Abstract
In advanced cancer, current conventional therapies or immunotherapies cannot eradicate all tumor cells for most patients. Integration of these two treatments for synergistic effects could eradicate more tumor cells and increase overall survival rates. But proper integration is a challenge, partly due to a poor understanding of the impact of conventional treatment on immune responses. Intensive chemo/radiotherapy may impair ongoing immune responses whilst lower intensity of therapy might not kill enough tumor cells, both leading to tumor relapse. Current understanding of mechanisms of resistance to conventional and targeted cancer therapies has focused on cell intrinsic pathways that trigger DNA damage/repair or signaling pathways related to cell growth. Recent reports show that host T cells properly primed against tumor specific antigens after conventional treatment can integrate with direct cytotoxic effects induced by radiation or chemotherapy to profoundly control tumors. Following cytotoxic anticancer treatment, tumor derived DAMPs (damage-associated molecular patterns) can be sensed by innate cells which drives type I interferon (IFNs production) for cross priming of CD8+ T cells. Some types and protocols of chemotherapy or radiation can increase tumor infiltrating lymphocytes that overcome resistance to immunotherapy. As such, a deeper understanding to the immune mechanisms of conventional and targeted cancer therapies will lead toward novel combinatorial anticancer strategies with improved clinical benefit.
Introduction
For metastatic or locally advanced solid tumors, neither current conventional treatments such as cytotoxic chemotherapy, radiotherapy nor the newer immunotherapies alone can effectively cure patients in many cases. Intensive chemotherapy reduces tumor cell burden hardly eradicate all cancer cells in adult solid tumors(1). Immunotherapy can improve T cell responses for many patients but it is only curative in a very small fraction of patients(2, 3). Integration of conventional treatment and immunotherapy with the goal of curing advanced cancers is a major challenge to improving both treatments. In most cases, DNA damage and modulation of oncogenic signals have long been considered the major mechanisms responsible for the effects of conventional and targeted therapies(4–6). As such, cell intrinsic signals involving in DNA repair, cell cycle checkpoints and signal transduction have been intensively studied as dominant mechanisms controlling the response to therapy. While manipulating cell intrinsic pathways has attracted much attention, several surprising studies implicated that chemotherapy and targeted treatment trigger immunity contributing to the eradication of tumor cells(7–9). The importance of the immune system in the responses to radiation, chemotherapy and targeted therapy has been observed in multiple syngeneic murine cancer models. Since then, many in depth mechanistic studies on multiple immune pathways that regulate the response to therapy have been explored and identified(10, 11). Here, we summarize the current state of mechanisms regarding treatment-induced adaptive immune responses. We place emphasis on recent data in radiation and targeted monoclonal antibodies (mAb) therapies implicating the cytosolic DNA sensing and host type I IFN production as the key innate immune steps in driving adaptive immunity.
The influence of the immune system on radiation efficacy
Local radiation modulates immunity for tumor control
The rationale for radiotherapy (RT) is based on inducing lethal DNA damage to tumor cells or tumor associated stroma. The most commonly administered RT regimens deliver small, fractionated doses of ionizing radiation over several weeks. Depending on the location of the tumor, its type, volume of normal tissue irradiated and tissue toxicity, a total dose of approximately 60–80 gray (Gy) is divided into relatively small doses of 2–3 Gy/day. However, protracted fractionation may lead to tumor re-growth between doses. Very large (>15 Gy) single radiation treatments or hypo-fractionated radiation treatments (10 Gy over 5 treatments) are administered to treat brain or spine metastasis(12). Single dose or hypo-fractionated regimen is employed with curative intent. In the treatment of oligo metastasis and localized lung cancer(13). Importantly, the volume of surrounding normal tissue must be limited to avoid complications.
It has been increasingly observed that the use of local radiotherapy stimulates anti-tumor immune responses. Most studies have focused on the immune-modulating effects directly induced on tumor cells. Radiation can modulate the peptide repertoire and enhance MHC class I expression on tumor cells, which boosts the efficacy of adoptive CTL immunotherapy(14). Other reports have illustrated that local radiation of tumors alters the phenotype of tumor cells, rendering them more susceptible to vaccine-mediated T-cell killing(15). Additionally, it has been proposed that local radiation changes the endothelium by reversing the non-adhesive phenotype of the tumor endothelium, contributing to increased infiltration of lymphocytes into tumor(16). Consistently, it has been shown that the generation of tumor antigen-specific effector cells that traffic to tumors increases after local radiation(17). By inducing apoptosis and necrosis of tumor cells, RT can promote increased antigen presentation through both increased antigen availability as well as “danger signal”-induced dendritic cell (DC) maturation(18). A pioneering study showed that the immune system contributes to both radiotherapy and chemotherapy via the release of the high-mobility-group box 1 (HMGB1) alarming protein from dying tumor cells and subsequently increase DC antigen(Ag)-processing and cross-presentation(19). Subsequent studies identified immunogenic cell death post RT via (1) cell surface translocation of calreticulin under ER stress (2) release of extracellular HMGB1 and (3) ATP during autophagy; (4) release of cellular RNA/DNA(20, 21). These danger signals are generally referred to as “damage-associated molecular patterns” (DAMPs) that alarm the innate immune response to phagocyte tumor cell and activate innate immune response post RT(22, 23). Although many studies demonstrate that RT can trigger innate immune activation, whether CD8+ T cell immune response contributes to tumor reduction in a meaningful way has remained unclear until recently.
Our group first reported that CD8+ T cells are essential for ablative radiation-induced tumor regression, that had changed the current understanding of how radiotherapy works(2, 8). Further investigation of the mechanisms revealed that ablative RT dramatically increases eradication of the primary tumor or distant metastasis (abscopal effect) in a CD8+ T cell dependent fashion, which can be further amplified by combined with local immunotherapy(8). Ablative RT dramatically increases T cell priming in draining lymphoid tissues, leading to reduction/eradication of the primary tumor or distant metastasis in a CD8+ T cell dependent fashion. Such immune activation effect of radiotherapy to reduce tumor burden can be sustained and elevated by subsequent immunotherapy(8). Noticeably, while large amount of neo-antigen may release post RT, the pre-existing immunosuppressive tumor microenvironment can restrict the antitumor immune response and the abscopal effect triggered by RT. Therefore, the understanding of the abscopal effect has the potential to change radiotherapy from a local and regional treatment modality to a modality that effectively treats distant metastasis and opens the door to combinations of radiotherapy and immunotherapy. In order to improve the efficacy of radiotherapy in conjunction with immunotherapy, the immunogenic characters of different radiotherapy schemes (e.g. stereotactic, fractionated) l are necessary to be considered(24). Some data have shown that stereo-tactic radiotherapy in combination with T cell immunotherapy was more effective than a fractionated scheme(25). Conversely, some illustrated that fractionated radiotherapy can completely eradicated tumors in a mouse model of lymphoma, while other regimes only slowed growth(26). There are some possibilities that can explain the different immune response resulted from different radiation fraction schemes, 1. Various rate of direct tumor cell killing led by different regimens; 2. A balance between immunostimulatory and immunoinhibitory effects of radiotherapy(27). In all, finding equilibrium to maximize anti-tumor T cell activation while minimizing immunoinhibitory effect is of great importance in optimizing the combination treatment effect.
Key for ideal integration between immunotherapy and radiotherapy
Radiation-induced equilibrium or dormancy is a common feature in both clinical and preclinical radiated-tumors. One view of late tumor recurrence is that the duration of progression-free/stable disease is determined by the very small number of surviving clones which survive cytotoxic treatment and slowly adapt and create proper environment to repopulate. Therefore the extent of massive cell death of tumor cell or stromal triggered by radiation is proposed to, predominantly mediate the time to tumor relapse(28). An alternative/complimentary explanation is the induction of tumor equilibrium which is a state whereby tumor proliferation is balanced by cell death, and lack of angiogenesis, mechanisms have been demonstrated to mediate tumor equilibrium (29, 30), suggesting that there is a balance between cell growth and cell death due to insufficient angiogenesis, or intrinsic cellular factors. Our recent study revealed the immunologically based equilibrium after RT in which the active balance of tumor cell proliferation and T cell-mediated killing is the major mechanism resulting in RT-induced dormancy and equilibrium(31). We observed an essential role for immune cells and their cytokines in maintaining RT-induced equilibrium. Depletion of T cells or neutralization of IFN-γ reversed such equilibrium, leading to tumor relapse. However, without any intervention, T cells are not functional enough to eradicate residual tumor cells over the course of achieving tumor equilibrium and tumors eventually relapse. We demonstrated that PD-L1 blockade could disrupt equilibrium by augmenting T cell responses, leading to complete tumors regression. Considering such interplay between tumor cells and immune cells occurs in radiation-induced tumor equilibrium, immunotherapy should be involved in combinational treatments that tip over the balance for complete eradication.
Innate immune sensing pathways serve as a bridge to an adaptive immune response in radiation therapy
The mechanism of increased cross priming and tumor-specific T cell activation inside tumor or DLN has not been well illustrated(32). Type I IFNs serve as a linker between innate responses and adaptive immunity(33, 34). Interestingly, it has been shown that endogenous type I IFN production plays a critical role not only in immune-editing of tumors but also in promoting DCs maturation and stimulating the cross-priming of tumor specific CD8+ T cells (35, 36). Therefore, type I IFNs are indispensable components of host defense during tumor initiation and progression. Tumor antigens and danger signals released after local high-dose radiation may potentially trigger IFN pathway in order to increase cross priming. Therefore we demonstrated an effective anti-tumor adaptive immune response by revealing the potential role of type I IFNs in bridging the initial inflammation induced by radiation(32). After radiotherapy, type I IFN inside tumor is increased for re-bridging the innate and adaptive immunity in the tumor microenvironment. More importantly, the diminished anti-tumor effect of radiotherapy- in IFN-α/β receptor knockout hosts in comparison with wild type mice demonstrated the potential physiological and clinical significance of our studies. A similar situation appears to be the case when RT is used in conjugated with immunotherapy strategies such as cancer vaccines. Consistent with our observations, type I IFN signaling is demonstrated to orchestrate the synergy of radiation and antigenic peptide vaccine(37). Collectively, these findings lead to investigations of type I IFN agonists which presumably contribute to eradicate the established radiation-resistant tumors.
When sufficient data points to type I IFNs, it is necessary to discern the mechanism responsible for type I IFN induction by radiation in order to develop potential therapeutics that targets this pathway. It is known that a diverse range of stimuli is able to generate type I IFN production. For example, a recently defined endoplasmic-reticulum-associated protein STING (stimulator of IFN genes) has been demonstrated to be a mediator for type I IFN induction by intracellular exogenous DNA in a TLR-independent manner(38, 39). Moreover, STING is also a mediator for autoimmune diseases, which are initiated by the aberrant cytoplasmic DNA(40). Following the recognition of cytosolic DNA, cGAMP synthase (cGAS) catalyzes the generation of 20 to 50 cyclic GMP-AMP, which binds to and activates STING signaling(41). More recently, cGAS has been considered as an universal cytosol DNA sensor for STING activation, such as in the setting of viral infection and lupus erythematosus(42, 43). Notably, our study demonstrated that STING signaling, instead of MYD88 or TRIF signaling (two major TLR associated pathways for IFN induction), is required for antitumor effects of radiation mediated type I IFN response in dendritic cells. Furthermore, STING signaling is required to elicit robust innate and adaptive immune response to radiation. Specifically, radiation creates stress for tumor cells, causing them to release danger signals that are recognized by patrolling dendritic cells (DCs) and in turn activates the cGAS-STING-IRF3-IFNβ axis(44, 45). These studies have not only pointed to the significance of nucleic acid sensing pathways in effective antitumor immune responses and type I IFN induction after radiotherapy, but also integrate current understanding of radiation-induced DNA damage into immune sensing. However, it is still unclear how tumor DNA can get into cytosolic compartment of DC. Directly providing naturally produced GMP-AMP fails to activate DC but additional local RT allows STING signaling, suggesting that radiation might change local environment to provide additional condition that allow tumor DNA to invade the cytosolic compartment of DC. An intriguing approach is to improve the physical and chemical properties of GMP-AMP that can actively enter cytosolic compartment and signal STING pathway(46, 47). The local use of the modified molecules that can target to cytosolic compartment can generate strong IFN and tumor regression. However, systemic delivery of such modified molecules might have higher toxicity and reduced tumor targeting. How to increase tumor targeting by systemic delivery remains to be determined.
Except for tumor-infiltrating macrophages and DCs, NK cells also contribute to antitumor effect by targeting malignant cells via direct cytolysis and secretion of potent immune mediators(48). NKG2D ligands, an activating receptor for NK cells are demonstrated to be up-regulated by radiotherapy therefore rendering radiated-tumor cells more susceptible to NK-cell mediated cytolysis(49). In pancreatic and colon carcinoma, when tumor cells exposed to radiation, Hsp70 will be induced thereby targeting them for lysis by NK cells(50).
The influence of the immune system on chemotherapeutic efficacy
The death of tumor cells after chemotherapy that elicit an antitumor immune response and immunological memory against tumor-associated antigens defines immunogenic cell death (ICD) and has been well reviewed somewhere else(23, 51–53). We only briefly summarize some key findings in this important field. It is reported that chemotherapeutic agents such as anthracyclines and oxaliplatincan induce ICD. The dying tumor cells alert the immune system through expressing or releasing damage-associated molecular patterns (DAMPs) via specific sensing pathways(54, 55). Well-characterized DAMPs include increased expression of the endoplasmic reticulum protein calreticulin (CRT) on the cell surface(54, 55), extracellular secretion of ATP(56, 57), and extracellular release of the non-histone chromatin protein HMGB1 (high mobility group box 1)(19, 58). Clinically, the expression of DAMPs, which are induced by radiotherapy or chemotherapy, have been shown to be related to patient’ survival in various cancers. For oxaliplatin-treated colorectal cancer patients, tumor cell apoptosis will induce CRT in tumor cells early apoptosis phase and HMGB1 in late apoptosis phase(59, 60). In Patients with Esophageal Squamous Cell Carcinoma, the degree of HMGB1 positively correlated with patient survival(61). Liu’s group found that soluble CRT (sCRT) level in serum samples from 58 lung cancer patients was significantly higher than that from 40 healthy individuals(62). The predictive impact of DAMPs for radiotherapy and chemotherapy in cancer patients enlightened the concept of utilizing ICD as biomarkers/ predictors of therapeutic responses and correlating them with immunological and/or clinical observations.
DAMPs such as HMGB1 may bind to TLR4 (Toll-like receptor 4) on APCs, which in turn can stimulate dendritic cells and contribute to the antitumor T cell response. In addition, tumor-derived nucleic acids released after chemotherapy activate TLRs or cytosolic nucleic acid–sensing pathways, which presumably contribute to antitumor immune responses by inducing production of type I IFNs(63, 64). These findings, including seminal work from Zitvogel and Kroemer (see their review elsewhere), have demonstrated in detail that DAMPs are determined factors driving innate immune recognition response and subsequent antitumor immunity.(52) Even though DAMPS are considered as a signal to alarm host immune system to get activated, whether it can always sufficiently mediate immunogenicity, leading to potent immune response, still remains a question. Caetano Reis e Sousa summarized that immunogenicity can be composed by two basic properties: antigenicity and adjuvanticity. Antigenicity is defined as a given immunogen which can be recognized by lymphocytes and adjuvanticity denotes a substance’s ability to promote the priming of lymphocytes(65). According to this characterization, most of DAMPs can be categorized as pro-inflammatory signals while these molecules cannot be considered equivalent to the ability of promoting DC activation and adaptive immunity to associated foreign antigens
However, there are some factors that compromise the interaction between chemo/radiotherapy and host immune system. First, the expression and binding sites of DAMPs will be regulated by conventional therapies. In breast cancer patients, Zitvogel and Kroemer’s group reported that a TLR4 polymorphism impairs HMGB1 binding to TLR4, which, leads to tumor early relapse after anthracycline-based chemotherapy(19). Second, some immunosuppressive cytokines induced by conventional therapies are shown to affect tumor microenvironment and host immune system. Transforming growth factor-beta ("TGF-beta") is known to be one of most potent immunosuppressor induced by radiotherapy through inhibiting APC maturation and effector CD8+T cells proliferation, and through promoting T-reg cells or generation pro-tumorigenic M2 macrophages(66). How to properly use combinational therapy to synergize their effects has become an outstanding issue. The dose and timing of each treatment could be important factors to consider.
The influence of the immune system on targeted mAb therapy
Adaptive immunity is essential for anti-tumor effects of targeted antibody therapy
Antagonists antibodies of the oncogenic receptor tyrosine kinases block tumor cell growth signaling and trigger apoptosis pathways. Targeted antibodies, such as trastuzumab, cetuximab and rituximab, are clinically efficacious targeted treatments with proven survival benefits in some patients with oncogene mutations or overexpression, respectively(67, 68). The action of these mAbs principally was attributed to programmed cell death induction, complement-dependent cytotoxicity (CDC), antibody-dependent cell cytotoxicity (ADCC), and phagocytosis of mAb-opsonized target cells through FcγRs (receptors for the Fc portion of IgG) expressed on macrophages, neutrophils, and natural killer (NK) cells. In contrast to the established direct cell death mechanisms, the long-term effects of targeted antibody therapy on the host adaptive response have received little attention. Early clinical trials noticed that patients who previously received trastuzumab therapy exhibited a substantial increase of HER2 E75 peptide specific CD8+ T cells response, which was correlated with improved clinical outcomes and highlight a positive correlation between the adaptive immune response and clinical benefits(69, 70). Similarly, two clinical observations suggest that rituximab therapy elicits a “vaccine” effect that might correlate to prolong survival but causative effect cannot be established in such observation(71). While many studies have a correlation between immune activation and prolonged survival, whether or what extend such adaptive immunity contribute to tumor regression has been difficulty to establish by clinical observation. Furthermore, the mechanism by which the adaptive immune responses mediate antibody-mediated tumor control is still unknown. For mechanistic studies after different therapies, xenograft transplanted tumor models were primarily used with a prolonged high dose of antibody.
Using a mouse mammary tumor line derived from Her2/neu transgenic mice in immune competent host, it was clearly demonstrated for the first time that the therapeutic effect of anti-HER2/neu antibody largely depends on the CD8+ T cell response(9). This study has been fully supported by others using different models(9, 72). Anti-Her2/neu antibody therapy can induce tumor regression in Myd88 and IFN dependent fashion. Stress proteins induced by oncogenic receptor blockade might contribute to MyD88 dependent innate sensing and subsequent T cell activation for tumor regression. Therefore, anti-oncogenic receptor antibody can be synergized by antibodies that promote T cell responses(9, 72). Similarly, when reconstituting immune cells in Rag mice, the antitumor effect of Cetuximab became more pronounced than immune deficient mice, and the EGFR(+) human tumor burden was more profoundly reduced in the presence of an adaptive immune system(73, 74). The consequent DC-mediated cross-priming of antigens derived from mAb-covered cancer cells elicited a robust CTL anti-tumor response. On the basis of our data, we suggest a possible involvement of CTL-dependent immunity in cetuximab anti-tumor effects.
In contrast, the effects of anti-CD20 on adaptive responses appear still obscure. The action of anti-CD20 antibody was principally attributed to CDC and ADCC. By using the receptor for the Fc region of immunoglobulin G (FcγRs) deficient mice, the essential role of ADCC has been confirmed in the therapeutic function of anti-CD20 in xenograft model(75). For lymphoma depletion, high-affinity FcγRI, low-affinity FcγRIII, and intermediate-affinity FcγRIV each contributed to lymphoma depletion when treated withanti-CD20 mAb, suggesting that the type and activity of the FcγRs explains much of the efficacy of anti-CD20(76, 77). Further study shows anti-CD20 mAb becomes internalized via FcγRIIB expressed on lymphoma cells, limiting the elimination of tumor cells via ADCC through innate immune effector cells.(78). In mantle cell lymphoma, patients with FcγRIIB-negative tumor biopsies showed a greater durable response when treated with a rituximab immune-chemotherapy compared with FcγRIIB-positive tumor biopsies(79), emphasizing that ADCC plays an important role in anti-CD20 therapy. Effective control of B cell lymphoma by anti-CD20 in xenograft models further suggests direct killing or innate-mediated killing may be sufficient for the control of this type of tumor while the role of adaptive immune system has not been defined. In xenograft model, innate immune cells might be overly activated and their role in clearing tumor cells might be overestimated(80). In contrast to the established direct cell death mechanisms, the long-term effects of targeted antibody therapy on the host adaptive response have received little attention. To study the effect of FDA approved Rituximab in the present of immunocompetent host, human CD20 was transfected into EL4, a T cell lymphoma model. Rituximab triggered an adaptive antitumor immune response in immunocompetent mice and provide long-lasting protection against(52). By using the same model, another group showed that anti-CD20 treatment generated protective memory T cell responses through different FcγRs, but the role of T cells in the primary treatment was not demonstrated(81). Since the anti-CD20 therapy is more potent in mediating the depletion of B cells and represents a breakthrough in the treatment of B cell lymphoma, the function of adaptive immune response, especially CD8+ T cells in the therapeutic activity of anti-CD20 mAb in B cell malignancies nonetheless still requires further elucidation.
Unlike other targeted mAbs, anti-CD47mAb binds to and blocks a “don’t eat me” molecule CD47(82). Since CD47 is expressed broadly across cancer types, it represents a novel potentially tractable and widely applicable target for therapeutic blockade in patients(80). Human CD47-blocking monoclonal antibodies (mAbs) have demonstrated efficacy in various preclinical tumor models and the therapeutic effects of anti-human CD47 are largely considered to enhance antitumor phagocytosis by macrophages(82, 83). However, these studies employed xenograft human tumors in T cell–deficient mice and innate cells might be overly activated. Thus, they were not able to evaluate the role of adaptive immunity in the effectiveness of CD47 blockade. Using syngeneic mouse models of cancer in immune competent host, rather than transplanted xenografts in T cell deficient mice, it was first demonstrated that most of the anti-tumor effect mediated by the CD47 blockade is T cell–dependent—specifically CD8+ cytotoxic T cell–dependent—as the efficacy of the CD47 antibody disappears in T cell–deficient or CD8+ cell–depleted, tumor-bearing hosts(84). Together with the observation of anti-HER2 and anti-EGFR, these findings reinstate that targeted mAb therapy can exert its therapeutic effects through the induction of an adaptive cellular immune response, aside from well-characterized tumor-intrinsic mechanisms.
Innate immune sensing pathways serve as a bridge to an adaptive immune response intargeted therapy
Dendritic cells (DCs) are crucial for generating tumor-specific T cell responses; these cells express an array of FcRs for internalization of antigen–antibody complexes and antigen presentation for cross-priming(85). Antigen uptake through opsonization of apoptotic tumor cells is also associated with enhanced antigen presentation by DCs(86). mAbs can facilitate the uptake of tumor antigens by DCs, which helps to stimulate the activation and expansion of CD4+ and CD8+ tumor-specific T cells. Insight into this process has arisen from the observation that targeted therapy-mediated cell death can release large amounts of tumor derived damage-associated molecular pattern molecules (DAMPs), which can augment antigen presentation by DCs to enhance the priming of cytotoxic T lymphocytes (CTLs)(9, 72). The major DAMP driving host antitumor immune response varies in different therapies. For example, the effect of anti-HER2 was demonstrated to depend on DC cross-priming through a HMGB1-MyD88-dependent mode in two distinct immune competent murine HER2 breast cancer models(9, 72). Neutralization of HMGB1 alone greatly abolished the efficacy of anti-HER2 therapy. In this context, HMGB1 as endogenous danger signal is essential for antibody mediated tumor control. These observations were confirmed and extended by another study demonstrating that anti-HER2 therapy depends on HMGB1-TLR axis through MyD88, thereby and causes the release of type I interferons to prime the adaptive immune response50. Using neutralizing antibodies, it was demonstrated that IFNAR1, which is the receptor of type I IFNs in mice, is required for anti–HER2 treatment(9, 72). Consistently, a requirement for type I IFNs upstream from T cell priming against tumor-associated antigens was further confirmed in anti-EGFR. Cetuximab was shown to promote opsonization and phagocytosis of colon cancer cells by human DCs, which were subsequently engaged in antigen cross-presentation and CTL activation through both the FcR and MyD88 pathways(73). In addition, as the critical cytokine for cross-priming, type I IFN levels were demonstrated that correlated with the sensitivity of tumors to anti-EGFR in mouse tumor models, which raised the possibility that lower levels of type I IFNs may limit immune responses and additional type I IFNs may control Ab-resistant tumor growth(87). In accordance with this, anti-EGFR mAb with IFNβ was more potent than the first generation of Ab for controlling Ab-resistant tumors. Thus, type I IFN production is a key output from whatever innate immune sensing pathways detect the presence of cancer in vivo. Anti-CD47 mAb also bridges innate immune DC activation and T cell priming through type I IFN. The ability of antigen cross-presentation by DCs has been enhanced after anti-CD47 therapy(84). As a consequence, priming or boosting CD8+ effector T cells with tumor specificity can be accomplished by blocking the CD47. Interestingly, unlike anti-HER2 and anti-EGFR, the major innate sensing pathway driving type I IFN production and adaptive immunity for tumor control is the cytosolic DNA sensor STING, expressed by DCs. However, signaling through MyD88, for example by Toll-like receptors, was not required. How DNA from irradiated-tumor cells is delivered and why DNA sensing through STING is essential in this model remain to be determined. Relapse eventually occurs even in the face of even integrated chemotherapy plus immunotherapy. This failure may result from immunotherapy assistance or chemo-induced immune downregulation. The integration of anticancer treatment with immunotherapy can not only decrease the size of the primary tumor, but also alter tumor microenvironment. Thus, it is important to carefully monitor the frequency of combined treatments, the concentration of agents and the sequence of administration. Our study suggests that sequential administration of anti-CD47 antibody after chemotherapy may allow chemotherapy to enhance the antibody mediated antitumor effect(84). Therefore, whether and which chemotherapy drugs can enhance antibody mediated anti-cancer effect in neoadjuvant, adjuvant, and metastasis settings needed further consideration.
Conclusions
In this review, we postulate that in addition to immune checkpoint inhibitors, enhancing innate immune sensing for type I IFN induction is an essential tool for tumor management via conventional therapy and targeted mAb therapy. Type I IFN signaling reinforces DC activity and then promotes CD8+ T cell cross-priming, leading to tumor control. Multiple nucleic acid-sensing pathways control the induction of type I IFN, and the corresponding agonists have displayed the potential ability to improve the overall response to combinational therapy. So far, the detailed mechanisms of the combinations are still not well-defined. To translate these discoveries into practice, it will be necessary to further determine the toxicity and synergy of conventional and targeted therapy with nucleic acid-sensing agonists in the clinic, as well as to develop carriers for effective delivery of agonists to tumor sites to improve overall response.
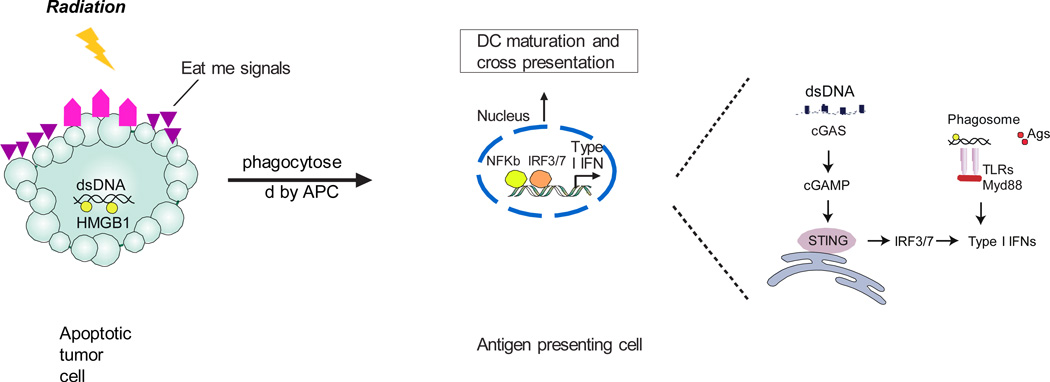
Figure 1. Irradiation mediated sensing of tumor-derived DNA for induction of type I interferons (IFNs) in antigen-presenting cells (APCs).
Radiation results in the release of “find-me” and “eat-me” signals from tumor cells. During phagocytosis in myeloid cells, the DNA fragments hidden in irradiated tumor cells are released from phagosomes to cytoplasm, acting as a danger signal. The cGAS binds this DNA and generates cGAMP as a second messenger. cGAMP binds to STING, which subsequently activates IFN regulatory factor 3 (IRF3) or IRF7 to induce type I IFN production. Alternatively, DNA released from dying tumor cells can theoretically activate endosomal TLR9 and induce type I IFN production through Myd88.
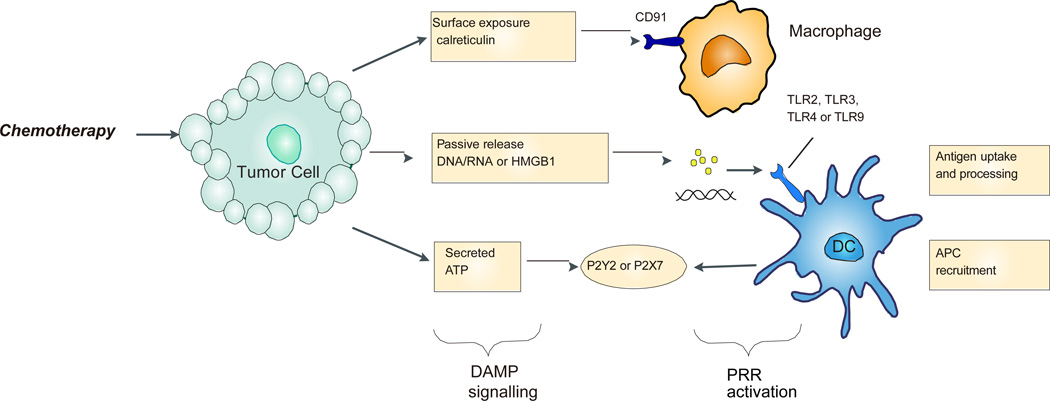
Figure 2. Damage-associated molecular patterns (DAMPs) for innate immune activation in response to chemotherapy-induced tumor cell stress or death.
Increased exposure of calreticulin on the surface of tumor cells facilitates their uptake through interaction with CD91 on antigen-presenting cells such as macrophage. Tumor released nucleic acid and high-mobility group box 1 protein (HMGB1) may bind to Toll-like receptors on antigen-presenting cells, which can subsequently increase antigen processing and induce maturation of dendritic cells. ATP secreted from dying tumor cells can bind to the P2X purinoceptor 7 receptor (P2X7R) of APCs, which resulting in production of IL-1β and chemotactic attraction of antigen-presenting cells.
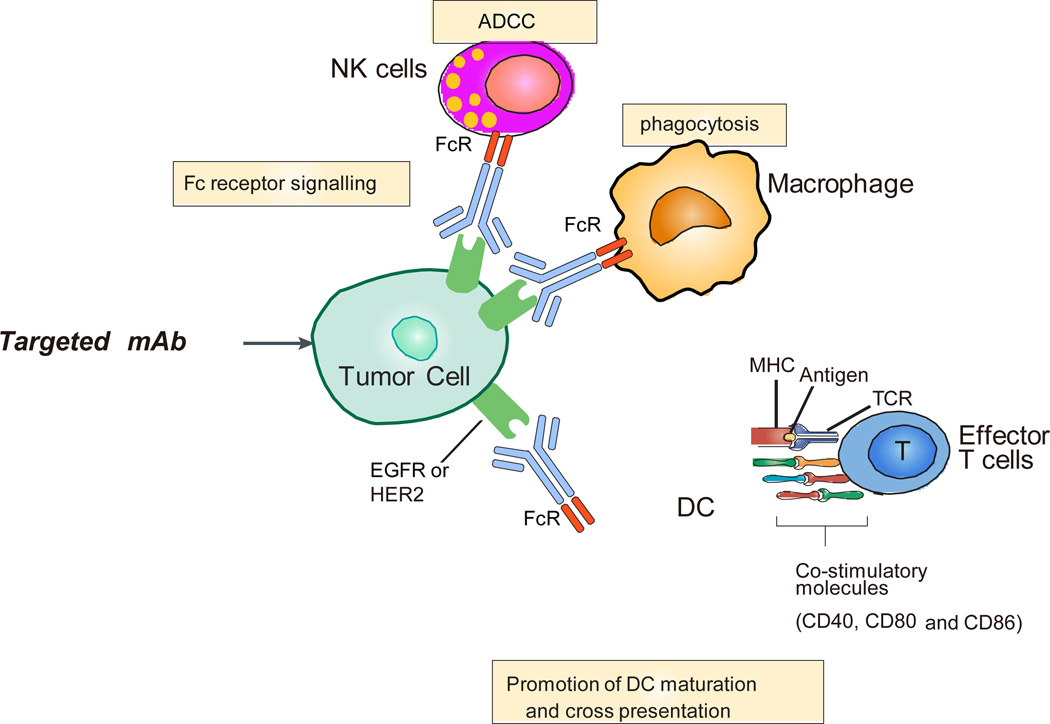
Figure 3. The immune aspects of antibody-based targeted therapy.
The effects of antibody-based targeted therapies on immune response include (1) FcRγ-dependent natural killer (NK) cell cytotoxicity and macrophage phagocytosis; (2) promoting dendritic cell (DC) priming and increasing the expression of co-stimulatory molecules, such as CD40, CD80, and CD86on the DC surface;(3) activating CD8+ T cells-dependent adaptive anti-tumor immunity.
Acknowledgments
This work was in part supported by US National Institutes of Health grants CA141975 to Y-X.F., a grant from the Ludwig Foundation to R.R.W., and a generous gift from The Foglia Foundation (Y-X.F., and R.R.W.).
References
- 1.Waun Ki Hong RCBJ, Hait William N, Kufe Donald W, Pollock Raphael E, Weichselbaum Ralph R, Holland James F, Frei Emil., III . Cancer Medicine. 8th. Shelton, Connecticut: People’s Medical Publishing House –USA; 2010. [Google Scholar]
- 2.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(13):1270–1271. doi: 10.1056/NEJMc1509660. [DOI] [PubMed] [Google Scholar]
- 4.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7(8):573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 5.Prise KM, O'Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer. 2009;9(5):351–360. doi: 10.1038/nrc2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet JM, Villanueva A, Lachenmayer A, Finn RS. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol. 2015;12(8):436. doi: 10.1038/nrclinonc.2015.121. [DOI] [PubMed] [Google Scholar]
- 7.Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202(12):1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee Y, Auh SL, Wang Y, Burnette B, Meng Y, Beckett M, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114(3):589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18(2):160–170. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nat Rev Immunol. 2015;15(7):405–414. doi: 10.1038/nri3845. [DOI] [PubMed] [Google Scholar]
- 11.Deng L, Liang H, Fu S, Weichselbaum RR, Fu YX. From DNA Damage to Nucleic Acid Sensing: A Strategy to Enhance Radiation Therapy. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-14-3110. [DOI] [PubMed] [Google Scholar]
- 12.Salama JK, Hasselle MD, Chmura SJ, Malik R, Mehta N, Yenice KM, et al. Stereotactic body radiotherapy for multisite extracranial oligometastases: final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer. 2012;118(11):2962–2970. doi: 10.1002/cncr.26611. [DOI] [PubMed] [Google Scholar]
- 13.Westover KD, Iyengar P, Sharma AN, Timmerman R. SABR for aggressive local therapy of metastatic cancer: A new paradigm for metastatic non-small cell lung cancer. Lung Cancer. 2015;89(2):87–93. doi: 10.1016/j.lungcan.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64(12):4328–4337. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 16.Ganss R, Ryschich E, Klar E, Arnold B, Hammerling GJ. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002;62(5):1462–1470. [PubMed] [Google Scholar]
- 17.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174(12):7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 18.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5(11):1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 19.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 20.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. Journal of the National Cancer Institute. 2013;105(4):256–265. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5(2):403–416. doi: 10.18632/oncotarget.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12(12):860–875. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 23.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annual review of immunology. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 24.Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. International journal of radiation oncology, biology, physics. 2012;83(4):1306–1310. doi: 10.1016/j.ijrobp.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114(3):589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dovedi SJ, Melis MH, Wilkinson RW, Adlard AL, Stratford IJ, Honeychurch J, et al. Systemic delivery of a TLR7 agonist in combination with radiation primes durable antitumor immune responses in mouse models of lymphoma. Blood. 2013;121(2):251–259. doi: 10.1182/blood-2012-05-432393. [DOI] [PubMed] [Google Scholar]
- 27.Gandhi SJ, Minn AJ, Vonderheide RH, Wherry EJ, Hahn SM, Maity A. Awakening the immune system with radiation: Optimal dose and fractionation. Cancer letters. 2015;368(2):185–190. doi: 10.1016/j.canlet.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 28.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501(7467):328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7(11):834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goss PE, Chambers AF. Does tumour dormancy offer a therapeutic target? Nat Rev Cancer. 2010;10(12):871–877. doi: 10.1038/nrc2933. [DOI] [PubMed] [Google Scholar]
- 31.Liang H, Deng L, Chmura S, Burnette B, Liadis N, Darga T, et al. Radiation-induced equilibrium is a balance between tumor cell proliferation and T cell-mediated killing. J Immunol. 2013;190(11):5874–5881. doi: 10.4049/jimmunol.1202612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71(7):2488–2496. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crouse J, Kalinke U, Oxenius A. Regulation of antiviral T cell responses by type I interferons. Nat Rev Immunol. 2015;15(4):231–242. doi: 10.1038/nri3806. [DOI] [PubMed] [Google Scholar]
- 34.Trinchieri G. Type I interferon: friend or foe? J Exp Med. 2010;207(10):2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208(10):2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208(10):1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu CY, Yang LH, Yang HY, Knoff J, Peng S, Lin YH, et al. Enhanced cancer radiotherapy through immunosuppressive stromal cell destruction in tumors. Clin Cancer Res. 2014;20(3):644–657. doi: 10.1158/1078-0432.CCR-13-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339(6121):786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, Shi H, Wu J, Sun L, Chen C, Chen ZJ. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell. 2013;51(2):226–235. doi: 10.1016/j.molcel.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahn GO, Tseng D, Liao CH, Dorie MJ, Czechowicz A, Brown JM. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc Natl Acad Sci U S A. 2010;107(18):8363–8368. doi: 10.1073/pnas.0911378107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu J, Chen ZJ. Innate immune sensing and signaling of cytosolic nucleic acids. Annual review of immunology. 2014;32:461–488. doi: 10.1146/annurev-immunol-032713-120156. [DOI] [PubMed] [Google Scholar]
- 42.Gao D, Wu J, Wu YT, Du F, Aroh C, Yan N, et al. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 2013;341(6148):903–906. doi: 10.1126/science.1240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahn J, Gutman D, Saijo S, Barber GN. STING manifests self DNA-dependent inflammatory disease. Proc Natl Acad Sci U S A. 2012;109(47):19386–19391. doi: 10.1073/pnas.1215006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41(5):830–842. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41(5):843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E, et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med. 2015;7(283):283ra52. doi: 10.1126/scitranslmed.aaa4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, et al. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 2015;11(7):1018–1030. doi: 10.1016/j.celrep.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orr MT, Lanier LL. Natural killer cell education and tolerance. Cell. 2010;142(6):847–856. doi: 10.1016/j.cell.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436(7054):1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gehrmann M, Marienhagen J, Eichholtz-Wirth H, Fritz E, Ellwart J, Jaattela M, et al. Dual function of membrane-bound heat shock protein 70 (Hsp70), Bag-4, and Hsp40: protection against radiation-induced effects and target structure for natural killer cells. Cell death and differentiation. 2005;12(1):38–51. doi: 10.1038/sj.cdd.4401510. [DOI] [PubMed] [Google Scholar]
- 51.Galluzzi L, Vacchelli E, Bravo-San Pedro JM, Buque A, Senovilla L, Baracco EE, et al. Classification of current anticancer immunotherapies. Oncotarget. 2014;5(24):12472–12508. doi: 10.18632/oncotarget.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abes R, Gelize E, Fridman WH, Teillaud JL. Long-lasting antitumor protection by anti-CD20 antibody through cellular immune response. Blood. 2010;116(6):926–934. doi: 10.1182/blood-2009-10-248609. [DOI] [PubMed] [Google Scholar]
- 53.Garg AD, Galluzzi L, Apetoh L, Baert T, Birge RB, Bravo-San Pedro JM, et al. Molecular and Translational Classifications of DAMPs in Immunogenic Cell Death. Front Immunol. 2015;6:588. doi: 10.3389/fimmu.2015.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 55.Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 2012;31(5):1062–1079. doi: 10.1038/emboj.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martins I, Tesniere A, Kepp O, Michaud M, Schlemmer F, Senovilla L, et al. Chemotherapy induces ATP release from tumor cells. Cell Cycle. 2009;8(22):3723–3728. doi: 10.4161/cc.8.22.10026. [DOI] [PubMed] [Google Scholar]
- 57.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334(6062):1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 58.Jube S, Rivera ZS, Bianchi ME, Powers A, Wang E, Pagano I, et al. Cancer cell secretion of the DAMP protein HMGB1 supports progression in malignant mesothelioma. Cancer Res. 2012;72(13):3290–3301. doi: 10.1158/0008-5472.CAN-11-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zitvogel L, Kepp O, Senovilla L, Menger L, Chaput N, Kroemer G. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res. 2010;16(12):3100–3104. doi: 10.1158/1078-0432.CCR-09-2891. [DOI] [PubMed] [Google Scholar]
- 60.Haynes NM, van der Most RG, Lake RA, Smyth MJ. Immunogenic anti-cancer chemotherapy as an emerging concept. Current opinion in immunology. 2008;20(5):545–557. doi: 10.1016/j.coi.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki Y, Mimura K, Yoshimoto Y, Watanabe M, Ohkubo Y, Izawa S, et al. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res. 2012;72(16):3967–3976. doi: 10.1158/0008-5472.CAN-12-0851. [DOI] [PubMed] [Google Scholar]
- 62.Liu R, Gong J, Chen J, Li Q, Song C, Zhang J, et al. Calreticulin as a potential diagnostic biomarker for lung cancer. Cancer immunology, immunotherapy : CII. 2012;61(6):855–864. doi: 10.1007/s00262-011-1146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20(11):1301–1309. doi: 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- 64.Hartlova A, Erttmann SF, Raffi FA, Schmalz AM, Resch U, Anugula S, et al. DNA damage primes the type I interferon system via the cytosolic DNA sensor STING to promote anti-microbial innate immunity. Immunity. 2015;42(2):332–343. doi: 10.1016/j.immuni.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 65.Zelenay S, Reis e Sousa C. Adaptive immunity after cell death. Trends Immunol. 2013;34(7):329–335. doi: 10.1016/j.it.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 66.Biswas S, Guix M, Rinehart C, Dugger TC, Chytil A, Moses HL, et al. Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest. 2007;117(5):1305–1313. doi: 10.1172/JCI30740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baselga J, Albanell J. Mechanism of action of anti-HER2 monoclonal antibodies. Ann Oncol. 2001;12(Suppl 1):S35–S41. doi: 10.1093/annonc/12.suppl_1.s35. [DOI] [PubMed] [Google Scholar]
- 68.Baselga J. The EGFR as a target for anticancer therapy--focus on cetuximab. Eur J Cancer. 2001;37(Suppl 4):S16–S22. doi: 10.1016/s0959-8049(01)00233-7. [DOI] [PubMed] [Google Scholar]
- 69.Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006;94(2):259–267. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Benavides LC, Gates JD, Carmichael MG, Patil R, Holmes JP, Hueman MT, et al. The impact of HER2/neu expression level on response to the E75 vaccine: from U.S. Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Clin Cancer Res. 2009;15(8):2895–2904. doi: 10.1158/1078-0432.CCR-08-1126. [DOI] [PubMed] [Google Scholar]
- 71.Cartron G, Watier H, Golay J, Solal-Celigny P. From the bench to the bedside: ways to improve rituximab efficacy. Blood. 2004;104(9):2635–2642. doi: 10.1182/blood-2004-03-1110. [DOI] [PubMed] [Google Scholar]
- 72.Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H, et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci U S A. 2011;108(17):7142–7147. doi: 10.1073/pnas.1016569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang X, Zhang X, Mortenson ED, Radkevich-Brown O, Wang Y, Fu YX. Cetuximab-mediated tumor regression depends on innate and adaptive immune responses. Mol Ther. 2013;21(1):91–100. doi: 10.1038/mt.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Correale P, Botta C, Cusi MG, Del Vecchio MT, De Santi MM, Gori Savellini G, et al. Cetuximab +/− chemotherapy enhances dendritic cell-mediated phagocytosis of colon cancer cells and ignites a highly efficient colon cancer antigen-specific cytotoxic T-cell response in vitro. Int J Cancer. 2012;130(7):1577–1589. doi: 10.1002/ijc.26181. [DOI] [PubMed] [Google Scholar]
- 75.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6(4):443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 76.Minard-Colin V, Xiu Y, Poe JC, Horikawa M, Magro CM, Hamaguchi Y, et al. Lymphoma depletion during CD20 immunotherapy in mice is mediated by macrophage FcgammaRI, FcgammaRIII, and FcgammaRIV. Blood. 2008;112(4):1205–1213. doi: 10.1182/blood-2008-01-135160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, et al. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med. 2004;199(12):1659–1669. doi: 10.1084/jem.20040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roghanian A, Teige I, Martensson L, Cox KL, Kovacek M, Ljungars A, et al. Antagonistic human FcgammaRIIB (CD32B) antibodies have anti-tumor activity and overcome resistance to antibody therapy in vivo. Cancer Cell. 2015;27(4):473–488. doi: 10.1016/j.ccell.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 79.Lim SH, Vaughan AT, Ashton-Key M, Williams EL, Dixon SV, Chan HT, et al. Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood. 2011;118(9):2530–2540. doi: 10.1182/blood-2011-01-330357. [DOI] [PubMed] [Google Scholar]
- 80.Liu J, Wang L, Zhao F, Tseng S, Narayanan C, Shura L, et al. Pre-Clinical Development of a Humanized Anti-CD47 Antibody with Anti-Cancer Therapeutic Potential. PLoS One. 2015;10(9):e0137345. doi: 10.1371/journal.pone.0137345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DiLillo DJ, Ravetch JV. Differential Fc-Receptor Engagement Drives an Anti-tumor Vaccinal Effect. Cell. 2015;161(5):1035–1045. doi: 10.1016/j.cell.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142(5):699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138(2):286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA, et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med. 2015;21(10):1209–1215. doi: 10.1038/nm.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rafiq K, Bergtold A, Clynes R. Immune complex-mediated antigen presentation induces tumor immunity. J Clin Invest. 2002;110(1):71–79. doi: 10.1172/JCI15640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kurts C, Robinson BW, Knolle PA. Cross-priming in health and disease. Nat Rev Immunol. 2010;10(6):403–414. doi: 10.1038/nri2780. [DOI] [PubMed] [Google Scholar]
- 87.Yang X, Zhang X, Fu ML, Weichselbaum RR, Gajewski TF, Guo Y, et al. Targeting the tumor microenvironment with interferon-beta bridges innate and adaptive immune responses. Cancer Cell. 2014;25(1):37–48. doi: 10.1016/j.ccr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]