0009-8981(00)00201-1. [DOI] [PubMed] [Google Scholar]
- 64.Nobre H.V.J., Cunha G.M., de Vasconcelos L.M., Magalhães H.I., Oliveira Neto R.N., Maia F.D. Caffeine and CSC, adenosine A2A antagonists, offer neuroprotection against 6-OHDA-induced neurotoxicity in rat mesencephalic cells. Neurochem Int. 2010;56:51–58. doi: 10.1016/j.neuint.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 65.Agostinho P., Caseiro P., Rego A.C., Duarte E.P., Cunha R.A., Oliveira C.R. Adenosine modulation of d-[3H] aspartate release in cultured retina cells exposed to oxidative stress. Neurochem Int. 2000;36:255–265. doi: 10.1016/s0197-0186(99)00113-8. [DOI] [PubMed] [Google Scholar]
- 66.Thakur S., Du J., Hourani S., Ledent C., Li J.M. Inactivation of adenosine A2Areceptor attenuates basal and angiotensin II-induced ROS production by Nox2 in endothelial cells. J Biol Chem. 2010;285:40104–40113. doi: 10.1074/jbc.M110.184606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ofluoglu E., Pasaoglu H., Pasaoglu A. The effects of caffeine on L-arginine metabolism in the brain of rats. Neurochem Res. 2009;34:395–399. doi: 10.1007/s11064-008-9790-x. [DOI] [PubMed] [Google Scholar]
- 68.Al Moutaery K., Al Deeb S., Ahmad Khan H., Tariq M. Caffeine impairs short-term neurological outcome after concussive head injury in rats. Neurosurg. 2003;53:704–711. doi: 10.1227/01.neu.0000079487.66013.6f. [DOI] [PubMed] [Google Scholar]