Abstract
Objective(s):
Neurotrophins (NTs) exert various effects on neuronal system. Growing evidence indicates that NTs are involved in the pathophysiology of neuropathic pain. However, the exact role of these proteins in modulating nociceptive signaling requires being defined. Thus, the aim of this study was to evaluate the effects of spinal nerve ligation (SNL) on NTs activation in the lumbar dorsal root.
Materials and Methods:
Ten male Wistar rats were randomly assigned to two groups: tight ligation of the L5 spinal nerve (SNL: n=5) and Sham (n=5). In order to produce neuropathic pain, the L5 spinal nerve was tightly ligated (SNL). Then, allodynia and hyperalgesia tests were conducted weekly. After 4 weeks, tissue samples were taken from the two groups for laboratory evaluations. Here, Real-Time PCR quantity method was used for measuring NTs gene expression levels.
Results:
SNL resulted in a significant weight loss in the soleus muscle (P<0.05), mechanical allodynia and thermal hyperalgesia thresholds (respectively, P<0.05; P<0.05). Also, NGF, NT-4, NT-3, TrkA, TrkB and TrkC expression were up-regulated following spinal nerve ligation group (respectively, P=0.025, P=0.013, P=0.001, P=0.002, P<0.001, P=001) (respectively, 4.7, 5.2, 7.5, 5.1, 7.2, 6.2 folds).
Conclusion:
The present study provides new evidence that neuropathic pain induced by spinal nerve ligation probably activates NTs and Trk receptors expression in DRG. However, further studies are needed to better elucidate the role of NTs in a neuropathic pain.
Keywords: Allodynia, Hyperalgesia, Neuropathic pain, Neurotrophins, Spinal nerve ligation
Introduction
Pain can be produced by activation of the nociceptive pathways following damage to tissues, or by damage to nerves (1). Neuropathic pain is defined by the International Association for the Study of Pain (IASP) as pain that arises as a direct consequence of a lesion or disease affecting the somatosensory system (2). Neuropathic pain is defined as having a lancinating or persistent burning property and is mostly correlated with the manifestation of dissonant sensory signs, e.g. allodynia (pain created by a stimulus which does not normally provoke pain) or hyperalgesia (a raised response to a stimulus which is normally painful) (3, 4). Pathobiology of neuropathic pain has not clearly been demonstrated so far, but one hypothesis that can explain why various pathological processes in the spinal cord are correlated with the onset of neuropathic pain is that neurotrophins (NTs) may have some kind of contribution.
The NTs nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and neurotrophin-4 (NT-4) are a subset of neurotrophic factors that are structurally and functionally related to each other (5). Mature NTs initiate their biological effects by binding to two major receptor types, neurotrophic tyrosine kinase (Trks) receptors and the pan- neurotrophin receptor at 75 KDa (p75NTR) (6). TrkA (also known as neurotrophic tyrosine kinase receptor, type 1 (NTRK1)) is the high-affinity receptor for NGF while for BDNF and NT-4, it is TrkB, and for NT-3, it is TrkC (6, 7). However, all of the above-mentioned NTs are able to activate the p75 receptor (3).
NTs regulate the growth, maintenance and apoptosis of neurons in the neurogenesis process as well as injured neurons (8–10). It was revealed that NGF, BDNF and NT-3 are synthesized mainly in the well as injured neurons (8–10). It was revealed that NGF, BDNF and NT-3 are synthesized mainly in the dorsal root ganglia (DRG) with anterograde transported into the dorsal horn of the spinal cord (11). Recently, NTs have been shown to be involved in the neuronal mechanism including neuropathic pain development and transmission (3). Apart from their roles in various physiological functions, they regulate central sensitization in the spinal cord that underpins maintenance of neuropathic pain (11). Since NTs have main roles in the wrapped mechanisms of central and peripheral sensitization, it seems that they contribute to the pathogenesis of neuropathic pain (12–14). However, the specific contribution of individual NTs signaling via a particular Trk receptor and/or the p75NTR in the pathobiology of neuropathic pain is remaining elusive (11).
The present study utilized spinal nerve ligation (SNL) as neuropathic pain and investigated whether NTs with their receptors were activated following SNL and then determined whether the activated NTs played a role in neuropathic pain. These findings increased the possibility of targeting NTs having a specific role in the treatment of neuropathic pain.
Materials and Methods
Animals
Ten male Wistar rats aged 10 weeks with weight range of 200-250 g were provided by Animal Maintenance Unit of Razi Research Center (Razi institute Animal Center, Karaj, Iran) and conveyed to Animal Laboratory of Tarbiat Modares University, Tehran, Iran. All rats were kept under a controlled environment condition with a mean temperature of 22±3 °C degrees, dark-light cycle of 12:12 hr, relative humidity of 40%, and free access to food and water ad libitum. The experimental protocols to perform this study were approved by the Ethics Committee for the Use of Animals of Tarbiat Modares University. All efforts were made to minimize the discomfort of the animals and reduce the number of experimental animals. All procedures conformed to the ethical guidelines for the care and use of laboratory animals, published by the IASP and the National Institutes of Health. After two weeks of acclimatization of the animals with the new environment, experimental protocols were initiated and the rats were randomly (simple randomization) allocated into two groups (5 rats in each group): (1) tight ligation of the L5 spinal nerve (SNL: n=5); (2) Sham: n=5. Calculated sample size by the following formula showed 3 animals in each group:
N = [(Z + Z) 2SD2] d2
Where Zα = 1.96, Zβ = 0.84, SD = 0.18 and d = 0.4. The expected power was considered at 80%.
Induction of neuropathic pain
Animals were anesthetized with pentobarbital sodium (60 mg/Kg, intraperitoneal). Then the L5 spinal nerve was tightly ligated according to the method of Kim and Chung (1992) (15). Briefly, the left Paraspinal muscles were separated at the L5-S2 levels and the left transverse process of the L6 vertebra was removed. The left L5 spinal nerve was identified and gently separated from the adjacent L4 spinal nerve. The L5 spinal nerve was tightly ligated using silk threads (6-10) and was transected just distal to ligature to ensure that all fibers were interrupted; then the wound was closed with 3-0 silk threads. Great care was taken to avoid any damages to the L4 nerve. In a control sham group, the surgical procedure was identical to that described above, except for the left L5 spinal nerve that was not ligated and transected. Only animals showing no signs of motor deficiencies were considered to be used for further experimentations. Only animals were chosen to continue the experiment with that had shown neuropathic pains in their behavioral tests. Then the rats were divided into 2 groups with 5 members in each: sham and neuropathic pain (SNL) groups.
Behavioral tests for measuring neuropathic pain
Radiation heat apparatus was used to measure hyperalgesia that the middle part of animal’s paw from Plexiglas level put to thermal constant radiations and paw withdraw threshold time (PWTs) was calculated. Heat excitations were repeated 3 times, with 5 to 10 min intervals. To measure mechanical allodynia, the animal was located on the wired network and inside the Plexiglas capsule with 20×20 dimensions and 30 cm height. After acclimatization with the new environment, Von Fery fibers with a weight range of 2 to 60 g (92-4-6-8-15-26-60) manufactured by Stolting Inc. were used. The experiment started with the lightest fiber and in the non-response cases heavier fibers were employed gradually. For excitation initiation, each fiber 3 times consecutively, with 5 sec intervals, and for one second was inserted into animal’s paw. If the response was positive (animal raising its foot) in 2 consecutive performances, that weight of the fiber was selected as the response threshold. If the animal had no response to any of the fibers, number 60 was considered as the response threshold (16).
L5 DRG and soleus removing
After 4 weeks, rats were anesthetized to take samples after injecting intraperitoneal ketamine (90 ml per kg) and xylazine (10 ml per kg), and L5 dorsal root ganglion (DRG) and soleus muscle were removed, quickly frozen in liquid nitrogen and stored at −80 °C.
Soleus muscles from the left leg were dissected from the bone quantitatively, immediately weighed, and frozen at 20 °C. At a later date, these muscles were lyophilized and weighed for dry weight. After excision of muscles the left tibia was removed and freed from connective tissue, and the maximal length was measured. Yin et al (17) showed that expressing muscle mass per unit of tibia length is a valid way to normalize mass when body weight differs between experimental groups.
RNA extraction and cDNA synthesis
RNA extraction was done using QIAzol® Lysis Reagent (Germany, Qiagen) and chloroform (Germany, Qiagen) and in accordance with its manufacturers’ instructions. Therefore, about 50 mg of the L5 DRG was homogenized separately in 1 to 10 portions in QIAzol® Lysis Reagent for total RNA extraction and for removing protein components. The final product was centrifuged at 12000×g for 10 min at 4 °C. Then mixed with chloroform in 1 to 5 portions and shaken severely for 15 sec. Then the supernatant was at 12000×g for 10 min at 4 °C and its mineral part and water were removed. Finally, its RNA containing portion was removed and mixed with isopropanol in 1 to 5 portions. It was left for 10 min at room temperature and then centrifuged at 4 °C for 10 min with 12000 g revolution. RNA containing pellet was washed and resolved in 20 microliters RNsa-free water. RNA concentration was measured by UV spectrophotometry method (Eppendorf, Germany), and 260 to 280 portions in 1.8 -2 were determined as the desired purification. cDNA synthesis was done by using Quanti Tect Reverse Transcription Kit (Qiagen, Germany) in accordance with the manufacturer’s manual.
Real-Time PCR
Real-Time PCR quantity method was used by Premix SYBR Green II (Qiagen, Germany) for measuring NT4, NT3, NGF, TrkA, TrkB, and TrkC mRNA expression levels (Applied Biosystems Step One, America). The reaction mixture was done in final volume in 20 microliters (includes 1 microliter of cDNA, 1 microliter of forward primer, 1 microliter of reverse primer, 7 microliters of DEPC water and 10 microliters of Syber Green) and each reaction in a duplicate form. Designing primers was done according to NT4, NT3, NGF, TrkA, TrkB, TrkC, and GAPDH genes in the NCBI gene bank and by a German company (Qiagen). Furthermore, GAPDH was used as the reference gene. The thermal program used in Real Time-PCR included: 95 °C for 10 min, 95 °C for 15 sec, and 60 °C for 1 min (40 cycle repetitions). Melt curve and standard curve were drawn and considered for evaluating data authenticity and optimization experiment conditions respectively and NT4, NT3, NGF, TrkA, TrkB and TrkC expression data were normalized using GAPDH (reference gene). Fold change of genes was measured by the R=2-ctΔΔ formula (18).
Statistical analysis
All statistical analyses were done by using SPSS software (version 19, SPSS Inc., Chicago, IL, USA). The normal assumption was examined using one-sample Kolmogorov-Smirnov test. For comparison of variables, one sample t-test and One-way repeated measures ANOVA were used. Significant level was determined at α=0.05.
Results
Neuropathic pain behavior
All rats that received L5 SNL developed mechanical and heat hypersensitivity on the ipsilateral hind paw. As shown in Figures 1 and 2, PWT as well as PWL in the SNL group were lower postoperatively on first, 2d, 3d and 4th weeks (P< 0.05 versus sham group), indicating that mechanical allodynia and thermal hyperalgesia had been induced by the SNL operation.
Figure 1.
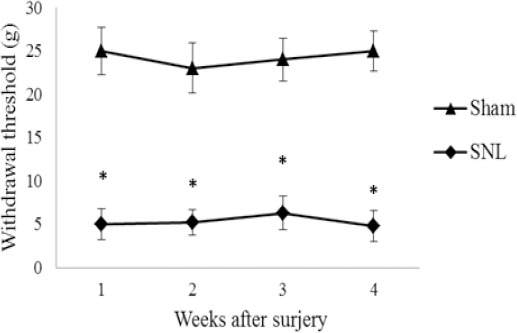
Mechanical withdrawal threshold of the left hind paws in SNL and sham groups. Data are shown as mean±SD
Sham: non-operated animals group. SNL: spinal nerve ligation group
(*): Significant difference with the sham group (P≤0.05)
PWT decreased in the SNL rats postoperatively on the 1st, 2nd, 3rd, and 4th weeks (P<0.05 versus sham group)
Figure 2.
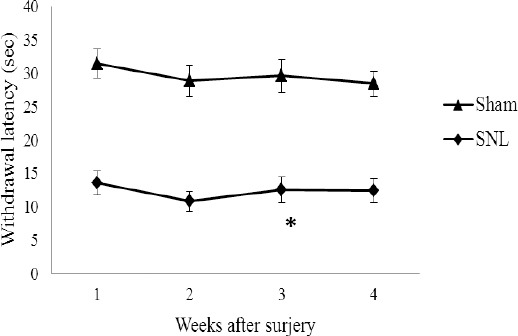
Thermal withdrawal latency of the left hind paws in SNL and sham groups. Data are shown as mean±SD
Sham: non-operated animals group. SNL: spinal nerve ligation group
(*): Significant difference with the sham group (P≤0.05)
PWT decreased in the SNL rats postoperatively on the 1st, 2nd, 3rd, and 4th weeks (P<0.05 versus sham group). Data are shown as the mean±SD
Soleus atrophy and up-regulation of NT4, NT3, NGF, TrkA, TrkB, and TrkC in the DRG at the mRNA level
Soleus weight (gram of muscle/tibial length, cm) decreased in SNL rats and this result indicates muscular atrophy as a consequence of tight ligation of the L5 spinal nerve (P≤0.05) (Figure 3). To elucidate the possible regulation of NT4, NT3, NGF, TrkA, TrkB, and TrkC at mRNA level in rat models of neuropathic pain, we examined NGF, NT4, NT3, TrkA, TrkB, and TrkC at mRNA level in DRG after 4 weeks of tight ligation of L5 spinal nerves. Statistical comparison between the SNL and the sham-operated animals showed that NGF, NT-4, NT-3, TrkA, TrkB, and TrkC expressions in the SNL group were significantly higher than that in the sham group (P=0.025, P=0.013, P=0.001, P=0.002, P<0.001, P=001, respectively) (4.7, 5.2, 7.5, 5.1, 7.2, 6.2, respectively) (Figure 4).
Figure 3.
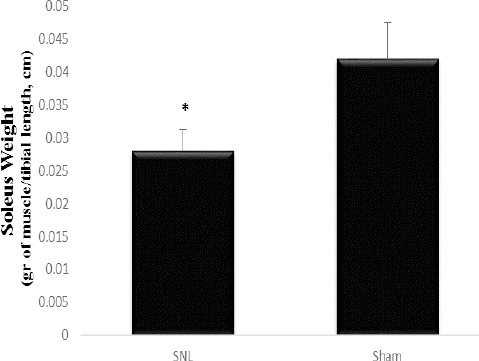
Weight of soleus muscle in SNL and sham groups
Sham: non-operated animals group. SNL: spinal nerve ligation group
Muscle mass was reduced in SNL rats. Data are shown as mean±SD * which indicates significant differences with other groups (P<0.05)
Figure 4.
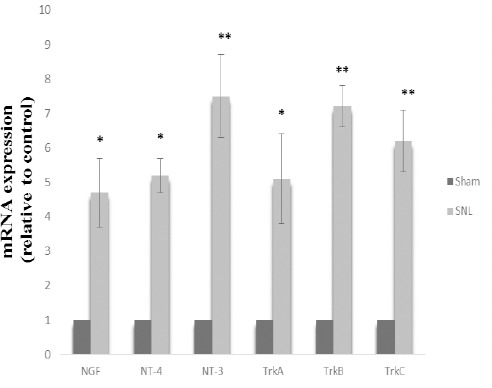
Neurotrophin mRNA expression in sham and SNL groups. Data are shown as mean±SD. Data analysis was done by one sample t-test
Sham: non-operated animals group. SNL: spinal nerve ligation group
(*): Significant difference with the sham group (P≤0.05)
(**): Significant difference with the sham group (P≤0.001)
Discussion
Peripheral nerve injuries often result in development of neuropathic pain symptoms, including spontaneous pain sensation, hyperalgesia and allodynia. Neuropathic pain symptoms are also related to axonal regeneration mechanisms and functional recovery. Following injury, mechanical allodynia impresses the use of the injured paw and compromises successful rehabilitation (19). However, only a small number of studies have investigated the effects of decreased level of activity as SNL on neuropathic pain and NTs expression in DRG. In this study, L5 SNL was used as a model of neuropathic pain. We observed that weight of Soleus muscle was decreased 4 weeks after SNL, which indicated ligation was operated correctively. Our results also have demonstrated mechanical and heat hypersensitivity on the ipsilateral hind paw following L5 SNL. Interestingly, there was a continuous reduction of allodynia and hyperalgesia for 4 weeks. On the other hand, SNL was shown to have had a considerable effect on NTs expression.
Our results showed that NGF and its receptor (TrkA) expression were up-regulated following L5 SNL. It has been demonstrated that NGF has key roles in the complex mechanisms that form peripheral and central sensitization, thus it could contribute to the pathogenesis of neuropathic pain (12–14). In parallel with this hypothesis, in the peripheral neuropathy model in form of chronic constriction injury (CCI), application of an antibody against the high affinity NGF receptor, TrkA, induced long-term pain palliation in the peripheral neuropathic pain created by slack ligatures looped around sciatic nerve (20). Also, the pronociceptive action of NGF was inverted by an NGF antagonist in rodent models of peripheral neuropathic pain created by peripheral nerve ligation (21–23).
The mechanism by which NGF acts on nociception and chronic pain is complex. NGF generated non-inflammatory and long-lasting thermal and mechanical hyperalgesia after its local or systemic administration in rodents and humans (24–26). Recent studies have reported that daily injection of NGF has led to more sensitization to heat and mechanical stimuli (27). Decreasing the NGF levels by blocking antibodies reduced the number of C-fibers that respond to heat, while increasing the NGF levels increased the number of heat sensitive C-fibers (28). It has been suggested that NGF can also increase transient receptor potential vanilloid 1 (TRPV1) expression levels through the Ras-MAPK pathway (29), which could facilitate the long-lasting heat hyperalgesia in the presence of NGF (27). In addition, a primary study utilizing rat DRG neurons introduced PKA as a key factor in NGF sensitization (30), but more recent studies illustrated that although protein kinase activity was involved in producing sensitization, it was PKC and PI3K that were responsible (31). Also, it has been shown that following NGF induced activation of TrkA, augmented p38 MAPK and ERK signaling in the spinal cord and lumbar and DRGs have been implicated in the pathobiology of chronic pain (32, 33).
Another mechanism for NGF is that it dynamically regulates the synthesis of multiple neurotrans mitters and neuropeptides in sensory and sympathetic neurons (34). For example, norepinephrine in sym-pathetic neurons (35), the pronociceptive neuropep-tides, calcitonin generelated peptide (CGRP), and substance P (SP) in sensory neurons in the DRG and spinal cord (36). Thus, it can be concluded that the NGF complex and its high affinity receptor, TrkA, serve as potential mediators in neuropathic pain induced by SNL.
The results also showed that NT-3 and its receptor (TrkC) expression increased in DRG following 4 weeks L5 SNL. NT-3 produces its biological effects via signaling that is initiated by TrkC connection. (37). NT-3 may also act on neurons that contain TrkA (38-39) and may have a role in the regulation of nociceptive signaling through anterograde transport of NT-3 from the lumbar DRGs into the spinal cord (40, 37). It has been reported that NT-3 could both prevent the start of thermal hyperalgesia and reverse established thermal hyperalgesia. Results also showed that NT-3 elevated TRPV1 expression one week after CCI (37). In this study, intrathecal infusion of NT-3 during 7 days both inhibited and reduced thermal hyperalgesia but not mechanical allodynia in the injured side hind paws of CCI-mice (37). Also, there was a correlation between this inhibition and a considerable decrease in up-regulated expression levels of TRPV1. As this pronociceptive mediator was induced by NGF, it can be illustrated that NT-3 is a main negative regulator of NGF and its pronociceptive activity (37).
In addition, NT-3 can affect a down-regulation in TrkA expression, NGF high-affinity binding sites, and associated nociceptive phenotype in intact sensory neurons (39, 41-42). Also, other findings revealed that in intact neurons, NT-3 causes a notable reduction in TrkA, high-affinity NGF binding sites, BDNF, SP, CGRP, and pituitary cAMP-activated peptide levels (39, 41-46). Thus, taken together, these findings are consistent with the concept that NT-3 plays an analgesic role in pain perception. In the present study, NT-3 expression was elevated more than other NTs, which may be related to the manner in which these NTs mediated neuropathic pain.
The results also indicated that NT-4 and its high- affinity receptors, TrkB, were elevated following L5 SNL. NT-4, like BDNF, is a ligand for the TrkB receptor (47). It was also revealed that NT-4 is expressed in the rat spinal cord and found in DRG (47). However, the biological effects of BDNF and NT-4 are different (48). It indicated that NT-4 plays a key role in the maintenance and survival of motor neurons, as it expressed predominantly by motor neurons in the ventral horn of the spinal cord (49-50). Up to now, the role of NT-4 in neuropathic pain physiology has remained unclear. In support of this idea, a study showed that NT-4 does not have a role in nociceptive transmission in ex vivo spinal cord preparations from mice null for NT-4 (50). Moreover, repeated administrations of an NT-4 antibody did not reverse the thermal hyperalgesia induced by sciatic nerve ligation in mice (47). These results concertedly certify the conception that NT-4-TrkB complex does not have a clarified role in the regulation of nociception. However, NT-4 expression levels were significantly reduced in the sciatic nerves of a rat model of diabetic peripheral neuropathy (51) and in the brain of experimental autoimmune encephalo-myelitis (EAE)-mice (52). Therefore, the exact role of NT-4 in modulating nociceptive signaling remains to be defined and further research is required to better explain the function of NT-4 in neuropathic pain.
In addition, NT-4 induces peripheral nerve regeneration through the adjustment of the expression of myelin basic protein, myelin-associated glycoprotein, and low-molecular-weight neurofilament protein (53). Therefore, in this study, increased NT-4 and TrkB expression may be as a result of myelin destruction caused by SNL. Moreover, NT-4 and BDNF serve as a semi-ligand peptide, so elevated TrkB expression can be attributed to the main role of BDNF in neuropathic pain regulation, as reported in several recent studies (54-55).
Conclusion
In summary, the present study provided new evidence that neuropathic pain induced by SNL could activate NTs and Trk receptors expression in DRG. However, this is effect thought to be partially attributed to NTs’ neural protection. Moreover, the results of this study provide the new interesting finding that the elevated NTs expression following SNL indicates its probable neuropathic pain mediator role or its neuroprotective role. Thus, further research is needed to better elucidate the role of NTs in neuropathic pain.
Acknowledgment
The authors would like to thank Council of Research, Neuroscience Research Center, Institute of Neuropharmacology of Medical Sciences University of Kerman, Kerman, Iran and of Vali E Asr University of Rafsanjan, Rafsanjan, Iran for providing financial support.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.Ashton JC. Neuropathic pain: An evolutionary hypothesis. Med Hypotheses. 2012;78:641–643. doi: 10.1016/j.mehy.2012.01.044. [DOI] [PubMed] [Google Scholar]
- 2.Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, et al. Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 3.Siniscalco D, Giordano C, Rossi F, Maione S, Novellis VD. Role of neurotrophins in neuropathic pain. Curr Neuropharmacol. 2011;9:523–529. doi: 10.2174/157015911798376208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg E. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- 5.Levi-Montalcini R. Tissue and nerve growth promoting factors. Biological aspects of specific growth promoting factors. Proc R Soc Med. 1965;58:357–360. [PMC free article] [PubMed] [Google Scholar]
- 6.Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11:272–280. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- 7.Klein R, Jing SQ, Nanduri V, O’Rourke E, Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991;65:189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- 8.Ceni C, Unsain N, Zeinieh MP, Barker PA. Neurotrophins in the regulation of cellular survival and death. Handb. Exp Pharmacol. 2014;220:193–221. doi: 10.1007/978-3-642-45106-5_8. [DOI] [PubMed] [Google Scholar]
- 9.Skaper SD. The neurotrophin family of neurotrophic factors: An overview. Methods Mol Biol. 2012;846:1–12. doi: 10.1007/978-1-61779-536-7_1. [DOI] [PubMed] [Google Scholar]
- 10.Davies AM. Neurotrophins giveth and they taketh away. Nat Neurosci. 2008;11:627–628. doi: 10.1038/nn0608-627. [DOI] [PubMed] [Google Scholar]
- 11.Khan N, Smith MT. Neurotrophins and neuropathic pain: role in pathobiology. Molecules. 2015;20:10657–10688. doi: 10.3390/molecules200610657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nijs J, Meeus M, Versijpt J, Moens M, Bos I, Knaepen K, et al. Brain-derived neurotrophic factor as a driving force behind neuroplasticity in neuropathic and central sensitization pain: A new therapeutic target? Expert Opin Ther Targets. 2015;19:565–576. doi: 10.1517/14728222.2014.994506. [DOI] [PubMed] [Google Scholar]
- 13.McMahon SB, Jones NG. Plasticity of pain signaling: Role of neurotrophic factors exemplified by acid-induced pain. J Neurobiol. 2004;61:72–87. doi: 10.1002/neu.20093. [DOI] [PubMed] [Google Scholar]
- 14.Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 16.Cruccu G, Anand P, Attal N, Garcia-Larrea L, Haanpää M, Jørum E, et al. EFNS guidelines on neuropathic pain assessment. Eur J Neurol. 2004;11:153–162. doi: 10.1111/j.1468-1331.2004.00791.x. [DOI] [PubMed] [Google Scholar]
- 17.Yin FC, Spurgeon HA, Rakusan K, Weisfeldt ML, Lakatta EG. Use of tibial length to quantify cardiac hypertrophy: application in the aging rat. Am J Physiol. 1982;243:H941–947. doi: 10.1152/ajpheart.1982.243.6.H941. [DOI] [PubMed] [Google Scholar]
- 18.Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29:e45–e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogelaar CF, Vrinten DH, Hoekman MF, M Brakkee JH, Burbach JP, Hamers FPT. Sciatic nerve regeneration in mice and rats: recovery of sensory innervation is followed by a slowly retreating neuropathic pain-like syndrome. Brain Res. 2004;1027:67–72. doi: 10.1016/j.brainres.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 20.Ugolini G, Marinelli S, Covaceuszach S, Cattaneo A, Pavone F. The function neutralizing anti-TrkA antibody MNAC13 reduces inflammatory and neuropathic pain. Proc Natl Acad Sci USA. 2007;104:2985–2990. doi: 10.1073/pnas.0611253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owolabi JB, Rizkalla G, Tehim A, Ross GM, Riopelle RJ, Kamboj R, et al. Characterization of antiallodynic actions of ALE-0540, a novel nerve growth factor receptor antagonist, in the rat. J Pharmacol Exp Ther. 1999;289:1271–1276. [PubMed] [Google Scholar]
- 22.Ro LS, Chen ST, Tang LM, Jacobs JM. Effect of NGF and anti-NGF on neuropathic pain in rats following chronic constriction injury of the sciatic nerve. Pain. 1999;79:265–274. doi: 10.1016/s0304-3959(98)00164-x. [DOI] [PubMed] [Google Scholar]
- 23.Wild KD, Bian D, Zhu D, Davis J, Bannon AW, Zhang TJ, et al. Antibodies to nerve growth factor reverse established tactile allodynia in rodent models of neuropathic pain without tolerance. J Pharmacol Exp Ther. 2007;322:282–287. doi: 10.1124/jpet.106.116236. [DOI] [PubMed] [Google Scholar]
- 24.Lewin GR, Ritter AM, Mendell LM. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J Neurosci. 1993;13:2136–2148. doi: 10.1523/JNEUROSCI.13-05-02136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rukwied R, Mayer A, Kluschina O, Obreja O, Schley M, Schmelz M. NGF induces non-inflammatory localized and lasting mechanical and thermal hypersensitivity in human skin. Pain. 2010;148:407–413. doi: 10.1016/j.pain.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 26.Thompson SW, Dray A, McCarson KE, Krause JE, Urban L. Nerve growth factor induces mechanical allodynia associated with novel a fibre-evoked spinal reflex activity and enhanced neurokinin-1 receptor activation in the rat. Pain. 1995;62:219–231. doi: 10.1016/0304-3959(94)00271-F. [DOI] [PubMed] [Google Scholar]
- 27.Lewin GR, Lechner SG, Smith EJ. Nerve growth factor and nociception: from experimental embryology to new analgesic therapy. Handb Exp Pharmacol. 2014;220:251–282. doi: 10.1007/978-3-642-45106-5_10. [DOI] [PubMed] [Google Scholar]
- 28.Lewin GR, Mendell LM. Regulation of cutaneous C-fiber heat nociceptors by nerve growth factor in the developing rat. J Neurophysiol. 1994;71:941–949. doi: 10.1152/jn.1994.71.3.941. [DOI] [PubMed] [Google Scholar]
- 29.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2008;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 30.Shu X, Mendell LM. Nerve growth factor acutely sensitizes the response of adult rat sensory neurons to capsaicin. Neurosci Lett. 1999;274:159–162. doi: 10.1016/s0304-3940(99)00701-6. [DOI] [PubMed] [Google Scholar]
- 31.Bonnington JK, McNaughton PA. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J Physiol. 2003;551:433–446. doi: 10.1113/jphysiol.2003.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pezet S, Malcangio M, McMahon SB. BDNF: A neuromodulator in nociceptive pathways? Brain Res Rev. 2002;40:240–249. doi: 10.1016/s0165-0173(02)00206-0. [DOI] [PubMed] [Google Scholar]
- 33.Zhuang ZY, Gerner P, Woolf CJ, Ji RR. Erk is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 34.Aloe L, Rocco M, Bianchi P, Manni L. Nerve growth factor: From the early discoveries to the potential clinical use. J Transl Med. 2012;10:239. doi: 10.1186/1479-5876-10-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otten U, Schwab M, Gagnon C, Thoenen H. Selective induction of tyrosine hydroxylase and dopamine beta-hydroxylase by nerve growth factor: Comparison between adrenal medulla and sympathetic ganglia of adult and newborn rats. Brain Res. 1977;133:291–303. doi: 10.1016/0006-8993(77)90765-x. [DOI] [PubMed] [Google Scholar]
- 36.Shadiack AM, Sun Y, Zigmond RE. Nerve growth factor antiserum induces axotomy-like changes in neuropeptide expression in intact sympathetic and sensory neurons. J Neurosci. 2001;21:363–371. doi: 10.1523/JNEUROSCI.21-02-00363.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson-Gerwing TD, Dmyterko MV, Zochodne DW, Johnston JM, Verge VM. Neurotrophin-3 suppresses thermal hyperalgesia associated with neuropathic pain and attenuates transient receptor potential vanilloid receptor-1 expression in adult sensory neurons. J Neurosci. 2005;25:758–767. doi: 10.1523/JNEUROSCI.3909-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ivanisevic L, Zheng W, Woo SB, Neet KE, Saragovi HU. TrkA receptor “hot spots” for binding of NT-3 as a heterologous ligand. J Biol Chem. 2007;282:16754–16763. doi: 10.1074/jbc.M701996200. [DOI] [PubMed] [Google Scholar]
- 39.Gratto KA, Verge VM. Neurotrophin-3 down-regulates TrkA mRNA, NGF high-affinity binding sites, and associated phenotype in adult DRG neurons. Eur J Neurosci. 2003;18:1535–1548. doi: 10.1046/j.1460-9568.2003.02881.x. [DOI] [PubMed] [Google Scholar]
- 40.Wang TH, Meng QS, Qi JG, Zhang WM, Chen J, Wu LF. NT-3 expression in spared DRG and the associated spinal laminae as well as its anterograde transport in sensory neurons following removal of adjacent DRG in cats. Neurochem Res. 2008;33:1–7. doi: 10.1007/s11064-007-9398-6. [DOI] [PubMed] [Google Scholar]
- 41.Jongsma WH, Danielsen N, Johnston JM, Gratto KA, Karchewski LA, Verge VM. Exogenous NT-3 and NGF differentially modulate PACAP expression in adult sensory neurons, suggesting distinct roles in injury and inflammation. Eur J Neurosci. 2001;14:267–282. doi: 10.1046/j.0953-816x.2001.01641.x. [DOI] [PubMed] [Google Scholar]
- 42.Karchewski LA, Gratto KA, Wetmore C, Verge VM. Dynamic patterns of BDNF expression in injured sensory neurons: differential modulation byNGFand NT-3. Eur J Neurosci. 2002;16:1449–1462. doi: 10.1046/j.1460-9568.2002.02205.x. [DOI] [PubMed] [Google Scholar]
- 43.Verge VM, Riopelle RJ, Richardson PM. Nerve growth factor receptors on normal and injured sensory neurons. J Neurosci. 1990;9:914–922. doi: 10.1523/JNEUROSCI.09-03-00914.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verge VM, Richardson PM, Benoit R, Riopelle RJ. Histochemical characterization of sensory neurons with high-affinity receptors for nerve growth factor. J Neurocytol. 1989;18:583–591. doi: 10.1007/BF01187079. [DOI] [PubMed] [Google Scholar]
- 45.Verge VM, Zhang X, Xu XJ, Wiesenfeld-Hallin Z, Hokfelt T. Marked increase in nitric oxide synthase mRNA in rat dorsal root ganglia after peripheral axotomy: in situ hybridization and functional studies. Proc Natl Acad Sci USA. 1992;89:11617–11621. doi: 10.1073/pnas.89.23.11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verge VM, Richardson PM, Wiesenfeld-Hallin Z, Hokfelt T. Differential influence of nerve growth factor on neuropeptide expression in vivo: a novel role in peptide suppression in adult sensory neurons. J Neurosci. 1995;15:2081–2096. doi: 10.1523/JNEUROSCI.15-03-02081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yajima Y, Narita M, Narita M, Matsumoto N, Suzuki T. Involvement of a spinal brain-derived neurotrophic factor/full-length TrkB pathway in the development of nerve injury-induced thermal hyperalgesia in mice. Brain Res. 2002;958:338–346. doi: 10.1016/s0006-8993(02)03666-1. [DOI] [PubMed] [Google Scholar]
- 48.Hibbert AP, Morris SJ, Seidah NG, Murphy RA. Neurotrophin-4, alone or heterodimerized with brain-derived neurotrophic factor, is sorted to the constitutive secretory pathway. J Biol Chem. 2003;278:48129–48136. doi: 10.1074/jbc.M300961200. [DOI] [PubMed] [Google Scholar]
- 49.Buck CR, Seburn KL, Cope TC. Neurotrophin expression by spinal motoneurons in adult and developing rats. J Comp Neurol. 2000;416:309–318. doi: 10.1002/(sici)1096-9861(20000117)416:3<309::aid-cne3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 50.Heppenstall PA, Lewin GR. BDNF but not NT-4 is required for normal flexion reflex plasticity and function. Proc Natl Acad Sci USA. 2001;98:8107–8112. doi: 10.1073/pnas.141015098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodriguez-Pena A, Botana M, Gonzalez M, Requejo F. Expression of neurotrophins and their receptors in sciatic nerve of experimentally diabetic rats. Neurosci Lett. 1995;200:37–40. doi: 10.1016/0304-3940(95)12067-e. [DOI] [PubMed] [Google Scholar]
- 52.Aharoni R, Eilam R, Domev H, Labunskay G, Sela M, Arnon R. The immunomodulator glatiramer acetate augments the expression of neurotrophic factors in brains of experimental autoimmune encephalomyelitis mice. Proc Natl Acad Sci USA. 2005;102:19045–19050. doi: 10.1073/pnas.0509438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yin Q, Kemp GJ, Yu LG, Wagstaff SC, Frostick SP. Expression of Schwann cell-specific proteins and low-molecularweight neurofilament protein during regeneration of sciatic nerve treated with neurotrophin-4. Neuroscience. 2001;105:779–783. doi: 10.1016/s0306-4522(01)00216-0. [DOI] [PubMed] [Google Scholar]
- 54.Geng SJ, Liao FF, Dang WH, Ding X, Liu XD, Cai J, et al. Contribution of the spinal cord BDNF to the development of neuropathic pain by activation of the NR2B-containing NMDA receptors in rats with spinal nerve ligation. Exp Neurol. 2010;222:256–266. doi: 10.1016/j.expneurol.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Li L, Xian CJ, Zhong JH, Zhou XF. Upregulation of brainderived neurotrophic factor in the sensory pathway by selective motor nerve injury in adult rats. Neurotox Res. 2006;9:269–283. doi: 10.1007/BF03033317. [DOI] [PubMed] [Google Scholar]


