Abstract
Japan promotes research related to intractable diseases and financially supports patients with these diseases. Intractable diseases are designated as those that fulfill the following criteria: (1) rarity (affecting less than 0.1% of the population in Japan), (2) unknown etiology, (3) lack of effective treatment, (4) necessity of long-term treatment, and (5) existence of objective diagnostic criteria and not necessarily equal to rare diseases in other countries. The construction of a national database is required to promote research to clarify the pathogenesis of these diseases and to develop pharmaceutical products and medical devices. The Ministry of Health, Labor, and Welfare launched an online registration system in 2001, but many problems associated with gathering and utilizing information on patients with intractable diseases remain. In this paper, we describe the present status of the national registry of designated intractable diseases in Japan and discuss future prospects.
Keywords: intractable diseases, registry, clinical research
Introduction
Recently, more than 6000 rare diseases have been identified (defined as affecting <5/10,000 individuals in Europe and <200,000 people in the United States).1) In Japan, a certain number of rare diseases were designated intractable diseases for promoting research to clarify the pathogenesis of these diseases and to develop pharmaceutical products and medical devices.2) The promotion of a clinical study on rare diseases is necessary to construct a national database because demographic as well as genomic data are required for evaluating the prognosis of patients with rare diseases.3) On the other hand, the diagnostic criteria for rare diseases should be revised according to the progress of medical diagnostic technologies; therefore, the standardization of a registration is necessary for re-thinking the classification and categorization of diseases.4) The Ministry of Health, Labor, and Welfare (MHLW) in Japan launched an online registry system for gathering designated rare and intractable diseases in 2001. Although the registration rate of these diseases has improved each year, a remarkable difference exists among prefectures. To address these issues, the MHLW established a new Act on intractable diseases that enables registration at public health centers as well as at hospitals that satisfy certain criteria. Herein, we describe the present status of the national registry of designated intractable diseases in Japan and discuss future prospects.
Japan’s Policy for Intractable Diseases
Japan’s policy for intractable diseases has its roots in the mid 1950s, when cases of an unknown illness with visual and neurological disturbance were reported. In 1970, a task-force research group determined that the disease, called subacute myelo-optic-neuropathy, was caused by the chronic administration of a large dose of clioquinol as an antiflatulent.5) Following this experience, the MHLW launched the following initiatives to promote the elucidation of the etiology of intractable diseases in 1972: Research Projects on Overcoming Intractable Diseases and the Treatment for Specified Diseases. These programs have been key components of Japan’s intractable disease policy.6)
The Research Project on Treatment for Specified Diseases provides assistance for selected diseases among the designated intractable diseases that are entitled to the research grant program. The aspects of this project include research promotion as well as financial assistance to avoid catastrophic expenditure as it covers the co-payment of eligible patients in return for providing data for research use. At the start of the program, four diseases were designated for the Research Project on Treatment for Specified Diseases, and the number of designated diseases gradually increased to 56 before a major reform for intractable diseases was carried out. With the enforcement of the Intractable Disease Health Care Act in 2015, 306 diseases are now eligible for financial assistance.
The criteria for designated intractable diseases are as follows: (1) rarity (affecting less than 0.1% of the population in Japan), (2) unknown etiology, (3) lack of effective treatment, (4) necessity of long-term treatment, and (5) existence of objective diagnostic criteria. 16 out of 306 designated intractable diseases are related to neurosurgery (Table 1).
Table 1.
Target diseases of neurosurgery in Japan
|
National Registry for Designated Intractable Diseases
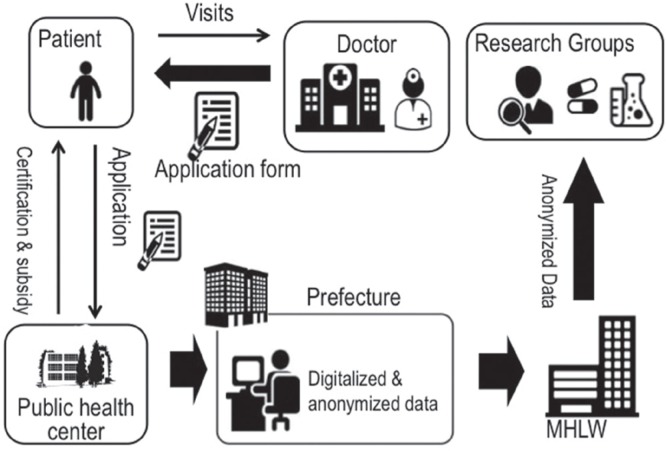
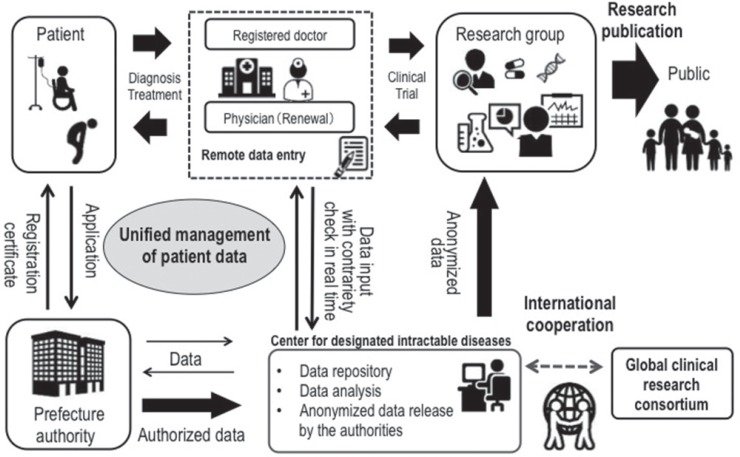
In Japan, when a doctor diagnoses a patient with a designated intractable disease, he/she fills out a registration form and gives the form to the patient along with an application form for a medical expense subsidy. This information on this form includes demographic data necessary for certificating the diagnosis and clinical stage. A patient takes this document to a public health center to request a medical expense subsidy, and the public health center gathers these applications and submits them to the governor of the prefecture. The governor decides whether patients are eligible for subsidy and sends them for claimant certifications. The MHLW gathered these registration forms for analyzing the etiology of designated intractable diseases, but the registration forms differed among prefectures. Therefore, the MHLW launched an online registration system for designated intractable diseases in 20007). The registration forms are digitalized and anonymized at a prefecture and are uploaded to the MHLW server (Fig. 1). The uploaded data are automatically classified as definite, probable, or possible intractable disease according to the clinical criteria of each disease. The MHLW research groups for designated intractable diseases begin to utilize this national database after the MHLW approves their research plan. Although the total cost of administrating online registration system is about a hundred million yen per year and the half of operating cost is supported by the MHLW, some prefectures have not submitted information to the registry system.
Fig. 1.
National registry of designated intractable diseases in Japan.
Utilization of a National Database on Designated Intractable Diseases for Clinical Research
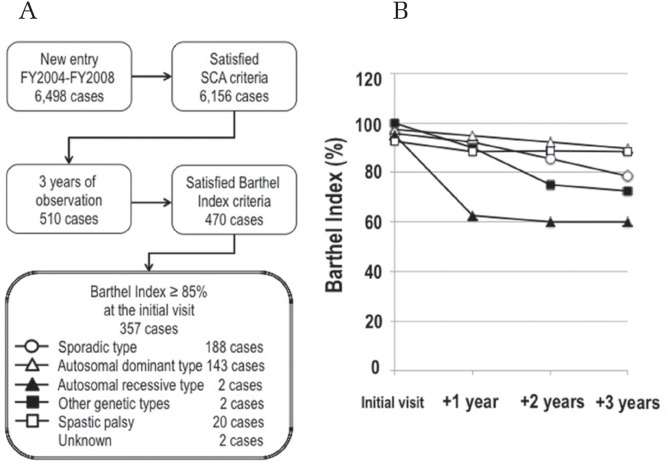
The national database enables clarification of the etiology of designated intractable diseases and understanding of the actual condition of patients with designated intractable diseases.8) The database covers over 90% of new designated intractable cases in Japan. For example, we evaluated the prognosis of patients with spinocerebellar ataxia (SCA). SCA is classified as sporadic, hereditary (autosomal dominant, autosomal recessive, and other genotypes), or spastic paraplegia. An evaluation of the SCA cases registered in the national database revealed that the clinical manifestation of SCA is heterogeneous, suggesting a different focus on the nervous system (Table 2). Because the SCA registration form contains queries related to the Barthel Index, we compared the prognoses of SCA cases according to the Barthel Index for 3 years.9) The Barthel Index of the autosomal dominant type of SCA and spastic paraplegia remained at over 85% for 3 years, but that of autosomal recessive and other SCA genotypes deteriorated to below 75% within 2 years of observation (Fig. 2). If a Barthel Index of less than 85% were adopted as criteria for medical expense subsidy, most cases with the autosomal dominant type of SCA and spastic paraplegia would not be covered. Therefore, the MHLW selected the modified Rankin Scale (mRS) as a criterion for medical expense subsidy.10)
Table 2.
Clinical manifestation of spinocerebellar ataxia in Japan*
| Types of SCA (n = 6,156) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Number of cases | Sporadic | Autosomal dominant | Autosomal recessive | Other genetic types | Spastic palsy | Unknown |
| 3,410 | 1,914 | 81 | 70 | 484 | 197 | |
| Neurological presentation | ||||||
| Dementia | 9.2% | 11.8% | 22.2% | 18.6% | 11.2% | 8.1% |
| Cerebellar dysarthria | 86.3% | 86.5% | 84.0% | 81.4% | 12.8% | 77.2% |
| Gait ataxia | 94.5% | 93.8% | 84.0% | 94.3% | 21.5% | 82.7% |
| Limb ataxia | 92.5% | 92.5% | 91.4% | 92.9% | 18.8% | 80.2% |
| Romberg sign | 23.3% | 19.5% | 19.8% | 22.9% | 9.5% | 21.3% |
| Babinski sign | 7.6% | 12.9% | 27.2% | 15.7% | 77.1% | 10.2% |
| Vertical supra-nuclear gaze palsy | 2.0% | 3.6% | 2.5% | 4.3% | 2.7% | 1.5% |
| Gaze-evoked nystagmus | 19.1% | 36.7% | 30.9% | 22.9% | 2.9% | 20.8% |
| Disturbance of slow | 24.5% | 29.3% | 24.7% | 30.0% | 5.2% | 21.3% |
| Eye movement | ||||||
| Parkinsonism | 5.7% | 3.7% | 1.2% | 10.0% | 2.3% | 3.0% |
| Limb reflex | ||||||
| Hyperactive | 22.1% | 34.3% | 30.9% | 20.0% | 92.1% | 23.9% |
| Sluggish | 16.9% | 15.5% | 42.0% | 17.1% | 0.6% | 16.8% |
| Normal | 59.5% | 48.6% | 25.9% | 62.9% | 7.2% | 46.2% |
From FY2004 to FY2008, 6,156 cases were registered in the national database for designated intractable diseases. We analyzed the clinical manifestation of SCA types by using the latest criteria: sporadic autosomal dominant, autosomal recessive, other genetic types, spastic palsy, and unknown.
Fig. 2.
Evaluation of the prognosis of designated intractable diseases. (A) Flow of cases enrolled in this study. (B) Evaluation of SCA prognosis by using the Barthel Index.
Standardization of Clinical Data Required for Registration
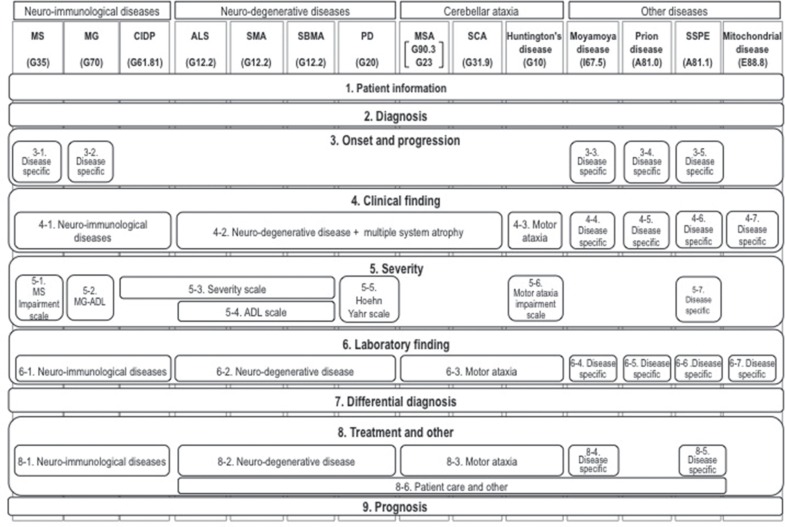
Before 2000, the registration forms for designated intractable diseases differed among prefectures. Therefore, the MHLW constructed an online registry system in which the registration forms must record the following items in order to standardize accreditation criteria for medical expense subsidy: (1) patient information, (2) diagnosis, (3) onset and progressive course, (4) clinical findings, (5) severity, (6) laboratory findings, (7) differential diagnosis, (8) treatment, and (9) prognosis. With the exception of patient information, the submission forms varied among the different disease types. Since some of the designated intractable diseases have a similar clinical presentation, distinguishing between these diseases is difficult without genetic analysis.11) Therefore, the data elements should be shared in the same disease categories or disease groups. We classified designated intractable diseases according to the ICD10 (International Statistical Classification of Diseases version 10); however, the registry forms used in Japanese nationwide surveillance are not necessarily based on clinical manifestation. We categorized 14 designated intractable neurological diseases into the following three groups based on clinical manifestation and pathogenesis: the neuro-immunological disease group, neuro-degenerative disease group, and cerebellar ataxia (Fig. 3). The queries consist of general queries and disease-specific queries, and some queries are shared by the same disease group. However, some queries require disease-specific elements, such as genetic analysis and/or activity of daily living (ADL) score. Recently, the standardization of electric heath records (EHRs) has been requested to improve the quality of clinical research, and we reconstructed the present registry system with a data model that satisfies the ISO13606 standard specification.12)
Fig. 3.
Standardization of clinical data required for registration of neurological diseases. MS: Multiple sclerosis, MG: myasthenia gravis, CIDP: chronic inflammatory demyelinating polyneuropathy, ALS: amyotrophic lateral sclerosis, SMA: spinal muscular atrophy, SBMA: spinobulbar muscular atrophy, PD: Parkinson’s disease, MSA: multiple system atrophy, SCA: spinocerebellar ataxia, SSPE: subacute sclerosing panencephalitis. Each disease is classified by ICD10.
Research Projects to Improve the Prognosis of Designated Intractable Diseases
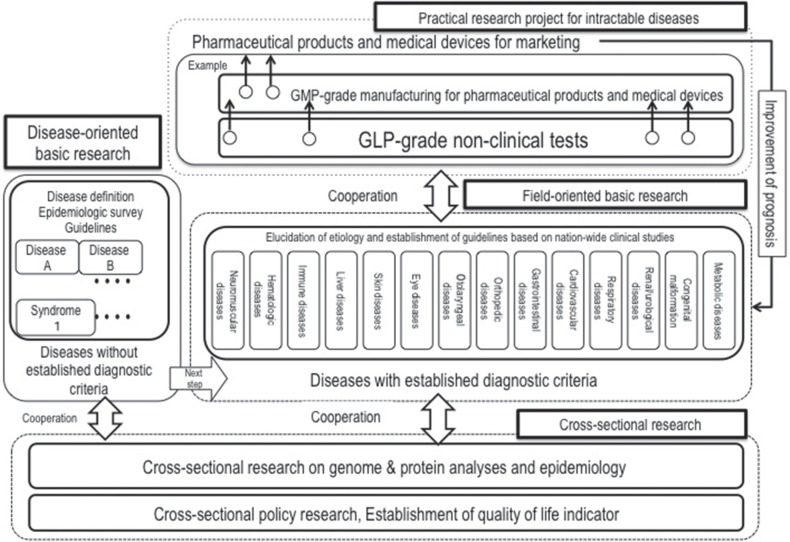
Updating the definition and related diagnostic guidelines is necessary for constructing a database for designated intractable diseases.4) The MHLW has launched Japan Intractable Diseases Information Center (http://www.nanbyou.or.jp), which provides up-to-date diagnostic guidelines on designated intractable diseases. Moreover, researchers involved in projects that to develop pharmaceutical products and medical devices are anticipated to utilize the national database for recruiting patients who have rare and intractable diseases. Therefore, the MHLW has integrated individual research projects in the following four categories: (1) disease-oriented basic research, (2) field-oriented basic research, (3) cross-sectional research, and (4) practical research projects for rare and intractable diseases (Fig. 4). The mission of these projects is to establish diagnostic criteria for diseases with unknown etiology (disease-oriented basic research), to elucidate the etiology and revise the guidelines based on nationwide clinical studies (field-oriented basic research), to support the bio-bank and find bio-markers related to prognosis (cross-sectional research), and to promote clinical studies on pharmaceutical products and medical devices (practical research projects for rare and intractable diseases).
Fig. 4.
Research projects to improve the prognosis of intractable diseases.
Future Prospects
In 2015, the Intractable Disease Health Care Act was initiated to secure a budget for maintaining medical expense subsidy for patients with intractable diseases and to promote research on clarifying the pathogenesis and promoting the development of pharmaceutical products and medical devices. With the new act, the number of designated intractable diseases was expanded from 56 to 306 by July 2015, and the MHLW has begun to designate doctors and hospitals that have the capability to diagnose and provide medical care for patients with intractable diseases. The online-registry system will be transferred from public health centers to hospitals, which makes it possible to share patient information among healthcare providers in the community (Fig. 5). Designated doctors are able to access the system and follow prognosis of their patients. However, the high cost for medical providers to construct an information platform for sharing and utilizing information on patients is an existing problem that must be addressed. This is due to the difficulty of up-loading patient information directly through EHRs since most of the EHRs in Japan are not accessible via the internet to prevent the leakage of information.13) Recently, an open EHR system has been considered to enable the sharing of health information in the community and mobile virtual private network seems to be an appropriate infrastructure.14) The new registry system is adopting ISO13606 and it is compatible with an open her system. In addition to constructing a nation-wide registry system to promote clinical research, educating doctors for high-quality diagnoses and further improvement of the health care system are important for patients with rare diseases. Especially, promoting research and the development of pharmaceutical products and medical devices on ultra-rare diseases (less than 1 person per 50,000 people) is difficult with respect to obtaining financial support and recruiting patients in a single country.15) Therefore, a global alliance is necessary for promoting research on ultra-rare diseases. In 2011, a world-wide clinical research alliance, the International Rare Diseases Research Consortium (IRDiRC), was established. In 2015, a headquarter organization promoting innovative medical research, the Japan Agency for Medical Research and Development (AMED), was launched in Japan; this organization participated in IRDiRC in 2016.
Fig. 5.
Future Prospects: New designated intractable diseases registry schema.
Acknowledgements
The necessary expenses for publication of this work were supported by a research grant from the Ministry of Health and Welfare of Japan (Research on Measures for Intractable Diseases).
Footnotes
Conflicts of Interest Disclosure
The authors declare no conflicts of interest.
References
- 1). Forman J, Taruscio D, Llera VA, Barrera LA, Coté TR, Edfjäll C, Gavhed D, Haffner ME, Nishimura Y, Posada M, Tambuyzer E, Groft SC, Henter JI, International Conference for Rare Diseases and Orphan Drugs (ICORD) : The need for worldwide policy and action plans for rare diseases. Acta Paediatr 101: 805– 807, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Hayashi S, Umeda T: 35 years of Japanese policy on rare diseases. Lancet 372: 889– 890, 2008. [DOI] [PubMed] [Google Scholar]
- 3). Gliklich RE, Dreyer NA, Leavy MB. (eds): Registries for Evaluating Patient Outcomes: A User’s Guide [Internet]. 3rd edition Rockville (MD): Agency for Healthcare Research and Quality (US); 2014. April. [PubMed] [Google Scholar]
- 4). Svenstrup D, Jørgensen HL, Winther O: Rare disease diagnosis: A review of web search, social media and large-scale data-mining approaches. Rare Dis 16: e1083145, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Egashira Y, Matsuyama H: Subacute myelo-opticoneuropathy (SMON) in Japan. With special reference to the autopsy cases. Acta Pathol Jpn 32 Suppl 1: 101– 116, 1982. [PubMed] [Google Scholar]
- 6). Nakatani H, Kondo T: Characteristics of a medical care program for specific diseases in Japan in an era of changing cost-sharing. Health Policy 64: 377– 389, 2003. [DOI] [PubMed] [Google Scholar]
- 7). Kimura E, Kobayashi S, Kanatani Y, Ishihara K, Mimori T, Takahashi R, Chiba T, Yoshihara H: Developing an electronic health record for intractable diseases in Japan. Stud Health Technol Inform 169: 255– 259, 2011. [PubMed] [Google Scholar]
- 8). Sato Y, Nakatani E, Watanabe Y, Fukushima M, Nakashima K, Kannagi M, Kanatani Y, Mizushima H: Prediction of prognosis of ALS: Importance of active denervation findings of the cervical-upper limb area and trunk area. Intractable Rare Dis Res 4: 181– 189, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Weyer A, Abele M, Schmitz-Hübsch T, Schoch B, Frings M, Timmann D, Klockgether T: Reliability and validity of the scale for the assessment and rating of ataxia: a study in 64 ataxia patients. Mov Disord 15: 1633– 1637, 2007. [DOI] [PubMed] [Google Scholar]
- 10). Notermans NC, van Dijk GW, van der Graaf Y, van Gijn J, Wokke JH: Measuring ataxia: quantification based on the standard neurological examination. J Neurol Neurosurg Psychiatry 57: 22– 26, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Liu Z, Shen B: Overcoming difficulty in diagnosis and differential diagnosis of Crohn’s disease: the potential role of serological and genetic tests. Expert Rev Mol Diagn 15: 1133– 1141, 2015. [DOI] [PubMed] [Google Scholar]
- 12). Fernandez-Breis JT, Menarguez-Tortosa M, Martinez-Costa C, Fernandez-Breis E, Herrero-Sempere J, Moner D, Sanchez J, Valencia-Garcia R, Robles M: A semantic web-based system for managing clinical archetypes. Conf Proc IEEE Eng Med Biol Soc 2008: 1482– 1485, 2008. [DOI] [PubMed] [Google Scholar]
- 13). Gomi Y, Nogawa H, Tanaka H: Toward secure distribution of electronic health records: quantitative feasibility study on secure E-mail systems for sharing patient records. J Med Dent Sci 52: 229– 236, 2005. [PubMed] [Google Scholar]
- 14). Ventola CL: Mobile devices and apps for health care professionals: uses and benefits. P T 39: 356– 364, 2014. [PMC free article] [PubMed] [Google Scholar]
- 15). Hughes DA, Tunnage B, Yeo ST: Drugs for exceptionally rare diseases: do they deserve special status for funding? QJM 98: 829– 836, 2005. [DOI] [PubMed] [Google Scholar]