ABSTRACT
The use of bacteriophages as antimicrobials against pathogenic bacteria offers a promising alternative to traditional antibiotics and disinfectants. Significantly, phages may help to remove biofilms, which are notoriously resistant to commonly used eradication methods. However, the successful development of novel antibiofilm strategies must take into account that real-life biofilms usually consist of mixed-species populations. Within this context, this study aimed to explore the effectiveness of bacteriophage-based sanitation procedures for removing polymicrobial biofilms from food industry surfaces. We treated dual-species biofilms formed by the food pathogenic bacterium Staphylococcus aureus in combination with Lactobacillus plantarum, Enterococcus faecium, or Lactobacillus pentosus with the staphylococcal phage phiIPLA-RODI. Our results suggest that the impact of bacteriophage treatment on S. aureus mixed-species biofilms varies depending on the accompanying species and the infection conditions. For instance, short treatments (4 h) with a phage suspension under nutrient-limiting conditions reduced the number of S. aureus cells in 5-h biofilms by ∼1 log unit without releasing the nonsusceptible species. In contrast, longer infection periods (18 h) with no nutrient limitation increased the killing of S. aureus cells by the phage (decrease of up to 2.9 log units). However, in some cases, these conditions promoted the growth of the accompanying species. For example, the L. plantarum cell count in the treated sample was up to 2.3 log units higher than that in the untreated control. Furthermore, phage propagation inside dual-species biofilms also depended greatly on the accompanying species, with the highest rate detected in biofilms formed by S. aureus-L. pentosus. Scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM) also showed changes in the three-dimensional structures of the mixed-species biofilms after phage treatment. Altogether, the results presented here highlight the need to study the impact of phage therapy on microbial communities that reflect a more realistic setting.
IMPORTANCE Biofilms represent a major source of contamination in industrial and hospital settings. Therefore, developing efficient strategies to combat bacterial biofilms is of the utmost importance from medical and economic perspectives. Bacteriophages have shown potential as novel antibiofilm agents, but further research is still required to fully understand the interactions between phages and biofilm-embedded bacteria. The results presented in this study contribute to achieving a better understanding of such interactions in a more realistic context, considering that most biofilms in the environment consist of mixed-species populations.
KEYWORDS: Staphylococcus aureus, biofilms, phage
INTRODUCTION
Biofilms are microbial communities attached to biotic or abiotic surfaces (1), whose structure is maintained by an extracellular matrix (2). Biofilms can be composed of single or multiple species, the latter scenario being the most common in both natural and man-made environments. The presence of different microorganisms renders the structure of the biofilm very complex, which has important implications for biofilm eradication and removal. Indeed, multispecies interactions in mixed biofilms are known to affect resistance to disinfectants (3). For instance, the presence of a curli-producing Escherichia coli strain was found to protect Salmonella enterica serovar Typhimurium biofilm cells from chlorine (4). Additionally, the matrix of mixed-species biofilms often presents greater biochemical complexity compared with that of the matrix of single-species communities (5). The presence of several microorganisms may also alter the spatial structure of the biofilm, as the different species tend to distribute according to their particular requirements along the gradients of nutrients, oxygen, and metabolites (6). Finally, quorum-sensing signals from one microorganism may interfere with the signaling of other species, thereby altering their gene expression and physiology (7).
In the food industry, biofilms are an important source of food contamination (8), posing a serious risk for the health of consumers. Indeed, biofilm-related contamination of equipment has been estimated to cause 59% of foodborne disease outbreaks (9). For this reason, efforts to find new biofilm control strategies have increased considerably over the last decade. Recently developed methods include the use of antibacterial surfaces (10), biofilm detachment procedures (11), and matrix degradation techniques (12). Within this context, numerous studies have explored the potential use of bacteriophages and phage-encoded proteins as antibiofilm agents. Phages, due to their antimicrobial activity, have been proposed as natural weapons for the control of pathogenic bacteria (“phage therapy”) in clinical, veterinary, food safety, and environmental settings (13, 14). Moreover, numerous studies have already shown the ability of phages to remove biofilms formed by a variety of bacteria, including staphylococcal species (15–18). Additionally, phage-encoded proteins, such as endolysins and exopolysaccharide depolymerases, can also be used for biofilm removal (19–21). Nevertheless, most studies available to date have focused on single-species communities, while only limited work has tackled the elimination of polymicrobial biofilms with phage-based products (22–26).
The Gram-positive bacterium Staphylococcus aureus is a major cause of foodborne illness due to its ability to synthesize enterotoxins. Indeed, staphylococcal food poisoning represented 7.5% of all outbreaks reported in the European Union in 2014 (27). Some studies described a high incidence of S. aureus on food industry surfaces (28, 29), where this bacterium often forms complex communities with other pathogenic and spoilage bacteria (30). In a previous work, we described the isolation and characterization of the myophage phiIPLA-RODI. Notably, this lytic phage has a wide host range among staphylococcal species, including S. aureus and S. epidermidis. Thus, exposure of 24-h biofilms to phage phiIPLA-RODI resulted in a 2-log decrease in the number of S. aureus cells in single-species biofilms and 4 log units in S. aureus-S. epidermidis dual-species biofilms (15).
The aim of this study was to assess the efficacy of bacteriophage phiIPLA-RODI against dual-species biofilms formed by S. aureus with strains of Lactobacillus plantarum, Enterococcus faecium, and Lactobacillus pentosus. All these accompanying bacteria have been isolated from food processing surfaces and are not sensitive to this phage. In this framework, we investigated the impact of different phage concentrations and incubation conditions on the behavior of S. aureus and the accompanying species. Additionally, we analyzed the ability of phiIPLA-RODI to propagate inside the dual-species biofilms. These data may help in the design of improved phage-based disinfection products for the food industry.
RESULTS
S. aureus forms biofilms with strains of L. plantarum, L. pentosus, and E. faecium.
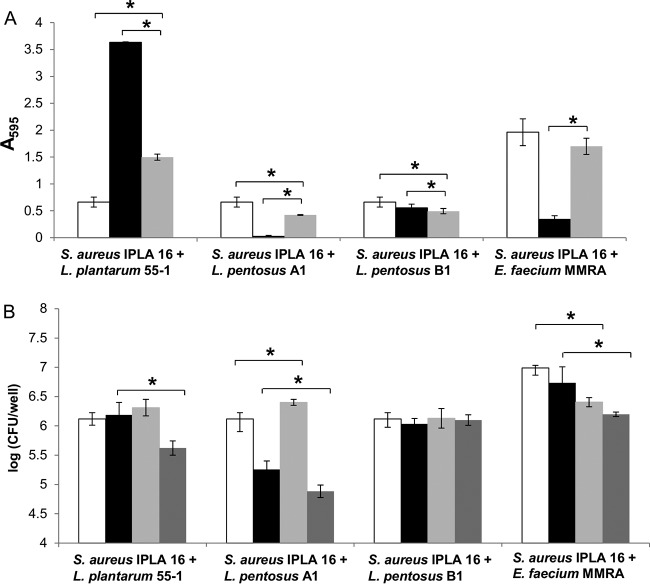
Before performing the phage-infection experiments, we examined the ability of S. aureus IPLA16 to establish biofilms in the presence of other microorganisms. To do that, four strains belonging to three different species (L. plantarum 55-1, L. pentosus A1, L. pentosus B1, and E. faecium MMRA) were selected based on previous data demonstrating their presence in biofilm samples from food industry surfaces (16). These strains were grown in mono- and dual-species biofilms and, after incubating for 5 h, the total biomass and viable cell counts were determined (Fig. 1). The data confirmed the formation of dual-species biofilms in all four cases. However, there were some differences in the behavior of S. aureus IPLA16 depending on the accompanying species. For instance, the total biomass of dual-species biofilms formed by S. aureus IPLA16 and L. plantarum 55-1 decreased 58% compared with that in L. plantarum 55-1 monospecies biofilms and increased 100% compared with that in S. aureus monospecies biofilms (Fig. 1A). In contrast, the biomass values of dual-species biofilms formed by S. aureus with L. pentosus A1 or L. pentosus B1 were slightly smaller compared with that of S. aureus IPLA16 monospecies biofilms (Fig. 1A). It must also be noted that, while L. pentosus B1 formed biofilms even in the absence of S. aureus, L. pentosus A1 showed negligible attached biomass levels in monocultures (Fig. 1A). Despite the absence of biofilm-forming capacity, L. pentosus A1 cells adhered to the well surface, as determined by viable cell counts of monospecies and dual-species biofilms involving this strain (Fig. 1B). Nonetheless, the number of L. pentosus A1 cells in dual-species biofilms was approximately 1.6 log units smaller than that of S. aureus IPLA16 cells (Fig. 1B). Finally, the biomass of dual-species biofilms formed by S. aureus IPLA16 and E. faecium MMRA was not significantly different than that of S. aureus IPLA16 monospecies biofilms (Fig. 1A). In contrast, the biomass of the E. faecium MMRA single-culture biofilm was significantly smaller than that of the mixed biofilm (Fig. 1A). Both species, S. aureus and E. faecium, showed small (<1 log unit) but significant decreases in cell counts in the mixed-species biofilm compared to those of the respective single cultures (Fig. 1B).
FIG 1.
Biofilms formed by S. aureus IPLA16 with L. plantarum 55-1, E. faecium MMRA, L. pentosus A1, and L. pentosus B1 after incubating 5 h at 32°C or 37°C. (A) Biomass of monospecies biofilms formed by S. aureus IPLA16 (white bars), accompanying species (black bars), and dual-species (gray bars). (B) Numbers of viable cells in monospecies (white bars, S. aureus IPLA16; black bars, accompanying species) and dual-species (light gray bars, S. aureus IPLA16; dark gray bars, accompanying species) biofilms. Means and standard deviations were calculated for three biological replicates. Data from dual-species biofilms were compared to those from single-species biofilms with a two-tailed Student's t test. *, P < 0.05.
Treatment of S. aureus dual-species biofilms with phage phiIPLA-RODI under nutrient-limiting conditions.
Once we characterized the dual-species biofilm models, we investigated the ability of bacteriophage phiIPLA-RODI to attack these structures. To do that, we established 5-h and 24-h dual-species biofilms formed by S. aureus IPLA16 with L. plantarum 55-1 or E. faecium MMRA and then treated them for 4 h with increasing concentrations of phage phiIPLA-RODI diluted in SM buffer (107, 108, and 109 PFU/well). These phage concentrations correspond, respectively, to multiplicity of infection (MOI) values of 10, 100, and 1,000 in 5-h biofilms and to MOIs of 1, 10, and 100 in 24-h biofilms. MOIs were calculated considering the number of cells present in the biofilms at the time of treatment.
The exposures of the mixed-species biofilms to phage concentrations of 107 and 108 PFU/well did not significantly affect bacterial cell counts or biomass (data not shown). In contrast, the treatment of 5-h biofilms with 109 PFU/well decreased the biomass of S. aureus IPLA16-L. plantarum 55-1 and S. aureus IPLA16-E. faecium MMRA biofilms by 31% and 67%, respectively (data not shown). In addition, the S. aureus IPLA16 cell counts were reduced by 0.8 and 0.7 log units, respectively (Fig. 2A). Similar results were obtained after treatments of 24-h biofilms. Thus, exposing S. aureus IPLA16-L. plantarum 55-1 and S. aureus IPLA16-E. faecium MMRA biofilms to the phage reduced the biomass by 18% and 63%, respectively (data not shown), and decreased viable S. aureus cell counts by 0.4 and 0.6 log units, respectively (Fig. 2B).
FIG 2.
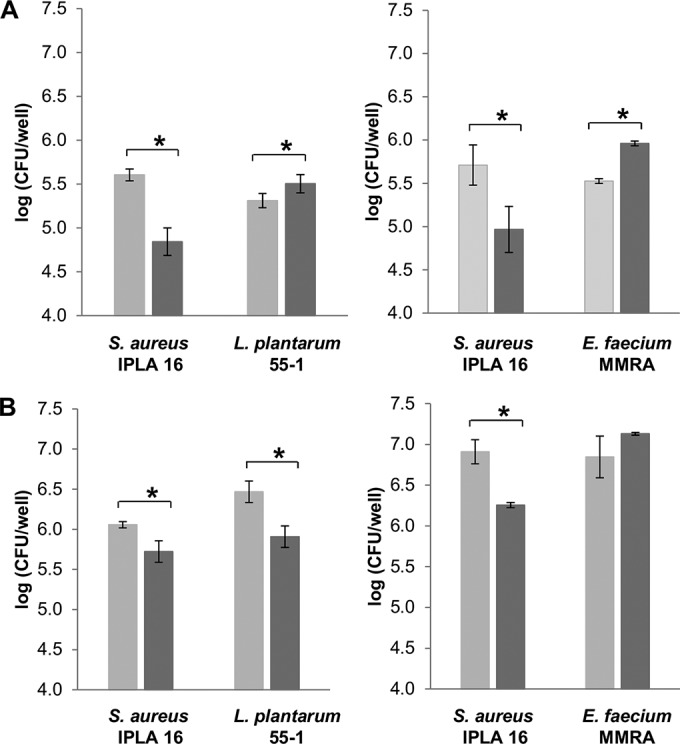
Effect of phage phiIPLA-RODI on 5 h (A) and 24 h (B) biofilms formed by S. aureus IPLA16-L. plantarum 55-1 and S. aureus IPLA16-E. faecium MMRA after 4 h of treatment with 109 PFU/well in SM buffer. Values represent the means ± standard deviations of bacterial counts performed in triplicate. Light gray and dark gray bars correspond to control and treated samples, respectively. *, P < 0.05.
Regarding the accompanying species, phage treatments of 5-h biofilms slightly but significantly increased viable cell counts of E. faecium MMRA and L. plantarum 55-1 (Fig. 2A). However, in 24-h biofilms, the L. plantarum 55-1 cell count was only slightly decreased, while the E. faecium MMRA cell count was not affected by phage treatment (Fig. 2B).
Analysis of dual-species biofilms by scanning and confocal microscopy.
The effect of phage phiIPLA-RODI on dual-species biofilms was also evaluated by microscopy (Fig. 3 and 4; see also Fig. S1 in the supplemental material). Images obtained by scanning electron microscopy of control biofilms treated with SM buffer showed well-organized cells belonging to both species attached to the coverslip surface (Fig. 3). Extracellular material was observed in all dual-species biofilms, although it was more evident in the sample corresponding to S. aureus IPLA16-L. plantarum 55-1 (Fig. 3C). The biofilm formed by S. aureus IPLA16 and L. pentosus A1 (Fig. 3E) contained fewer apparent cells of the accompanying species than did the other three dual-species biofilms (Fig. 3A, C, and G). This was consistent with the viable cell count results (Fig. 1B). Additionally, the biofilms formed by S. aureus IPLA16-L. pentosus A1 (Fig. 3E) and S. aureus IPLA16-L. pentosus B1 (Fig. 3G) were less dense than the other two (Fig. 3A and C). Similarly, the dual-species biofilms involving the two L. pentosus strains displayed the lowest biomass values in polystyrene (Fig. 1A). All four dual-species biofilms treated with phiIPLA-RODI contained lower numbers of cells than their respective control samples (Fig. 3B, D, F, and H). Also, the remaining cells appeared embedded in amorphous material, which likely resulted from S. aureus cell lysis (Fig. 3B, D, F, and H).
FIG 3.
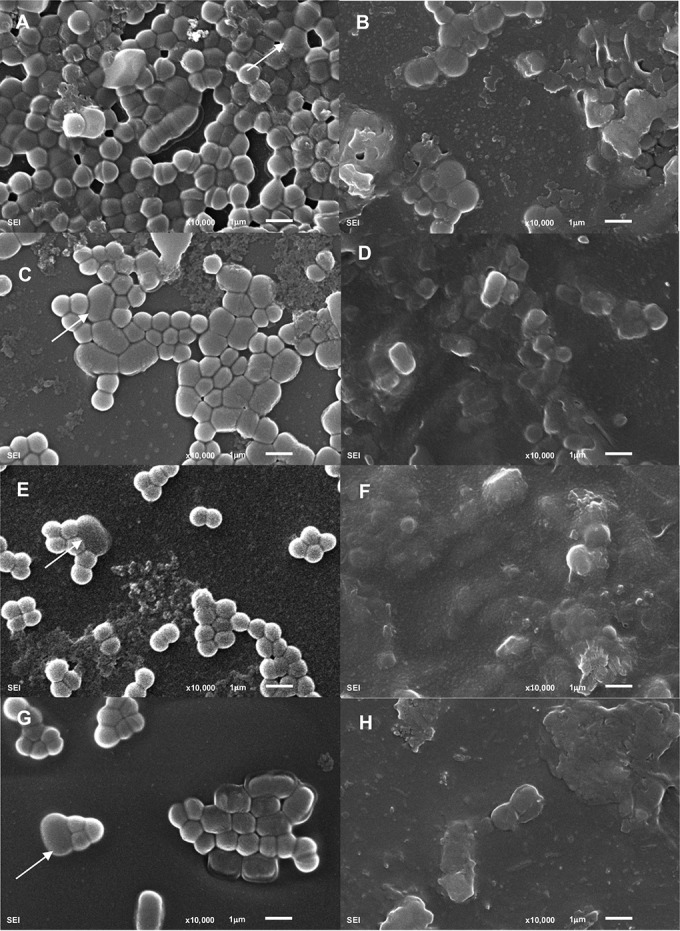
Scanning electron micrographs of S. aureus dual-species biofilms. Images correspond to 5-h biofilms formed by S. aureus IPLA16 with E. faecium MMRA (A and B), L. plantarum 55-1 (C and D), L. pentosus A1 (E and F), or L. pentosus B1 (G and H) following a 4-h treatment with SM buffer (left) or phiIPLA-RODI (right). White arrows indicate cells from the accompanying species.
FIG 4.
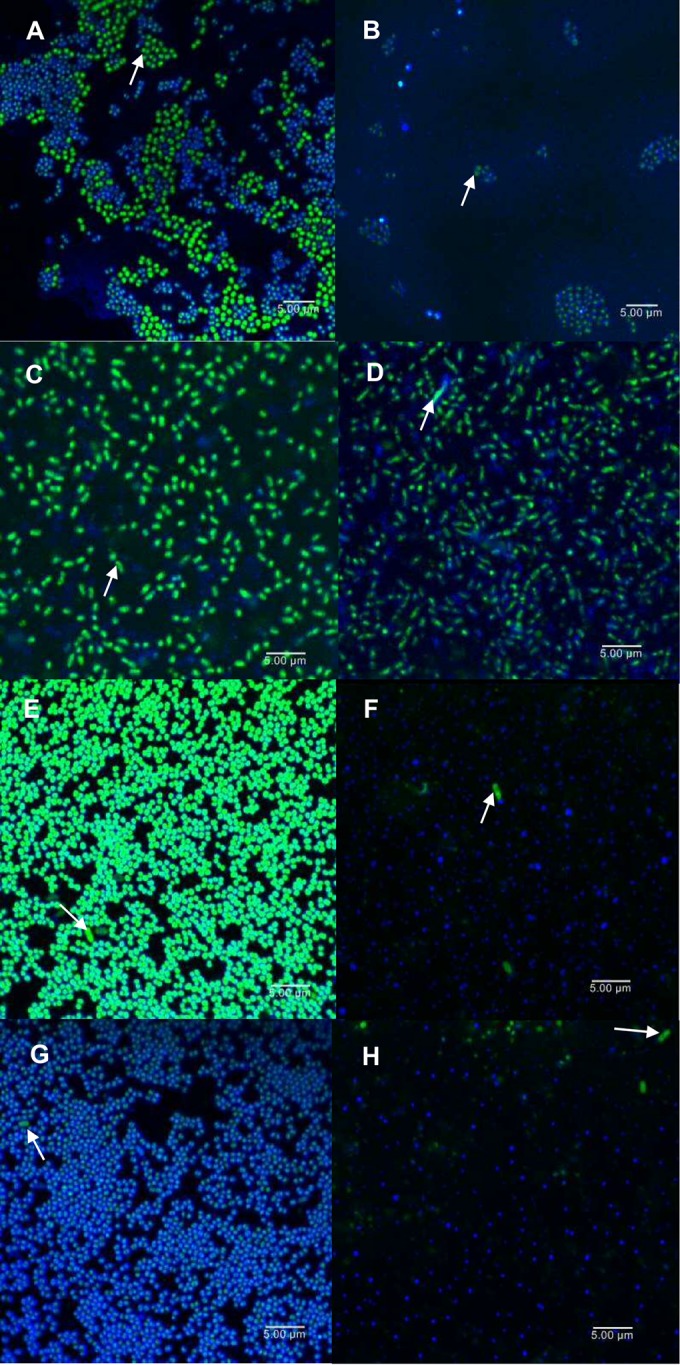
CLSM images of S. aureus IPLA16 and E. faecium MMRA (A and B), L. plantarum 55-1 (C and D), L. pentosus A1 (E and F), or L. pentosus B1 (G and H) dual-species biofilms. Images correspond to 5-h biofilms untreated (A, C, E, and G) or treated with phiIPLA-RODI for 4 h (B, D, F, and H). All samples were stained with SYTO 9 (green), which dyes cells of all species, and WGA Alexa Fluor 647 (blue), which stains the extracellular polysaccharide in the biofilm matrix. White arrows indicate cells from the accompanying species.
Similar results were observed by confocal laser scanning microscopy (CLSM). In this case, biofilms were dyed with SYTO 9, which stains cells of all species, and wheat germ agglutinin (WGA) Alexa Fluor 647 to detect the presence of the extracellular polysaccharide poly-beta-1,6-N-acetylglucosamine (PIA/PNAG). Each of the untreated biofilm samples exhibited microcolony-like groups of bacteria (Fig. 4A, C, E, and G), although these structures were organized differently depending on the accompanying species. Moreover, staining of PIA/PNAG revealed the presence of extracellular material in all mixed-species biofilms, particularly surrounding the S. aureus cells (Fig. 4A, C, E, and G). Phage treatment disaggregated the biofilm structures and decreased the amount of S. aureus intact cells (Fig. 4; see also Fig. S1). Regarding the accompanying species, the numbers of cells were not apparently changed in the cases of the two L. pentosus strains (Fig. 4F and H). In contrast, there were fewer E. faecium and more L. plantarum cells evident in the treated biofilms (Fig. 4B and D). Treatment with phage phiIPLA-RODI also altered the three-dimensional structures of the mixed biofilms (see Fig. S1). Biofilms formed by S. aureus IPLA16-E. faecium MMRA, S. aureus IPLA16-L. pentosus A1, and S. aureus IPLA16-L. pentosus B1 were flatter and less organized compared with the biofilms of the untreated samples (see Fig. S1B, F, and H). Phage treatment also reduced the thickness of S. aureus IPLA16-L. plantarum 55-1 biofilms (see Fig. S1D).
Infection of S. aureus dual-species biofilms by phiIPLA-RODI impacts the accompanying species.
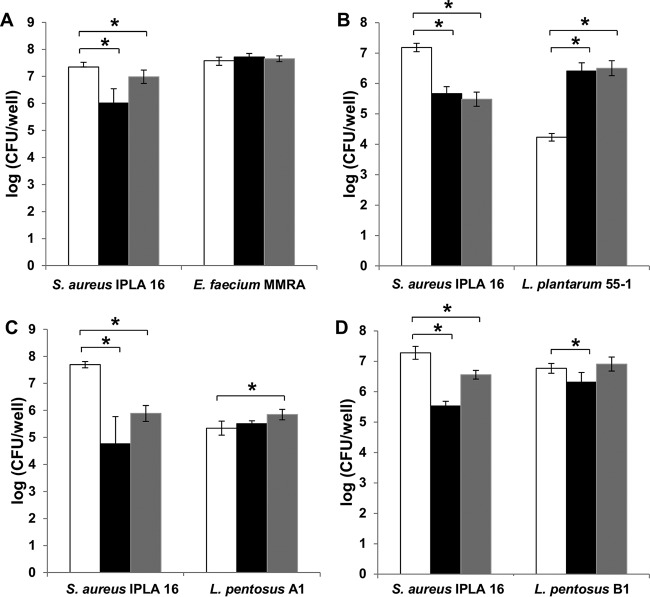
In addition to infection experiments using SM buffer as a diluting agent, we assessed the efficacy of the phage when applied in a rich growth medium. Our rationale was that efficient phage propagation, which relies on the availability of nutrients to sustain active growth of the host, may improve biofilm removal. To test this hypothesis, we infected dual-species biofilms formed by S. aureus IPLA16 and the different accompanying species with phiIPLA-RODI (109 and 106 PFU/well, corresponding to MOIs of 1000 and 1, respectively) diluted in tryptic soy broth supplemented with glucose (TSBG). We determined the total adhered biomass and viable cell counts after incubating the biofilms with the phage for 18 h at 37°C or 32°C. The biomass of S. aureus IPLA16-E. faecium MMRA biofilms treated with 109 PFU/well and 106 PFU/well decreased by 61% and 21%, respectively (see Fig. S2). Similarly, viable cell counts of S. aureus decreased by 1.3 and 0.4 log units after treatments with high and low phage concentrations, respectively. However, the number of viable E. faecium MMRA cells was not affected by phage treatment (Fig. 5A).
FIG 5.
Bacterial counts of 5-h biofilms formed by S. aureus IPLA16 with other species under proliferation conditions and treated with phage phiIPLA-RODI. Values represent the means ± standard deviations of two independent biological replicates. The accompanying species were E. faecium MMRA (A), L. plantarum (B), L. pentosus A1 (C), and L. pentosus B1 (D). White bars, untreated controls; black bars, samples treated with 109 PFU/well; gray bars, samples treated with 106 PFU/well. *, P < 0.05.
Unexpectedly, the total biomass of biofilms formed by S. aureus IPLA16 and L. plantarum 55-1 was 120% higher after treatment with 109 PFU/well compared with that of the untreated control (see Fig. S2). Regarding viable counts, treatments with each of the phage concentrations reduced the viable cell counts for S. aureus IPLA16 (about 2 log units) and increased the counts for L. plantarum 55-1 (about 2.3 log units) (Fig. 5B).
Finally, the biomass of 5-h biofilms formed by S. aureus IPLA16 and L. pentosus A1 decreased 86% with phiIPLA-RODI infection, while no significant difference was observed in S. aureus IPLA16-L. pentosus B1 biofilms (see Fig. S2). Viable cell counts of S. aureus IPLA16 in S. aureus IPLA16-L. pentosus A1 biofilms decreased 2.9 and 1.8 log units after treatment with 109 and 106 PFU/well, respectively (Fig. 5C). A lesser impact was observed in S. aureus IPLA16-L. pentosus B1 biofilms, in which cell counts were reduced 1.7 and 0.7 log units following treatment with 109 and 106 PFU/well, respectively (Fig. 5D). A slight increase in the L. pentosus A1 population (approximately 0.5 log units) was detected after treatment with 106 PFU/well (Fig. 5C). In the case of L. pentosus B1, treatment with 106 PFU/well did not change the cell number, whereas a slightly lower count was observed for the samples treated with 109 PFU/well.
Propagation of phiIPLA-RODI in S. aureus mixed biofilms depends on the accompanying species.
To maximize the efficacy of phages as antibiofilm agents, it is important to determine if the viral particles can reach the target bacteria and propagate inside the biofilms. Here, we estimated propagation by titrating the number of phages in the planktonic phase and in the biofilm, differentiating between viral particles associated with cells (infectious centers) and those present as free virions.
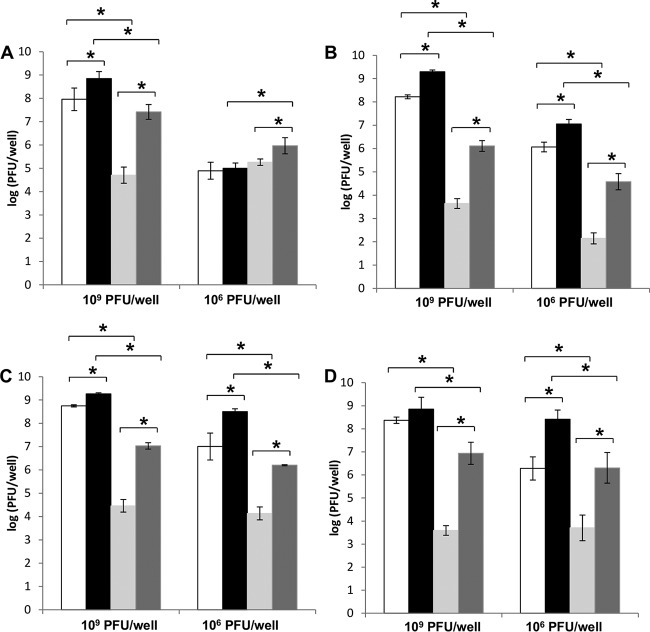
The titration of phiIPLA-RODI isolated from dual-species biofilms challenged with 109 PFU/well showed the same trends for all species combinations. Thus, the planktonic phase had the highest number of viral particles, as the number of free virions was 0.5 to 1 log unit higher than that of infective centers. Biofilms contained a smaller viral load and most phage particles were free in the extracellular matrix. Consequently, the cell-associated fraction corresponding to the biofilm had the lowest phage titer. The total phage titer per well was approximately 109 PFU/well for each of the dual-species biofilms, which is equal to that of the starting inoculum. However, there were differences in the abilities of phiIPLA-RODI to multiply inside the biofilms depending on the accompanying species. Indeed, the numbers of free and cell-associated viral particles in dual-species biofilms formed by S. aureus IPLA16 with E. faecium MMRA and L. pentosus A1 were approximately 1 log unit higher than in those formed by S. aureus IPLA16 with L. plantarum 55-1 and with L. pentosus B1 (Fig. 6 A to D).
FIG 6.
Propagation of phage phiIPLA-RODI on 5-h dual-species biofilms of S. aureus IPLA16 with other bacteria treated for 18 h with 109 PFU/well or 106 PFU/well. The accompanying species tested were E. faecium MMRA (A), L. plantarum 55-1 (B), L. pentosus A1 (C), and L. pentosus B1 (D). White bars, phages present in the planktonic phase associated with cells; black bars, phages present as free virions in the planktonic phase; light gray bars, infectious centers in the adhered (biofilm) phase; dark gray bars, free phages present in the adhered phase. These results represent the means ± standard deviations of three replicates. The following data sets were compared by statistical analyses: free phages versus cell-associated phages (biofilm), free phages versus cell-associated phages (planktonic phase), biofilm versus planktonic phase (free phages), and biofilm versus planktonic phase (cell-associated phages). *, P < 0.05.
The propagation of phiIPLA-RODI in S. aureus dual-species biofilms treated with 106 PFU/well showed different patterns depending on the accompanying strain. In the case of biofilms involving the three lactobacillus strains, the trend was fairly similar to the one described for samples treated with 109 PFU/well. Indeed, phage titers obtained in samples from the planktonic phase were higher than those from biofilms (Fig. 6 B-D). Also, both phases (planktonic and biofilm) contained higher numbers of free phage particles than cell-associated virions (Fig. 6 B-D). All three dual-species biofilms formed by S. aureus with lactobacilli and treated with 106 PFU/well showed significant increases in phage particles compared with that of the starting inoculum. Thus, the calculated P values were 0.001, 3.15 × 10−5, and 0.001 for biofilms formed by S. aureus with L. plantarum, with L. pentosus A1, and with L. pentosus B1, respectively. Interestingly, the total phage titer (biofilm and planktonic phase) corresponding to S. aureus-L. plantarum biofilms (7.1 log PFU/well) was significantly lower than those estimated for sessile communities formed by S. aureus-L. pentosus A1 and S. aureus-L. pentosus B1 (8.5 and 8.4 log PFU/well, respectively). The respective P values were 3.97 × 10−4 and 4.40 × 10−4 for comparisons of S. aureus-L. plantarum with S. aureus-L. pentosus A1 and S. aureus-L. pentosus B1 biofilms treated with 106 PFU/well. Unexpectedly, similar numbers of phages were obtained in the planktonic and biofilm phases of communities formed by S. aureus and E. faecium that were treated with 106 PFU/well. Moreover, the numbers of infective centers and free particles in the planktonic phase were similar. However, the phage titer inside the biofilm structure was slightly higher than in the planktonic phase, and there were more phage particles (about 106 log units) free in the extracellular matrix than there were associated with host cells (Fig. 6A). Interestingly, the phage titer in S. aureus-E. faecium biofilms treated with 106 PFU/well was not significantly increased compared with the starting inoculum (P value = 0.93).
DISCUSSION
Recently, a number of articles reported the potential of bacteriophages for the control of bacterial biofilms. However, most of these studies have focused on monospecies cultures, which do not reflect the majority of biofilms found in nature. This study addressed the use of phage phiIPLA-RODI to control dual-species biofilms composed of S. aureus together with other species from food-related environments (L. plantarum, L. pentosus, and E. faecium). None of the selected microorganisms was susceptible to phiIPLA-RODI infection, and importantly, all four strains formed stable dual-species biofilms with S. aureus. In most cases, the numbers of cells of S. aureus and the accompanying species were fairly similar, except for with L. pentosus A1. This might be due to the poor adhesion to polystyrene and glass displayed by strain A1, which was observed even in the absence of S. aureus. Interestingly, there were fewer cells of L. plantarum in dual-species biofilms compared with in monocultures. In this case, it appears that S. aureus might exert a negative impact on the presence of this bacterium.
In a previous study performed in our laboratory, we described that treatment with phiIPLA-RODI reduced the number S. aureus IPLA16 cells by 2.4 log units in monospecies biofilms and by 4.2 log units in dual-species biofilms formed with the phage-sensitive strain S. epidermidis LO5081 (15). Thus, the presence of a second species, also susceptible to the phage, seemed to enhance the efficacy of phage treatment. In contrast, the results of this study show lower efficacy rates for short phage treatment of mixed-species biofilms performed under nutrient-limiting conditions. Indeed, cell counts of S. aureus IPLA16 remained below 1 log unit for dual-species biofilms formed with nonsensitive bacteria, such as L. plantarum and E. faecium, regardless of the biofilm maturation stage or the phage concentration used. Therefore, it appears that the presence of cells from a nonsensitive species in the biofilm may hinder the ability of the phage to reach and lyse the target cells. It is well known that mixed-species biofilms frequently display a higher resistance to antibiotics than do monospecies biofilms (31, 32). This phenomenon may involve multiple mechanisms, including interspecies signaling, spatial distribution of physiologically different bacteria, and interference from the matrix (3). Similarly, the low efficacy of phages to infect dual-species biofilms might be due to the protection of the sensitive host by the nonsensitive species (33, 34), coaggregation of biofilm communities (35), limited penetration of phages (36, 37), or changes in the availability of phage receptors on the cell surface of the host species (38).
Despite the low efficacy from applying short phage treatments to dual-species cultures, microscopy revealed changes in the structures of treated biofilms compared with those of their respective controls. Indeed, all mixed-species biofilms displayed decreased numbers of intact S. aureus cells and disaggregated three-dimensional structures of the biofilms. However, a possibility is that the use of a different substrate for these experiments (glass instead of polystyrene) influenced the final results. Therefore, it may be more effective to apply the phage suspension to biofilms formed on glass coverslips than to those formed on polystyrene wells.
With regard to the treatment duration, previous studies reported that biofilm removal with phage-based products does not require incubation times longer than 5 h (25, 39). However, our results indicate that the control of biofilms consisting of several species might benefit from longer incubation times. Additionally, it appears that treatment is more effective under conditions that allow active growth of the host. Nonetheless, it must be considered that incubation under nutrient-rich conditions may also affect the proliferation of the accompanying species. This effect would be undesirable when the accompanying species are pathogenic or spoilage bacteria, especially if they are good biofilm producers. Conversely, there may be a synergistic effect of interspecies competition between the phage and the nonsensitive bacteria during active growth, thereby favoring elimination of the target pathogen. This may also limit phage-resistance acquisition, which generally confers a loss of competitive ability of the bacteria (30). This seemed to be the case, for example, of dual-species biofilms formed by E. coli and Pseudomonas aeruginosa (22). Here, there were differences in the behaviors of the four accompanying strains studied following antistaphylococcal phage treatments. Thus, the reduction in the number of S. aureus IPLA16 cells due to phage infection led to an increase in the number of L. plantarum cells. Also, S. aureus-L. plantarum biofilms displayed increased biomass after phage treatment. These results were not surprising given the inhibitory effect of S. aureus over L. plantarum that was observed in the preliminary experiments. As mentioned previously, the number of viable L. plantarum 55-1 cells in dual-species biofilms formed with S. aureus was significantly lower than in the L. plantarum single-species community. In contrast, partial elimination of the S. aureus population by phage treatment seems to enable the L. plantarum population to develop in a manner similar to that of the single-species biofilm. This is reflected in the higher L. plantarum cell number and increased adhered biomass. Additionally, growth of L. plantarum may be facilitated by the greater availability of nutrients resulting from phage-induced bacterial lysis or the reduced growth of S. aureus IPLA16. Nevertheless, the interactions between bacterial species in a biofilm are very complex and not always easy to explain. Although phage predation has been shown to promote biofilm formation in certain bacteria growing in monospecies biofilms (40), our results suggest that the increase in biomass in S. aureus-L. plantarum cocultures is due to increases in the accompanying species rather than inducing the surviving S. aureus cells to form a biofilm.
With regard to the other three strains studied, namely, L. pentosus A1, L. pentosus B1, and E. faecium MMRA, no major increases in cell counts were observed after treatments with phiIPLA-RODI. Nevertheless, comparing the cell counts obtained here with previous results of biofilm formation growth curves (unpublished data) indicates that the populations in treated and control samples reached the maximum cell numbers for the aforementioned strains. Interestingly, phage treatment did not release cells from the accompanying species to the planktonic phase. Similar results were described for biofilms formed by Pseudomonas fluorescens and Staphylococcus lentus under static conditions. However, under dynamic conditions, phage ΦIBB-PF7A caused the release of the nonsusceptible S. lentus cells (25).
Even though it is widely recognized that bacteriophages can replicate inside biofilms, data concerning phage propagation in multiple species communities are scarce. The phage life cycle inside the biofilm requires attachment to bacterial cells embedded in an extracellular matrix and, after production of the phage progeny, the diffusion of new phages to other areas of the biofilm to reach new susceptible cells (41). Depending on the bacterial species, the metabolic and physicochemical conditions of the biofilm (matrix density, number of cells, and metabolic state) can be quite different. Phage propagation in the four dual-species biofilms studied here was evaluated using two different phage concentrations. Not surprisingly, the phage titer in the planktonic phase was considerably higher than the number of phages inside the adhered phase for all dual-species biofilms. Moreover, our results showed that dual-species biofilms support phage multiplication, since a significant percentage of phage particles was isolated as cell-associated infectious centers. Overall, it seems that the adsorption rate of phages to host cells is favored under planktonic conditions compared to that in a biofilm environment. One explanation for this phenomenon is that the production and migration of phage particles in biofilms are limited considerably by extracellular material (42). Interestingly, phage propagation in the biofilm phase was lower in S. aureus-L. plantarum biofilms than in the dual species biofilms involving either of the two L. pentosus strains. As mentioned previously, L. plantarum forms biofilms better than the L. pentosus strains and is known to produce extracellular material. Therefore, it is possible that the greater complexity of the extracellular matrix in the S. aureus-L. plantarum biofilms hinders phage propagation in the biofilm. Nonetheless, some studies seem to indicate that phage penetration into biofilms is not completely blocked by the extracellular matrix. For instance, it has been shown that c2 phage can diffuse into biofilms through water-filled channels, although some phage particles may interact with their specific binding sites on bacteria and are immobilized there (37). However, the interaction of phages with extracellular components cannot be completely discarded (22, 43). In addition to the role played by the matrix, the lower infectivity detected in biofilms may also be due to the heterogeneity of these communities with regard to the structure and metabolic state of the host cells. Thus, phage infection may be more prevalent in metabolically active cells located on the biofilm surface than in the slow-growing cells located in the inner layers of the biofilm (1, 41). Overall, we conclude that successful propagation of phages in mixed-species biofilms depends on the accompanying nonsusceptible species. This is likely due to the differences in the composition of the extracellular matrix, which can affect the ability of the viral particles to reach susceptible cells.
In conclusion, this study provides evidence that phage infection in dual-species biofilms is a complex process likely subject to population dynamics. Hence, phage treatment may require the use of phage cocktails or phages combined with other antimicrobials to target the accompanying species. Overall, our results contribute to the understanding of interspecies interactions in the context of developing phage-based strategies for the control of mixed-species biofilms.
MATERIALS AND METHODS
Bacterial strains, bacteriophage, and growth conditions.
All of the bacterial strains used in this study are listed in Table 1. The staphylococcal strains and E. faecium MMRA were routinely grown in TSB (tryptic soy broth; Scharlau, Barcelona, Spain) at 37°C with shaking or on TSB plates containing 2% (wt/vol) bacteriological agar (TSA). L. plantarum 55-1 (kindly supplied by R. Jiménez-Díaz, IG-CSIC, Sevilla, Spain) and the exopolysaccharide producers L. pentosus A1 (EPS A and EPS B) and L. pentosus B1 (EPS B) were grown in MRS (Scharlab S.L., Spain) broth or agar at 32°C (44). For selective growth of the different species, the following media were used: Baird-Parker agar (BP) for S. aureus, Kenner Fecal (KF) agar for E. faecium, and MRS agar plates for the other three strains. Bacteriophage phiIPLA-RODI was propagated on S. aureus IPLA1 as previously described (15).
TABLE 1.
Bacterial strains used in this study
| Species | Strain | Origin | Reference |
|---|---|---|---|
| Staphylococcus aureus | IPLA1 | Dairy industry surface | 30 |
| S. aureus | IPLA16 | Meat industry surface | 30 |
| Enterococcus faecium | MMRA | Dairy product | 46 |
| Lactobacillus plantarum | 55-1 | Natural fermentation of olives | 47 |
| L. pentosus | LPS26 | Natural fermentation of olives | 44 |
| L. pentosus | A1 | Derivative from LPS26 | 44 |
| L. pentosus | B1 | Derivative from LPS26 | 44 |
Biofilm formation.
Overnight cultures of the different strains were diluted to 106 CFU/ml into fresh TSB supplemented with 0.25% glucose (TSBG). Mixtures of 100 μl aliquots from each single culture and 100 μl of TSBG or mixtures of two strains (100 μl of each strain) were poured into each well of 96-well microtiter plates (TC Microwell 96U with lid, Nunclon D SI; Thermo Scientific, Nunc, Madrid, Spain). These plates were incubated for 5 or 24 h at 37°C for S. aureus-E. faecium biofilms or at 32°C for S. aureus-L. plantarum and S. aureus-L. pentosus biofilms. After biofilm formation, the planktonic phase was removed and wells were washed twice with phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 2 mM KH2PO4, pH 7.4). The adhered cells were collected by scratching the bottom of the well with a sterile swab and then suspended in 9 ml of PBS by vigorously vortexing the sample for 1 min. Serial dilutions of these suspensions were plated on selective medium for bacterial counting. Alternatively, the total biomass adhered to the well was determined by crystal violet (0.1%, wt/vol) staining as described previously (20). Each experiment was repeated at least three times.
Infection of established biofilms with phage phiIPLA-RODI.
To perform short infection period experiments, biofilms were grown for 5 h or 24 h and washed with PBS. Then, 100 μl of SM buffer and 100 μl of phiIPLA-RODI were added to each well (107, 108, or 109 PFU/well). A volume of 200 μl of SM buffer was added to the control wells. The plates were incubated for 4 h at 32°C or 37°C depending on the accompanying species. To test the effect of long infection periods under nutrient-rich conditions, biofilms were established for 5 h, washed with TSBG, and incubated with phage diluted in TSBG (109 PFU/well or 106 PFU/well). TSBG was added to the control wells. Biofilms were treated for 18 h at 32°C or 37°C depending on the species. Afterwards, the planktonic phase was collected and the wells were washed twice with PBS. The number of cells attached to the well surface and the total adhered biomass were determined as described above.
The titrations of “free” and “cell-associated” phages (or infective centers) in the planktonic phase and the biofilm were performed as follows. First, the planktonic phase was taken directly from the wells and adhered cells were collected, after washing, by scratching the wells as indicated above. Then, all samples were centrifuged at 16,100 × g for 5 min to separate the supernatant containing the “free” viral particles from the pellets containing the “cell-associated” phages. Both supernatants and pellets were stored at 4°C until further processing. To determine the titer of “free” phages, the supernatants were filtered (0.45 μm; VWR, Spain), diluted in SM buffer, and plated on the reference strain S. aureus IPLA1 by the double-layer technique (45). Prior to titrating the infective centers, the pellets from planktonic and biofilm samples were resuspended in PBS. Then, 50 μl of chloroform was added to each sample to lyse the bacterial cells and release the viral particles. After vortexing, the cell debris was separated further by centrifugation. The resulting supernatants were then titrated by the double-layer technique.
Microscopy techniques.
For microscopy analyses, biofilms were formed on borosilicate glass coverslips (18-mm diameter; VWR International, UK) previously placed into the wells of a 12-well microtiter plate (Thermo Scientific, Nunc, Madrid, Spain). To prepare the inoculum for each biofilms, overnight cultures of the different strains were diluted to 106 CFU/ml into fresh TSB supplemented with 0.25% glucose (TSBG). Mixtures of 1 ml aliquots from each single culture and 1 ml of TSBG or mixtures of two strains (1 ml of each strain) were poured into each coverslip-containing well. These plates were incubated for 5 h at 37°C for S. aureus-E. faecium biofilms or at 32°C for S. aureus-L. plantarum and S. aureus-L. pentosus biofilms. The planktonic phase was then removed and the established biofilms were treated with phage phiIPLA-RODI (109 PFU/ml) or SM buffer for 4 h. After treatment, the coverslips were washed twice with PBS and dried for 20 h at 25°C.
For scanning electron microscopy (SEM), the samples were subsequently covered in gold (gold sputter coating) and examined using a scanning electron microscope (JSM-6610LV; JEOL Ltd., Tokyo, Japan).
For confocal laser scanning microscopy (CLSM), samples were washed twice with PBS and stained with SYTO 9 from the Live/Dead BacLight kit (Invitrogen AG, Basel, Switzerland) and wheat germ agglutinin (WGA) Alexa Fluor 647 conjugate (Life Technologies, Oregon, USA) following the manufacturer's instructions. SYTO 9 and the WGA Alexa Fluor 647 conjugate stain bacterial cells of all species and the exopolysaccharide PIA/PNAG, respectively. Images were acquired with a confocal scanning laser microscope (DMi8; Leica Microsystems) using a 100× oil objective.
Statistical analysis.
All experiments were performed with at least three biological replicates and the means ± standard deviations were obtained and further analyzed to establish significant differences. Statistical analyses were performed by a two-tailed Student′s t test and significance was considered at a P of <0.05.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the Ministry of Science and Innovation, Spain (AGL2012-40194-C02-01), the Program of Science, Technology and Innovation 2013–2017 (AGL2015-65673-R), and the FEDER EU funds, Principado de Asturias, Spain (GRUPIN14-139). L.F. was awarded a Marie Curie Clarin-Cofund grant.
P.G., B.M., and A.R. are members of the FWO Vlaanderen-funded “Phagebiotics” research community (WO.016.14) and the bacteriophage network FAGOMA. We thank S. Simón Vinagre and R. Calvo for their collaboration. We acknowledge A. Rehaiem and R. Jiménez for the E. faecium and Lactobacillus strains, respectively. We thank D. M. Donovan (Animal and Natural Resources Institute, BARC, ARS, U.S. Department of Agriculture, Beltsville, Maryland, USA) for English-language revisions and helpful suggestions for improving the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02821-16.
REFERENCES
- 1.Stoodley P, Sauer K, Davies DG, Costerton JW. 2002. Biofilms as complex differentiated communities. Annu Rev Microbiol 56:187–209. doi: 10.1146/annurev.micro.56.012302.160705. [DOI] [PubMed] [Google Scholar]
- 2.Flemming HC, Wingender J. 2010. The biofilm matrix. Nat Rev Microbiol 8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Vizuete P, Orgaz B, Aymerich S, Le Coq D, Briandet R. 2015. Pathogens protection against the action of disinfectants in multispecies biofilms. Front Microbiol 6:705. doi: 10.3389/fmicb.2015.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang R, Kalchayanand N, Schmidt JW, Harhay DM. 2013. Mixed biofilm formation by Shiga toxin-producing Escherichia coli and Salmonella enterica serovar Typhimurium enhanced bacterial resistance to sanitization due to extracellular polymeric substances. J Food Prot 76:1513–1522. doi: 10.4315/0362-028X.JFP-13-077. [DOI] [PubMed] [Google Scholar]
- 5.Andersson S, Dalhammar G, Kuttuva Rajarao G. 2011. Influence of microbial interactions and EPS/polysaccharide composition on nutrient removal activity in biofilms formed by strains found in wastewater treatment systems. Microbiol Res 166:449–457. doi: 10.1016/j.micres.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Stewart PS, Franklin MJ. 2008. Physiological heterogeneity in biofilms. Nat Rev Microbiol 6:199–210. doi: 10.1038/nrmicro1838. [DOI] [PubMed] [Google Scholar]
- 7.Zhang LH, Dong YH. 2004. Quorum sensing and signal interference: diverse implications. Mol Microbiol 53:1563–1571. doi: 10.1111/j.1365-2958.2004.04234.x. [DOI] [PubMed] [Google Scholar]
- 8.DeVita MD, Whadhera RK, Theis ML, Ingham SC. 2007. Assessing the potential of Streptococcus pyogenes and Staphylococcus aureus transfer to foods and customers via a survey of hands, hand-contact surfaces and food-contact surfaces at foodservice facilities. J Foodservice 18:76–79. doi: 10.1111/j.1745-4506.2007.00049.x. [DOI] [Google Scholar]
- 9.Midelet G, Carpentier B. 2004. Impact of cleaning and disinfection agents on biofilm structure and on microbial transfer to a solid model food. J Appl Microbiol 97:262–270. doi: 10.1111/j.1365-2672.2004.02296.x. [DOI] [PubMed] [Google Scholar]
- 10.Pang LQ, Zhong LJ, Zhou HF, Wu XE, Chen XD. 2015. Grafting of ionic liquids on stainless steel surface for antibacterial application. Colloids Surf B Biointerfaces 126:162–168. doi: 10.1016/j.colsurfb.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 11.Cerca N, Gomes F, Bento JC, Franca A, Rolo J, Miragaia M, Teixeira P, Oliveira R. 2013. Farnesol induces cell detachment from established S. epidermidis biofilms. J Antibiot (Tokyo) 66:255–258. doi: 10.1038/ja.2013.11. [DOI] [PubMed] [Google Scholar]
- 12.Ramasubbu N, Thomas LM, Ragunath C, Kaplan JB. 2005. Structural analysis of dispersin B, a biofilm-releasing glycoside hydrolase from the periodontopathogen Actinobacillus actinomycetemcomitans. J Mol Biol 349:475–486. doi: 10.1016/j.jmb.2005.03.082. [DOI] [PubMed] [Google Scholar]
- 13.O'Flaherty S, Ross RP, Coffey A. 2009. Bacteriophage and their lysins for elimination of infectious bacteria. FEMS Microbiol Rev 33:801–819. doi: 10.1111/j.1574-6976.2009.00176.x. [DOI] [PubMed] [Google Scholar]
- 14.García P, Rodríguez L, Rodríguez A, Martínez B. 2010. Food biopreservation: promising strategies using bacteriocins, bacteriophages and endolysins. Trends Food Sci Technol 21:373–382. doi: 10.1016/j.tifs.2010.04.010. [DOI] [Google Scholar]
- 15.Gutiérrez D, Vandenheuvel D, Martínez B, Rodríguez A, Lavigne R, García P. 2015. Two phages, phiIPLA-RODI and phiIPLA-C1C, lyse mono- and dual-species staphylococcal biofilms. Appl Environ Microbiol 81:3336–3348. doi: 10.1128/AEM.03560-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutiérrez D, Martínez B, Rodríguez A, García P. 2012. Genomic characterization of two Staphylococcus epidermidis bacteriophages with anti-biofilm potential. BMC Genomics 13:228. doi: 10.1186/1471-2164-13-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly D, McAuliffe O, Ross RP, Coffey A. 2012. Prevention of Staphylococcus aureus biofilm formation and reduction in established biofilm density using a combination of phage K and modified derivatives. Lett Appl Microbiol 54:286–291. doi: 10.1111/j.1472-765X.2012.03205.x. [DOI] [PubMed] [Google Scholar]
- 18.Cerca N, Oliveira R, Azeredo J. 2007. Susceptibility of Staphylococcus epidermidis planktonic cells and biofilms to the lytic action of Staphylococcus bacteriophage K. Lett Appl Microbiol 45:313–317. doi: 10.1111/j.1472-765X.2007.02190.x. [DOI] [PubMed] [Google Scholar]
- 19.Cornelissen A, Ceyssens PJ, T'Syen J, Van Praet H, Noben JP, Shaburova OV, Krylov VN, Volckaert G, Lavigne R. 2011. The T7-related Pseudomonas putida phage phi15 displays virion-associated biofilm degradation properties. PLoS One 6:e18597. doi: 10.1371/journal.pone.0018597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutiérrez D, Ruas-Madiedo P, Martínez B, Rodríguez A, García P. 2014. Effective removal of staphylococcal biofilms by the endolysin LysH5. PLoS One 9:e107307. doi: 10.1371/journal.pone.0107307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutiérrez D, Briers Y, Rodríguez-Rubio L, Martínez B, Rodríguez A, Lavigne R, García P. 2015. Role of the pre-neck appendage protein (Dpo7) from phage vB_SepiS-phiIPLA7 as an anti-biofilm agent in staphylococcal species. Front Microbiol 6:1315. doi: 10.3389/fmicb.2015.01315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kay MK, Erwin TC, McLean RJ, Aron GM. 2011. Bacteriophage ecology in Escherichia coli and Pseudomonas aeruginosa mixed-biofilm communities. Appl Environ Microbiol 77:821–829. doi: 10.1128/AEM.01797-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carson L, Gorman SP, Gilmore BF. 2010. The use of lytic bacteriophages in the prevention and eradication of biofilms of Proteus mirabilis and Escherichia coli. FEMS Immunol Med Microbiol 59:447–455. doi: 10.1111/j.1574-695X.2010.00696.x. [DOI] [PubMed] [Google Scholar]
- 24.Coulter LB, McLean RJ, Rohde RE, Aron GM. 2014. Effect of bacteriophage infection in combination with tobramycin on the emergence of resistance in Escherichia coli and Pseudomonas aeruginosa biofilms. Viruses 6:3778–3786. doi: 10.3390/v6103778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sillankorva S, Neubauer P, Azeredo J. 2010. Phage control of dual species biofilms of Pseudomonas fluorescens and Staphylococcus lentus. Biofouling 26:567–575. doi: 10.1080/08927014.2010.494251. [DOI] [PubMed] [Google Scholar]
- 26.Harcombe WR, Bull JJ. 2005. Impact of phages on two-species bacterial communities. Appl Environ Microbiol 71:5254–5259. doi: 10.1128/AEM.71.9.5254-5259.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.European Food Safety Authority, European Centre for Disease Prevention and Control (ECDC). 2015. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2014. EFSA J 13:4329. doi: 10.2903/j.efsa.2015.4329. [DOI] [Google Scholar]
- 28.Gounadaki AS, Skandamis PN, Drosinos EH, Nychas GJ. 2008. Microbial ecology of food contact surfaces and products of small-scale facilities producing traditional sausages. Food Microbiol 25:313–323. doi: 10.1016/j.fm.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Palá TR, Sevilla A. 2004. Microbial contamination of carcasses, meat, and equipment from an Iberian pork cutting plant. J Food Prot 67:1624–1629. [DOI] [PubMed] [Google Scholar]
- 30.Gutiérrez D, Delgado S, Vázquez-Sánchez D, Martínez B, Cabo ML, Rodríguez A, Herrera JJ, García P. 2012. Incidence of Staphylococcus aureus and analysis of associated bacterial communities on food industry surfaces. Appl Environ Microbiol 78:8547–8554. doi: 10.1128/AEM.02045-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leriche V, Briandet R, Carpentier B. 2003. Ecology of mixed biofilms subjected daily to a chlorinated alkaline solution: spatial distribution of bacterial species suggests a protective effect of one species to another. Environ Microbiol 5:64–71. doi: 10.1046/j.1462-2920.2003.00394.x. [DOI] [PubMed] [Google Scholar]
- 32.Burmølle M, Webb JS, Rao D, Hansen LH, Sorensen SJ, Kjelleberg S. 2006. Enhanced biofilm formation and increased resistance to antimicrobial agents and bacterial invasion are caused by synergistic interactions in multispecies biofilms. Appl Environ Microbiol 72:3916–3923. doi: 10.1128/AEM.03022-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tait K, Sutherland IW. 2002. Antagonistic interactions amongst bacteriocin-producing enteric bacteria in dual species biofilms. J Appl Microbiol 93:345–352. doi: 10.1046/j.1365-2672.2002.01692.x. [DOI] [PubMed] [Google Scholar]
- 34.Bridier A, Sanchez-Vizuete MDP, Le Coq D, Aymerich S, Meylheuc T, Maillard JY, Thomas V, Dubois-Brissonnet F, Briandet R. 2012. Biofilms of a Bacillus subtilis hospital isolate protect Staphylococcus aureus from biocide action. PLoS One 7:e44506. doi: 10.1371/journal.pone.0044506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rickard AH, McBain AJ, Ledder RG, Handley PS, Gilbert P. 2003. Coaggregation between freshwater bacteria within biofilm and planktonic communities. FEMS Microbiol Lett 220:133–140. doi: 10.1016/S0378-1097(03)00094-6. [DOI] [PubMed] [Google Scholar]
- 36.Sutherland IW, Hughes KA, Skillman LC, Tait K. 2004. The interaction of phage and biofilms. FEMS Microbiol Lett 232:1–6. doi: 10.1016/S0378-1097(04)00041-2. [DOI] [PubMed] [Google Scholar]
- 37.Briandet R, Lacroix-Gueu P, Renault M, Lecart S, Meylheuc T, Bidnenko E, Steenkeste K, Bellon-Fontaine MN, Fontaine-Aupart MP. 2008. Fluorescence correlation spectroscopy to study diffusion and reaction of bacteriophages inside biofilms. Appl Environ Microbiol 74:2135–2143. doi: 10.1128/AEM.02304-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoyland-Kroghsbo NM, Maerkedahl RB, Svenningsen SL. 2013. A quorum-sensing-induced bacteriophage defense mechanism. mBio 4:e00362-12. doi: 10.1128/mBio.00362-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hughes KA, Sutherland IW, Jones MV. 1998. Biofilm susceptibility to bacteriophage attack: the role of phage-borne polysaccharide depolymerase. Microbiology 144:3039–3047. doi: 10.1099/00221287-144-11-3039. [DOI] [PubMed] [Google Scholar]
- 40.Hosseinidoust Z, Tufenkji N, van de Ven TG. 2013. Formation of biofilms under phage predation: considerations concerning a biofilm increase. Biofouling 29:457–468. doi: 10.1080/08927014.2013.779370. [DOI] [PubMed] [Google Scholar]
- 41.Abedon ST. 2016. Bacteriophage exploitation of bacterial biofilms: phage preference for less mature targets? FEMS Microbiol Lett 363:pii=fnv246. doi: 10.1093/femsle/fnv246. [DOI] [PubMed] [Google Scholar]
- 42.Gallet R, Shao Y, Wang IN. 2009. High adsorption rate is detrimental to bacteriophage fitness in a biofilm-like environment. BMC Evol Biol 9:241. doi: 10.1186/1471-2148-9-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forde A, Fitzgerald GF. 2003. Molecular organization of exopolysaccharide (EPS) encoding genes on the lactococcal bacteriophage adsorption blocking plasmid, pCI658. Plasmid 49:130–142. doi: 10.1016/S0147-619X(02)00156-7. [DOI] [PubMed] [Google Scholar]
- 44.Sánchez JI, Martinez B, Guillen R, Jimenez-Diaz R, Rodriguez A. 2006. Culture conditions determine the balance between two different exopolysaccharides produced by Lactobacillus pentosus LPS26. Appl Environ Microbiol 72:7495–7502. doi: 10.1128/AEM.01078-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gutiérrez D, Martínez B, Rodríguez A, García P. 2010. Isolation and characterization of bacteriophages infecting Staphylococcus epidermidis. Curr Microbiol 61:601–608. doi: 10.1007/s00284-010-9659-5. [DOI] [PubMed] [Google Scholar]
- 46.Rehaiem A, Martinez B, Manai M, Rodriguez A. 2010. Production of enterocin A by Enterococcus faecium MMRA isolated from ‘Rayeb’, a traditional Tunisian dairy beverage. J Appl Microbiol 108:1685–1693. doi: 10.1111/j.1365-2672.2009.04565.x. [DOI] [PubMed] [Google Scholar]
- 47.Ruiz-Barba JL, Cathcart DP, Warner PJ, Jimenez-Diaz R. 1994. Use of Lactobacillus plantarum LPCO10, a bacteriocin producer, as a starter culture in Spanish-style green olive fermentations. Appl Environ Microbiol 60:2059–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.