Abstract
Effective treatment regimens for elderly acute myeloid leukemia (AML) patients harboring internal tandem duplication mutations in the FLT3 kinase gene (FLT3/ITD) are lacking and represent a significant unmet need. Recent data on the effects of FLT3 tyrosine kinase inhibitors on FLT3/ITD+ AML showed promising clinical activity, including in elderly patients. DNA methyltransferase (DNMT) inhibitors such as decitabine (DEC) and 5-azacitidine (AZA) demonstrated clinical benefit in AML, are well tolerated, and are associated with minimal increases in FLT3 ligand, which can represent a potential resistance mechanism to FLT3 inhibitors. In addition, both FLT3 and DNMT inhibition are associated with the induction of terminal differentiation of myeloid blasts. Consequently, there is a strong theoretical rationale for combining FLT3 and DNMT inhibition for FLT3/ITD+ AML. We therefore sought to study the anti-leukemic effects of DEC, AZA and FLT3 inhibitors, either as single agents or in combination, on AML cell lines and primary cells derived from newly diagnosed and relapsed AML patients. Our studies indicate that combined treatment using FLT3 inhibition and hypomethylation confers synergistic anti-leukemic effects, including apoptosis, growth inhibition, and differentiation. The simultaneous administration of 5-azacitidine and FLT3 inhibition appears to be the most efficacious combination in this regard. These drugs may provide a novel therapeutic approach for FLT3/ITD+ AML, particularly for older patients.
Introduction
Internal tandem duplication mutations in the receptor tyrosine kinase FMS-Like Tyrosine Kinase-3 (FLT3/ITD mutations) are present in a substantial proportion of AML patients of all age groups and have been shown to be an important negative prognostic indicator of disease outcome, as mirrored in high relapse and poor survival rates(1). The poor prognostic impact, along with the observation that FLT3 is frequently overexpressed in the majority of AML cases, has formed the platform for the development of FLT3-targeted strategies. To date, several FLT3 kinase inhibitors have been investigated in pre-clinical and clinical studies(2). Agents such as quizartinib (formerly AC220) and sorafenib demonstrated selectivity and potency in cell culture assays and animal models(3, 4). Recently, Sexauer et al. demonstrated that inhibition of FLT3 signaling with either quizartinib or sorafenib results in rapid clearance of peripheral blasts by induction of apoptotic cell death and terminal myeloid differentiation of bone marrow blasts, which, in most cases, is clinically represented by a surge of leukemia-derived neutrophils occurring 30 to 60 days after initiation of treatment(5). Others have shown similar findings for patients treated with FLT3 inhibitors(6-8). Despite the fact that FLT3 inhibitors induce clinical remissions in the majority of patients, drug resistance is frequently encountered within the first 12 months of treatment, either due to acquired mutations in the tyrosine kinase domain (TKD) of FLT3, increases in FLT3 ligand (FL) levels, or through other incompletely understood mechanisms(7, 9, 10). In order to improve the therapeutic outcome for treatment-naive and -resistant FLT3/ITD+ AML several strategies, including combination therapy with traditional chemotherapeutic regimens and allogeneic stem cell transplant (SCT), have been employed(11-13). While younger patients with FLT3/ITD+ AML seem to benefit from allogeneic SCT, this is frequently not an option for many older patients due to preexisting comorbidities and the inability to tolerate intensive induction and consolidation protocols. Hence, effective treatment approaches for elderly patients are lacking and represent a significant unmet need in this field.
In recent years, therapeutic approaches targeting epigenetic changes in hematologic malignancies have received considerable attention in light of the potential role of these alterations in leukemogenesis(14, 15). To this end, the cytidine derivatives and DNA methyltransferase inhibitors (DNMTis) decitabine (5-aza-2-deoxycytidine, DEC) and azacitidine (5-azacytidine, AZA) have shown anti-leukemic potential in several preclinical and clinical studies, largely mediated by inhibition of proliferation, induction of apoptosis, and myeloid differentiation(16-19). Importantly, these agents do not appear to induce large magnitude increases in FL levels and are therefore unlikely to hinder the effects of FLT3 inhibitors(20). DEC and AZA confer their epigenetic effects by incorporating into newly formed DNA where they irreversibly bind to DNA-methyltransferases in an S-phase dependent fashion. Notably, besides being converted to decitabine triphosphate and subsequent incorporation into DNA, the majority of AZA (approximately 80-90%) is incorporated into RNA, thereby disrupting nucleic acid and protein synthesis(21). It has been suggested that these additional effects of AZA contribute to the different outcomes between DEC and AZA observed in clinical trials (22-24), although the exact mechanisms have not yet been clearly defined.
Due to their different modes of action but similar, and partially complementary, effects on leukemic blasts, there is a strong theoretical rationale to combine FLT3 inhibitors with DNMTis. Single arm studies combining AZA with either sorafenib or quizartinib have recently been carried out and demonstrated both safety and a promising response rate in FLT3/ITD AML patients(20, 25). In the present study, we investigated the effects of DEC and AZA on AML cell lines and primary cells to determine if the combination of either drug with FLT3 inhibition bears synergistic anti-leukemic potential, and, if so, which drug and which sequence of administration would be most effective. Our findings suggest that AZA administered simultaneously with FLT3 inhibition leads to the highest degree of anti-leukemic activity, as manifest by inhibition of growth and induction of both apoptosis and differentiation. This represents a novel approach to target FLT3/ITD mutated AML, particularly in elderly AML patients.
Methods
Cell culture and reagents
AML cell lines and primary blasts were cultured as previously described(26). Molm14 cells were obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ; Braunschweig, Germany). Quizartinib and sorafenib (LC labs Woburn, MA) was dissolved in dimethyl sulfoxide (DMSO) at stock concentrations of 10 mM. DEC and AZA were purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in phosphate-buffered saline (PBS) to stock concentrations of 5 mM and 10 mM and stored at − 20°C. Depending on the incubation period for each experiment, quizartinib or sorafenib was added every 5 days, and DEC and AZA were added every 24 hours. L-glutamine and Penicillin/Streptomycin were purchased from Invitrogen (Carlsbad, CA, USA). Hydrocortisone was purchased from Sigma-Aldrich (St. Louis, MO, USA).
The following antibodies were used for Western blotting: Anti-phosphotyrosine clone 4G10 (Millipore), FLT3 (S-18, Santa Cruz), and beta-actin (#4967, Cell Signaling Technologies). The following antibodies used for flow cytometry were obtained from BD Pharmingen: CD34-FITC (#555821), CD117-PE (#340529), FITC-Annexin V (#556419), and Propidium Iodide (#556463).
Patient samples
AML blasts from whole blood or bone marrow of newly diagnosed (ND) and relapsed (REL) FLT3/ITD mutated patients and bone marrow aspirates from healthy donors were collected and banked separately as part of an institutional protocol supported by the Regional Oncology Research Center Grant # 2 P30 CA 006973-44. All patients gave informed consent according to the Declaration of Helsinki. Blasts were separated by centrifugation over a layer of Ficoll-paque PLUS (GE Healthcare, Fairfield, CT, USA), collected as the buffy coat and then either stored in freezing medium (fetal bovine serum [FBS] with 10% DMSO) in liquid nitrogen or plated immediately for experiments. The clinical characteristics of the patients from whom samples were derived are listed in Supplemental Table 1. For the experiments involving patient blasts incorporated into figure 2, three replicates were performed (n = 3), and the differentiation experiments (n = 3 for samples from different patients) were exploratory and dependent upon patient sample availability and cell yield.
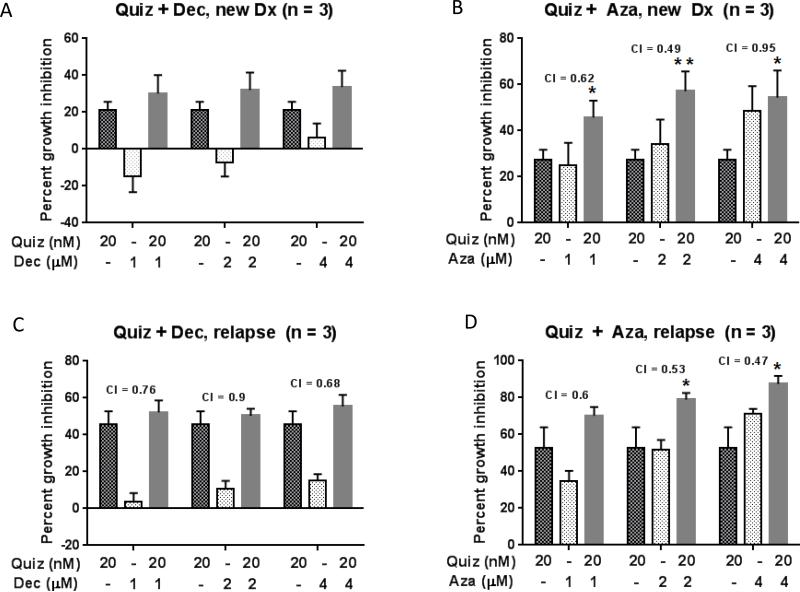
Figure 2. Combined treatment confers additive to synergistic growth inhibitory effects on primary FLT3 positive AML cells.
Primary blasts derived from newly diagnosed (A, B) and relapsed (C, D) FLT3/ITD+ AML patients were incubated in drug supplemented medium at the indicated concentrations in the presence of stroma. Growth inhibition was assessed after 48 hours using the MTT assay. The mean ± SEM is based on replicate experiments (n= 3). Significant changes in the percentage of growth inhibition are indicated (*p<.05), (**p<.01), (***p<.001).
Bone marrow stromal culture
Normal human bone marrow stroma was harvested from patients as previously described(5). Mononuclear cells were isolated via centrifugation with Ficoll-Paque PLUS (GE Healthcare Life Sciences), plated into T75 flasks, and incubated in stromal medium (RPMI 1640/ 10%FBS/ 1% L-glutamine, 1% Penicillin/Streptomycin, 1μmol/l hydrocortisone) at 33°C in 5% CO2. The first media change was performed 24-48 hours after the initial plating, and every subsequent media change was performed every 1-2 weeks. Once the adherent stromal cells growing in the flasks were at least 80% confluent, cells were passaged using Trypsin-EDTA (Sigma) to be plated into 6-, 12-, or 96-well plates and T75 flasks.
MTT Cell viability assays
Cell growth and cytotoxicity were assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Roche, Indianapolis, USA) in accordance to the manufacturer's recommended protocols in the presence and absence of inhibitors as outlined for each experiment. When performing assays with bone marrow stromal co-culture, approximately 8000 stromal cells/well were seeded in 96-well plates. For MTTs with both cell lines and patient blasts (Figs. 1-2), sample sizes are provided in the corresponding figure legends.
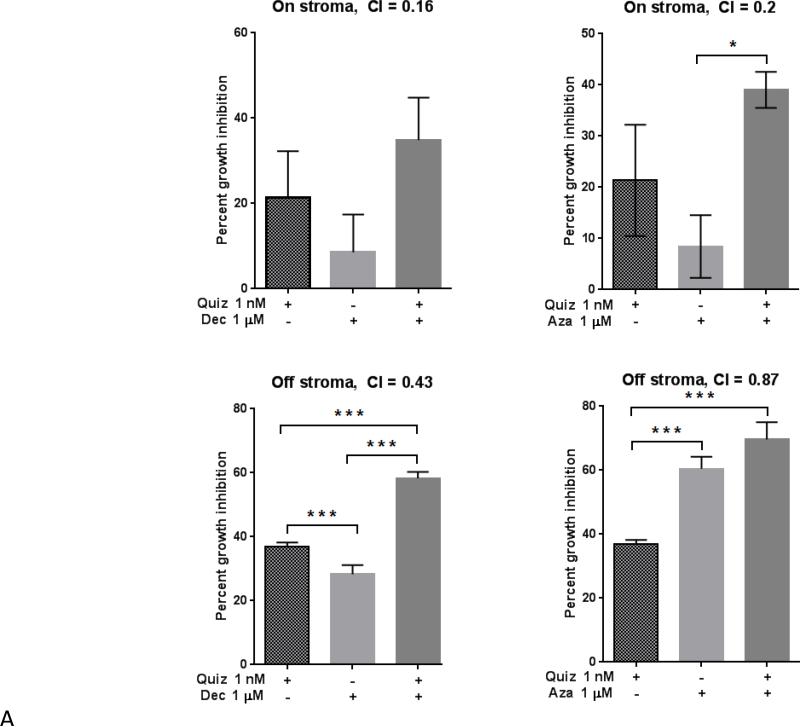
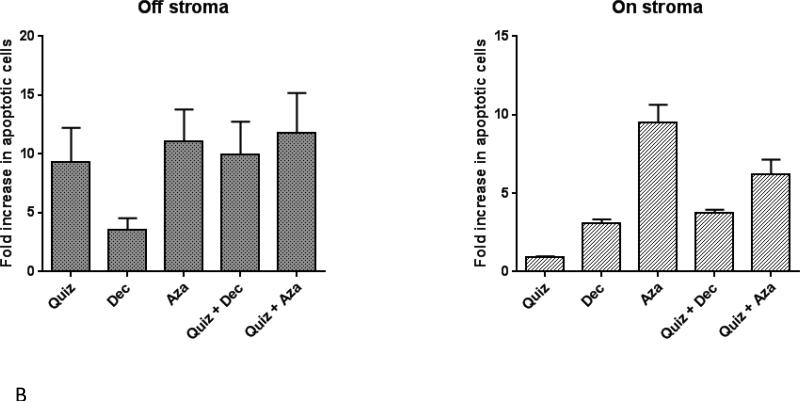
Figure 1. Combined treatment results in synergistic anti-leukemic effects in the presence and absence of stroma.
Molm14 cells were cultured in drug supplemented medium at the indicated concentrations, both in the presence and absence of stroma. Growth inhibition was assessed after 48 hours using MTT cell viability assays (A). Combination Index (CI) values are shown for each experiment. The mean ± SEM is based on replicate experiments (n= 3-12). Significant changes in the percentage of growth inhibition are indicated (*p<.05), (**p<.01), (***p<.001). Flow cytometry was used to measure apoptosis in these cells via Annexin V/propidium iodide and quantified as shown in (B).
For MTT assays used to assess drug regimen sequence, cells (Molm14 or FLT3-ITD+ patient blasts, one replicate each) were plated on Day 0. After an overnight incubation on stroma, cells were treated with drug (Day 1) – either a single agent or with both agents simultaneously. For the Molm14 experiments, wells were re-spiked with AZA or DEC on Days 2 and 3 of the experiment, and quizartinib or sorafenib were added to wells on Day 3. MTT reagents were added on Day 5, and plates were read on Day 6 at 595 nm with a plate reader (iMark Microplate reader, Bio-Rad). For patient blasts, wells were re-spiked with AZA or DEC on Days 2, 3, and 4 of the experiment, and the FLT3 inhibitor was added to wells on Day 4. MTT reagents were added on Day 7, and plates were read on Day 8 at 595 nm.
Flow cytometry
Flow cytometric analysis was performed on Molm14 cells and primary AML blasts as described(5). Cell line data reflect a sample size (n = 3). Experiments with primary patient blasts reflect data from three separate patient samples and were performed based on patient sample availability and cell yield. Cells were treated with quizartinib or sorafenib, DEC and AZA, either as single agents or in combination, in the presence and absence of bone marrow stromal cells. Stromal cells were seeded into 6- or 12-well plates. Leukemia cells were seeded in 6- or 12-well plates in the presence or absence of bone marrow stromal cells. At the appropriate time, leukemia cells were harvested from the plates (the stromal cells in each well remain adherent) and assayed on a FACSCalibur machine (BD Biosciences) as described(27).
Immunoblotting
In each well of a 6- or 12-well plate, Molm14 (5 × 106) cells were incubated in RPMI medium in the presence or absence of stroma as outlined for each experiment. After incubation at 37°C the cells were harvested at the appropriate time and analyzed by immunoblotting as described previously(26). After cell lysis and prior to running SDS-PAGE, protein concentrations in each sample were normalized via the BCA protein assay (Pierce).
Differentiation assay
FLT3-ITD+ patient blasts (4 × 106) were plated (Day 0) on normal human bone marrow stroma growing in 6-well plates. Blasts were incubated on stroma overnight before adding drug the following day (Day 1). DEC and AZA were added to plates every day and quizartinib or sorafenib was added every five days. On Day 12, cells were harvested off stroma and analyzed by flow cytometry with a BD FACSCalibur and by Western blotting for FLT3.
DNA sequencing
Libraries were prepared using the Agilent SureSelect-XT Target Enrichment Kit. Briefly, 1 μg of DNA was fragmented to a size of 250-300 bp. Each library was then hybridized to a SureSelect custom panel 2.8M bait set (Agilent) according to the manufacturer's protocol. The custom panel was designed as a clinical leukemia panel, and covers 637 genes important in oncogenesis. The gene manifest is available upon request. Captured samples were clustered in groups of five on a cBOT system and sequenced on a single lane of a PE-flow cell on a HiSeq2500 (Illumina), using a 2 × 150 bp PE protocol. All reads were aligned to the human genome (GRCh37/hg19), using the Burrows–Wheeler alignment (BWA) algorithm. The final BAM file was used for variant calling with our custom variant caller pipeline. The sequencing coverage exceeded 300 reads in more than 94% of all regions.
Statistical analysis
Data obtained from independent experiments on multiple samples were reported as the mean ± SEM. Significance levels were determined by Student's t-test analysis. Synergy calculations were performed using CalcuSyn software (Biosoft, Cambridge, UK).
Results
The combination of FLT3 inhibition and DNMT inhibition results in synergistic cytotoxicity
Our previous work indicated that mesenchymal stromal cells derived from normal human bone marrow conferred on leukemia cells a protective effect against FLT3 inhibition, and represented a better alternative to suspension culture for modeling responses to cytotoxic agents(5, 28). The Molm14 cell line is an AML cell line derived from a patient harboring a FLT3/ITD mutation(29). We exposed Molm14 cells to different concentrations of inhibitors, alone and in combination (simultaneous treatment) in the presence or absence of bone marrow stromal cells. Quizartinib was added at time = 0 on day 0 whereas AZA or DEC were added at time = 0 and 24 hours due to the reported short half-life of these agents(23). After 48 hours, we performed MTT assays to measure cell proliferation and reduction in cell viability. As shown in Figure 1A, growth inhibitory effects were observed for FLT3 and DNMT inhibition, both in single and combined treatment. In line with our previous findings, stromal cells consistently conferred protection against the cytotoxic effects of all treatments (Figure 1A). The combination of either AZA or DEC with quizartinib was particularly synergistic at impeding growth in the setting of stromal co-culture. These experiments were repeated using 3 nM sorafenib in place of 1 nM quizartinib, and the results were essentially the same (data not shown). This is consistent with our previous findings of sorafenib exerting a similar cytotoxic effect as quizartinib(5, 28).
In order to investigate the basis for this cytotoxicity, we incubated Molm14 cells with quizartinib, DEC or AZA in single or combined treatment mode in the presence or absence of stromal cells, and after 48 hours, the cells were harvested, labeled with Annexin V, and analyzed by flow cytometry (Figure 1B). Combined treatment of Molm14 cells with quizartinib and DEC or AZA for 48 hours did not result in a higher level of apoptosis inducing-effects when compared to single agent treatment. In the presence of stroma, AZA conferred more pro-apoptotic effects than DEC, but the effects of both agents were modest. While the pro-apoptotic effects of AZA and quizartinib were similar in the absence of stroma, AZA induced significantly more induction of apoptosis than quizartinib when stromal cells were present. These findings imply that the synergistic effect of FLT3 inhibition and DNMT inhibition on growth inhibition is not exclusively mediated through a synergistic induction of apoptosis.
We next wished to confirm that the synergistic cytotoxicity we observed with combined FLT3 and DNMT inhibition in Molm14 cells could be reproduced in primary cells isolated from AML patients harboring FLT3/ITD mutations. Because blasts isolated from newly diagnosed versus relapsed FLT3/ITD AML patients display differential responses to FLT3 inhibition in vitro (e.g. samples from relapsed patients are more responsive)(26), we tested blasts from both types of patients. The anti-leukemic effects of quizartinib, DEC and AZA against primary AML blasts derived from newly diagnosed and relapsed FLT3/ITD mutated patients were assessed after 48 hours of exposure in culture in the presence of stroma. Consistent with the data obtained in the Molm14 cell line, MTT assays indicated that each DEC and AZA exerted dose dependent cytotoxicity as shown in Figure 2. As expected, quizartinib demonstrated significantly greater growth inhibition in relapsed compared to newly diagnosed samples, which is consistent with the greater addiction to FLT3 signaling commonly observed in relapsed samples. Furthermore, in both newly diagnosed and relapsed samples, AZA demonstrated significantly greater cytotoxicity than DEC as depicted in Figure 2 (A and C versus B and D).
Simultaneous versus sequential treatment
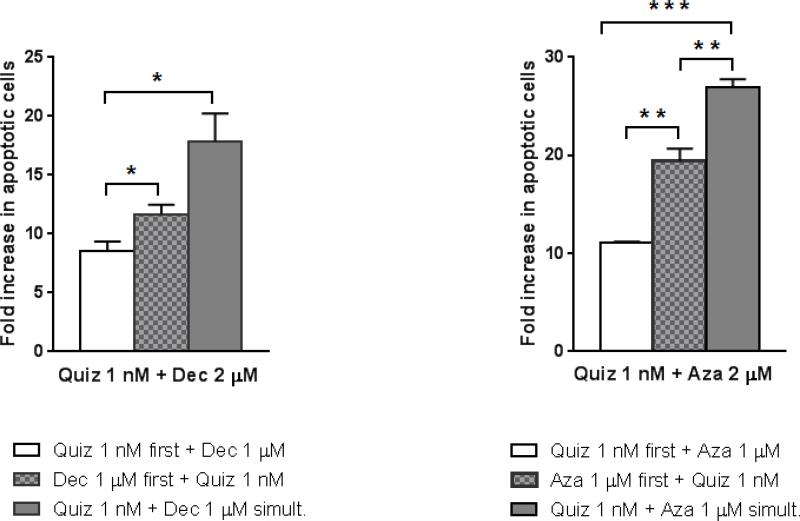
Our previous studies suggested that the sequence of administration of FLT3 inhibitors and chemotherapy was important for maximizing cytotoxic effect(27). However, DNMT inhibitors have a mechanism of action that is unique from conventional DNA-damaging agents (e.g., anthracyclines) in that they induce epigenetic changes. Therefore, we wished to determine if the sequence of administration (e.g., simultaneous or sequential) of FLT3 inhibitors and DNMT inhibitors influenced the cytotoxic effect. We first confirmed that for both AZA and DEC, pre-treatment with FLT3 inhibition was the least effective sequence for inducing apoptosis (Figure 3) and thus focused on 2 approaches: 1) simultaneous exposure to AZA or DEC and quizartinib; and 2) exposure to AZA or DEC followed by quizartinib. These dose response studies were carried out using Molm14 cells cultured on bone marrow stroma, combination indices were calculated by median effect analysis, and the results are shown in Table 1. In contrast to what we expected, the most effective sequence for either AZA or DEC with quizartinib was simultaneous, not sequential, with CI values indicating additive or synergistic effects at most concentrations. Extending these studies to primary AML cells, a similar pattern was observed (Table 2), in that simultaneous treatment was the most effective way to combine the agents. In fact, DEC or AZA followed by quizartinib was mostly antagonistic with this primary sample.
Figure 3. The sequence of administration for combining FLT3 inhibitors and DNMT inhibitors is crucial.
Molm14 cells were cultured in drug supplemented medium in suspension. The different drugs (the FLT3 inhibitor quizartinib and the two DNMT inhibitors) were given in either sequentially or simultaneously: white bars denote sequential treatment with Quiz first followed by DEC/AZA, black bars represent sequential treatment with DEC/AZA first followed by Quiz, and gray bars denote simultaneous administration of both types of drugs. Following pretreatment, cells were incubated for an additional 48 hours before being harvested for flow cytometry analysis with Annexin V/propidium iodide staining. The mean ± SEM is based on replicate experiments (n= 3). Significant changes in the percentage of growth inhibition are indicated (*p<.05), (**p<.01), (***p<.001).
Table 1.
| Simultaneous treatment: | ||||||||
|---|---|---|---|---|---|---|---|---|
| Decitabine |
Azacytidine |
|||||||
| 0.2 uM | 0.5 uM | 1 uM | 2 uM | 0.2 uM | 0.5 uM | 1 uM | 2 uM | |
| 1 nm Quiz | 0.172 * | 0.19 * | 0.158 * | 0.166 * | 0.431 * | 0.487 * | 0.652 * | 0.986 |
| 5 nM Quiz | 0.555 * | 0.53 * | 0.553 * | 0.556 * | 0.727 * | 0.716 * | 0.907 | 1.269 |
| 10 nM Quiz | 1.1 | 1.117 | 1.24 | 1.029 | 1.266 | 1.4 | 1.474 | 1.787 |
| Sequential treatment: | ||||||||
|---|---|---|---|---|---|---|---|---|
| Decitabine |
Azacytidine |
|||||||
| 0.2 uM | 0.5 uM | 1 uM | 2 uM | 0.2 uM | 0.5 uM | 1 uM | 2 uM | |
| 1 nm Quiz | 1.079 | 0.958 | 0.466 * | 0.433 * | 1.711 | 1.219 | 0.873 * | 1.014 |
| 5 nM Quiz | 0.647 * | 0.63 * | 0.601 * | 0.625 * | 0.919 | 0.1008 | 1.088 | 1.218 |
| 10 nM Quiz | 1.29 | 1.178 | 1.145 | 1.072 | 1.538 | 1.545 | 1.505 | 1.909 |
denotes synergy (0 < CI < 0.9)
Table 2.
| Simultaneous treatment: | ||||||||
|---|---|---|---|---|---|---|---|---|
| Decitabine |
Azacytidine |
|||||||
| 0.2 uM | 0.5 uM | 1 uM | 2 uM | 0.2 uM | 0.5 uM | 1 uM | 2 uM | |
| 5 nm Quiz | 0.514 * | 0.945 | 0.744 * | 0.618 * | 0.459 | 0.401 * | 0.469 * | 0.753 * |
| 10 nM Quiz | 0.655 * | 0.943 | 0.328 * | 0.434* | 0.495 * | 0.467 * | 0.55 * | 0.715 * |
| 20 nM Quiz | 0.333 * | 0.465 * | 0.674 * | 0.51 * | 1.399 | 0.842 * | 0.532 * | 0.741 * |
| Sequential treatment: | ||||||||
|---|---|---|---|---|---|---|---|---|
| Decitabine |
Azacytidine |
|||||||
| 0.2 uM | 0.5 uM | 1 uM | 2 uM | 0.2 uM | 0.5 uM | 1 uM | 2 uM | |
| 5 nm Quiz | 5.31 | 4.725 | 1.913 | 0.953 | 3.731 | 1.152 | 0.594 * | 0.781 * |
| 10 nM Quiz | 5.906 | 3.874 | 1.087 | 0.78 * | 3.919 | 2.518 | 0.795 * | 1.053 |
| 20 nM Quiz | 3.891 | 2.499 | 1.219 | 2.401 | 9.797 | 2.042 | 0.635 * | 0.737 * |
denotes synergy (0 < CI < 0.9)
DNMT and FLT3 inhibition induce differentiation
According to the results of the assays for cell proliferation and apoptosis described above, simultaneous treatment of FLT3/ITD AML cells with quizartinib and either AZA or DEC results in synergistic inhibition of growth, with induction of apoptosis contributing to this effect. Because both classes of agents are associated with the induction of differentiation, we wished to determine if differentiation could be contributing to the overall cytotoxic effect as well. Because the simultaneous treatment model yielded the greatest magnitude of cytotoxicity, we focused on this approach, using a primary FLT3/ITD AML sample (collected at relapse) cultured on stroma.
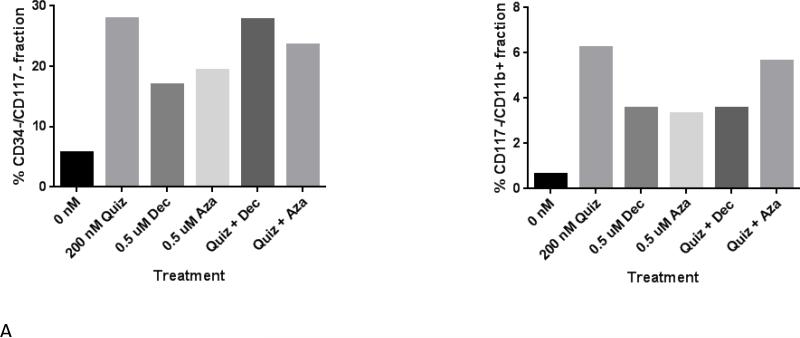
The degree of cytotoxic effect induced by these combinations at 12 days rendered it problematic to identify differentiated cells by morphology (there were too few cells remaining to quantify a differentiation effect by microscopy). However, we analyzed blasts for expression of cell surface markers of differentiation following treatment with single agent AZA, DEC, or quizartinib, or the combination (Figure 4A). Using loss of CD34, CD117, and CD11b expression as markers of differentiation, we found that all 3 agents induced differentiation of this sample after cells were exposed for 12 days. However, combination therapy did not appear to result in synergy, or even additivity, although median effect analysis was not performed because we did not carry out a dose-response experiment. We looked at these markers in a second primary sample (Supplemental Figure 1) and also saw a differentiation effect. However, for this sample, the differentiation effects of AZA were much more prominent than those of the other agents. This variation is most likely due to the patient-to-patient differences that would be expected in experiments utilizing primary blasts. This experiment was repeated with a third primary sample, with similar results (not shown), although down-regulation of CD117 was not prominent until Day 15 of exposure.
Figure 4. Treating FLT3/ITD blasts with a FLT3 inhibitor, a DNMT inhibitor, or both simultaneously induces differentiation.
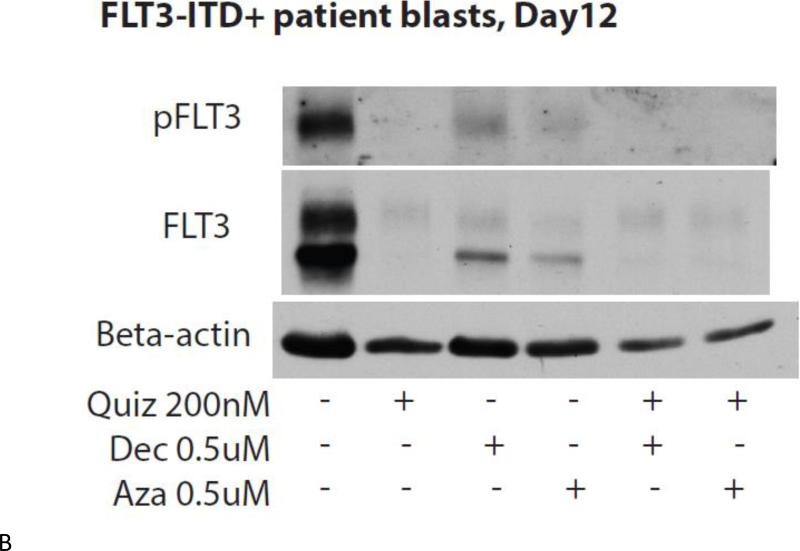
FLT3/ITD primary blasts co-cultured on stroma were treated with drug at the specified concentrations. Cells were harvested on Day 12 after starting drug treatment, stained for the cell-surface markers CD34, CD117, and CD11b, and analyzed via flow cytometry (A). The remaining cells were lysed and analyzed via immunoblotting (B).
If leukemia cells are undergoing terminal myeloid differentiation in response to either FLT3 or DNMT inhibition, expression of the FLT3 receptor should be down-regulated as a part of that process, as we have previously observed(5). To confirm this, we carried out immunoblots of total and phosphorylated FLT3 in the cells used in the above experiment shown in Figure 4A. As shown in Figure 4B, expression of both phosphorylated and total FLT3 is nearly absent in cells treated with single or combination therapy. We examined two other markers of neutrophil differentiation, lactoferrin and matrix metalloproteinase-9 (not shown); however, the immunoblots for these markers did not show any clear trends towards differentiation.
The combination of sorafenib and 5-azacitidine induces differentiation in vivo
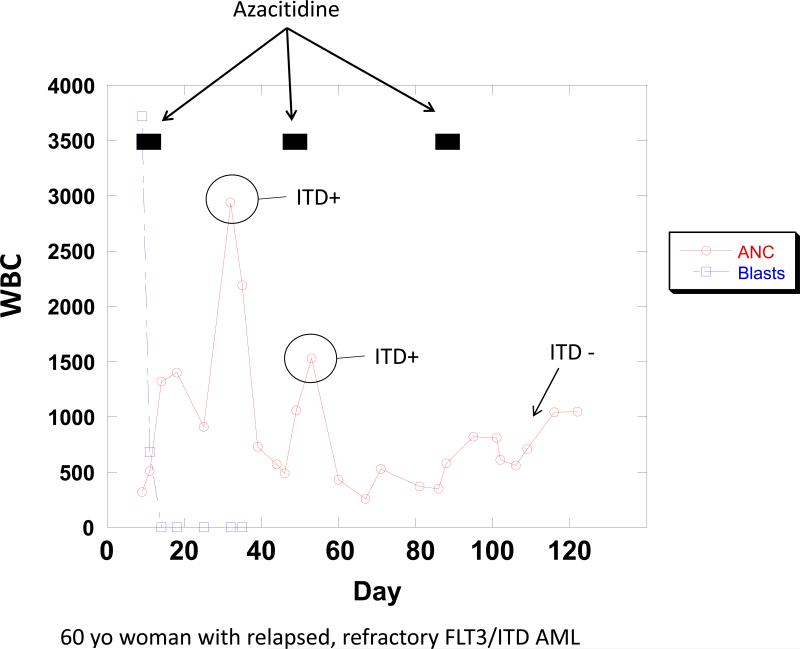
Both quizartinib and sorafenib have been used in combination with 5-azacitidine in single arm clinical trials(20, 25). However, the endpoints of these trials were clinical, and correlative laboratory studies did not focus on any in vivo differentiation that might have been induced by this combination. A 60 year old woman with FLT3/ITD AML in first remission was scheduled for an allogeneic transplant, but relapsed just prior to starting preparative chemotherapy. She was enrolled on a trial of PLX3397, a novel FLT3 inhibitor(30), but was deemed unresponsive after 2 cycles. We therefore opted to treat her with sorafenib (off-label, off-protocol) 400 mg orally twice daily continuously, plus 5-azacitidine 75 mg/m2 IV days 1-7 of each 28 day cycle of sorafenib, as per the published protocol(20). We performed mutation analysis on her peripheral blood cells at the start of therapy, and at different time points during therapy (Figure 5). Each of her first 2 cycles of 5-azacitidine were followed with a surge of neutrophils which harbored the FLT3/ITD mutation noted to be in blasts at the start of therapy. After the third cycle of 5-azacitidine, the circulating neutrophils increased more gradually, and the FLT3/ITD mutation was no longer detectable in these cells. A bone marrow biopsy confirmed a complete remission by IWG standards.
Figure 5. Peripheral blood absolute blast and neutrophil count of patient treated with 5-azacitidine and sorafenib.
The patient was treated continuously with sorafenib 400 mg twice daily beginning on Day 1, and with 5-azacitidine 75 mg/meter-squared IV daily for 7 days during the time periods indicated by black bars. At each of the indicated time points, the cellular fraction from the peripheral blood was analyzed for the presence of the FLT3/ITD mutation.
Discussion
In the present study, we used in vitro models to assess the effects of either DEC or AZA combined with Quiz on the FLT3/ITD+ cell line Molm14 and primary cells derived from newly diagnosed and relapsed patients. Consistent with our previous studies, bone marrow stromal cells conferred significant protection against the effects of all treatments(28). Our data shows that all 3 agents have cytotoxic activity against FLT3/ITD mutated cells within the tested dose range. The synergistic cytotoxic effect observed with the combination of FLT3 inhibition and DNMT inhibition was due to the induction of immediate apoptosis as well as terminal myeloid differentiation. The FLT3 allelic burden for these differentiated blasts were not analyzed post-treatment, as the blast population used in our experiments were peripheral blasts that most likely had very few non-malignant stem/progenitor cells in them. Thus, normal hematopoiesis would not occur within the context of our experiments, and the allelic burden would not change, as shown by Sexauer et al.(5). The efficacy of this combination is already apparent from clinical studies, and the case presented in this report illustrates that the in vitro processes we have investigated can occur in vivo. Our data, therefore, establishes a mechanistic rationale for this treatment regimen.
The differentiation induced by FLT3 inhibition seems to be a class effect, as it has been observed with multiple different TKIs, both in vitro and in vivo. The choice of which demethylating agent should be combined with a FLT3 inhibitor is perhaps less clear. Our data would suggest that AZA is the better choice, with the obvious caveat that in vitro studies have significant limitations in predicting responses in vivo. Both in the presence and absence of stroma, AZA was significantly more cytotoxic than equimolar concentrations of DEC, at least from the standpoint of inducing growth inhibition and apoptosis. This differential effect in anti-leukemic activity may at least in part be due to the additional incorporation of AZA into RNA, and the resulting interference with nucleic acid and protein synthesis, whereas DEC is solely incorporated into DNA. Indeed, although DEC and AZA are structurally related and share related mechanisms of action, differences in their potency have been reported in clinical and preclinical studies(18, 22, 23). Viability assays on AML cell lines demonstrated that AZA is much more potent that DEC whereas a higher level of activity has been reported for DEC when assessing molecular endpoints such as incorporation into DNA, DNA hypomethylation and DNA damage(31). Both drugs exert their anti-leukemic activity via the regulation of multiple, partially interconnected mechanisms, ranging from DNA methylation, gene expression, cell cycle progression and differentiation to different modes of cell death. While some of these mechanisms are shared or overlapping, others are specific to each drug, and yet other mechanisms have not been unveiled to date. Clearly, our data and that of others indicate that the effects of DEC and AZA are dose and time dependent and therefore offer a broad range of therapeutic options, including long term treatment courses at low concentrations either as single modality or in combination with other agents(32, 33). We are currently exploring the mechanism of cooperation between FLT3 and DNMT inhibition, focusing on alterations in expression of myeloid-relevant genes. For example, DNMT inhibition might lead to the reversal of aberrant methylation in the promoter regions of myeloid transcription factors(34). In the setting of cell cycle arrest induced by FLT3 inhibition, combined with and the presence of stromal-derived cytokines, de-methylation could enhance induction of differentiation. An interesting point to note is that all nine patient samples contained mutations in genes that are involved in epigenetic modifications (i.e. IDH1, WT1). The role of these genes in affecting DNA methylation could explain the response of these patient blasts to the hypomethylating agents. The prevalence of these genes in AML suggests that a combination therapy of FLT3 inhibitors and demethylating agents could be broadly applied to treating AML patients.
The prognosis for AML patients harboring a FLT3/ITD mutation remains generally poor, and while younger patients with FLT3/ITD AML seem to benefit from allogeneic transplant, this is frequently not an option for many older patients. Our study demonstrates that the combination of a hypomethylating agent and FLT3 inhibitor is a potentially important new therapeutic regimen for FLT3/ITD+ AML, and further suggests that this regimen could be used in patients of any age group.
Supplementary Material
Acknowledgements
This work was supported by the NCI Leukemia SPORE P50 CA100632-11.
Footnotes
Conflicts of Interest: EC, SG, TR, CG and HK do not report any conflicts of interest.
References
- 1.Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17(9):1738–52. doi: 10.1038/sj.leu.2403099. [DOI] [PubMed] [Google Scholar]
- 2.Konig H, Levis M. Targeting FLT3 to treat leukemia. Expert Opin Ther Targets. 2015;19(1):37–54. doi: 10.1517/14728222.2014.960843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang W, Konopleva M, Shi YX, McQueen T, Harris D, Ling X, et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008;100(3):184–98. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- 4.Zarrinkar PP, Gunawardane RN, Cramer MD, Gardner MF, Brigham D, Belli B, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML). Blood. 2009;114(14):2984–92. doi: 10.1182/blood-2009-05-222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sexauer A, Perl A, Yang X, Borowitz M, Gocke C, Rajkhowa T, et al. Terminal myeloid differentiation in vivo is induced by FLT3 inhibition in FLT3/ITD AML. Blood. 2012;120(20):4205–14. doi: 10.1182/blood-2012-01-402545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng R, Friedman AD, Levis M, Li L, Weir EG, Small D. Internal tandem duplication mutation of FLT3 blocks myeloid differentiation through suppression of C/EBPalpha expression. Blood. 2004;103(5):1883–90. doi: 10.1182/blood-2003-06-1978. [DOI] [PubMed] [Google Scholar]
- 7.Man CH, Fung TK, Ho C, Han HH, Chow HC, Ma AC, et al. Sorafenib treatment of FLT3-ITD(+) acute myeloid leukemia: favorable initial outcome and mechanisms of subsequent nonresponsiveness associated with the emergence of a D835 mutation. Blood. 2012;119(22):5133–43. doi: 10.1182/blood-2011-06-363960. [DOI] [PubMed] [Google Scholar]
- 8.Fathi AT, Le L, Hasserjian RP, Sadrzadeh H, Levis M, Chen YB. FLT3 inhibitor-induced neutrophilic dermatosis. Blood. 2013;122(2):239–42. doi: 10.1182/blood-2013-01-478172. [DOI] [PubMed] [Google Scholar]
- 9.Smith CC, Wang Q, Chin CS, Salerno S, Damon LE, Levis MJ, et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature. 2012;485(7397):260–3. doi: 10.1038/nature11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grunwald MR, Levis MJ. FLT3 inhibitors for acute myeloid leukemia: a review of their efficacy and mechanisms of resistance. Int J Hematol. 2013;97(6):683–94. doi: 10.1007/s12185-013-1334-8. [DOI] [PubMed] [Google Scholar]
- 11.Levis M, Ravandi F, Wang ES, Baer MR, Perl A, Coutre S, et al. Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse. Blood. 2011;117(12):3294–301. doi: 10.1182/blood-2010-08-301796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serve H, Krug U, Wagner R, Sauerland MC, Heinecke A, Brunnberg U, et al. Sorafenib in combination with intensive chemotherapy in elderly patients with acute myeloid leukemia: results from a randomized, placebo-controlled trial. J Clin Oncol. 2013;31(25):3110–8. doi: 10.1200/JCO.2012.46.4990. [DOI] [PubMed] [Google Scholar]
- 13.Chen YB, Li S, Lane AA, Connolly C, Del Rio C, Valles B, et al. Phase I trial of maintenance sorafenib after allogeneic hematopoietic stem cell transplantation for fms-like tyrosine kinase 3 internal tandem duplication acute myeloid leukemia. Biol Blood Marrow Transplant. 2014;20(12):2042–8. doi: 10.1016/j.bbmt.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plimack ER, Kantarjian HM, Issa JP. Decitabine and its role in the treatment of hematopoietic malignancies. Leuk Lymphoma. 2007;48(8):1472–81. doi: 10.1080/10428190701471981. [DOI] [PubMed] [Google Scholar]
- 15.Pollyea DA, Kohrt HE, Gallegos L, Figueroa ME, Abdel-Wahab O, Zhang B, et al. Safety, efficacy and biological predictors of response to sequential azacitidine and lenalidomide for elderly patients with acute myeloid leukemia. Leukemia. 2012;26(5):893–901. doi: 10.1038/leu.2011.294. [DOI] [PubMed] [Google Scholar]
- 16.Sudan N, Rossetti JM, Shadduck RK, Latsko J, Lech JA, Kaplan RB, et al. Treatment of acute myelogenous leukemia with outpatient azacitidine. Cancer. 2006;107(8):1839–43. doi: 10.1002/cncr.22204. [DOI] [PubMed] [Google Scholar]
- 17.Lubbert M, Ruter BH, Claus R, Schmoor C, Schmid M, Germing U, et al. A multicenter phase II trial of decitabine as first-line treatment for older patients with acute myeloid leukemia judged unfit for induction chemotherapy. Haematologica. 2012;97(3):393–401. doi: 10.3324/haematol.2011.048231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cashen AF, Schiller GJ, O'Donnell MR, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28(4):556–61. doi: 10.1200/JCO.2009.23.9178. [DOI] [PubMed] [Google Scholar]
- 19.Saunthararajah Y, Triozzi P, Rini B, Singh A, Radivoyevitch T, Sekeres M, et al. p53-Independent, normal stem cell sparing epigenetic differentiation therapy for myeloid and other malignancies. Semin Oncol. 2012;39(1):97–108. doi: 10.1053/j.seminoncol.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravandi F, Alattar ML, Grunwald MR, Rudek MA, Rajkhowa T, Richie MA, et al. Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood. 2013;121(23):4655–62. doi: 10.1182/blood-2013-01-480228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aimiuwu J, Wang H, Chen P, Xie Z, Wang J, Liu S, et al. RNA-dependent inhibition of ribonucleotide reductase is a major pathway for 5-azacytidine activity in acute myeloid leukemia. Blood. 2012;119(22):5229–38. doi: 10.1182/blood-2011-11-382226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Gattermann N, Germing U, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28(4):562–9. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 23.Hollenbach PW, Nguyen AN, Brady H, Williams M, Ning Y, Richard N, et al. A comparison of azacitidine and decitabine activities in acute myeloid leukemia cell lines. PLoS One. 2010;5(2):e9001. doi: 10.1371/journal.pone.0009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670–7. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borthakur GKHM, O'Brien S, Garcia-Manero G, Jabbour E, Daver N, Kadia TM, Gborogen R, Konopleva M, Andreeff M, Ravandi F, Cortes J. The Combination of Quizartinib with Azacitidine or Low Dose Cytarabine Is Highly Active in Patients (Pts) with FLT3-ITD Mutated Myeloid Leukemias: Interim Report of a Phase I/II Trial. Blood. 2014 56th ASH Annual Meeting (abstract #388) [Google Scholar]
- 26.Pratz KW, Sato T, Murphy KM, Stine A, Rajkhowa T, Levis M. FLT3-mutant allelic burden and clinical status are predictive of response to FLT3 inhibitors in AML. Blood. 2010;115(7):1425–32. doi: 10.1182/blood-2009-09-242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levis M, Pham R, Smith BD, Small D. In vitro studies of a FLT3 inhibitor combined with chemotherapy: sequence of administration is important to achieve synergistic cytotoxic effects. Blood. 2004;104(4):1145–50. doi: 10.1182/blood-2004-01-0388. [DOI] [PubMed] [Google Scholar]
- 28.Yang X, Sexauer A, Levis M. Bone marrow stroma-mediated resistance to FLT3 inhibitors in FLT3-ITD AML is mediated by persistent activation of extracellular regulated kinase. Br J Haematol. 2014;164(1):61–72. doi: 10.1111/bjh.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuo Y, MacLeod RA, Uphoff CC, Drexler HG, Nishizaki C, Katayama Y, et al. Two acute monocytic leukemia (AML-M5a) cell lines (MOLM-13 and MOLM-14) with interclonal phenotypic heterogeneity showing MLL-AF9 fusion resulting from an occult chromosome insertion, ins(11;9)(q23;p22p23). Leukemia. 1997;11(9):1469–77. doi: 10.1038/sj.leu.2400768. [DOI] [PubMed] [Google Scholar]
- 30.Burton EWB, Zhang J, West B, Bollag G, Habets G, Galanis A, Nguyen H, Arowojolu O, Rajhowa T, Levis MJ. The Novel Inhibitor PLX3397 Effectively Inhibits FLT3-Mutant AML. Blood. 2011 53rd ASH Annual Meeting(abstract #3632) [Google Scholar]
- 31.Flotho C, Claus R, Batz C, Schneider M, Sandrock I, Ihde S, et al. The DNA methyltransferase inhibitors azacitidine, decitabine and zebularine exert differential effects on cancer gene expression in acute myeloid leukemia cells. Leukemia. 2009;23(6):1019–28. doi: 10.1038/leu.2008.397. [DOI] [PubMed] [Google Scholar]
- 32.Wong YF, Jakt LM, Nishikawa S. Prolonged treatment with DNMT inhibitors induces distinct effects in promoters and gene-bodies. PLoS One. 2013;8(8):e71099. doi: 10.1371/journal.pone.0071099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saunthararajah Y, Sekeres M, Advani A, Mahfouz R, Durkin L, Radivoyevitch T, et al. Evaluation of noncytotoxic DNMT1-depleting therapy in patients with myelodysplastic syndromes. J Clin Invest. 2015;125(3):1043–55. doi: 10.1172/JCI78789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agrawal S, Hofmann WK, Tidow N, Ehrich M, van den Boom D, Koschmieder S, et al. The C/EBPdelta tumor suppressor is silenced by hypermethylation in acute myeloid leukemia. Blood. 2007;109(9):3895–905. doi: 10.1182/blood-2006-08-040147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.