Abstract
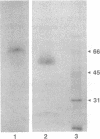
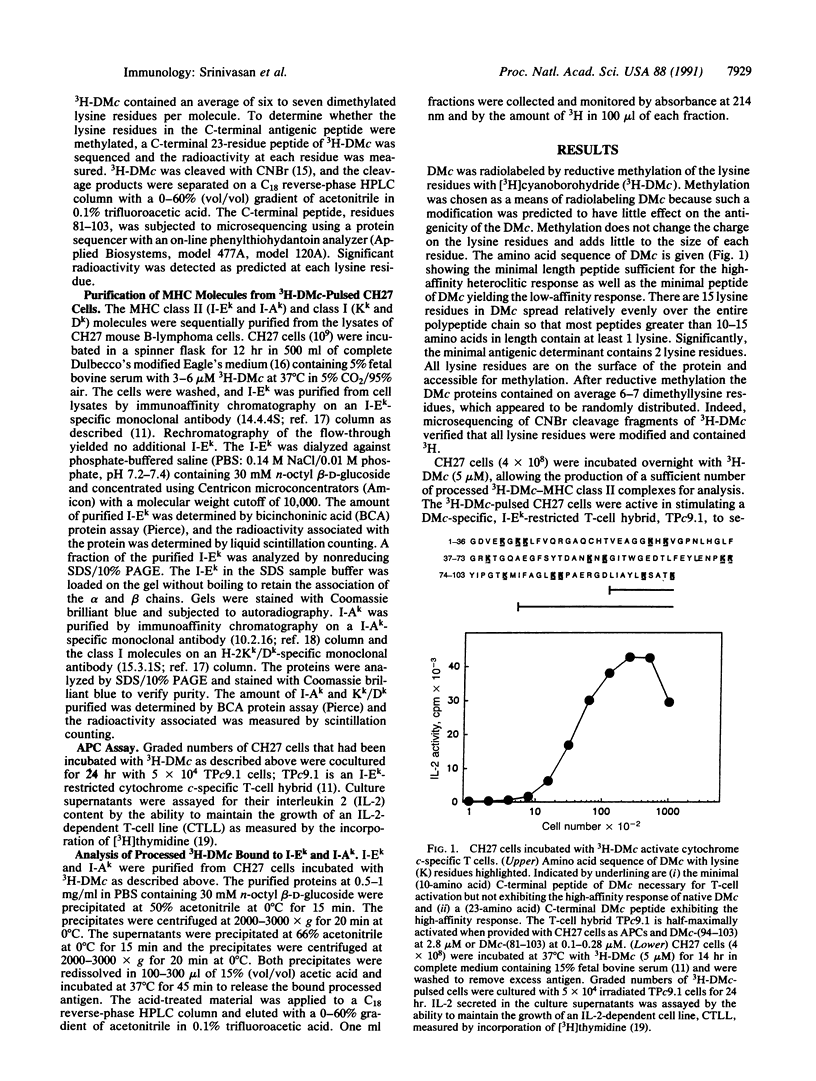
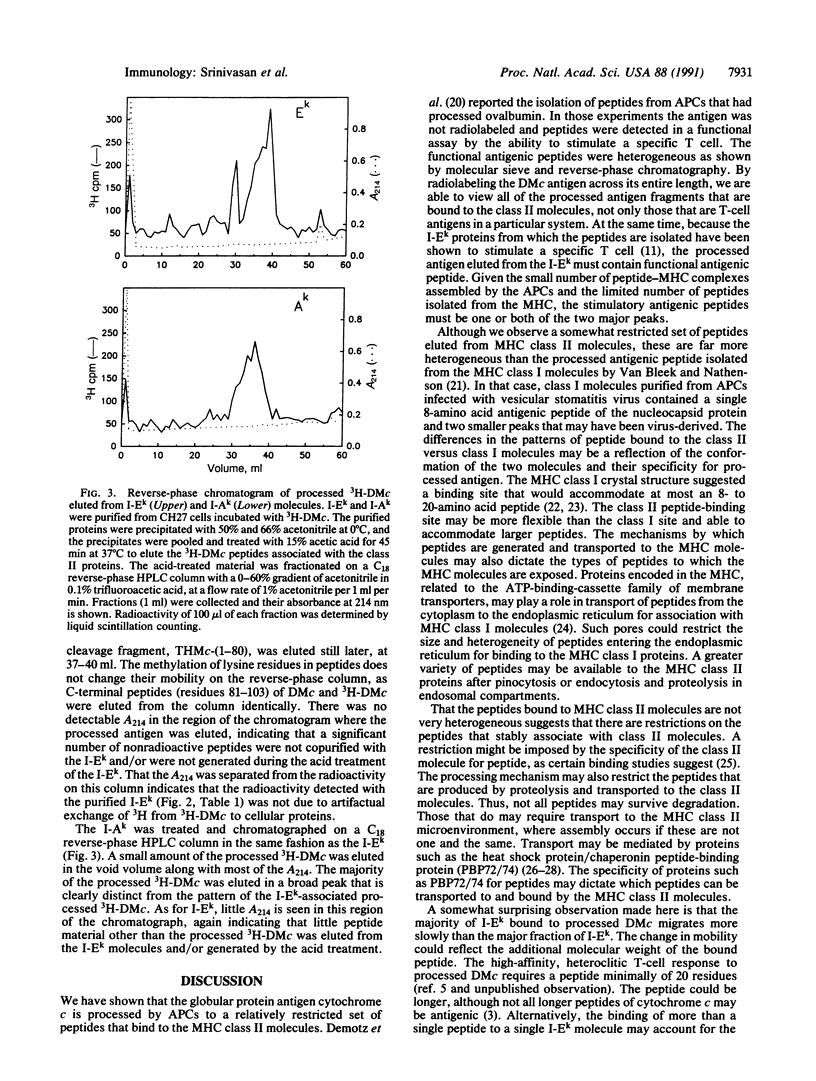
Helper T lymphocytes recognize peptide fragments of antigen bound to major histocompatibility complex (MHC) class II molecules presented on the surface of antigen-presenting cells (APCs). Previous studies showed that the MHC class II, I-Ek molecules purified from APCs that had processed Drosophila melanogaster cytochrome c (DMc) contained functional, processed antigen-I-Ek complexes. This was demonstrated by the ability of purified I-Ek, incorporated into liposomes, to stimulate DMc-specific T cells in the absence of any additional antigen. Here we describe the isolation and characterization of the processed antigen bound to I-Ek. This was accomplished using DMc radiolabeled across its entire length by reductive methylation of its lysine residues, allowing an analysis of the totality of processed antigen bound to MHC class II molecules. After processing, only about 0.2% of the APC I-Ek molecules contained processed DMc (approximately 800 per cell), yet these were sufficient to stimulate specific T cells. The DMc peptides isolated from the I-Ek molecules showed only two predominant radioactive peaks as analyzed by reverse-phase chromatography. Less processed antigen was bound to purified I-Ak molecules, and these peptides were distinct from those bound to I-Ek. The association of processed DMc with the I-Ek and I-Ak molecules appears highly specific in that no radiolabeled peptides were isolated from purified MHC class I molecules, Kk and Dk, or from the B-cell differentiation antigen B220. The majority of processed antigen-I-Ek complexes migrated more slowly than the majority of the I-Ek protein as analyzed by SDS/PAGE under nonreducing conditions without heating of the sample. This form of I-Ek may be analogous to the earlier described "floppy" form of MHC class II molecules [Dormair, K., Rothenhausler, B. & McConnell, H. M. (1990) Cold Spring Harbor Symp. Quant. Biol. 54, 409-416]. Since newly processed antigen binds nearly exclusively to this slow-migrating form, it may be of functional significance.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Matsueda G. R., Evans R. J., Dunbar J. B., Jr, Marshall G. R., Unanue E. R. Identification of the T-cell and Ia contact residues of a T-cell antigenic epitope. 1987 Jun 25-Jul 1Nature. 327(6124):713–715. doi: 10.1038/327713a0. [DOI] [PubMed] [Google Scholar]
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Jenis D. M., Grey H. M. Isolation and characterization of antigen-Ia complexes involved in T cell recognition. Cell. 1986 Dec 26;47(6):1071–1077. doi: 10.1016/0092-8674(86)90822-6. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Miles C., Grey H. M. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science. 1987 Mar 13;235(4794):1353–1358. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- Corradin G., Harbury H. A. Cleavage of cytochrome c with cyanogen bromide. Biochim Biophys Acta. 1970 Dec 22;221(3):489–496. doi: 10.1016/0005-2795(70)90219-9. [DOI] [PubMed] [Google Scholar]
- Demotz S., Grey H. M., Appella E., Sette A. Characterization of a naturally processed MHC class II-restricted T-cell determinant of hen egg lysozyme. Nature. 1989 Dec 7;342(6250):682–684. doi: 10.1038/342682a0. [DOI] [PubMed] [Google Scholar]
- Demotz S., Grey H. M., Sette A. The minimal number of class II MHC-antigen complexes needed for T cell activation. Science. 1990 Aug 31;249(4972):1028–1030. doi: 10.1126/science.2118680. [DOI] [PubMed] [Google Scholar]
- Dornmair K., Rothenhäusler B., McConnell H. M. Structural intermediates in the reactions of antigenic peptides with MHC molecules. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):409–416. doi: 10.1101/sqb.1989.054.01.050. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Harding C. V., Leyva-Cobian F., Unanue E. R. Mechanisms of antigen processing. Immunol Rev. 1988 Dec;106:77–92. doi: 10.1111/j.1600-065x.1988.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Harding C. V., Unanue E. R. Quantitation of antigen-presenting cell MHC class II/peptide complexes necessary for T-cell stimulation. Nature. 1990 Aug 9;346(6284):574–576. doi: 10.1038/346574a0. [DOI] [PubMed] [Google Scholar]
- Jelachich M. L., Grusby M. J., Clark D., Tasch D., Margoliash E., Pierce S. K. Synergistic effects of antigen and soluble T-cell factors in B-lymphocyte activation. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5537–5541. doi: 10.1073/pnas.81.17.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentoft N., Dearborn D. G. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J Biol Chem. 1979 Jun 10;254(11):4359–4365. [PubMed] [Google Scholar]
- Jentoft N., Dearborn D. G. Protein labeling by reductive alkylation. Methods Enzymol. 1983;91:570–579. doi: 10.1016/s0076-6879(83)91052-2. [DOI] [PubMed] [Google Scholar]
- Lakey E. K., Margoliash E., Pierce S. K. Identification of a peptide binding protein that plays a role in antigen presentation. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1659–1663. doi: 10.1073/pnas.84.6.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Parham P. Antigen processing. Transporters of delight. Nature. 1990 Dec 20;348(6303):674–675. doi: 10.1038/348674a0. [DOI] [PubMed] [Google Scholar]
- Pierce S. K., Morris J. F., Grusby M. J., Kaumaya P., van Buskirk A., Srinivasan M., Crump B., Smolenski L. A. Antigen-presenting function of B lymphocytes. Immunol Rev. 1988 Dec;106:149–180. doi: 10.1111/j.1600-065x.1988.tb00778.x. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H., Fox B. S., Fraga E., Chen C., Singh B. The T lymphocyte response to cytochrome c. V. Determination of the minimal peptide size required for stimulation of T cell clones and assessment of the contribution of each residue beyond this size to antigenic potency. J Immunol. 1985 Oct;135(4):2598–2608. [PubMed] [Google Scholar]
- Solinger A. M., Ultee M. E., Margoliash E., Schwartz R. H. T-lymphocyte response to cytochrome c. I. Demonstration of a T-cell heteroclitic proliferative response and identification of a topographic antigenic determinant on pigeon cytochrome c whose immune recognition requires two complementing major histocompatibility complex-linked immune response genes. J Exp Med. 1979 Oct 1;150(4):830–848. doi: 10.1084/jem.150.4.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M., Pierce S. K. Isolation of a functional antigen-Ia complex. Proc Natl Acad Sci U S A. 1990 Feb;87(3):919–922. doi: 10.1073/pnas.87.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bleek G. M., Nathenson S. G. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990 Nov 15;348(6298):213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- VanBuskirk A. M., DeNagel D. C., Guagliardi L. E., Brodsky F. M., Pierce S. K. Cellular and subcellular distribution of PBP72/74, a peptide-binding protein that plays a role in antigen processing. J Immunol. 1991 Jan 15;146(2):500–506. [PubMed] [Google Scholar]
- Vanbuskirk A., Crump B. L., Margoliash E., Pierce S. K. A peptide binding protein having a role in antigen presentation is a member of the HSP70 heat shock family. J Exp Med. 1989 Dec 1;170(6):1799–1809. doi: 10.1084/jem.170.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts T. H., McConnell H. M. High-affinity fluorescent peptide binding to I-Ad in lipid membranes. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9660–9664. doi: 10.1073/pnas.83.24.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]