Abstract
The kidney collecting duct is an important renal tubular segment for regulation of body water homeostasis and urine concentration. Water reabsorption in the collecting duct principal cells is controlled by vasopressin, a peptide hormone that induces the osmotic water transport across the collecting duct epithelia through regulation of water channel proteins aquaporin-2 (AQP2) and aquaporin-3 (AQP3). In particular, vasopressin induces both intracellular translocation of AQP2-bearing vesicles to the apical plasma membrane and transcription of the Aqp2 gene to increase AQP2 protein abundance. The signaling pathways, including AQP2 phosphorylation, RhoA phosphorylation, intracellular calcium mobilization, and actin depolymerization, play a key role in the translocation of AQP2. This review summarizes recent data demonstrating the regulation of AQP2 as the underlying molecular mechanism for the homeostasis of water balance in the body.
Keywords: arginine vasopressin, aquaporin-2, body water balance, intracellular trafficking
Body water homeostasis is mainly established by the kidney function, such as tubular reabsorption of water and sodium through water channel proteins (aquaporins: AQPs) and sodium cotransporters (3, 34, 58, 61, 82, 98). As water can slowly diffuse through biomembranes composed of lipid bilayers, all membranes exhibit some degree of water permeability (27). Nonetheless, the plasma membranes of the renal tubular epithelia have distinctly high water permeability for water transport. Water reabsorption in the kidney tubule depends on the driving force (i.e., high interstitial osmolality/tonicity) and osmotic equilibration of water across the tubular epithelia (i.e., high osmotic water permeability of the membrane). The majority of fluid filtered in the glomerulus is constitutively reabsorbed in the proximal tubules and descending thin limbs (60, 115). The subsequent renal tubular segments, i.e., ascending thin limbs, thick ascending limbs, and distal convoluted tubules, are relatively water impermeable and hence the tubular fluid could be delivered into the connecting tubules and collecting ducts (11, 120).
The connecting tubule and collecting duct are important tubular segments for the regulation of body water homeostasis, where vasopressin regulates water reabsorption (24, 59, 121, 127, 128). Vasopressin is a peptide hormone that controls plasma osmolality and extracellular fluid volume. It is synthesized in the hypothalamus, stored and released from the neurohypophysis, and has a physiological role in the kidney connecting tubules and collecting ducts via vasopressin V2 receptor (V2R) (58, 59). Epithelial water permeability in the collecting duct principal cells is low in the absence of vasopressin stimulation, but it increases substantially to the high levels, when the principal cells are stimulated by vasopressin. Vasopressin binds to the G protein-linked V2R in the basolateral plasma membrane and promotes osmotic water reabsorption across the epithelia of the collecting duct via osmotic equilibrium with the hyperosmotic interstitium (64, 97, 98). Previously, expression of V2R mRNA was found in medullary and cortical thick ascending limb, macula densa, distal convoluted tubule, connecting tubule, and cortical and medullary collecting duct in rat, mouse, and human kidney (23, 90). In the present review, we mainly focused on the short-term regulation of water channel protein aquaporin-2 (AQP2) through AQP2 phosphorylation and intracellular trafficking of AQP2, which are induced by vasopressin stimulation in the kidney collecting duct principal cells.
Vasopressin-Regulated AQP2
Aquaporin is water channel protein that transports water molecules across the biomembrane (109, 110). In particular, AQP2 is the critical water channel protein for vasopressin-mediated water reabsorption, which is localized in the kidney connecting tubules and collecting ducts (29, 121). Vasopressin induces a rapid increase of the osmotic water permeability in the collecting duct principal cells by promoting the translocation or trafficking of AQP2 between an intracellular reservoir in vesicles and the apical plasma membrane, i.e., short-term regulation of AQP2 (10, 93, 145, 153). V2R-mediated stimulation of adenylyl cyclases (ACs), elevation of cAMP, and activation of protein kinase A (PKA) are the principal signaling pathways for triggering both the subsequent increases of AQP2 trafficking and AQP2 protein abundance (17, 64, 98, 99, 156; summarized in Fig. 1). AC6 is the principal AC mediating these responses, as only AC6-deficient mice have urine concentration defects (54, 55, 118, 122). However, cAMP-independent mechanisms for AQP2 trafficking also exist, as previously demonstrated, e.g., stimulation of prostanoid receptors EP2 and EP4 and short-term exposure to peroxisomal proliferator-activated receptor subtype γ-agonist (102, 111).
Fig. 1.
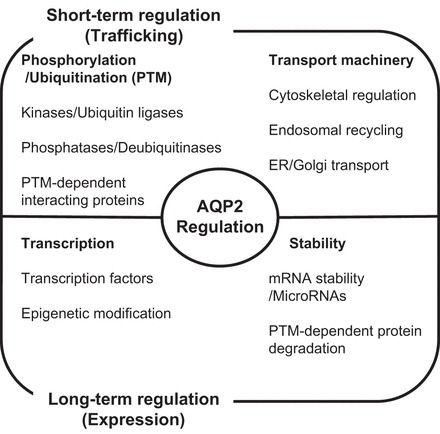
Summary of intracellular molecular mechanisms for aquaporin 2 (AQP2) regulation in renal collecting duct cells. Water permeability of collecting duct cells is regulated via AQP2 under various physiological and pathophysiological conditions through 2 mechanisms. Short-term regulation: trafficking between intracellular vesicles and plasma membrane is associated with posttranslational modification of AQP2 and vesicle-transporting systems dependent on cell signaling; long-term regulation: change of protein abundance is regulated by transcription and mRNA/protein stability. PTM, posttranslational modification.
In the renal collecting duct cells without vasopressin stimulation, AQP2 is present mainly in the recycling endosome where AQP2 and Rab11 proteins are colocalized (4, 91). In contrast, when the collecting ducts are stimulated by vasopressin, AQP2 is translocated to the apical plasma membrane, associated with an increase of osmotic water permeability (97). Nevertheless, AQP2 was also found at the basolateral plasma membrane, as previously demonstrated in rats with lithium-induced nephrogenic diabetes insipidus (NDI) and normal rats after aldosterone treatment (19, 96). Moreover, the AQP2 water channel is not only just for water moving but also involved in cell migration and epithelial morphogenesis (13). The actions of vasopressin are accompanied by the changes in AQP2 phosphorylation at four different serine sites (S256, S261, S264, and S269) in the carboxy terminus (40, 41, 81). On the contrary, long-term regulation or adaptation of AQP2 is presented by changing AQP2 protein abundance (98). Recently Schenk et al. (129) quantified vasopressin-induced changes in the nuclear proteome of cortical collecting duct cells by large-scale proteomics profiling and demonstrated a number of transcription factors that have putative binding sites in the 5′-flanking region of the gene coding for the AQP2. In addition, nuclear receptors affecting AQP2 expression are also recently summarized (160). When vasopressin stimulation is removed, endocytosis and intracellular degradation of AQP2 could occur (49, 66). AQP2 degradation was inhibited by either MG132 (a proteasome inhibitor) or chloroquine (lysosomal pathway blocker) treatment in primary cultured inner medullary collecting duct (IMCD) cells of the rat kidney (66). This finding suggests that ubiquitination and subsequent proteosomal and/or lysosomal degradation of AQP2 could also be important in regulation of AQP2 abundance (49).
In clinical conditions, a number of previous studies demonstrated that dysregulation of AQP2 plays a critical role in the pathophysiology of both water-losing disorders with polyuria and water retention disorders with dilutional hyponatremia (6, 7, 58, 62, 64, 98, 100). Moreover, mutations in the AQP2 gene lead to autosomal recessive NDI in human patients (71, 87, 88, 147).
Role of cAMP/PKA Pathway on AQP2 Phosphorylation and Intracellular Trafficking of AQP2
The signaling transduction pathways involved in the apical trafficking or endocytosis of AQP2 and the changes of AQP2 protein abundance have been extensively studied (summarized in Table 1) (25). In the process of exocytosis and endocytosis of AQP2, several phosphorylation sites in the carboxy terminus of AQP2 (40, 81–83) are targeted by various kinases. Among them, cAMP and PKA signaling pathways have widely been studied for AQP2 trafficking. Vasopressin binding to the G protein-linked V2R stimulates ACs, leading to elevation of cAMP levels and activation of PKA. This leads to the recruitment of PKA to AQP2-bearing vesicles by PKA-anchoring proteins (AKAPs) (56). Consistent with this, AQP2 is colocalized with AKAP 18 delta in the intracellular vesicles (38). Moreover, rolipram treatment, an inhibitor of cAMP-specific phosphodiesterase-4D, increases AKAP-tethered PKA activity in the AQP2-bearing vesicles and hence AQP2 is subsequently translocated to the apical plasma membrane (134). A recent study demonstrated that AKAP220 also interacts with the Rho-family GTPase effector protein IQGAP and enhances the actin polymerization (151). Accordingly, AKAP220 null mice revealed that RhoA and AQP2 accumulate at the apical membrane domains of the renal collecting duct cells with inappropriate water reabsorption (151).
Table 1.
Intracellular signaling pathways for AQP2 trafficking or endocytosis
| Pathways | Protein Modification of AQP2* | Reference |
|---|---|---|
| Trafficking | ||
| cAMP/PKA | Phosphorylation (S256) | Christensen et al. (17) |
| Katsura et al. (51) | ||
| Intracellular calcium (Ca2+) mobilization (calcium-calmodulin-mediated myosin activation) | Phosphorylation (S256) | Chou et al. (16) |
| PI3K-dependent activation of AKT | Phosphorylation (S256) | Pisitkun et al. (108) |
| AS160 phosphorylation | N.A. | Kim et al. (52) |
| RhoA-dependent cytoskeletal dynamics | Phosphorylation (S256) | Tamma et al. (139) |
| Endocytosis | ||
| Clathrin-mediated endocytosis | Sun et al. (136) | |
| Ubiquitination of AQP2 | Ubiquitination (K270) | Kamsteeg et al. (49) |
| PGE2 | Phosphorylation (S256) | Olesen et al. (103) |
| Phosphorylation (S264) | ||
| Phosphorylation (S269) | Zelenina et al. (159) | |
| Dopamine | Ubiquitination (K270) | Boone et al. (8) |
| Phosphorylation (S261) | Nejsum et al. (94) |
AS160, Akt substrate of 160 kDa; AQP2, aquaporin-2; PGE2, prostaglandin E2; PI3K, phosphatidylinositol-3-kinase.
Protein modification of AQP2 is found within the pathway.
Recruitment of PKA to the AQP2-bearing vesicles results in phosphorylation of AQP2. One of phosphorylated residues of AQP2 is serine 256 (pS256-AQP2), part of consensus motif (RRQS) for phosphorylation by PKA (28, 51). In the kidney collecting duct principal cells, the observed immunolocalization of pS256-AQP2 at the plasma membrane and intracellular vesicles (17) suggests that it is constitutively phosphorylated even in response to low circulating vasopressin levels. Vasopressin stimulates the translocation of AQP2 from the intracellular vesicle to the apical plasma membrane, where AQP2 exists as a tetramer, with minimally three monomers in an AQP2 tetramer to be phosphorylated (48). Semiquantitative immunoelectron microscopy of AQP2 in rat kidney demonstrated that 11% of total AQP2 observed in the apical plasma membrane in the absence of dDAVP stimulation was increased to 25% in the apical plasma membrane following dDAVP injection (152). Importantly, vasopressin or forskolin treatment failed to induce translocation of AQP2 when AQP2-S256A mutant (from Ser to Ala) was transfected to LLC-PK1 cells (28, 51). Moreover, in a mouse strain with an amino-acid substitution at S256 (from Ser to Leu) for preventing phosphorylation, congenital progressive hydronephrosis, polyuria, and urinary concentrating defect were observed, in which vasopressin treatment was not effective (78).
Phosphoproteomics study in the rat IMCD cells has determined that AQP2 is further phosphorylated on S264 and S269 in the carboxy terminus after vasopressin stimulation (41). While AQP2 trafficking is mainly associated with S256 phosphorylation (51), S269 phosphorylation was exclusively found in the apical plasma membrane of the collecting duct cells and was dependent on the prior phosphorylation of S256 (40). Semiquantitative analysis revealed that phosphorylation of S269 was increased from 3 to 26% of total AQP2 in rat IMCD cells after treatment of dDAVP (152). S269 phosphorylation reduces K270 polyubiquitination-mediated AQP2 endocytosis, which could accumulate AQP2 in the plasma membrane (79). Moreover, interaction of AQP2-S269D (mutant protein mimicking S269 phosphorylation) with proteins involved in the endocytosis, e.g., Hsc70 and dynamin, was decreased (117), suggesting that S269 phosphorylation may play a role in AQP2 retention in the apical plasma membrane. The role of pS264-AQP2 is unknown, but immunohistochemistry revealed that pS264-AQP2 was translocated to both the apical and basolateral plasma membrane of the collecting duct cells in Brattleboro rats after dDAVP treatment in a time-dependent manner (26). In addition, pS264-AQP2 was seen in early endosomes but not in lysosomes during withdrawal of vasopressin stimulation, suggesting that phosphorylation of AQP2 could affect intracellular compartmentalization of AQP2 (26).
In contrast, vasopressin decreases S261 phosphorylation by decreasing the activity of MAP kinases (92, 119), which was associated with reduced stability of AQP2 (92). It was also demonstrated that phosphorylation of S261 stabilizes AQP2 ubiquitination and intracellular localization (142). These results suggest that vasopressin-induced AQP2 accumulation in the plasma membrane is likely to be regulated by both AQP2 phosphorylation and protein-protein interactions.
Role of Phosphatidylinositol-3-Kinase/Akt, GSK3, and Cyclin-Dependent Kinases on AQP2 Phosphorylation and Intracellular Trafficking of AQP2
cAMP treatment in rat kidney IMCD cells increased phosphorylation of AQP2 at S256, S264, and S269, whereas the phosphorylation was inhibited by the PKA inhibitor H-89 (40). Interestingly, when a nonphosphorylated synthetic peptide corresponding to the AQP2 carboxy terminus was incubated with PKA in the presence of ATP in vitro, phosphorylation at S256 occurred, whereas phosphorylation at the other three sites (S261, S264, and S269) was not observed (40). PKA, therefore, phosphorylates S256; however, it is unlikely that PKA phosphorylates the other three serine sites directly (40). This finding suggested that other kinases could also be involved in the phosphorylation of AQP2. Bradford et al. (9) proposed probable kinase candidates for the S256 phosphorylation by integrating information extracted from multiple experimental data sets that had been conducted in rat IMCD cells. Among total kinases that were evaluated, top likely kinases were Ca2+/calmodulin-dependent protein kinase II (CAMK2) and protein kinase B (Akt) as well as PKA. These vasopressin-responsive kinases were also suggested by other previous experimental studies (52, 108, 119). However, it should be emphasized that while other kinases are involved as mentioned, PKA is the principal kinase for AQP2 trafficking and body fluid homeostasis, which was also demonstrated by a study exploiting a dominant negative PKA regulatory subunit (RIαB) to disrupt kinase activity in vivo (30). The results showed that dehydration in RIαB-expressing mice did not significantly increase AQP2 protein expression levels and urine was not fully concentrated (30).
G protein-coupled receptor (GPCR)-mediated signaling pathways, including V2R-signaling pathway, are complex and multiple downstream signaling pathways could be associated, e.g., the cross talk between cAMP/PKA signaling pathway and phosphatidylinositol-3-kinase (PI3K) pathway (104). Dimer of G protein subunit-β and -γ is associated with PI3K signaling pathway (67), suggesting that the PI3K/Akt signaling pathway is likely to play a role in V2R-mediated AQP2 trafficking. An in vitro study on recycling mechanism of AQP2 in Madin-Darby canine kidney (MDCK) cells stably expressing human AQP2 revealed that apical AQP2 was retrieved to subapical storage compartment through EEA1-positive endosomes within 90 min after withdrawal of forskolin (137). PI3K inhibitors, wortmannin and LY294002, markedly prolonged this retrieval process to 120 min (137). Moreover, vasopressin stimulates the PI3K/Akt signaling pathway in renal collecting ducts. For example, studies on global network of kinases associated with vasopressin signaling in IMCD tubule suspension showed that Akt phosphorylation (T308 and S473) was increased within 5 min of vasopressin stimulation, resulting in Akt activation (108). Akt signaling was also stimulated by both cAMP and increased osmolality, even in the absence of vasopressin stimulation (108). Consistent with this, immunoblotting and fluorescence resonance energy transfer (FRET)-based imaging analysis in mouse collecting duct cell lines demonstrated an increase of the Akt phosphorylation (S473) and Akt activity within 10 min of dDAVP stimulation (52).
Although these arguments provide an evidence of vasopressin-induced PI3K/Akt signaling in the renal collecting duct cells, direct regulation of AQP2 phosphorylation sites and the intersected components at the downstream of cross talk between cAMP/PKA and PI3K/Akt signaling pathways still need to be explored. In addition to the collecting ducts, vasopressin acting through the V2R can also stimulate PI3K pathways in the distal convoluted tubule and subsequently activate AC and increase cAMP (14).
AQP2 trafficking to the apical plasma membrane was decreased in the lithium-induced nephrogenic diabetes insipidus (NDI) (63, 74). Lithium is an inhibitor of GSK3β, which is a crucial component of Wnt signaling pathway (114, 133). A proteomics study demonstrated that lithium-induced inactivation of GSK was associated with intracellular accumulation of β-catenin, which could affect the Wnt signaling cascade (95). Dysfunction of GSK3, two isoforms (GSK3α and GSK3β), by gene deletion and inhibitor showed reduction of urine concentrating ability, accompanied by decreased expression of AQP2 and pS256-AQP2 (101, 113). Since diminution of AC activity was observed in GSK3α- or GSK3β-knockout mice, further studies are required to figure out the cross talk between GSK activity and AQP2 phosphorylation. Although several studies proposed potential roles of components of Wnt signaling in vasopressin-mediated AQP2 regulation (46, 73, 113, 129), regulatory mechanisms associated with GSK family of Wnt signaling in AQP2 trafficking have not been well elucidated yet.
Cell cycle-related proteins could be involved with vasopressin signaling of collecting duct cells due to rapid turnover of protein abundance (126). Accumulation of pS256-AQP2 at the cell surface was observed when pharmaceutical inhibitor of cyclin-dependent kinases (CDKs) was treated in MDCK cells (119, 141). Increase of apical AQP2 by CDK inhibitor is likely to be induced by diminished activity of protein phosphatase 2A (PP2A), which is counteracting protein of CDK as well as intracellular Ca2+ level. In addition to the involvement of PP2A in AQP2 regulation, protein phosphatase 2B (PP2B), one of components of AKAPs-containing multiple complex has also been proposed to dephosphorylate endosome-bounded AQP2 (44). A recent study demonstrated that multiple phosphatases are involved in the subcellular localization of phosphorylated AQP2, and particularly PP1/PP2A plays a role in the AQP2 phosphorylation and AQP2 expression in the apical plasma membrane (116). Comprehensive understanding of the effects of multiple kinases under different conditions, such as activity and specificity against each phosphorylation site of AQP2 and the interaction with other proteins, could provide information for better understanding of the phosphorylation-mediated AQP2 trafficking.
Role of Cytoskeleton and Small GTPase on AQP2 Phosphorylation and Intracellular Trafficking of AQP2
Earlier observation in toad urinary bladder demonstrated that microtubular network was involved in the vasopressin-stimulated translocation of particle aggregates from the cytoplasm to the membrane (89). The cytoskeleton has been demonstrated to be involved in the AQP2 trafficking in kidney collecting duct cells. This was demonstrated by the findings showing that chemical disruption of microtubules inhibited the vasopressin-induced osmotic water permeability in both the toad bladder and the mammalian collecting duct (21, 106, 107, 124). Intracellular translocation of vesicles occurred along microtubules is likely to be driven by microtubule-associated motor proteins (130). Consistent with this, the motor proteins dynactin and dynein were present in the immunoisolated AQP2-bearing vesicles (76), suggesting that microtubule motor proteins play a role in AQP2 trafficking. Actin-based motor proteins, e.g., myosin 1C, nonmuscle myosins IIA and IIB, and myosin VI were identified by proteomics analysis in AQP2-immunoisolated vesicles from IMCD suspension of rat kidney (4). A recent proteomics study in the apical plasma membrane of the mouse cortical collecting duct cells further highlights the role of the actin cytoskeleton following vasopressin (70). In addition, Rab proteins control vesicle trafficking via regulation of cytoskeleton-based motor proteins (4, 45, 47, 135).
Vasopressin- or forskolin-stimulated AQP2 trafficking is associated with depolymerization of actin cytoskeleton (20, 36, 132). Okadaic acid, a phosphatase inhibitor, induced actin depolymerization and AQP2 translocation to the plasma membrane in CD8 cells, which were similar to the findings induced by forskolin (144). Interestingly, okadaic acid-induced actin depolymerization and AQP2 translocation were visible in CD8 cells despite pretreatment of H89 (a selective PKA-inhibitor) (144). This suggests that the reorganization of the actin network per se plays an important role in the enhancement of AQP2 translocation to the plasma membrane, even though PKA pathway was inhibited. Consistently, in primary cultured IMCD cells of rat kidney, arginine vasopressin-induced redistribution of AQP2 to the plasma membrane was observed when microtubules were depolymerized by nocodazole (148). Interestingly, perinuclear positioning of AQP2 was prevented by the depolymerization of microtubules in the cells during AQP2 internalization after removal of vasopressin stimulation (148). This suggests that microtubules play a role in the regulation of AQP2 compartmentalization.
The Rho GTPases are small GTP-binding proteins, including RhoA, Rac1, and Cdc42 proteins, and play a role in a variety of cellular functions such as cytoskeleton organization and cell migration. Small GTPase Rho affects AQP2 trafficking via reorganization of the actin network (139). For example, in primary cultured rat IMCD cells, inactivation of Rho by Clostridium toxin or Rho-kinase inhibitor treatment induced both actin depolymerization and translocation of AQP2 to the membrane despite an absence of vasopressin (46). Conversely, transfection of these cells with a constitutively active RhoA mutant induced formation of actin stress fibers and inhibited the cAMP-induced AQP2 translocation (46). The data indicate that active Rho is likely to act as an inhibitor of AQP2 trafficking through its induction of actin polymerization.
Vasopressin could inactivate RhoA by serine phosphorylation and increased formation of the Rho-GDP dissociation inhibitor (RhoA-RhoGDI) complex, resulting in actin depolymerization. Based on the differential centrifugation methods for isolating AQP2-bearing vesicles (31, 32, 42, 75), proteomics analysis in native IMCD cells isolated from rat kidneys and cultured collecting duct cells identified the proteins associated with intracellular vesicles (4, 66, 125, 154, 155). Exposure to vasopressin for 30 min induced a change of AQP2 expression ratio between 17,000-g plasma membrane fractions and 200,000-g intracellular vesicle fractions (66, 125). The increased expression of AQP2 in 17,000-g membrane fractions by vasopressin stimulation was due to the activation of exocytic processes in which myosin and GTPase proteins were involved (91). Vasopressin induced actin depolymerization (20), which allowed AQP2-bearing vesicles to access to the apical plasma membrane through inhibition of the GTPase activity of the Rho family proteins, Rac/Cdc42 exchange factor complex, and Rho A (57, 138). On the other hand, liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) proteomics analysis in AQP2-bearing vesicles isolated from 200,000-g fractions of rat IMCD cells identified several Rab GTPases, Rab4, Rab5, Rab7, Rab11, Rab18, Rab21, and Rab25 as the Rab proteins enriched in AQP2-bearing vesicles (4). Existence of various Rab GTPases in the fractions containing AQP2-bearing vesicles indicates that AQP2-bearing vesicles are distributed from early endosomes to recycling endosomes (5, 149).
Both clathrin- and caveolin-mediated endocytosis were examined for AQP2 translocation. AQP2 is located in the clathrin-coated pits in the apical membrane domain in the principal cells and it was demonstrated that AQP2 was endocytosed by a clathrin-mediated mechanism (136). Colocalization of clathrin with AQP2 in the recycling endosome was observed during endocytosis (33, 136). However, caveolin-mediated endocytosis of AQP2 was also suggested. For example, AQP2 internalization was dynamin dependent (136), which is also involved in caveolin-1-mediated endocytosis (37). The association between AQP2 and caveolin-1 was also demonstrated by proteomics analysis of detergent-resistant membrane (caveolin-1 containing lipid raft) proteins from rat kidney collecting duct (157). Moreover, AQP2 and caveolin-1 coimmunoprecipitated in the MDCK cells (2). In rat kidney in vivo, caveolin-1 is localized in the basolateral membrane of collecting duct principal cells, where it is associated with caveolae (105). In response to vasopressin stimulation, caveolin-1 was translocated to the apical plasma membrane in rat kidney and was colocalized with AQP2 (105). However, importantly caveolae were not observed in the apical plasma membrane of principal cells (105), suggesting that caveolae are unlikely to play a major role in the internalization of AQP2 in vivo after removal of vasopressin stimulation.
Role of Altered Microenvironment (e.g., Osmotic Stress, pH, and Fluid Shear Stress) on AQP2 Phosphorylation and Intracellular Trafficking of AQP2
The characteristic phenotype of renal medullary cells is that they survive and functionally adapt to high osmolality/tonicity in the interstitium. Despite the absence of vasopressin stimulation, hypertonicity alone induced a rapid accumulation of AQP2 in the plasma membrane of collecting duct principal cells in rat kidney in vivo (35). Moreover, the hypertonic condition for the cultured AQP2-expressing LLC-PK1 and mCCDc11 cells attenuates AQP2 endocytosis by activation of MAP kinases, independent of intracellular cAMP concentration (35). Accumulation of the pS256-AQP2 at the plasma membrane by osmotic stress was disrupted by MAP kinase inhibitors (35), indicating that acute hypertonicity significantly alters AQP2 trafficking and hypertonicity-induced AQP2 accumulation at the plasma membrane is in part dependent on MAP kinase activity. However, additional studies on the expression levels of other phosphorylation sites (S261, S264, and S269) of AQP2 under the different osmotic stress conditions are required. This could provide more detailed regulatory mechanisms of AQP2 phosphorylation by p38, ERK1/2, and JNK MAPK pathways, which could be activated by osmotic stress. Inhibition of p38, ERK1/2, and JNK MAPK pathways by vasopressin is likely to be part of cAMP/PKA-dependent cascade (92, 108). Quantitative phosphoproteomics analysis by Rinschen et al. (119) revealed that vasopressin decreases phosphorylation of ERK1/2 and JNK 1/2. Concurrence of the abolished p38-MAP kinase activity and the decreased phosphorylation of AQP2 (S261) in response to vasopressin suggests the potential correlation between these events in vasopressin signaling.
Kidney collecting duct cells are continuously exposed to the changes of extracellular pH. Thus it is interesting to examine the effects of altered luminal or interstitial pH on the AQP2 phosphorylation and apical trafficking of AQP2 in collecting duct cells. Previous studies demonstrated the decrease of vasopressin binding affinity to V2R under acidic pH, compared with that at neutral pH (158). Urinary excretion of AQP2 was decreased in rats with metabolic acidosis, whereas AQP2 mRNA and protein expression in the kidney was increased (86). Moreover, urine alkalinization was associated with higher excretion of urinary exosomal AQP2, which was independent of vasopressin stimulation in rats (39). Consistently, a recent study demonstrated that phosphorylation levels at the S256, S264, and S269 and vasopressin-induced AQP2 trafficking were significantly decreased in primary cultured IMCD cells under acidic conditions (15). Vasopressin-induced increase of PKA activity was attenuated when LLC-PK1 cells were exposed to acidic pH, compared with neutral or alkaline pH. In contrast, forskolin-induced PKA activation was not affected under acidic pH, suggesting that exposure to acidic pH attenuates vasopressin-induced phosphorylation and trafficking of AQP2, likely via an inhibition of V2R-G protein-cAMP-PKA actions. A recent study showed that the water permeability through AQP4 can be increased at conditions of low pH, and AQP4 is directly gated by pH changes (50), which have not been examined for AQP2 yet.
Renal tubular epithelia including collecting duct principal cells are exposed to fluid shear stress. Interestingly, a simple collecting-duct-on-a-chip approach was recently demonstrated that luminal fluid shear stress induces AQP2 translocation associated with actin depolymerization in primary cultured IMCD cells of rat kidney in the absence of vasopressin stimulation (43). It was revealed that fluid shear stress modulates the activity of small GTPases in the endothelial cells (69, 143). Thus further studies are needed to understand the mechanistic details of how small GTPases regulate the response of collecting duct cells to the luminar fluid shear stress. In addition, fluid shear stress has been demonstrated to modulate nitric oxide production and increase intracellular concentration of Ca2+ in the IMCD cells (12), both of which play a role in AQP2 trafficking.
AQP2-Binding Protein Complex in AQP2 Trafficking
Changes in the posttranslational modification of the functional region of AQP2 affect AQP2 trafficking to the plasma membrane or internalization. This could be through interaction with regulatory proteins that facilitate the intracellular movement of the vesicles containing membrane-bound proteins. Previous studies exploiting yeast two-hybrid system identified Hsc70 and Hsp70 as the binding partners of AQP2 (72). Vasopressin stimulation increases the interaction of Hsc70 and AQP2, and membrane accumulation of AQP2 with reduced endocytosis was observed in the cells with Hsc70 knockdown (72). Consistent with yeast two-hybrid screening assay, these heat shock proteins have been found consistently in proteomics analysis, which identified proteins interacting with carboxy-terminal peptides of AQP2 (161). Importantly, these studies showed that the binding affinity between AQP2 and its binding proteins could be influenced by the phosphorylation status of AQP2.
The 14–3–3 proteins, which are phospho-serine/phospho-threonine binding proteins (1, 85), have also been reported as AQP2-binding partners, depending on the phosphorylation of AQP2 (84). The levels of two isoforms of 14–3–3 proteins, 14–3–3θ and 14–3–3ζ were increased by short-term vasopressin stimulation. However, the levels of ubiquitination, half-life, and the expression levels of AQP2 were changed differently, when each isoform was knock-downed, separately (84). Moreover, in the degradation process of AQP2, lysosomal trafficking regulator-interacting protein 5 (LIP5) interacted with the carboxy-terminal region of AQP2, independent on phosphorylation (S256) and ubiquitination (K270) of AQP2 (146).
Extracellular loop of AQP2 protein has binding domains that interact with other proteins in the plasma membrane. Unlike cytosolic proteins that interact with the cytoplasmic region of AQP2 protein, the interaction between extracellular domains of AQP2 and other membrane proteins is less likely to regulate AQP2 trafficking directly. RGD domain in the extracellular loop of AQP2 stimulates the binding partners controlling AQP2 through activation of intracellular cAMP and Ca2+ signaling (140). RGD-binding integrins are associated with various G protein-coupled receptor signaling affecting cAMP and Ca2+ signaling pathway (22, 123, 131).
Long-term Regulation of AQP2 Protein Abundance
The long-term adaptation of AQP2 occurs as a result of a vasopressin-induced increase in total abundance of the AQP2 protein in collecting duct cells, which is associated with regulatory processes at the transcriptional or posttranscriptional level. Transcription of the Aqp2 gene is significantly increased by vasopressin stimulation, resulting in increased cellular mRNA levels and translation of AQP2 (77). In cultured mpkCCD cells, vasopressin stimulation increases the half-life of AQP2 protein from 9 to 14 h (126). Stability of AQP2 protein also affects AQP2 protein abundance. Vasopressin increases AQP2 protein abundance by regulating the proteasomal degradation through PKA- and p38-MAP kinase-dependent pathway (92). The process of AQP2 endocytosis and subsequent degradation of AQP2 in the proteasome and lysosome could be mediated by ubiquitination of AQP2 protein at lysine 270 (49, 66).
MicroRNA (miRNA) is a posttranscriptional regulator, inhibiting the translation of target mRNA via translational regression of the RNA-induced silencing complex (65). Recently, miRNAs targeting AQP2 expression were predicted by in silico analysis, and hence the predicted AQP2-targeting miRNAs (miR-32 and miR-137) have gained focus to understand the novel cellular and molecular mechanisms of AQP2 protein regulation (53).
Summary and Conclusions
This review highlights some of new understanding in the regulation of AQP2 trafficking and AQP2 protein abundance. Vasopressin induces both intracellular translocation of AQP2-bearing vesicles to the apical plasma membrane and transcription of Aqp2 gene to increase AQP2 protein abundance. In particular, for the AQP2 trafficking, the main underlying signaling pathways are AQP2 phosphorylation, RhoA phosphorylation, intracellular Ca2+ mobilization, and actin depolymerization. Additional signaling pathways including angiotensin II, aldosterone, prostaglandins, and vesicle-targeting receptors have been reported in previous studies and reviews (18, 34, 64, 68, 80, 98, 100, 102, 103, 112, 145, 150). Detailed studies on the AQP2 in the kidney collecting ducts will provide new insights in the treatment of patients with body water balance disorders, including NDI, and water retention conditions, such as congestive heart failure and liver cirrhosis.
GRANTS
This study was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning, Korea (2014R1A5A2009242 and 2016R1A2B4009365).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.J.J. and T.-H.K. conception and design of research; H.J.J. and T.-H.K. analyzed data; H.J.J. and T.-H.K. interpreted results of experiments; H.J.J. and T.-H.K. prepared figures; H.J.J. and T.-H.K. drafted manuscript; H.J.J. and T.-H.K. edited and revised manuscript; H.J.J. and T.-H.K. approved final version of manuscript.
REFERENCES
- 1.Aitken A. 14-3-3 proteins: a historic overview. Semin Cancer Biol 16: 162–172, 2006. doi: 10.1016/j.semcancer.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Aoki T, Suzuki T, Hagiwara H, Kuwahara M, Sasaki S, Takata K, Matsuzaki T. Close association of aquaporin-2 internalization with caveolin-1. Acta Histochem Cytochem 45: 139–146, 2012. doi: 10.1267/ahc.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bankir L, Bichet DG, Bouby N. Vasopressin V2 receptors, ENaC, and sodium reabsorption: a risk factor for hypertension? Am J Physiol Renal Physiol 299: F917–F928, 2010. doi: 10.1152/ajprenal.00413.2010. [DOI] [PubMed] [Google Scholar]
- 4.Barile M, Pisitkun T, Yu MJ, Chou CL, Verbalis MJ, Shen RF, Knepper MA. Large scale protein identification in intracellular aquaporin-2 vesicles from renal inner medullary collecting duct. Mol Cell Proteomics 4: 1095–1106, 2005. doi: 10.1074/mcp.M500049-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhuin T, Roy JK. Rab proteins: the key regulators of intracellular vesicle transport. Exp Cell Res 328: 1–19, 2014. doi: 10.1016/j.yexcr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 6.Bockenhauer D, Bichet DG. Pathophysiology, diagnosis and management of nephrogenic diabetes insipidus. Nat Rev Nephrol 11: 576–588, 2015. doi: 10.1038/nrneph.2015.89. [DOI] [PubMed] [Google Scholar]
- 7.Boone M, Deen PM. Physiology and pathophysiology of the vasopressin-regulated renal water reabsorption. Pflugers Arch 456: 1005–1024, 2008. doi: 10.1007/s00424-008-0498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boone M, Kortenoeven ML, Robben JH, Tamma G, Deen PM. Counteracting vasopressin-mediated water reabsorption by ATP, dopamine, and phorbol esters: mechanisms of action. Am J Physiol Renal Physiol 300: F761–F771, 2011. doi: 10.1152/ajprenal.00247.2010. [DOI] [PubMed] [Google Scholar]
- 9.Bradford D, Raghuram V, Wilson JL, Chou CL, Hoffert JD, Knepper MA, Pisitkun T. Use of LC-MS/MS and Bayes’ theorem to identify protein kinases that phosphorylate aquaporin-2 at Ser256. Am J Physiol Cell Physiol 307: C123–C139, 2014. doi: 10.1152/ajpcell.00377.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown D. The ins and outs of aquaporin-2 trafficking. Am J Physiol Renal Physiol 284: F893–F901, 2003. doi: 10.1152/ajprenal.00387.2002. [DOI] [PubMed] [Google Scholar]
- 11.Burg MB, Green N. Function of the thick ascending limb of Henle’s loop. Am J Physiol 224: 659–668, 1973. [DOI] [PubMed] [Google Scholar]
- 12.Cai Z, Xin J, Pollock DM, Pollock JS. Shear stress-mediated NO production in inner medullary collecting duct cells. Am J Physiol Renal Physiol 279: F270–F274, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Rice W, Gu Z, Li J, Huang J, Brenner MB, Van Hoek A, Xiong J, Gundersen GG, Norman JC, Hsu VW, Fenton RA, Brown D, Lu HA. Aquaporin 2 promotes cell migration and epithelial morphogenesis. J Am Soc Nephrol 23: 1506–1517, 2012. doi: 10.1681/ASN.2012010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng L, Wu Q, Kortenoeven ML, Pisitkun T, Fenton RA. A systems level analysis of vasopressin-mediated signaling networks in kidney distal convoluted tubule cells. Sci Rep 5: 12829, 2015. doi: 10.1038/srep12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi HJ, Jung HJ, Kwon TH. Extracellular pH affects phosphorylation and intracellular trafficking of AQP2 in inner medullary collecting duct cells. Am J Physiol Renal Physiol 308: F737–F748, 2015. doi: 10.1152/ajprenal.00376.2014. [DOI] [PubMed] [Google Scholar]
- 16.Chou CL, Yip KP, Michea L, Kador K, Ferraris JD, Wade JB, Knepper MA. Regulation of aquaporin-2 trafficking by vasopressin in the renal collecting duct. Roles of ryanodine-sensitive Ca2+ stores and calmodulin. J Biol Chem 275: 36839–36846, 2000. doi: 10.1074/jbc.M005552200. [DOI] [PubMed] [Google Scholar]
- 17.Christensen BM, Zelenina M, Aperia A, Nielsen S. Localization and regulation of PKA-phosphorylated AQP2 in response to V2-receptor agonist/antagonist treatment. Am J Physiol Renal Physiol 278: F29–F42, 2000. [DOI] [PubMed] [Google Scholar]
- 18.de Groot T, Sinke AP, Kortenoeven ML, Alsady M, Baumgarten R, Devuyst O, Loffing J, Wetzels JF, Deen PM. Acetazolamide attenuates lithium-induced nephrogenic diabetes insipidus. J Am Soc Nephrol 27: 2082–2091, 2016. doi: 10.1681/ASN.2015070796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Seigneux S, Nielsen J, Olesen ET, Dimke H, Kwon TH, Frøkiaer J, Nielsen S. Long-term aldosterone treatment induces decreased apical but increased basolateral expression of AQP2 in CCD of rat kidney. Am J Physiol Renal Physiol 293: F87–F99, 2007. doi: 10.1152/ajprenal.00431.2006. [DOI] [PubMed] [Google Scholar]
- 20.Ding GH, Franki N, Condeelis J, Hays RM. Vasopressin depolymerizes F-actin in toad bladder epithelial cells. Am J Physiol Cell Physiol 260: C9–C16, 1991. [DOI] [PubMed] [Google Scholar]
- 21.Dousa TP, Barnes LD. Effects of colchicine and vinblastine on the cellular action of vasopressin in mammalian kidney. A possible role of microtubules. J Clin Invest 54: 252–262, 1974. doi: 10.1172/JCI107760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erb L, Liu J, Ockerhausen J, Kong Q, Garrad RC, Griffin K, Neal C, Krugh B, Santiago-Pérez LI, González FA, Gresham HD, Turner JT, Weisman GA. An RGD sequence in the P2Y(2) receptor interacts with alpha(V)beta(3) integrins and is required for G(o)-mediated signal transduction. J Cell Biol 153: 491–501, 2001. doi: 10.1083/jcb.153.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fenton RA, Brønd L, Nielsen S, Praetorius J. Cellular and subcellular distribution of the type-2 vasopressin receptor in the kidney. Am J Physiol Renal Physiol 293: F748–F760, 2007. doi: 10.1152/ajprenal.00316.2006. [DOI] [PubMed] [Google Scholar]
- 24.Fenton RA, Knepper MA. Mouse models and the urinary concentrating mechanism in the new millennium. Physiol Rev 87: 1083–1112, 2007. doi: 10.1152/physrev.00053.2006. [DOI] [PubMed] [Google Scholar]
- 25.Fenton RA, Moeller HB. Recent discoveries in vasopressin-regulated aquaporin-2 trafficking. Prog Brain Res 170: 571–579, 2008. doi: 10.1016/S0079-6123(08)00444-5. [DOI] [PubMed] [Google Scholar]
- 26.Fenton RA, Moeller HB, Hoffert JD, Yu MJ, Nielsen S, Knepper MA. Acute regulation of aquaporin-2 phosphorylation at Ser-264 by vasopressin. Proc Natl Acad Sci USA 105: 3134–3139, 2008. doi: 10.1073/pnas.0712338105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finkelstein A. Water and nonelectrolyte permeability of lipid bilayer membranes. J Gen Physiol 68: 127–135, 1976. doi: 10.1085/jgp.68.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fushimi K, Sasaki S, Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem 272: 14800–14804, 1997. doi: 10.1074/jbc.272.23.14800. [DOI] [PubMed] [Google Scholar]
- 29.Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature 361: 549–552, 1993. doi: 10.1038/361549a0. [DOI] [PubMed] [Google Scholar]
- 30.Gilbert ML, Yang L, Su T, McKnight GS. Expression of a dominant negative PKA mutation in the kidney elicits a diabetes insipidus phenotype. Am J Physiol Renal Physiol 308: F627–F638, 2015. doi: 10.1152/ajprenal.00222.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goel M, Sinkins WG, Zuo CD, Hopfer U, Schilling WP. Vasopressin-induced membrane trafficking of TRPC3 and AQP2 channels in cells of the rat renal collecting duct. Am J Physiol Renal Physiol 293: F1476–F1488, 2007. doi: 10.1152/ajprenal.00186.2007. [DOI] [PubMed] [Google Scholar]
- 32.Gooch JL, Guler RL, Barnes JL, Toro JJ. Loss of calcineurin Aalpha results in altered trafficking of AQP2 and in nephrogenic diabetes insipidus. J Cell Sci 119: 2468–2476, 2006. doi: 10.1242/jcs.02971. [DOI] [PubMed] [Google Scholar]
- 33.Gustafson CE, Katsura T, McKee M, Bouley R, Casanova JE, Brown D. Recycling of AQP2 occurs through a temperature- and bafilomycin-sensitive trans-Golgi-associated compartment. Am J Physiol Renal Physiol 278: F317–F326, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Hasler U, Leroy V, Martin PY, Féraille E. Aquaporin-2 abundance in the renal collecting duct: new insights from cultured cell models. Am J Physiol Renal Physiol 297: F10–F18, 2009. doi: 10.1152/ajprenal.00053.2009. [DOI] [PubMed] [Google Scholar]
- 35.Hasler U, Nunes P, Bouley R, Lu HA, Matsuzaki T, Brown D. Acute hypertonicity alters aquaporin-2 trafficking and induces a MAPK-dependent accumulation at the plasma membrane of renal epithelial cells. J Biol Chem 283: 26643–26661, 2008. doi: 10.1074/jbc.M801071200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hays RM, Condeelis J, Gao Y, Simon H, Ding G, Franki N. The effect of vasopressin on the cytoskeleton of the epithelial cell. Pediatr Nephrol 7: 672–679, 1993. doi: 10.1007/BF00852577. [DOI] [PubMed] [Google Scholar]
- 37.Henley JR, Krueger EW, Oswald BJ, McNiven MA. Dynamin-mediated internalization of caveolae. J Cell Biol 141: 85–99, 1998. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henn V, Edemir B, Stefan E, Wiesner B, Lorenz D, Theilig F, Schmitt R, Vossebein L, Tamma G, Beyermann M, Krause E, Herberg FW, Valenti G, Bachmann S, Rosenthal W, Klussmann E. Identification of a novel A-kinase anchoring protein 18 isoform and evidence for its role in the vasopressin-induced aquaporin-2 shuttle in renal principal cells. J Biol Chem 279: 26654–26665, 2004. doi: 10.1074/jbc.M312835200. [DOI] [PubMed] [Google Scholar]
- 39.Higashijima Y, Sonoda H, Takahashi S, Kondo H, Shigemura K, Ikeda M. Excretion of urinary exosomal AQP2 in rats is regulated by vasopressin and urinary pH. Am J Physiol Renal Physiol 305: F1412–F1421, 2013. doi: 10.1152/ajprenal.00249.2013. [DOI] [PubMed] [Google Scholar]
- 40.Hoffert JD, Fenton RA, Moeller HB, Simons B, Tchapyjnikov D, McDill BW, Yu MJ, Pisitkun T, Chen F, Knepper MA. Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J Biol Chem 283: 24617–24627, 2008. doi: 10.1074/jbc.M803074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffert JD, Pisitkun T, Wang G, Shen RF, Knepper MA. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci USA 103: 7159–7164, 2006. doi: 10.1073/pnas.0600895103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inoue T, Terris J, Ecelbarger CA, Chou CL, Nielsen S, Knepper MA. Vasopressin regulates apical targeting of aquaporin-2 but not of UT1 urea transporter in renal collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 276: F559–F566, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Jang KJ, Cho HS, Kang DH, Bae WG, Kwon TH, Suh KY. Fluid-shear-stress-induced translocation of aquaporin-2 and reorganization of actin cytoskeleton in renal tubular epithelial cells. Integr Biol 3: 134–141, 2011. doi: 10.1039/C0IB00018C. [DOI] [PubMed] [Google Scholar]
- 44.Jo I, Ward DT, Baum MA, Scott JD, Coghlan VM, Hammond TG, Harris HW. AQP2 is a substrate for endogenous PP2B activity within an inner medullary AKAP-signaling complex. Am J Physiol Renal Physiol 281: F958–F965, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Jordens I, Marsman M, Kuijl C, Neefjes J. Rab proteins, connecting transport and vesicle fusion. Traffic 6: 1070–1077, 2005. doi: 10.1111/j.1600-0854.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 46.Jung HJ, Kim SY, Choi HJ, Park EJ, Lim JS, Frøkiaer J, Nielsen S, Kwon TH. Tankyrase-mediated β-catenin activity regulates vasopressin-induced AQP2 expression in kidney collecting duct mpkCCDc14 cells. Am J Physiol Renal Physiol 308: F473–F486, 2015. doi: 10.1152/ajprenal.00052.2014. [DOI] [PubMed] [Google Scholar]
- 47.Kamal A, Goldstein LS. Connecting vesicle transport to the cytoskeleton. Curr Opin Cell Biol 12: 503–508, 2000. doi: 10.1016/S0955-0674(00)00123-X. [DOI] [PubMed] [Google Scholar]
- 48.Kamsteeg EJ, Heijnen I, van Os CH, Deen PM. The subcellular localization of an aquaporin-2 tetramer depends on the stoichiometry of phosphorylated and nonphosphorylated monomers. J Cell Biol 151: 919–930, 2000. doi: 10.1083/jcb.151.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamsteeg EJ, Hendriks G, Boone M, Konings IB, Oorschot V, van der Sluijs P, Klumperman J, Deen PM. Short-chain ubiquitination mediates the regulated endocytosis of the aquaporin-2 water channel. Proc Natl Acad Sci USA 103: 18344–18349, 2006. doi: 10.1073/pnas.0604073103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaptan S, Assentoft M, Schneider HP, Fenton RA, Deitmer JW, MacAulay N, de Groot BL. H95 is a pH-dependent gate in qquaporin 4. Structure 23: 2309–2318, 2015. doi: 10.1016/j.str.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 51.Katsura T, Gustafson CE, Ausiello DA, Brown D. Protein kinase A phosphorylation is involved in regulated exocytosis of aquaporin-2 in transfected LLC-PK1 cells. Am J Physiol Renal Electrolyte Physiol 272: F817–F822, 1997. [PubMed] [Google Scholar]
- 52.Kim HY, Choi HJ, Lim JS, Park EJ, Jung HJ, Lee YJ, Kim SY, Kwon TH. Emerging role of Akt substrate protein AS160 in the regulation of AQP2 translocation. Am J Physiol Renal Physiol 301: F151–F161, 2011. doi: 10.1152/ajprenal.00519.2010. [DOI] [PubMed] [Google Scholar]
- 53.Kim JE, Jung HJ, Lee YJ, Kwon TH. Vasopressin-regulated miRNAs and AQP2-targeting miRNAs in kidney collecting duct cells. Am J Physiol Renal Physiol 308: F749–F764, 2015. doi: 10.1152/ajprenal.00334.2014. [DOI] [PubMed] [Google Scholar]
- 54.Kittikulsuth W, Stuart D, Kohan DE. Adenylyl cyclase 4 does not regulate collecting duct water and sodium handling. Physiol Rep 2: e00277, 2014. doi: 10.1002/phy2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kittikulsuth W, Stuart D, Van Hoek AN, Stockand JD, Bugaj V, Mironova E, Blount MA, Kohan DE. Lack of an effect of collecting duct-specific deletion of adenylyl cyclase 3 on renal Na+ and water excretion or arterial pressure. Am J Physiol Renal Physiol 306: F597–F607, 2014. doi: 10.1152/ajprenal.00505.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klussmann E, Maric K, Wiesner B, Beyermann M, Rosenthal W. Protein kinase A anchoring proteins are required for vasopressin-mediated translocation of aquaporin-2 into cell membranes of renal principal cells. J Biol Chem 274: 4934–4938, 1999. doi: 10.1074/jbc.274.8.4934. [DOI] [PubMed] [Google Scholar]
- 57.Klussmann E, Tamma G, Lorenz D, Wiesner B, Maric K, Hofmann F, Aktories K, Valenti G, Rosenthal W. An inhibitory role of Rho in the vasopressin-mediated translocation of aquaporin-2 into cell membranes of renal principal cells. J Biol Chem 276: 20451–20457, 2001. doi: 10.1074/jbc.M010270200. [DOI] [PubMed] [Google Scholar]
- 58.Knepper MA, Kwon TH, Nielsen S. Molecular physiology of water balance. N Engl J Med 372: 1349–1358, 2015. doi: 10.1056/NEJMra1404726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knepper MA, Nielsen S, Chou CL, DiGiovanni SR. Mechanism of vasopressin action in the renal collecting duct. Semin Nephrol 14: 302–321, 1994. [PubMed] [Google Scholar]
- 60.Kokko JP. Sodium chloride and water transport in the descending limb of Henle. J Clin Invest 49: 1838–1846, 1970. doi: 10.1172/JCI106401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kortenoeven ML, Pedersen NB, Rosenbaek LL, Fenton RA. Vasopressin regulation of sodium transport in the distal nephron and collecting duct. Am J Physiol Renal Physiol 309: F280–F299, 2015. doi: 10.1152/ajprenal.00093.2015. [DOI] [PubMed] [Google Scholar]
- 62.Kwon TH, Hager H, Nejsum LN, Andersen ML, Frøkiaer J, Nielsen S. Physiology and pathophysiology of renal aquaporins. Semin Nephrol 21: 231–238, 2001. doi: 10.1053/snep.2001.21647. [DOI] [PubMed] [Google Scholar]
- 63.Kwon TH, Laursen UH, Marples D, Maunsbach AB, Knepper MA, Frokiaer J, Nielsen S. Altered expression of renal AQPs and Na+ transporters in rats with lithium-induced NDI. Am J Physiol Renal Physiol 279: F552–F564, 2000. [DOI] [PubMed] [Google Scholar]
- 64.Kwon TH, Nielsen J, Møller HB, Fenton RA, Nielsen S, Frøkiaer J. Aquaporins in the kidney. Handb Exp Pharmacol 190: 95–132, 2009. doi: 10.1007/978-3-540-79885-9_5. [DOI] [PubMed] [Google Scholar]
- 65.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75: 843–854, 1993. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 66.Lee YJ, Lee JE, Choi HJ, Lim JS, Jung HJ, Baek MC, Frøkiær J, Nielsen S, Kwon TH. E3 ubiquitin-protein ligases in rat kidney collecting duct: response to vasopressin stimulation and withdrawal. Am J Physiol Renal Physiol 301: F883–F896, 2011. doi: 10.1152/ajprenal.00117.2011. [DOI] [PubMed] [Google Scholar]
- 67.Leopoldt D, Hanck T, Exner T, Maier U, Wetzker R, Nürnberg B. Gbetagamma stimulates phosphoinositide 3-kinase-gamma by direct interaction with two domains of the catalytic p110 subunit. J Biol Chem 273: 7024–7029, 1998. doi: 10.1074/jbc.273.12.7024. [DOI] [PubMed] [Google Scholar]
- 68.Li C, Wang W, Rivard CJ, Lanaspa MA, Summer S, Schrier RW. Molecular mechanisms of angiotensin II stimulation on aquaporin-2 expression and trafficking. Am J Physiol Renal Physiol 300: F1255–F1261, 2011. doi: 10.1152/ajprenal.00469.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li S, Chen BP, Azuma N, Hu YL, Wu SZ, Sumpio BE, Shyy JY, Chien S. Distinct roles for the small GTPases Cdc42 and Rho in endothelial responses to shear stress. J Clin Invest 103: 1141–1150, 1999. doi: 10.1172/JCI5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Loo CS, Chen CW, Wang PJ, Chen PY, Lin SY, Khoo KH, Fenton RA, Knepper MA, Yu MJ. Quantitative apical membrane proteomics reveals vasopressin-induced actin dynamics in collecting duct cells. Proc Natl Acad Sci USA 110: 17119–17124, 2013. doi: 10.1073/pnas.1309219110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loonen AJ, Knoers NV, van Os CH, Deen PM. Aquaporin 2 mutations in nephrogenic diabetes insipidus. Semin Nephrol 28: 252–265, 2008. doi: 10.1016/j.semnephrol.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 72.Lu HA, Sun TX, Matsuzaki T, Yi XH, Eswara J, Bouley R, McKee M, Brown D. Heat shock protein 70 interacts with aquaporin-2 and regulates its trafficking. J Biol Chem 282: 28721–28732, 2007. doi: 10.1074/jbc.M611101200. [DOI] [PubMed] [Google Scholar]
- 73.Lu X, Wang F, Xu C, Soodvilai S, Peng K, Su J, Zhao L, Yang KT, Feng Y, Zhou SF, Gustafsson JA, Yang T. Soluble (pro)renin receptor via β-catenin enhances urine concentration capability as a target of liver X receptor. Proc Natl Acad Sci USA 113: E1898–E1906, 2016. doi: 10.1073/pnas.1602397113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marples D, Christensen S, Christensen EI, Ottosen PD, Nielsen S. Lithium-induced downregulation of aquaporin-2 water channel expression in rat kidney medulla. J Clin Invest 95: 1838–1845, 1995. doi: 10.1172/JCI117863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marples D, Knepper MA, Christensen EI, Nielsen S. Redistribution of aquaporin-2 water channels induced by vasopressin in rat kidney inner medullary collecting duct. Am J Physiol 269: C655–C664, 1995. [DOI] [PubMed] [Google Scholar]
- 76.Marples D, Schroer TA, Ahrens N, Taylor A, Knepper MA, Nielsen S. Dynein and dynactin colocalize with AQP2 water channels in intracellular vesicles from kidney collecting duct. Am J Physiol 274: F384–F394, 1998. [DOI] [PubMed] [Google Scholar]
- 77.Matsumura Y, Uchida S, Rai T, Sasaki S, Marumo F. Transcriptional regulation of aquaporin-2 water channel gene by cAMP. J Am Soc Nephrol 8: 861–867, 1997. [DOI] [PubMed] [Google Scholar]
- 78.McDill BW, Li SZ, Kovach PA, Ding L, Chen F. Congenital progressive hydronephrosis (cph) is caused by an S256L mutation in aquaporin-2 that affects its phosphorylation and apical membrane accumulation. Proc Natl Acad Sci USA 103: 6952–6957, 2006. doi: 10.1073/pnas.0602087103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moeller HB, Aroankins TS, Slengerik-Hansen J, Pisitkun T, Fenton RA. Phosphorylation and ubiquitylation are opposing processes that regulate endocytosis of the water channel aquaporin-2. J Cell Sci 127: 3174–3183, 2014. doi: 10.1242/jcs.150680. [DOI] [PubMed] [Google Scholar]
- 80.Moeller HB, Fenton RA. Cell biology of vasopressin-regulated aquaporin-2 trafficking. Pflugers Arch 464: 133–144, 2012. doi: 10.1007/s00424-012-1129-4. [DOI] [PubMed] [Google Scholar]
- 81.Moeller HB, MacAulay N, Knepper MA, Fenton RA. Role of multiple phosphorylation sites in the COOH-terminal tail of aquaporin-2 for water transport: evidence against channel gating. Am J Physiol Renal Physiol 296: F649–F657, 2009. doi: 10.1152/ajprenal.90682.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moeller HB, Olesen ET, Fenton RA. Regulation of the water channel aquaporin-2 by posttranslational modification. Am J Physiol Renal Physiol 300: F1062–F1073, 2011. doi: 10.1152/ajprenal.00721.2010. [DOI] [PubMed] [Google Scholar]
- 83.Moeller HB, Praetorius J, Rützler MR, Fenton RA. Phosphorylation of aquaporin-2 regulates its endocytosis and protein-protein interactions. Proc Natl Acad Sci USA 107: 424–429, 2010. doi: 10.1073/pnas.0910683107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moeller HB, Slengerik-Hansen J, Aroankins T, Assentoft M, MacAulay N, Moestrup SK, Bhalla V, Fenton RA. Regulation of the water channel aquaporin-2 via 14-3-3θ and -ζ. J Biol Chem 291: 2469–2484, 2016. doi: 10.1074/jbc.M115.691121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morrison DK. The 14-3-3 proteins: integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol 19: 16–23, 2009. doi: 10.1016/j.tcb.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mouri T, Inoue T, Nonoguchi H, Nakayama Y, Miyazaki H, Matsuzaki T, Saito H, Nakanishi T, Kohda Y, Tomita K. Acute and chronic metabolic acidosis interferes with aquaporin-2 translocation in the rat kidney collecting ducts. Hypertens Res 32: 358–363, 2009. doi: 10.1038/hr.2009.19. [DOI] [PubMed] [Google Scholar]
- 87.Mulders SM, Bichet DG, Rijss JP, Kamsteeg EJ, Arthus MF, Lonergan M, Fujiwara M, Morgan K, Leijendekker R, van der Sluijs P, van Os CH, Deen PM. An aquaporin-2 water channel mutant which causes autosomal dominant nephrogenic diabetes insipidus is retained in the Golgi complex. J Clin Invest 102: 57–66, 1998. doi: 10.1172/JCI2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mulders SM, Knoers NV, Van Lieburg AF, Monnens LA, Leumann E, Wühl E, Schober E, Rijss JP, Van Os CH, Deen PM. New mutations in the AQP2 gene in nephrogenic diabetes insipidus resulting in functional but misrouted water channels. J Am Soc Nephrol 8: 242–248, 1997. [DOI] [PubMed] [Google Scholar]
- 89.Muller J, Kachadorian WA, DiScala VA. Evidence that ADH-stimulated intramembrane particle aggregates are transferred from cytoplasmic to luminal membranes in toad bladder epithelial cells. J Cell Biol 85: 83–95, 1980. doi: 10.1083/jcb.85.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mutig K, Paliege A, Kahl T, Jöns T, Müller-Esterl W, Bachmann S. Vasopressin V2 receptor expression along rat, mouse, and human renal epithelia with focus on TAL. Am J Physiol Renal Physiol 293: F1166–F1177, 2007. doi: 10.1152/ajprenal.00196.2007. [DOI] [PubMed] [Google Scholar]
- 91.Nedvetsky PI, Stefan E, Frische S, Santamaria K, Wiesner B, Valenti G, Hammer JA III, Nielsen S, Goldenring JR, Rosenthal W, Klussmann E. A role of myosin Vb and Rab11-FIP2 in the aquaporin-2 shuttle. Traffic 8: 110–123, 2007. doi: 10.1111/j.1600-0854.2006.00508.x. [DOI] [PubMed] [Google Scholar]
- 92.Nedvetsky PI, Tabor V, Tamma G, Beulshausen S, Skroblin P, Kirschner A, Mutig K, Boltzen M, Petrucci O, Vossenkämper A, Wiesner B, Bachmann S, Rosenthal W, Klussmann E. Reciprocal regulation of aquaporin-2 abundance and degradation by protein kinase A and p38-MAP kinase. J Am Soc Nephrol 21: 1645–1656, 2010. doi: 10.1681/ASN.2009111190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nedvetsky PI, Tamma G, Beulshausen S, Valenti G, Rosenthal W, Klussmann E. Regulation of aquaporin-2 trafficking. Handb Exp Pharmacol 190: 133–157, 2009. doi: 10.1007/978-3-540-79885-9_6. [DOI] [PubMed] [Google Scholar]
- 94.Nejsum LN, Zelenina M, Aperia A, Frøkiaer J, Nielsen S. Bidirectional regulation of AQP2 trafficking and recycling: involvement of AQP2-S256 phosphorylation. Am J Physiol Renal Physiol 288: F930–F938, 2005. doi: 10.1152/ajprenal.00291.2004. [DOI] [PubMed] [Google Scholar]
- 95.Nielsen J, Hoffert JD, Knepper MA, Agre P, Nielsen S, Fenton RA. Proteomic analysis of lithium-induced nephrogenic diabetes insipidus: mechanisms for aquaporin 2 down-regulation and cellular proliferation. Proc Natl Acad Sci USA 105: 3634–3639, 2008. doi: 10.1073/pnas.0800001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nielsen J, Kwon TH, Praetorius J, Frøkiaer J, Knepper MA, Nielsen S. Aldosterone increases urine production and decreases apical AQP2 expression in rats with diabetes insipidus. Am J Physiol Renal Physiol 290: F438–F449, 2006. doi: 10.1152/ajprenal.00158.2005. [DOI] [PubMed] [Google Scholar]
- 97.Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci USA 92: 1013–1017, 1995. doi: 10.1073/pnas.92.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nielsen S, Frøkiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82: 205–244, 2002. doi: 10.1152/physrev.00024.2001. [DOI] [PubMed] [Google Scholar]
- 99.Nishimoto G, Zelenina M, Li D, Yasui M, Aperia A, Nielsen S, Nairn AC. Arginine vasopressin stimulates phosphorylation of aquaporin-2 in rat renal tissue. Am J Physiol 276: F254–F259, 1999. [DOI] [PubMed] [Google Scholar]
- 100.Noda Y, Sohara E, Ohta E, Sasaki S. Aquaporins in kidney pathophysiology. Nat Rev Nephrol 6: 168–178, 2010. doi: 10.1038/nrneph.2009.231. [DOI] [PubMed] [Google Scholar]
- 101.Nørregaard R, Tao S, Nilsson L, Woodgett JR, Kakade V, Yu AS, Howard C, Rao R. Glycogen synthase kinase 3α regulates urine concentrating mechanism in mice. Am J Physiol Renal Physiol 308: F650–F660, 2015. doi: 10.1152/ajprenal.00516.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Olesen ET, Moeller HB, Assentoft M, MacAulay N, Fenton RA. The vasopressin type 2 receptor and prostaglandin receptors EP2 and EP4 can increase aquaporin-2 plasma membrane targeting through a cAMP-independent pathway. Am J Physiol Renal Physiol 311: F935–F944, 2016. doi: 10.1152/ajprenal.00559.2015. [DOI] [PubMed] [Google Scholar]
- 103.Olesen ET, Rützler MR, Moeller HB, Praetorius HA, Fenton RA. Vasopressin-independent targeting of aquaporin-2 by selective E-prostanoid receptor agonists alleviates nephrogenic diabetes insipidus. Proc Natl Acad Sci USA 108: 12949–12954, 2011. doi: 10.1073/pnas.1104691108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Osmond RI, Crouch MF, Dupriez VJ. An emerging role for kinase screening in GPCR drug discovery. Curr Opin Mol Ther 12: 305–315, 2010. [PubMed] [Google Scholar]
- 105.Păunescu TG, Lu HA, Russo LM, Pastor-Soler NM, McKee M, McLaughlin MM, Bartlett BE, Breton S, Brown D. Vasopressin induces apical expression of caveolin in rat kidney collecting duct principal cells. Am J Physiol Renal Physiol 305: F1783–F1795, 2013. doi: 10.1152/ajprenal.00622.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Phillips ME, Taylor A. Effect of colcemid on the water permeability response to vasopressin in isolated perfused rabbit collecting tubules. J Physiol 456: 591–608, 1992. doi: 10.1113/jphysiol.1992.sp019355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Phillips ME, Taylor A. Effect of nocodazole on the water permeability response to vasopressin in rabbit collecting tubules perfused in vitro. J Physiol 411: 529–544, 1989. doi: 10.1113/jphysiol.1989.sp017588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pisitkun T, Jacob V, Schleicher SM, Chou CL, Yu MJ, Knepper MA. Akt and ERK1/2 pathways are components of the vasopressin signaling network in rat native IMCD. Am J Physiol Renal Physiol 295: F1030–F1043, 2008. doi: 10.1152/ajprenal.90339.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Preston GM, Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci USA 88: 11110–11114, 1991. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256: 385–387, 1992. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- 111.Procino G, Gerbino A, Milano S, Nicoletti MC, Mastrofrancesco L, Carmosino M, Svelto M. Rosiglitazone promotes AQP2 plasma membrane expression in renal cells via a Ca-dependent/cAMP-independent mechanism. Cell Physiol Biochem 35: 1070–1085, 2015. doi: 10.1159/000373933. [DOI] [PubMed] [Google Scholar]
- 112.Ranieri M, Tamma G, Di Mise A, Russo A, Centrone M, Svelto M, Calamita G, Valenti G. Negative feedback from CaSR signaling to aquaporin-2 sensitizes vasopressin to extracellular Ca2. J Cell Sci 128: 2350–2360, 2015. doi: 10.1242/jcs.168096. [DOI] [PubMed] [Google Scholar]
- 113.Rao R, Patel S, Hao C, Woodgett J, Harris R. GSK3beta mediates renal response to vasopressin by modulating adenylate cyclase activity. J Am Soc Nephrol 21: 428–437, 2010. doi: 10.1681/ASN.2009060672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rao R, Zhang MZ, Zhao M, Cai H, Harris RC, Breyer MD, Hao CM. Lithium treatment inhibits renal GSK-3 activity and promotes cyclooxygenase 2-dependent polyuria. Am J Physiol Renal Physiol 288: F642–F649, 2005. doi: 10.1152/ajprenal.00287.2004. [DOI] [PubMed] [Google Scholar]
- 115.Rector FC, Jr. Sodium, bicarbonate, and chloride absorption by the proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol 244: F461–F471, 1983. [DOI] [PubMed] [Google Scholar]
- 116.Ren H, Yang B, Ruiz JA, Efe O, Ilori TO, Sands JM, Klein JD. Phosphatase inhibition increases AQP2 accumulation in the rat IMCD apical plasma membrane. Am J Physiol Renal Physiol. First published August 3, 2016; doi: 10.1152/ajprenal.00150.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rice WL, Zhang Y, Chen Y, Matsuzaki T, Brown D, Lu HA. Differential, phosphorylation dependent trafficking of AQP2 in LLC-PK1 cells. PLoS One 7: e32843, 2012. doi: 10.1371/journal.pone.0032843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rieg T, Tang T, Murray F, Schroth J, Insel PA, Fenton RA, Hammond HK, Vallon V. Adenylate cyclase 6 determines cAMP formation and aquaporin-2 phosphorylation and trafficking in inner medulla. J Am Soc Nephrol 21: 2059–2068, 2010. doi: 10.1681/ASN.2010040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rinschen MM, Yu MJ, Wang G, Boja ES, Hoffert JD, Pisitkun T, Knepper MA. Quantitative phosphoproteomic analysis reveals vasopressin V2-receptor-dependent signaling pathways in renal collecting duct cells. Proc Natl Acad Sci USA 107: 3882–3887, 2010. doi: 10.1073/pnas.0910646107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rocha AS, Kokko JP. Sodium chloride and water transport in the medullary thick ascending limb of Henle. Evidence for active chloride transport. J Clin Invest 52: 612–623, 1973. doi: 10.1172/JCI107223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rojek A, Füchtbauer EM, Kwon TH, Frøkiaer J, Nielsen S. Severe urinary concentrating defect in renal collecting duct-selective AQP2 conditional-knockout mice. Proc Natl Acad Sci USA 103: 6037–6042, 2006. doi: 10.1073/pnas.0511324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roos KP, Strait KA, Raphael KL, Blount MA, Kohan DE. Collecting duct-specific knockout of adenylyl cyclase type VI causes a urinary concentration defect in mice. Am J Physiol Renal Physiol 302: F78–F84, 2012. doi: 10.1152/ajprenal.00397.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rozengurt E. Signal transduction pathways in the mitogenic response to G protein-coupled neuropeptide receptor agonists. J Cell Physiol 177: 507–517, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 124.Sabolić I, Katsura T, Verbavatz JM, Brown D. The AQP2 water channel: effect of vasopressin treatment, microtubule disruption, and distribution in neonatal rats. J Membr Biol 143: 165–175, 1995. doi: 10.1007/BF00233445. [DOI] [PubMed] [Google Scholar]
- 125.Sachs AN, Pisitkun T, Hoffert JD, Yu MJ, Knepper MA. LC-MS/MS analysis of differential centrifugation fractions from native inner medullary collecting duct of rat. Am J Physiol Renal Physiol 295: F1799–F1806, 2008. doi: 10.1152/ajprenal.90510.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sandoval PC, Slentz DH, Pisitkun T, Saeed F, Hoffert JD, Knepper MA. Proteome-wide measurement of protein half-lives and translation rates in vasopressin-sensitive collecting duct cells. J Am Soc Nephrol 24: 1793–1805, 2013. doi: 10.1681/ASN.2013030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sands JM, Nonoguchi H, Knepper MA. Vasopressin effects on urea and H2O transport in inner medullary collecting duct subsegments. Am J Physiol Renal Fluid Electrolyte Physiol 253: F823–F832, 1987. [DOI] [PubMed] [Google Scholar]
- 128.Schafer JA, Andreoli TE. Cellular constraints to diffusion. The effect of antidiuretic hormone on water flows in isolated mammalian collecting tubules. J Clin Invest 51: 1264–1278, 1972. doi: 10.1172/JCI106921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schenk LK, Bolger SJ, Luginbuhl K, Gonzales PA, Rinschen MM, Yu MJ, Hoffert JD, Pisitkun T, Knepper MA. Quantitative proteomics identifies vasopressin-responsive nuclear proteins in collecting duct cells. J Am Soc Nephrol 23: 1008–1018, 2012. doi: 10.1681/ASN.2011070738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schroer TA, Sheetz MP. Functions of microtubule-based motors. Annu Rev Physiol 53: 629–652, 1991. doi: 10.1146/annurev.ph.53.030191.003213. [DOI] [PubMed] [Google Scholar]
- 131.Shen B, Estevez B, Xu Z, Kreutz B, Karginov A, Bai Y, Qian F, Norifumi U, Mosher D, Du X. The interaction of Gα13 with integrin β1 mediates cell migration by dynamic regulation of RhoA. Mol Biol Cell 26: 3658–3670, 2015. doi: 10.1091/mbc.E15-05-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Simon H, Gao Y, Franki N, Hays RM. Vasopressin depolymerizes apical F-actin in rat inner medullary collecting duct. Am J Physiol 265: C757–C762, 1993. [DOI] [PubMed] [Google Scholar]
- 133.Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol 6: 1664–1668, 1996. doi: 10.1016/S0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 134.Stefan E, Wiesner B, Baillie GS, Mollajew R, Henn V, Lorenz D, Furkert J, Santamaria K, Nedvetsky P, Hundsrucker C, Beyermann M, Krause E, Pohl P, Gall I, MacIntyre AN, Bachmann S, Houslay MD, Rosenthal W, Klussmann E. Compartmentalization of cAMP-dependent signaling by phosphodiesterase-4D is involved in the regulation of vasopressin-mediated water reabsorption in renal principal cells. J Am Soc Nephrol 18: 199–212, 2007. doi: 10.1681/ASN.2006020132. [DOI] [PubMed] [Google Scholar]
- 135.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10: 513–525, 2009. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 136.Sun TX, Van Hoek A, Huang Y, Bouley R, McLaughlin M, Brown D. Aquaporin-2 localization in clathrin-coated pits: inhibition of endocytosis by dominant-negative dynamin. Am J Physiol Renal Physiol 282: F998–F1011, 2002. doi: 10.1152/ajprenal.00257.2001. [DOI] [PubMed] [Google Scholar]
- 137.Tajika Y, Matsuzaki T, Suzuki T, Aoki T, Hagiwara H, Kuwahara M, Sasaki S, Takata K. Aquaporin-2 is retrieved to the apical storage compartment via early endosomes and phosphatidylinositol 3-kinase-dependent pathway. Endocrinology 145: 4375–4383, 2004. doi: 10.1210/en.2004-0073. [DOI] [PubMed] [Google Scholar]
- 138.Tamma G, Klussmann E, Maric K, Aktories K, Svelto M, Rosenthal W, Valenti G. Rho inhibits cAMP-induced translocation of aquaporin-2 into the apical membrane of renal cells. Am J Physiol Renal Physiol 281: F1092–F1101, 2001. doi: 10.1152/ajprenal.0091.2001. [DOI] [PubMed] [Google Scholar]
- 139.Tamma G, Klussmann E, Procino G, Svelto M, Rosenthal W, Valenti G. cAMP-induced AQP2 translocation is associated with RhoA inhibition through RhoA phosphorylation and interaction with RhoGDI. J Cell Sci 116: 1519–1525, 2003. doi: 10.1242/jcs.00355. [DOI] [PubMed] [Google Scholar]
- 140.Tamma G, Lasorsa D, Ranieri M, Mastrofrancesco L, Valenti G, Svelto M. Integrin signaling modulates AQP2 trafficking via Arg-Gly-Asp (RGD) motif. Cell Physiol Biochem 27: 739–748, 2011. doi: 10.1159/000330082. [DOI] [PubMed] [Google Scholar]
- 141.Tamma G, Lasorsa D, Trimpert C, Ranieri M, Di Mise A, Mola MG, Mastrofrancesco L, Devuyst O, Svelto M, Deen PM, Valenti G. A protein kinase A-independent pathway controlling aquaporin 2 trafficking as a possible cause for the syndrome of inappropriate antidiuresis associated with polycystic kidney disease 1 haploinsufficiency. J Am Soc Nephrol 25: 2241–2253, 2014. doi: 10.1681/ASN.2013111234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tamma G, Robben JH, Trimpert C, Boone M, Deen PM. Regulation of AQP2 localization by S256 and S261 phosphorylation and ubiquitination. Am J Physiol Cell Physiol 300: C636–C646, 2011. doi: 10.1152/ajpcell.00433.2009. [DOI] [PubMed] [Google Scholar]
- 143.Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J 20: 4639–4647, 2001. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Valenti G, Procino G, Carmosino M, Frigeri A, Mannucci R, Nicoletti I, Svelto M. The phosphatase inhibitor okadaic acid induces AQP2 translocation independently from AQP2 phosphorylation in renal collecting duct cells. J Cell Sci 113: 1985–1992, 2000. [DOI] [PubMed] [Google Scholar]
- 145.Valenti G, Procino G, Tamma G, Carmosino M, Svelto M. Minireview: aquaporin 2 trafficking. Endocrinology 146: 5063–5070, 2005. doi: 10.1210/en.2005-0868. [DOI] [PubMed] [Google Scholar]
- 146.van Balkom BW, Boone M, Hendriks G, Kamsteeg EJ, Robben JH, Stronks HC, van der Voorde A, van Herp F, van der Sluijs P, Deen PM. LIP5 interacts with aquaporin 2 and facilitates its lysosomal degradation. J Am Soc Nephrol 20: 990–1001, 2009. doi: 10.1681/ASN.2008060648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.van Lieburg AF, Verdijk MA, Knoers VV, van Essen AJ, Proesmans W, Mallmann R, Monnens LA, van Oost BA, van Os CH, Deen PM. Patients with autosomal nephrogenic diabetes insipidus homozygous for mutations in the aquaporin 2 water-channel gene. Am J Hum Genet 55: 648–652, 1994. [PMC free article] [PubMed] [Google Scholar]
- 148.Vossenkämper A, Nedvetsky PI, Wiesner B, Furkert J, Rosenthal W, Klussmann E. Microtubules are needed for the perinuclear positioning of aquaporin-2 after its endocytic retrieval in renal principal cells. Am J Physiol Cell Physiol 293: C1129–C1138, 2007. doi: 10.1152/ajpcell.00628.2006. [DOI] [PubMed] [Google Scholar]
- 149.Wandinger-Ness A, Zerial M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb Perspect Biol 6: a022616, 2014. doi: 10.1101/cshperspect.a022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang W, Luo R, Lin Y, Wang F, Zheng P, Levi M, Yang T, Li C. Aliskiren restores renal AQP2 expression during unilateral ureteral obstruction by inhibiting the inflammasome. Am J Physiol Renal Physiol 308: F910–F922, 2015. doi: 10.1152/ajprenal.00649.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Whiting JL, Ogier L, Forbush KA, Bucko P, Gopalan J, Seternes OM, Langeberg LK, Scott JD. AKAP220 manages apical actin networks that coordinate aquaporin-2 location and renal water reabsorption. Proc Natl Acad Sci USA 113: E4328–E4337, 2016. doi: 10.1073/pnas.1607745113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Xie L, Hoffert JD, Chou CL, Yu MJ, Pisitkun T, Knepper MA, Fenton RA. Quantitative analysis of aquaporin-2 phosphorylation. Am J Physiol Renal Physiol 298: F1018–F1023, 2010. doi: 10.1152/ajprenal.00580.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yamamoto T, Sasaki S, Fushimi K, Ishibashi K, Yaoita E, Kawasaki K, Marumo F, Kihara I. Vasopressin increases AQP-CD water channel in apical membrane of collecting duct cells in Brattleboro rats. Am J Physiol Cell Physiol 268: C1546–C1551, 1995. [DOI] [PubMed] [Google Scholar]
- 154.Yang CR, Raghuram V, Emamian M, Sandoval PC, Knepper MA. Deep proteomic profiling of vasopressin-sensitive collecting duct cells. II. Bioinformatic analysis of vasopressin signaling. Am J Physiol Cell Physiol 309: C799–C812, 2015. doi: 10.1152/ajpcell.00214.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang CR, Tongyoo P, Emamian M, Sandoval PC, Raghuram V, Knepper MA. Deep proteomic profiling of vasopressin-sensitive collecting duct cells. I. Virtual Western blots and molecular weight distributions. Am J Physiol Cell Physiol 309: C785–C798, 2015. doi: 10.1152/ajpcell.00213.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yasui M, Zelenin SM, Celsi G, Aperia A. Adenylate cyclase-coupled vasopressin receptor activates AQP2 promoter via a dual effect on CRE and AP1 elements. Am J Physiol 272: F443–F450, 1997. [DOI] [PubMed] [Google Scholar]
- 157.Yu MJ, Pisitkun T, Wang G, Aranda JF, Gonzales PA, Tchapyjnikov D, Shen RF, Alonso MA, Knepper MA. Large-scale quantitative LC-MS/MS analysis of detergent-resistant membrane proteins from rat renal collecting duct. Am J Physiol Cell Physiol 295: C661–C678, 2008. doi: 10.1152/ajpcell.90650.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zalyapin EA, Bouley R, Hasler U, Vilardaga JP, Lin HY, Brown D, Ausiello DA. Effects of the renal medullary pH and ionic environment on vasopressin binding and signaling. Kidney Int 74: 1557–1567, 2008. doi: 10.1038/ki.2008.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zelenina M, Christensen BM, Palmér J, Nairn AC, Nielsen S, Aperia A. Prostaglandin E2 interaction with AVP: effects on AQP2 phosphorylation and distribution. Am J Physiol Renal Physiol 278: F388–F394, 2000. [DOI] [PubMed] [Google Scholar]
- 160.Zhang XY, Wang B, Guan YF. Nuclear receptor regulation of aquaporin-2 in the kidney. Int J Mol Sci 17: E1105, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zwang NA, Hoffert JD, Pisitkun T, Moeller HB, Fenton RA, Knepper MA. Identification of phosphorylation-dependent binding partners of aquaporin-2 using protein mass spectrometry. J Proteome Res 8: 1540–1554, 2009. doi: 10.1021/pr800894p. [DOI] [PMC free article] [PubMed] [Google Scholar]


