Abstract
Regulation and specificity of membrane trafficking are required to maintain organelle integrity while performing essential cellular transport. Membrane fusion events in all eukaryotic cells are facilitated by the formation of specific SNARE (soluble N-ethylmaleimide-sensitive fusion protein-attachment protein receptor) complexes between proteins on opposing lipid bilayers. Although regulation of SNARE complex assembly is not well understood, it is clear that two conserved protein families, the Sx (syntaxin) and the SM (Sec1p/Munc18) proteins, are central to this process. Sxs are a subfamily of SNARE proteins; in addition to the coiled-coil SNARE motif, Sxs possess an N-terminal, autonomously folded, triple-helical (Habc) domain. For some Sxs, it has been demonstrated that this Habc domain exerts an autoinhibitory effect on SNARE complex assembly by making intramolecular contacts with the SNARE motif. SM proteins regulate membrane fusion through interactions with their cognate Sxs. One hypothesis for SM protein function is that they facilitate a switch of the Sx from a closed to an open conformation, thus lifting the inhibitory action of the Habc domain and freeing the SNARE motif to participate in SNARE complexes. However, whether these regulatory mechanisms are conserved throughout the Sx/SM protein families remains contentious as it is not clear whether the closed conformation represents a universal feature of Sxs.
Keywords: closed conformation, membrane traffic, Sec1p/Munc18 (SM), soluble N-ethylmaleimide-sensitive fusion protein-attachment protein receptor (SNARE), syntaxin
Introduction
A defining feature of eukaryotic cells is their compartmentalization into discrete membrane-bound cytosolic organelles. Non-disruptive transportation of molecules between these organelles and to the plasma membrane is extremely important, and each trafficking event is tightly regulated both spatially and temporally. SNARE (soluble N-ethylmaleimide-sensitive fusion protein-attachment protein receptor) proteins are central to this process, facilitating fusion by formation of specific complexes through their highly conserved α-helical cytosolic SNARE motifs. For vesicular transport, three α-helical coiled-coil domains from SNARE proteins on the target membrane bind to a final helix from a SNARE protein on the incoming vesicle to form the functional SNARE complex [1,2]. Formation of this parallel four-helix bundle is sufficient to drive the fusion of liposomes in vitro and thus SNAREs appear to be the minimal machinery for membrane fusion [3]. Regulating SNARE complex assembly therefore provides the cell with a means to regulate membrane traffic.
Regulation of SNARE complex assembly through an Sx (syntaxin) conformational switch
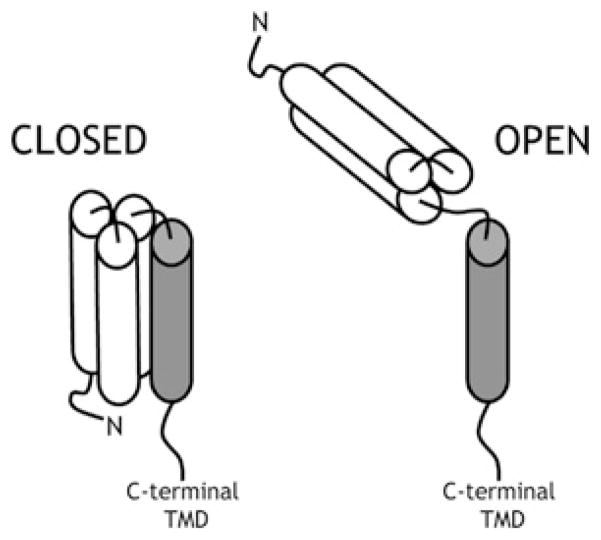
One of the helices in every functional SNARE core complex is contributed by the SNARE motif from a member of the Sx family. Sx1a was originally identified as part of the SNARE complex responsible for neuronal exocytosis [1]. NMR analysis of the N-terminal region of Sx1a identified an independently folded domain consisting of a three-helix bundle [4]. This domain, termed the Habc domain, is connected to the highly conserved SNARE motif by a flexible, unstructured linker region [4,5]. The Habc domain forms intramolecular contacts with the SNARE motif in a closed conformation that is incompatible with SNARE complex formation because of the lack of availability of the SNARE motif [6] (Figure 1). In order to form SNARE complexes, the Sx must adopt an open conformation to free the SNARE motif from the autoinhibition exerted by the Habc domain [6].
Figure 1. Model for the conformational switch of Sxs.
In the open conformation (right), the Habc domain (white cylinders) is released from the SNARE motif (grey cylinder) and is therefore free to interact with its cognate SNAREs to form a core complex. In the closed conformation (left), the Habc domain folds back on the SNARE motif, rendering it inaccessible to the cognate SNAREs, thus inhibiting SNARE complex assembly. TMD, transmembrane domain.
Structural and biochemical studies of the yeast exocytic Sx, Sso1p, revealed that it also adopts a closed conformation and that the Habc domain regulates SNARE complex formation, both in vitro and in vivo [7–9]. Similarly, other plasma membrane Sxs, including Sx4 in adipocytes [10,11] and UNC-64 in worms [12,13], are subject to regulation via a conformational switch. Together, these studies suggest that regulating the switch between the Sx closed and open conformations provides the cell with a mechanism to regulate SNARE complex assembly and consequently regulate membrane fusion.
SM (Sec1p/Munc18) proteins regulate SNARE-mediated membrane traffic
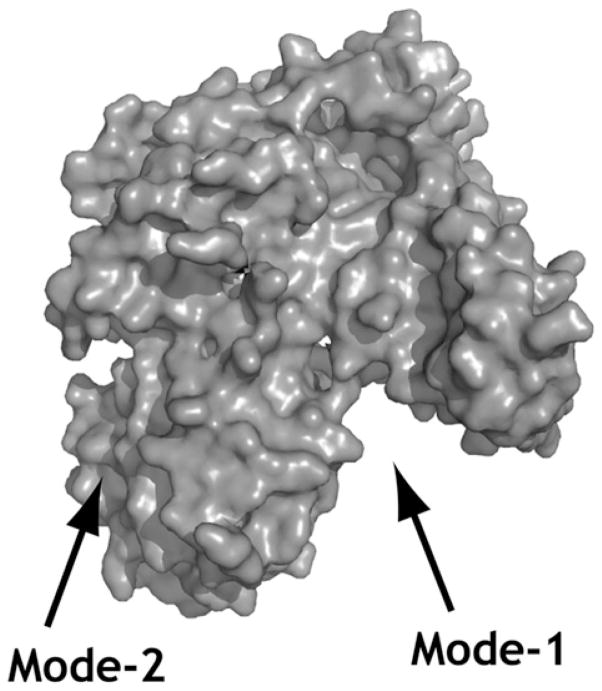
Although the SNARE complex represents the minimal machinery for membrane fusion in vitro [14], it is clear that a host of factors are involved in regulating this process in vivo. One family of proteins that plays an important role in SNARE-mediated membrane trafficking is the SM family [15]. Munc18a (also known as n-Sec1) was originally identified as an Sx1a-binding protein from rat brain homogenate [16]. The crystal structure of Munc18a in complex with Sx1a revealed that Munc18a is an arch-shaped molecule that cradles the Sx in its closed conformation [17] (Figure 2; mode-1). The closed conformation of Sx1a can be disrupted through mutations in the linker region; these mutations severely abrogate binding to Munc18a [6]. Taken together with the finding that Munc18a is essential for neuronal exocytosis [18], this suggests that the SM protein facilitates a switch of the Sx from a closed to an open conformation [6].
Figure 2. SM/Sx-binding sites.
Surface representation of the mammalian neuronal SM protein Munc18a (PDB code 3C98). The closed conformation of Sx1a binds in the cleft of the SM protein (mode-1). The N-terminal peptide of the Sx binds in a hydrophobic pocket on the surface (mode-2).
In support of this hypothesis, defects in SNARE complex assembly and traffic through the endosomal system due to the loss of Vps45p (Vps is vacuolar protein sorting) are bypassed by a version of the yeast endocytic Sx, Tlg2p, lacking its Habc domain [19]. These results support a model whereby Vps45p activates Tlg2p for entry into functional SNARE complexes by facilitating its switch from a closed to an open conformation. The version lacking the Habc domain is unable to adopt the closed conformation and therefore does not require ‘activation’ (opening) by Vps45p. Although these functional studies indicate that, like the plasma membrane Sxs described above, Tlg2p adopts a closed conformation that is incompatible with SNARE complex formation, NMR spectroscopy studies have been used to argue that this endosomal Sx does not adopt a closed conformation [20]. However, the bacterially produced version of Tlg2p used in these studies lacked almost half of the SNARE motif. Through analogy with the closed conformations of Sx1a and Sso1p, it is likely that these missing residues are required to stabilize a closed conformation of Tlg2p and therefore the physiological relevance of the NMR studies performed on the truncated Sx is not clear.
Definitive evidence of whether Tlg2p adopts a closed conformation will require high-resolution structural analyses. The intramolecular interactions between the SNARE motif and the Habc domain in trans may not be detectable using experimental approaches such as in vitro mixing studies or yeast two-hybrid assays, due to the inherent nature of the interaction. When the Habc domain and the SNARE motif are present on the same molecule, they are at extremely high effective concentrations, and therefore the Sx forms a closed conformation. However, if the closed conformation of an Sx provides a regulatory switch for SNARE complex assembly, an effective low-affinity interaction between the SNARE motif and the Habc domain is required so that the inhibition may be readily released for SNARE complex assembly. Thus it is often challenging to detect interactions between the two elements in trans.
The observations that different Sxs bind their cognate SM protein via strikingly different mechanisms led to the suggestion that the closed conformation may not act as a regulatory switch for all Sxs. In contrast with the binding of Sx1a in its closed conformation to the inner cavity of Munc18a (Figure 2; mode-1), the binding of other Sxs, including Tlg2p, to their cognate SM proteins does not rely on the closed conformation. This second mode of binding (mode-2) was first characterized for binding of the yeast Golgi SM protein Sly1p to its cognate Sx, Sed5p. In vitro binding experiments revealed that a short, evolutionarily conserved N-terminal peptide of Sed5p is sufficient to bind Sly1p [21]. The crystal structure of Sly1p in complex with the N-terminal peptide of Sed5p revealed that Sly1p is an arch-shaped molecule similar to Munc18a, underscoring the contention that the SM proteins form a closely related family. However, in contrast with the binding of Sx1a by Munc18a, the central cavity of Sly1p is not involved in mode-2 binding to the N-terminal peptide of Sed5p [22]. A small hydrophobic pocket on the exterior of Sly1p is the point of closest contact with the N-terminal peptide of Sed5p [22] (Figure 2; mode-2). Other SM proteins, including Vps45p and Munc18c, have also been shown to bind their Sxs via mode-2 [20,23].
Unlike mode-1 binding of Munc18a to Sx1a, which precludes complex formation [16], in vitro studies show that Sly1p can remain bound to Sed5p during SNARE complex assembly [24]. Seemingly conflicting results such as these have made the formulation of unifying hypotheses for the action of SM proteins challenging, as it was originally thought that different SM/Sx pairs used either mode-1 or mode-2 binding. However, an increasing number of Sxs have been found to use both modes to bind their cognate SM proteins. Of particular note is the finding that Sx1a also utilizes mode-2 binding to the N-terminal peptide of Sx1a [25–29].
In addition to the two modes of binding described above, other types of interactions between SM proteins and their cognate SNAREs have been described, including binding to the assembled SNARE complex [29–32]. Functionally, SM proteins have been suggested to regulate multiple stages of the SNARE assembly/disassembly cycle (pre-fusion, post-fusion and during fusion) [32]. This might explain why the formulation of unifying hypotheses for SM protein function has been so challenging. The membrane trafficking steps controlled by different Sx/SM pairs are subject to different levels of regulation; some fusion events occur in response to specific stimuli, whereas others occur constitutively. The binding mode characterized for a specific Sx/SM pair might reflect the step in the SNARE assembly/disassembly cycle that is rate-limiting for the trafficking pathway regulated by that Sx/SM pair. Sx1a controls a tightly regulated fusion pathway (regulated exocytosis), and the preferential binding of Munc18a to Sx1a in the closed conformation with high affinity in vitro could reflect a requirement for tight regulation of SNARE complex assembly. In support of this, an ‘open’ version of the Caenorhabditis elegans Sx1a homologue UNC-64 does not compensate for the loss of the SM protein UNC-18, indicating that other molecules, such as UNC-13, are required for the transition of this Sx from its closed to its open conformation [33]. In contrast with Sx1a, Sed5p and Tlg2p are involved in constitutive trafficking pathways and are likely to be regulated in a distinct manner, with the formation of their SNARE complexes not subject to acute regulation. In these cases, the regulation afforded by mode-2 binding may predominate and is therefore more readily detected in vitro.
Does Tlg2p/Sx16 adopt a closed conformation?
It is not only plasma membrane Sxs that are regulated by their N-terminal domains. The Habc domain of endosomal Sx7 folds back on to the SNARE motif and inhibits SNARE complex formation [34]. Our finding that a version of Tlg2p lacking its Habc domain bypasses the requirement of SNARE complex assembly on Vps45p supports a model in which Tlg2p adopts a closed conformation that is opened by Vps45p [19]. While our results cannot exclude other models, such as the Habc domain binding an unidentified inhibitory factor that is released by Vps45p, our interpretation is supported by studies on the mammalian orthologue of Tlg2p, Sx16. Like Tlg2p, Sx16 uses mode-2 binding to bind its SM protein (mVps45) via an N-terminal peptide [20]. ITC (isothermal titration calorimetry) studies of Sx16–mVps45 interactions noted markedly tighter affinity of the SM protein for the entire cytosolic region of Sx16 than for the N-terminal region alone [25]. Furthermore, a point mutation that abolishes mode-2 binding (F10A mutation in Sx16) abolishes all detectable binding to the N-terminal peptide of Sx16, but does not completely abolish binding to the full-length cytosolic region [25]. This revealed the presence of a second binding site for Sx16 on mVps45. Since this second mode of binding requires the SNARE motif, the simplest interpretation of these results is that Sx16 adopts a closed conformation that binds mVps45 via mode-1 binding [25]. Given the high degree of similarity between Tlg2p/Vps45p and Sx16/mVps45, it is likely that Tlg2p also utilizes both of these two distinct modes of binding. Support for this comes from the observation that, although the first 60 unstructured residues of Tlg2p are sufficient for interaction with Vps45p using a yeast two-hybrid assay, a more intense reporter signal is detected with the entire cytosolic domain [20]. A model in which Vps45p uses both modes-1 and -2 to bind to Tlg2p may explain why mutant versions of Vps45p and Sly1p abrogated for mode-2 binding to their Sxs appears to be functional in vivo [30,35].
Future directions
Although it is clear that interaction(s) between SM proteins and their cognate Sxs plays a key role in regulating membrane fusion, the precise molecular mechanism(s) by which this is achieved is not well understood. Using the yeast endosomal SM/Sx pair, Vps45p/Tlg2p, as our model system, our studies are directed towards elucidating the mechanisms by which Vps45p regulates Tlg2p-mediated fusion. We are currently investigating the importance of Tlg2p’s Habc domain in the regulation of complex assembly and also the role that different Vps45p interactions play in this regulation. We include analyses of the mammalian homologue of Tlg2p, Sx16, in our studies, since a major goal of our work is to identify conserved regulatory mechanisms involved in SNARE-mediated fusion in all eukaryotes.
Acknowledgments
Funding
Our experimental work was funded by the Biotechnology and Biological Science Research Council [grant number BB/E024904/1 to N.J.B. and Doctoral Training Grant to C.M.] and the National Institutes of Health [grant number GM068803 to M.M.]. N.J.B. is the recipient of a Prize Fellowship from the Lister Institute of Preventive Medicine.
Abbreviations used
- SM
Sec1p/Munc18
- SNARE
soluble N-ethylmaleimide-sensitive fusion protein-attachment protein receptor
- Sx
syntaxin
- Vps
vacuolar protein sorting
References
- 1.Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 2.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 3.Weber T, Parlati F, McNew JA, Johnston RJ, Westermann B, Sollner TH, Rothman JE. SNAREpins are functionally resistant to disruption by NSF and αSNAP. J Cell Biol. 2000;149:1063–1072. doi: 10.1083/jcb.149.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez I, Ubach J, Dulubova I, Zhang X, Sudhof TC, Rizo J. Three-dimensional structure of an evolutionarily conserved N-terminal domain of syntaxin 1A. Cell. 1998;94:841–849. doi: 10.1016/s0092-8674(00)81742-0. [DOI] [PubMed] [Google Scholar]
- 5.Lerman JC, Robblee J, Fairman R, Hughson FM. Structural analysis of the neuronal SNARE protein syntaxin-1A. Biochemistry. 2000;39:8470–8479. doi: 10.1021/bi0003994. [DOI] [PubMed] [Google Scholar]
- 6.Dulubova I, Sugita S, Hill S, Hosaka M, Fernandez I, Sudhof TC, Rizo J. A conformational switch in syntaxin during exocytosis: role of munc18. EMBO J. 1999;18:4372–4382. doi: 10.1093/emboj/18.16.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munson M, Chen X, Cocina AE, Schultz SM, Hughson FM. Interactions within the yeast t-SNARE Sso1p that control SNARE complex assembly. Nat Struct Biol. 2000;7:894–902. doi: 10.1038/79659. [DOI] [PubMed] [Google Scholar]
- 8.Munson M, Hughson FM. Conformational regulation of SNARE assembly and disassembly in vivo. J Biol Chem. 2002;277:9375–9381. doi: 10.1074/jbc.M111729200. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson KL, Munson M, Miller RB, Filip TJ, Fairman R, Hughson FM. Regulation of SNARE complex assembly by an N-terminal domain of the t-SNARE Sso1p. Nat Struct Biol. 1998;5:793–802. doi: 10.1038/1834. [DOI] [PubMed] [Google Scholar]
- 10.Aran V, Brandie FM, Boyd AR, Kantidakis T, Rideout EJ, Kelly SM, Gould GW, Bryant N. Characterisation of two distinct binding modes between Syntaxin 4 and Munc18c. Biochem J. 2009;419:655–660. doi: 10.1042/BJ20082293. [DOI] [PubMed] [Google Scholar]
- 11.D’Andrea-Merrins M, Chang L, Lam AD, Ernst SA, Stuenkel EL. Munc18c interaction with syntaxin 4 monomers and SNARE complex intermediates in GLUT4 vesicle trafficking. J Biol Chem. 2007;282:16553–16566. doi: 10.1074/jbc.M610818200. [DOI] [PubMed] [Google Scholar]
- 12.Johnson JR, Ferdek P, Lian LY, Barclay JW, Burgoyne RD, Morgan A. Binding of UNC-18 to the N-terminus of syntaxin is essential for neurotransmission in Caenorhabditis elegans. Biochem J. 2009;418:73–80. doi: 10.1042/BJ20081956. [DOI] [PubMed] [Google Scholar]
- 13.Richmond JE, Weimer RM, Jorgensen EM. An open form of syntaxin bypasses the requirement for UNC-13 in vesicle priming. Nature. 2001;412:338–341. doi: 10.1038/35085583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 15.Halachmi N, Lev Z. The Sec1 family: a novel family of proteins involved in synaptic transmission and general secretion. J Neurochem. 1996;66:889–897. doi: 10.1046/j.1471-4159.1996.66030889.x. [DOI] [PubMed] [Google Scholar]
- 16.Hata Y, Slaughter CA, Sudhof TC. Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature. 1993;366:347–351. doi: 10.1038/366347a0. [DOI] [PubMed] [Google Scholar]
- 17.Misura KM, Scheller RH, Weis WI. Three-dimensional structure of the neuronal Sec1–syntaxin 1a complex. Nature. 2000;404:355–362. doi: 10.1038/35006120. [DOI] [PubMed] [Google Scholar]
- 18.Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 19.Bryant NJ, James DE. Vps45p stabilizes the syntaxin homologue Tlg2p and positively regulates SNARE complex formation. EMBO J. 2001;20:3380–3388. doi: 10.1093/emboj/20.13.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dulubova I, Yamaguchi T, Gao Y, Min SW, Huryeva I, Sudhof TC, Rizo J. How Tlg2p/syntaxin 16 ‘snares’ Vps45. EMBO J. 2002;21:3620–3631. doi: 10.1093/emboj/cdf381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamaguchi T, Dulubova I, Min SW, Chen X, Rizo J, Sudhof TC. Sly1 binds to Golgi and ER syntaxins via a conserved N-terminal peptide motif. Dev Cell. 2002;2:295–305. doi: 10.1016/s1534-5807(02)00125-9. [DOI] [PubMed] [Google Scholar]
- 22.Bracher A, Weissenhorn W. Structural basis for the Golgi membrane recruitment of Sly1p by Sed5p. EMBO J. 2002;21:6114–6124. doi: 10.1093/emboj/cdf608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Latham CF, Lopez JA, Hu SH, Gee CL, Westbury E, Blair DH, Armishaw CJ, Alewood PF, Bryant NJ, James DE, Martin JL. Molecular dissection of the Munc18c/syntaxin4 interaction: implications for regulation of membrane trafficking. Traffic. 2006;7:1408–1419. doi: 10.1111/j.1600-0854.2006.00474.x. [DOI] [PubMed] [Google Scholar]
- 24.Peng R, Gallwitz D. Sly1 protein bound to Golgi syntaxin Sed5p allows assembly and contributes to specificity of SNARE fusion complexes. J Cell Biol. 2002;157:645–655. doi: 10.1083/jcb.200202006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burkhardt P, Hattendorf DA, Weis WI, Fasshauer D. Munc18a controls SNARE assembly through its interaction with the syntaxin N-peptide. EMBO J. 2008;27:923–933. doi: 10.1038/emboj.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dulubova I, Khvotchev M, Liu S, Huryeva I, Sudhof TC, Rizo J. Munc18-1 binds directly to the neuronal SNARE complex. Proc Natl Acad Sci USA. 2007;104:2697–2702. doi: 10.1073/pnas.0611318104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khvotchev M, Dulubova I, Sun J, Dai H, Rizo J, Sudhof TC. Dual modes of Munc18-1/SNARE interactions are coupled by functionally critical binding to syntaxin-1 N-terminus. J Neurosci. 2007;27:12147–12155. doi: 10.1523/JNEUROSCI.3655-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rickman C, Medine CN, Bergmann A, Duncan RR. Functionally and spatially distinct modes of munc18-syntaxin 1 interaction. J Biol Chem. 2007;282:12097–12103. doi: 10.1074/jbc.M700227200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell. 2007;128:183–195. doi: 10.1016/j.cell.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Carpp LN, Ciufo LF, Shanks SG, Boyd A, Bryant NJ. The Sec1p/Munc18 protein Vps45p binds its cognate SNARE proteins via two distinct modes. J Cell Biol. 2006;173:927–936. doi: 10.1083/jcb.200512024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carr CM, Grote E, Munson M, Hughson FM, Novick PJ. Sec1p binds to SNARE complexes and concentrates at sites of secretion. J Cell Biol. 1999;146:333–344. doi: 10.1083/jcb.146.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burgoyne RD, Morgan A. Membrane trafficking: three steps to fusion. Curr Biol. 2007;17:255–258. doi: 10.1016/j.cub.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Weimer RM, Richmond JE, Davis WS, Hadwiger G, Nonet ML, Jorgensen EM. Defects in synaptic vesicle docking in unc-18 mutants. Nat Neurosci. 2003;6:1023–1030. doi: 10.1038/nn1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antonin W, Fasshauer D, Becker S, Jahn R, Schneider TR. Crystal structure of the endosomal SNARE complex reveals common structural principles of all SNAREs. Nat Struct Biol. 2002;9:107–111. doi: 10.1038/nsb746. [DOI] [PubMed] [Google Scholar]
- 35.Peng R, Gallwitz D. Multiple SNARE interactions of an SM protein: Sed5p/Sly1p binding is dispensable for transport. EMBO J. 2004;23:3939–3949. doi: 10.1038/sj.emboj.7600410. [DOI] [PMC free article] [PubMed] [Google Scholar]