Abstract
Although it is generally assumed that herbivores have more voluminous body cavities due to larger digestive tracts required for the digestion of plant fiber, this concept has not been addressed quantitatively. We estimated the volume of the torso in 126 terrestrial tetrapods (synapsids including basal synapsids and mammals, and diapsids including birds, non‐avian dinosaurs and reptiles) classified as either herbivore or carnivore in digital models of mounted skeletons, using the convex hull method. The difference in relative torso volume between diet types was significant in mammals, where relative torso volumes of herbivores were about twice as large as that of carnivores, supporting the general hypothesis. However, this effect was not evident in diapsids. This may either reflect the difficulty to reliably reconstruct mounted skeletons in non‐avian dinosaurs, or a fundamental difference in the bauplan of different groups of tetrapods, for example due to differences in respiratory anatomy. Evidently, the condition in mammals should not be automatically assumed in other, including more basal, tetrapod lineages. In both synapsids and diapsids, large animals showed a high degree of divergence with respect to the proportion of their convex hull directly supported by bone, with animals like elephants or Triceratops having a low proportion, and animals such as rhinoceros having a high proportion of bony support. The relevance of this difference remains to be further investigated.
Keywords: anatomy, carnivory, digestive tract, herbivory, photogrammetry, ribcage
Introduction
Tetrapods have diversified into an enormous variety of body forms that display convergent evolution at various levels of organismal design. For example, the gastrointestinal tract (GIT) is adapted in size and shape to an animal's diet (Cuvier & Duméril, 1838; Treves, 1886). In broad terms, the diets of herbivorous animals are less easily digested than those of carnivores, and require both the presence of a large number of symbiotic gut microbes and time for these microbes to perform their digestive function (Stevens & Hume, 1998). Therefore, in order to accommodate this large microbiome, and to delay digesta passage, the GITs of herbivores are typically considered to be particularly long and/or voluminous (Cuvier & Duméril, 1838; Orr, 1976).
Differences in the length of the intestinal tract according to diet have been repeatedly shown for fish (Wagner et al. 2009; Karachle & Stergiou, 2010), lizards (O'Grady et al. 2005), and in other animal lineages such as invertebrates (Griffen & Mosblack, 2011), but not convincingly in birds (DeGolier et al. 1999; Lavin et al. 2008). In mammals, similar evidence is questionable and mostly limited to small body sizes (Barry, 1977; Wang et al. 2003). Chivers & Hladik (1980) calculated lower volumes of the combined stomach, caecum and colon (from linear GIT dimensions) for mammalian carnivores as compared with herbivores of similar cubic body length, and Schiek & Millar (1985) found more GIT tissue mass in herbivorous than carnivorous small mammals up to about 1 kg. However, Starck (1982) doubted that trophic groups can really be distinguished by the length of their intestinal tracts, and Lavin et al. (2008) did not detect a difference in the small intestinal length or volume in small mammals of different diet types. A major difficulty in such comparisons may be that the most relevant characteristic, a measure of gut fill, is available for a large number of herbivore species (Clauss et al. 2013) because their digestive tract usually always contains a relatively constant amount of digesta, but is not similarly available for carnivores where gut contents may vary enormously (Potgieter & Davies‐Mostert, 2012).
Nevertheless, a voluminous torso that can host a voluminous GIT is considered a prerequisite for high‐fiber herbivory (Hotton et al. 1997), and the appearance of the torso – as judged from articulated skeletons or the shape of ribs – is considered an indication for a diet type in fossil and extant tetrapods (Hotton et al. 1997; Sues & Reisz, 1998; Reisz & Sues, 2000), including hominids (Bryant, 1915; Aiello & Wheeler, 1995). However, quantitative tests of this concept are lacking. In this manuscript, we intended to test whether the volume of the body cavity (coelomic or the combination of thorax and abdomen), as reconstructed from mounted skeletons of various terrestrial tetrapods, differs systematically with the diet typically ascribed to these species. We hypothesized herbivores to have larger body cavities for a given body size than carnivores. Additionally, we expected that among herbivorous non‐avian dinosaurs, species without adaptations for ingesta particle size comminution (such as a grinding mastication or a gizzard) should have more voluminous body cavities than species with such adaptations, because a voluminous gut and the corresponding long digesta retention times can compensate for a lack of particle size reduction (Clauss et al. 2009; Hummel & Clauss, 2011).
Materials and methods
We compiled a dataset of digital 3D models of 11 mounted mammal skeletons available from Sellers et al. (2012), from 19 previously performed scans (Gunga et al. 1999, 2007, 2008; Stoinski et al. 2011), and additionally from our own reconstruction of 96 specimens based on photogrammetry. If, for a species available from Sellers et al. (2012) we also had a skeleton model of our own, we used our own model. All skeletal material was photographed with permission of the respective museum or institution. Although rarely discussed in detail (Bates et al. 2009b; Hutchinson et al. 2011; Sellers et al. 2012; Brassey & Sellers, 2014), a typical issue in dealing with mounted skeletons is the quality of the mount; whenever discussed, the positioning of the ribs and the intervertebral spaces are among the characteristics considered particularly critical. Because for our study the torso was the main target, we did not focus on the quality of other mounted parts (such as the neck, head or tail). For the torso, we only chose mounts in which the ribs were in a fixed position (as opposed to ‘dangling loosely’), where the rib cage did not have a ‘compressed’ appearance (such as in mounts where the osseous ventral ends of the ribs appeared too close to allow for a cartilaginous part or a sternum), and where the articular facets of the ribs and the thoracic vertebrae apposed each other. This resulted in 126 digital skeletons of tetrapods including 86 synapsids (10 ‘mammal‐like reptiles’ or basal synapsids and 76 fossil and extant mammals), 38 diapsids (six extant birds, 27 non‐avian dinosaurs, five fossil and extant reptiles), and two amphibians. Of these, 31 were categorized as carnivores and 95 as herbivores (Table S1).
For reconstruction from multiple images, we first made a series of overlapping photographs from a large number of positions in a circle around the specimen. The images were acquired with a Canon 600D DSLR camera, in most of the cases mounted on a tripod. For the majority of reconstructions we used an image resolution of 2592 × 1728 pixels, because we found this quality to be sufficient for our purposes. The 3D models were computed from these image sequences using publicly available structure‐from‐motion software Visual SFM (Wu, 2007, 2012; Wu et al. 2011) and Bundler (Snavely et al. 2006), and multiview stereo software PMVS2 (Furukawa & Ponce, 2010). The resulting reconstructions (Fig. 1a) were then scaled to true size. For this purpose, we measured several distances on the skeletal specimens or its location (such as the length of boards on which specimens were mounted), identified them in the point cloud and scaled the reconstruction accordingly. We cleaned the point clouds from the background, from supporting structures (such as poles on which bones were mounted) that would interfere with the reconstruction of the convex hull of the torso, and reconstruction artefacts (Fig. 1b). The 3D reconstructions used from previous sources resembled, in their state, those produced during the present study at this stage.
Figure 1.
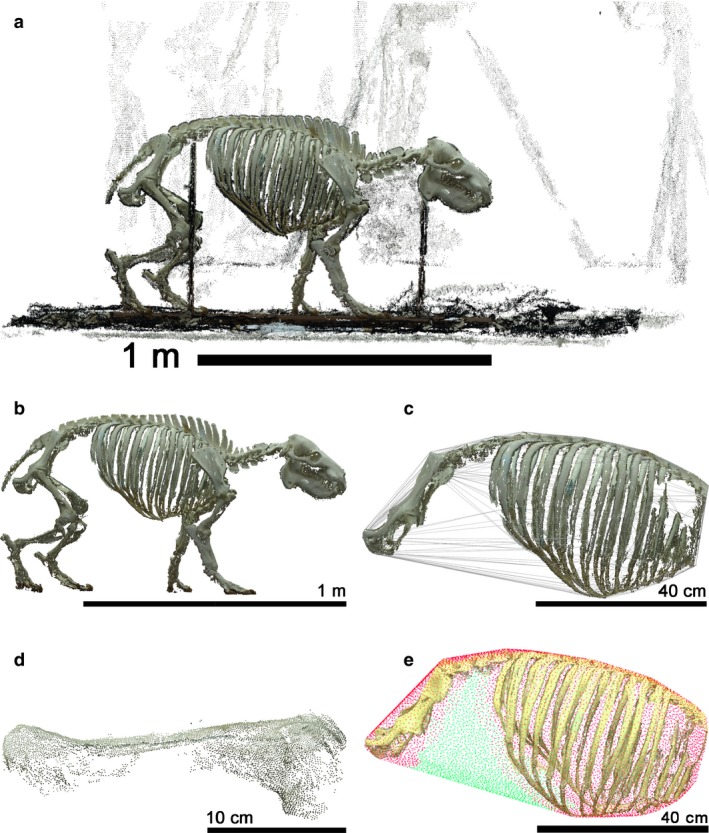
Illustration of the image processing for Hexaprotodon liberiensis. The raw data (a) were scaled, cleaned of background and supporting structures (b). The torso was isolated, removing structures that would influence the convex hull in a way not corresponding to the actual body cavity, for example the spinal processes. Then the convex hull was calculated (c). Note the absence of ribs in the area where they had been covered by the scapula. Finally, the femur was isolated (d) to measure its length. The convex hull was later divided (e) into parts that are supported by bone (red dots) and parts that are not (green dots), to estimate the ‘free‐hull ratio’.
From this stage onwards, the workflow was identical for 3D reconstructions from previous sources and the ones generated for the present study. Side views of all 3D reconstructions used in this study are given as Figs S1–S5, and the original 3D reconstructions can be accessed at Morphobank (www.morphobank.org, Project P2404). The torsos were segmented out using open source software Meshlab (Cignoni et al. 2008). In doing so, care was taken to remove from torsos all aspects that do not contribute to the volume of the body cavity, such as the spinal processes of the vertebrae. Then, the volumes of convex hulls (Sellers et al. 2012; Brassey & Sellers, 2014; Fig. 1c) of the torsos were calculated using Point Cloud Library (Aldoma et al. 2012). Five torsos that were reconstructed mainly from one side were digitally mirrored (indicated in Table S1). In eight cases, the convex hull of the torso was not plausible and included additional space, for example lateral to the ribcage; in these cases, the torso was digitally cut into two parts (typically at the level of the last rib) and the convex hull calculated for each part, and the resulting individual volumes added together (specimens indicated in Table S1).
In comparative analyses, it is necessary to correct for body size. Typically, this is done using body mass (Peters, 1983; Calder, 1996; Sibly et al. 2012), and alternatives are mostly only resorted to if body mass itself is not available. Body mass measures were not available for the specimens from which the skeletons for the present study had been taken and, therefore, a skeletal proxy for body mass had to be found. However, also methodological considerations argue against using body mass in this case: the volume of the torso represents a major proportion of overall body mass and, therefore, differences in torso volume most certainly are reflected in body mass differences already. ‘Correcting’ for body mass (rather than for body size) would hence most likely diminish any potential trophic signal. On the other hand, body mass itself might serve as a proxy for body cavity volume when compared with another size proxy. Please refer to Supporting Information for a more detailed discussion and a demonstration of this concept in Tables S3 and S4. Because body mass itself is not a useful proxy for the question of our study, mass reconstructions from convex hull volumes of the complete skeletons were not considered a valid option. Given the nature of our data, the most promising candidate was femur length (Campione & Evans, 2012). The femur length was calculated as the length of the bounding box of the thighbone (Fig. 1d). For this, we aligned the bone to the axis using principal component analysis (Jolliffe, 2002). The first principal axis, which is the axis of the largest variation of the data, for the thighbone usually corresponds to the main direction in which the bone is elongated.
As a proxy for the proportion of the convex hull of the abdominal cavity that was not ‘supported’ by bony structures (i.e. a proxy for how much of the abdominal wall reconstructed as the convex hull spanned ‘open distances’ in the mounted skeleton), we calculated the ‘free‐hull ratio’. We sampled 8000 evenly distributed points (with constant distance between the points for a given skeleton) on the convex hull, labeled every sample of it as ‘supported’ or ‘non‐supported’ (purple and green dots, respectively, in Fig. 1e), and calculated the ratio of the number of ‘non‐supported’ points to the number of all points. Labels were ascribed by the following procedure. For each 3D point on the skeleton we determined the closest point on the convex hull and marked all sampled points within a certain distance of it as ‘supported’. This distance had to be adapted to the size of the animal; we took 3% of the diagonal of the bounding box of the total animal model as determined by principal component analysis (Jolliffe, 2002). We used the region growing method from Point Cloud Library (Aldoma et al. 2012) to cluster the points with the same labels together. We took the largest cluster of ‘non‐supported’ points, which usually corresponded to the area of the abdominal wall (and discarded the cases when it did not). A higher ‘free‐hull ratio’ indicates that a larger proportion of the body cavity is delineated by soft tissue (i.e. the abdominal wall).
Species were classified as herbivores or carnivores (thus omitting more subtle categories such as omnivores) based on the main category of diet items, using a variety of sources (Walls, 1981; Losos & Greene, 1988; Rand et al. 1990; Weishampel et al. 1990; Reisz & Sues, 2000; Reisz, 2006; Wilman et al. 2014), including the Paleobiology Database (www.paleobiodb.org). Herbivorous dinosaurs were classified as chewers or non‐chewers following Weishampel et al. (1990), and considering sauropods as neither chewing nor grinding ingesta in a gizzard (Wings & Sander, 2007; classifications in Table S1).
We analyzed the influence of diet on the volume of the torso or the free‐hull ratio as related to femur length, accounting for phylogeny based on a tree constructed from literature data [the basic topology of tetrapod groups is based on tree of life project (Maddison & Schulz, 2007) supplemented with specific references]. See Supporting Information for a detailed description of Data S1.
Data were evaluated as
and
using log‐transformed data and diet type (carnivore or herbivore), chewing type (in non‐avian dinosaur herbivores: chewers and non‐chewers) or various taxonomic factors in addition, as indicated in Tables 1 and 2. When using an additional factor, first a model that included the femur length‐factor interaction was used; if the interaction was not significant, the same model without the interaction was used. For example, if the (factor) term was coded, for diet, as carnivore = 0 and herbivore = 1, then the resulting factor estimate z can be translated into ‘herbivores have a z times larger torso volume than carnivores’. To account for the phylogenetic non‐independence of data, analyses were performed using phylogenetic generalized least squares (PGLS). The phylogenetic signal (λ) was estimated using maximum likelihood (Revell, 2010). λ can vary between 0 (no phylogenetic signal) and 1 (strong phylogenetic signal; similarity among species scales in proportion to their shared evolutionary time), i.e. we assumed Pagel's correlation structure (Pagel, 1999; Freckleton et al. 2002). Statistical tests were performed using the package CAPER (Orme et al. 2010) in R 2.15.0 (Team RDC, 2011). Results of analyses with ordinary least squares (OLS), i.e. without accounting for the phylogenetic structure of the data, using the package nlme (Pinheiro et al. 2011), are also reported. Note that for some analyses that specifically address a question linked to phylogeny, such as the question whether basal synapsids differ from all other groups, analyses that ‘correct’ for the phylogenetic relationships cannot provide a relevant answer. The significance level was set to 0.05. Based on the general geometric relationship between a length and a volume measure, we expected torso volumes to scale approximately with femur length to the cubic power (length3).
Table 1.
Results of statistical analyses according to torso volume = a (factor) femur lengthb (and the corresponding factor × femur length interaction) in OLS and PGLS
| Stats | λ | a (95% CI) | P | b (95% CI) | P | Factord (95% CI) | P | Interactione P |
|---|---|---|---|---|---|---|---|---|
| All specimens (n = 126) | ||||||||
| OLS | (0) | 2.23 (1.38, 3.59) | 0.001 | 2.97 (2.84, 3.11) | < 0.001 | – | – | – |
| PGLS | 0.906b | 5.20 (2.47, 10.93) | < 0.001 | 3.04 (2.88, 3.21) | < 0.001 | – | – | – |
| Synapsid/Diapsid | ||||||||
| OLS | (0) | 1.75 (0.97, 3.16) | 0.067 | 3.01 (2.86, 3.15) | < 0.001 | 1.21 (0.92, 1.60) | 0.178 | n.s. |
| PGLS | 0.904b | 7.59 (2.94, 19.61) | < 0.001 | 3.03 (2.87, 3.20) | < 0.001 | 0.70 (0.41, 1.22) | 0.215 | n.s. |
| Basal synapsid | ||||||||
| OLS | (0) | 1.70 (1.13, 2.57) | 0.013 | 3.02 (2.90, 3.13) | < 0.001 | 3.64 (2.52, 5.26) | < 0.001 | n.s. |
| PGLS | 0.907b | 5.29 (2.51, 11.17) | < 0.001 | 3.04 (2.87, 3.21) | < 0.001 | 0.81 (0.47, 1.40) | 0.449 | n.s. |
| Diet | ||||||||
| OLS | (0) | 1.94 (1.21, 3.09) | 0.007 | 2.92 (2.78, 3.05) | < 0.001 | 1.57 (1.19, 2.08) | 0.002 | n.s. |
| PGLS | 0.872b | 4.81 (2.39, 9.66) | < 0.001 | 3.01 (2.84, 3.17) | < 0.001 | 1.48 (1.13, 1.95) | 0.005 | n.s. |
| All carnivores (n = 31) | ||||||||
| OLS | (0) | 2.38 (0.92, 6.16) | 0.085 | 2.85 (2.55, 3.15) | < 0.001 | – | – | – |
| PGLS | 0.922a | 8.93 (2.66, 29.95) | 0.001 | 2.79 (2.45, 3.13) | < 0.001 | – | – | – |
| All herbivores (n = 95) | ||||||||
| OLS | (0) | 2.83 (1.64, 4.90) | < 0.001 | 2.94 (2.79, 3.08) | < 0.001 | – | – | – |
| PGLS | 0.918b | 5.74 (2.58, 12.74) | < 0.001 | 3.06 (2.88, 3.25) | < 0.001 | – | – | – |
| Synapsids (n = 86) | ||||||||
| OLS | (0) | 1.71 (0.89, 3.28) | 0.112 | 3.07 (2.87, 3.26) | < 0.001 | – | – | – |
| PGLS | 0.926b | 4.47 (2.14, 9.34) | < 0.001 | 3.13 (2.93, 3.33) | < 0.001 | – | – | – |
| Basal synapsid | ||||||||
| OLS | (0) | 1.35 (0.78, 2.33) | 0.285 | 3.09 (2.93, 3.26) | < 0.001 | 3.45 (2.34, 5.08) | < 0.001 | n.s. |
| PGLS | 0.920b | 1.68 (0.32, 8.73) | 0.539 | 3.13 (2.93, 3.33) | < 0.001 | 2.66 (0.61, 11.66) | 0.199 | n.s. |
| Diet | ||||||||
| OLS | (0) | 1.51 (0.81, 2.81) | 0.202 | 2.98 (2.79, 3.18) | < 0.001 | 1.72 (1.23, 2.40) | 0.002 | n.s. |
| PGLS | 0.926b | 13.12 (4.10, 42.02) | < 0.001 | 2.73 (2.35, 3.10) | < 0.001 | 0.31 (0.09, 1.14) | 0.082 | 0.028 |
| Basal synapsids (n = 10) | ||||||||
| OLS | (0) | 0.31 (0.01, 7.79) | 0.499 | 3.96 (2.94, 4.98) | < 0.001 | – | – | – |
| PGLS | 0 | 0.50 (0.02, 12.99) | 0.685 | 3.83 (2.77, 4.89) | < 0.001 | – | – | – |
| Mammals (n = 76) | ||||||||
| OLS | (0) | 1.45 (0.84, 2.50) | 0.189 | 3.07 (2.91, 3.24) | < 0.001 | – | – | – |
| PGLS | 0.703b | 1.44 (0.72, 2.88) | 0.300 | 3.07 (2.90, 3.24) | < 0.001 | – | – | – |
| Diet | ||||||||
| OLS | (0) | 1.12 (0.70, 1.81) | 0.640 | 2.98 (2.84, 3.12) | < 0.001 | 2.08 (1.58, 2.73) | < 0.001 | n.s. |
| PGLS | 0.476 | 1.19 (0.63, 2.24) | 0.598 | 3.02 (2.86, 3.19) | < 0.001 | 1.56 (1.06, 2.29) | 0.027 | n.s. |
| Mammal carnivores (n = 18) | ||||||||
| OLS | (0) | 1.93 (0.89, 4.19) | 0.117 | 2.79 (2.53, 3.05) | < 0.001 | – | – | – |
| PGLS | 0 | 1.91 (0.88, 4.17) | 0.122 | 2.80 (2.54, 3.05) | < 0.001 | – | – | – |
| Mammal herbivores (n = 58) | ||||||||
| OLS | (0) | 1.95 (1.09, 3.49) | 0.028 | 3.03 (2.86, 3.20) | < 0.001 | – | – | – |
| PGLS | 0.755 | 1.25 (0.55, 2.83) | 0.592 | 3.15 (2.95, 3.35) | < 0.001 | – | – | – |
| Diapsids (n = 38) | ||||||||
| OLS | (0) | 2.01 (0.95, 4.23) | 0.075 | 2.96 (2.78, 3.15) | < 0.001 | – | – | – |
| PGLS | 0 | 2.88 (1.24, 6.69) | 0.019 | 2.88 (2.68, 3.09) | 0.127 | – | – | – |
| Diet | ||||||||
| OLS | (0) | 1.84 (0.85, 3.97) | 0.131 | 2.94 (2.75, 3.13) | < 0.001 | 1.25 (0.78, 2.03) | 0.363 | n.s. |
| PGLS | 0 | 2.32 (0.95, 5.67) | 0.074 | 2.87 (2.66, 3.07) | < 0.001 | 1.42 (0.84, 2.38) | 0.197 | n.s. |
| Diapsid carnivores (n = 8) | ||||||||
| OLS | (0) | 2.18 (0.53, 8.99) | 0.324 | 2.89 (2.50, 3.29) | < 0.001 | – | – | – |
| PGLS | 1c | 1.72 (0.43, 6.79) | 0.471 | 3.01 (2.66, 3.37) | < 0.001 | – | – | – |
| Diapsid herbivores (n = 30) | ||||||||
| OLS | (0) | 2.12 (0.84, 5.39) | 0.124 | 2.96 (2.74, 3.18) | < 0.001 | – | – | – |
| PGLS | 0 | 3.31 (1.21, 9.09) | 0.027 | 2.86 (2.62, 3.11) | < 0.001 | – | – | – |
| Non‐avian dinosaurs (n = 27) | ||||||||
| OLS | (0) | 2.87 (0.67, 12.30) | 0.168 | 2.89 (2.56, 3.21) | < 0.001 | – | – | – |
| PGLS | 0.651b | 2.05 (0.54, 7.83) | 0.303 | 2.96 (2.65, 3.27) | < 0.001 | – | – | – |
| Diet | ||||||||
| OLS | (0) | 2.15 (0.47, 9.84) | 0.333 | 2.89 (2.57, 3.21) | < 0.001 | 1.37 (0.81, 2.31) | 0.248 | n.s. |
| PGLS | 0.604 | 1.43 (0.32, 6.49) | 0.647 | 2.97 (2.66, 3.29) | < 0.001 | 1.40 (0.74, 2.66) | 0.317 | n.s. |
| Non‐avian dinosaur herbivores (n = 23) | ||||||||
| OLS | (0) | 3.19 (0.68, 14.86) | 0.155 | 2.87 (2.53, 3.22) | < 0.001 | – | – | – |
| PGLS | 0.639 | 2.00 (0.47, 8.45) | 0.358 | 2.97 (2.64, 3.31) | < 0.001 | – | – | – |
| Chewer | ||||||||
| OLS | (0) | 2.87 (0.63, 13.05) | 0.189 | 2.84 (2.50, 3.18) | < 0.001 | 1.39 (0.87, 2.23) | 0.187 | n.s. |
| PGLS | 0.649 | 2.14 (0.31, 14.61) | 0.445 | 2.97 (2.60, 3.34) | < 0.001 | 0.96 (0.45, 2.02) | 0.907 | n.s. |
Torso volume in cm3, femur length in cm.
λ significantly different from 0.
λ significantly different from 0 and 1.
λ not significantly different from 0 and 1.
Factor coding: diet (carnivore = 0, herbivore = 1), synapsid/diapsid (diapsid = 0, synapsid = 1), basal synapsid (no basal synapsid = 0, basal synapsid = 1), chewer (chewer = 0, non‐chewer = 1).
Models were calculated with interaction term first; if this was not significant, the model was again calculated without the interaction term; estimates for the factor in this table always represent the models where either the interaction was significant or excluded.
OLS, ordinary least squares; PGLS, phylogenetic generalized least squares.
Table 2.
Results of statistical analyses according to free‐hull ratio = a (factor) femur lengthb (and the corresponding factor × femur length interaction) in OLS and PGLS
| Stats | λ | a (95% CI) | P | b (95% CI) | P | Factorb (95% CI) | P | Interactionc P |
|---|---|---|---|---|---|---|---|---|
| All specimens (n = 126) | ||||||||
| OLS | (0) | 0.37 (0.29, 0.48) | < 0.001 | −0.19 (−0.26, −0.11) | < 0.001 | – | – | – |
| PGLS | 0.693a | 0.32 (0.21, 0.49) | < 0.001 | −0.17 (−0.27, −0.06) | 0.002 | – | – | – |
| Synapsid/Diapsid | ||||||||
| OLS | (0) | 0.38 (0.27, 0.52) | < 0.001 | −0.19 (−0.27, −0.11) | < 0.001 | 0.99 (0.85, 1.15) | 0.891 | n.s. |
| PGLS | 0.687a | 0.28 (0.16, 0.47) | < 0.001 | −0.16 (−0.27, −0.06) | 0.003 | 1.16 (0.84, 1.61) | 0.373 | n.s. |
| Basal synapsid | ||||||||
| OLS | (0) | 0.39 (0.30, 0.50) | < 0.001 | −0.19 (−0.27, −0.12) | < 0.001 | 0.85 (0.67, 1.08) | 0.182 | n.s. |
| PGLS | 0.694a | 0.32 (0.21, 0.49) | < 0.001 | −0.17 (−0.27, −0.06) | 0.002 | 1.02 (0.72, 1.44) | 0.929 | n.s. |
| Diet | ||||||||
| OLS | (0) | 0.37 (0.28, 0.48) | < 0.001 | −0.19 (−0.27, −0.12) | < 0.001 | 1.05 (0.90, 1.23) | 0.527 | n.s. |
| PGLS | 0.709a | 0.31 (0.21, 0.47) | < 0.001 | −0.18 (−0.29, −0.08) | 0.001 | 1.18 (0.99, 1.41) | 0.066 | n.s. |
| All carnivores (n = 31) | ||||||||
| OLS | (0) | 0.28 (0.19, 0.43) | < 0.001 | −0.11 (−0.24, 0.02) | 0.112 | – | – | – |
| PGLS | 1.000a | 0.22 (0.12, 0.40) | < 0.001 | −0.08 (−0.23, 0.07) | 0.290 | – | – | – |
| All herbivores (n = 95) | ||||||||
| OLS | (0) | 0.43 (0.30, 0.59) | < 0.001 | −0.22 (−0.31, −0.13) | < 0.001 | – | – | – |
| PGLS | 0.511a | 0.39 (0.25, 0.59) | < 0.001 | −0.22 (−0.33, −0.10) | < 0.001 | – | – | – |
| Synapsids (n = 86) | ||||||||
| OLS | (0) | 0.41 (0.29, 0.59) | < 0.001 | −0.22 (−0.32, −0.11) | < 0.001 | – | – | – |
| PGLS | 0.882a | 0.19 (0.11, 0.33) | < 0.001 | −0.17 (−0.31, −0.02) | 0.028 | – | – | – |
| Basal synapsid | ||||||||
| OLS | (0) | 0.43 (0.30, 0.61) | < 0.001 | −0.22 (−0.33, −0.12) | < 0.001 | 0.83 (0.65, 1.06) | 0.140 | n.s. |
| PGLS | 0.796a | 0.28 (0.11, 0.74) | 0.012 | −0.21 (−0.36, −0.06) | 0.006 | 0.19 (0.04, 0.90) | 0.040 | 0.031 |
| Diet | ||||||||
| OLS | (0) | 0.41 (0.29, 0.59) | < 0.001 | −0.22 (−0.33, −0.11) | < 0.001 | 1.02 (0.84, 1.22) | 0.876 | n.s. |
| PGLS | 0.826a | 0.20 (0.12, 0.33) | < 0.001 | −0.21 (−0.35, −0.07) | 0.005 | 1.33 (1.08, 1.64) | 0.010 | n.s. |
| Basal synapsids (n = 10) | ||||||||
| OLS | (0) | 0.09 (0.01, 0.81) | 0.064 | 0.22 (−0.48, 0.91) | 0.563 | – | – | – |
| PGLS | 0a | 0.04 (0.00, 0.44) | 0.031 | 0.46 (−0.34, 1.26) | 0.292 | – | – | – |
| Mammals (n = 76) | ||||||||
| OLS | (0) | 0.45 (0.31, 0.63) | < 0.001 | −0.23 (−0.34, −0.13) | < 0.001 | – | – | – |
| PGLS | 0.180 | 0.46 (0.31, 0.67) | < 0.001 | −0.22 (−0.33, −0.11) | < 0.001 | – | – | – |
| Diet | ||||||||
| OLS | (0) | 0.46 (0.32, 0.65) | < 0.001 | −0.23 (−0.33, −0.12) | < 0.001 | 0.93 (0.76, 1.14) | 0.509 | n.s. |
| PGLS | 0.171 | 0.47 (0.31, 0.70) | < 0.001 | −0.22 (−0.33, −0.10) | < 0.001 | 0.96 (0.76, 1.22) | 0.756 | n.s. |
| Mammal carnivores (n = 18) | ||||||||
| OLS | (0) | 0.31 (0.22, 0.43) | < 0.001 | −0.09 (−0.21, 0.03) | 0.146 | – | – | – |
| PGLS | 0.709a | 0.32 (0.22, 0.46) | < 0.001 | −0.11 (−0.23, 0.01) | 0.084 | – | – | – |
| Mammal herbivores (n = 58) | ||||||||
| OLS | (0) | 0.48 (0.31, 0.77) | 0.031 | −0.26 (−0.40, −0.13) | < 0.001 | – | – | – |
| PGLS | 0.147 | 0.53 (0.32, 0.87) | 0.015 | −0.26 (−0.40, −0.12) | 0.001 | – | – | – |
| Diapsids (n = 38) | ||||||||
| OLS | (0) | 0.31 (0.19, 0.50) | < 0.001 | −0.14 (−0.26, −0.02) | 0.029 | – | – | – |
| PGLS | 0.609a | 0.30 (0.16, 0.54) | < 0.001 | −0.17 (−0.33, −0.02) | 0.036 | – | – | – |
| Diet | ||||||||
| OLS | (0) | 0.28 (0.17, 0.47) | < 0.001 | −0.16 (−0.28, −0.04) | 0.015 | 1.24 (0.91, 1.68) | 0.186 | n.s. |
| PGLS | 0.600a | 0.29 (0.15, 0.58) | 0.001 | −0.17 (−0.33, −0.01) | 0.039 | 1.03 (0.72, 1.48) | 0.866 | n.s. |
| Diapsid carnivores (n = 8) | ||||||||
| OLS | (0) | 0.20 (0.11, 0.37) | 0.002 | −0.06 (−0.23, 0.11) | 0.503 | – | – | – |
| PGLS | 0.613a | 0.21 (0.11, 0.38) | 0.003 | −0.08 (−0.24, 0.08) | 0.369 | – | – | – |
| Diapsid herbivores (n = 30) | ||||||||
| OLS | (0) | 0.41 (0.22, 0.77) | 0.010 | −0.20 (−0.35, −0.05) | 0.015 | – | – | – |
| PGLS | 0.633a | 0.35 (0.16, 0.73) | 0.010 | −0.20 (−0.38, −0.01) | 0.050 | – | – | – |
| Non‐avian dinosaurs (n = 27) | ||||||||
| OLS | (0) | 1.57 (0.57, 4.34) | 0.391 | −0.49 (−0.72, −0.27) | < 0.001 | – | – | – |
| PGLS | 0.764a | 0.39 (0.11, 1.39) | 0.161 | −0.24 (−0.53, 0.06) | 0.127 | – | – | – |
| Diet | ||||||||
| OLS | (0) | 1.38 (0.47, 4.07) | 0.562 | −0.49 (−0.72, −0.26) | < 0.001 | 1.15 (0.79, 1.67) | 0.464 | n.s. |
| PGLS | 0.766a | 0.48 (0.11, 2.10) | 0.338 | −0.25 (−0.55, 0.05) | 0.122 | 0.84 (0.43, 1.66) | 0.621 | n.s. |
| Non‐avian dinosaur herbivores (n = 23) | ||||||||
| OLS | (0) | 1.70 (0.57, 5.08) | 0.355 | −0.51 (−0.75, −0.26) | 0.001 | – | – | – |
| PGLS | 0.857a | 0.44 (0.11, 1.79) | 0.264 | −0.27 (−0.60, 0.06) | 0.122 | – | – | – |
| Chewer | ||||||||
| OLS | (0) | 1.92 (0.71, 5.18) | 0.212 | −0.47 (−0.69, −0.25) | 0.001 | 0.68 (0.50, 0.93) | 0.025 | n.s. |
| PGLS | 0.713a | 0.93 (0.15, 5.70) | 0.938 | −0.35 (−0.69, 0.00) | 0.063 | 0.64 (0.31, 1.30) | 0.233 | n.s. |
Free‐hull ratio represents the proportion of the convex hull reconstruction of the torso not immediately supported by bone; femur length in cm.
λ significantly different from 0.
λ significantly different from 0 and 1.
λ not significantly different from 0 and 1.
Factor coding: diet (carnivore = 0, herbivore = 1), synapsid/diapsid (diapsid = 0, synapsid = 1), basal synapsid (no basal synapsid = 0, basal synapsid = 1), chewer (chewer = 0, non‐chewer = 1).
Models were calculated with interaction term first; if this was not significant, the model was again calculated without the interaction term; estimates for the factor in this table always represent the models where either the interaction was significant or excluded.
OLS, ordinary least squares; PGLS, phylogenetic generalized least squares.
Results
Generally, torso volume scaled to femur length at an exponent that included the cubic power (i.e. femur length3.0) in the 95% confidence interval, as expected for a geometric scaling of a volume–distance relationship (Table 1). This overall scaling did not differ between synapsids and diapsids (Table 1). However, the basal synapsids had torso volumes about 3.5 times larger than all the other clades (Table 1; Fig. 2a).
Figure 2.
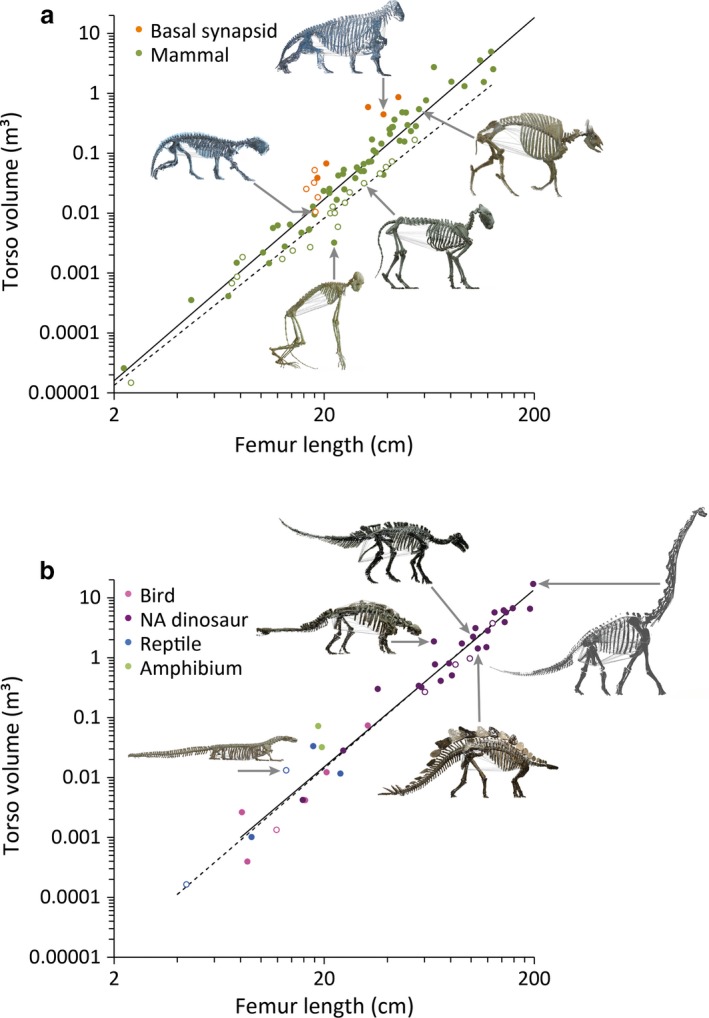
Relationship between the femur length (as proxy for body size) and the reconstructed volume of the body cavity in (a) synapsids and (b) diapsids. Closed symbols and full regression lines (cf. Table 1) indicate herbivores (except for the Amphibia), open symbols and dotted line indicate carnivores. Skeletal models with the estimated convex hull of the torso depicted include: (a, from left to right) Lycaenops, Moschops, Nasalis, Panthera leo, Bos gaurus; (b, from left to right) Varanus, Euoplocephalus, Giraffatitan, Stegosaurus, Iguanodon. Regression lines in (a) for mammals, in (b) for all diapsids.
In the overall dataset, diet had a significant effect on the torso volume, with herbivores having about 1.5 times larger torso volumes than carnivores (Table 1). This was due to a clear effect of diet in mammals – the largest clade in our dataset. In mammals, herbivores again had about 1.5 times larger torso volumes than carnivores (Table 1; Fig. 2a). We did not have a sufficient number of basal synapsids to test for a difference between diet types; the visual pattern does not suggest a clear distinction between carnivores and herbivores in this group (Fig. 2a).
In contrast to mammals, there was no significant effect of diet on torso volume in all diapsids or in non‐avian dinosaurs only (Table 1; Fig. 2b). We did not have a sufficient number of birds or reptiles to test for a difference between diet types in these diapsid clades; the visual patterns, however, did not suggest a clear distinction between carnivores and herbivores in these groups, nor in non‐avian dinosaurs (Fig. 2b). Among herbivorous non‐avian dinosaurs, there was no difference in relative torso volume between species with or without a grinding mastication (Table 1, as exemplified by the non‐chewers Giraffatitan, Stegosaurus and Euoplocephalus compared with the chewer Iguanodon in Fig. 2b).
The relationship of the free‐hull ratio and femur length was generally negative, indicating that larger animals had a lower proportion of their body cavity delineated by soft tissue (Table 2). This was evident in both synapsids (Fig. 3a) and diapsids (Fig. 3b). Diet did not have an effect on this relationship (Table 2). Variation in the free‐hull ratio increased with body size (Fig. 3a,b), some animals having a low contribution of bony support to the delineation of the body cavity (such as proboscideans amongst mammals in Fig. 3a or Triceratops among non‐avian dinosaurs in Fig. 3b), and some animals with a ribcage nearly delineating the complete ventral body cavity (such as giraffe or rhinoceros among mammals in Fig. 3a or Diplodocus among non‐avian dinosaurs in Fig. 3b).
Figure 3.
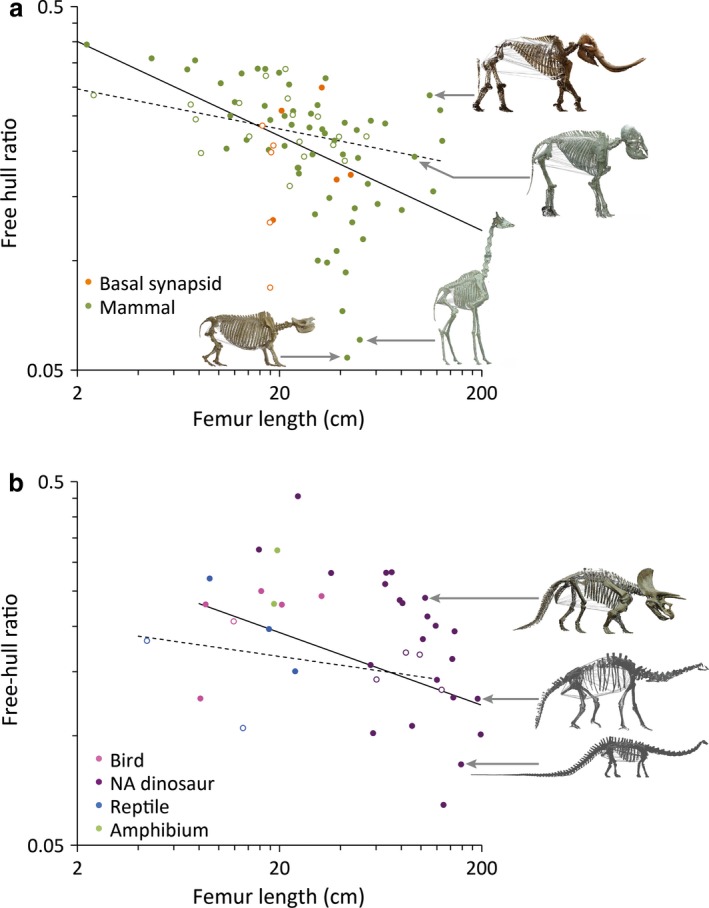
Relationship between the femur length (as proxy for body size) and the proportion of the torso not supported by bone (free‐hull ratio) in (a) synapsids and (b) diapsids. Closed symbols and full regression lines (cf. Table 2) indicate herbivores (except for the Amphibia), open symbols and dotted lines indicate carnivores. Skeletal models with the estimated convex hull of the torso depicted include: (a, from top to bottom) Mammutus, Elephas, Giraffa, Diceros; (b, top to bottom) Triceratops, Atlasaurus, Diplodocus. Regression lines in (a) for mammals, in (b) for all diapsids.
Discussion
The hypothesis that herbivores have more voluminous body cavities than carnivores was confirmed for the mammals in our dataset. However, no diet effect was detected in diapsids and non‐avian dinosaurs. Considering the overrepresentation of mammals in our dataset, and in particular the low number of birds, reptiles and carnivorous non‐avian dinosaurs, this finding may be due to a restricted sample size, and should be considered explorative for these groups. In this respect, we hope that making our digital skeletons accessible at Morphobank will facilitate similar tests with increased sample sizes as more digital skeletons become available. However, individual findings, such as a particularly large body cavity in a carnivorous varanid (Fig. 2b), possibly indicate that the diet effect observed in mammals need not necessarily be reflected in other groups.
Several important methodological constraints of our study need to be mentioned. The use of femur length as a proxy for body size might not be considered ideal, also because measurements were not taken on the original skeletons but, to grant consistency across all 3D models used, on the digitally isolated 3D reconstruction of the femur. Inaccurate measurements, such as underestimation of femur length due to overlap of other skeletal structures such as the acetabulum, may evidently occur. Yet, the question about a more suitable proxy than femur length is difficult to answer. As stated in the methods, because the torso volume represents a major proportion of overall body mass, it appears probable that differences in the torso volume–femur length relationship should be mirrored in the body mass–femur length relationship. See Supporting Information for an explorative analysis suggesting support for this hypothesis (using literature body mass data in connection with our own measurements). An even more important constraint of studies such as ours is the quality of the skeletal mounts used (Bates et al. 2009b; Hutchinson et al. 2011; Sellers et al. 2012; Brassey & Sellers, 2014; Claessens, 2015). Incorrect reconstructions of rib shape and rib position, exacerbated by a lack of conservation of cartilaginous components of the torso (such as costal and sternal cartilage and intervertebral disks) or small osseous structures (such as components of the pectoral girdle), will greatly influence any measurements derived from skeletal mounts, and are more likely to occur the less familiar a curator is with the species in question. Inherently, this means that fossil specimens underlie a greater uncertainty in this respect than representatives of extant species. Ultimately, concurrent measurements of gut tissue, gut content and body mass as well as body cavity volume in healthy, non‐fasted animals will be required to empirically prove the assumption that extant herbivores carry more weight at similar body size than extant carnivores.
The absence of a diet effect in non‐avian dinosaurs could on the one hand reflect these difficulties in correctly reconstructing skeletal appearance in fossil organisms, in particular the rib cage (Bates et al. 2009a; Claessens, 2015). On the other hand, the absence of a clear diet signal in diapsids could be linked to the bauplan heterogeneity within lineages (e.g. bipedal vs. quadrupedal, which in non‐avian dinosaurs mostly mirrors the herbivore/carnivore dichotomy); or due to an ectothermic or mesothermic metabolism in reptiles and (some) non‐avian dinosaurs (Grady et al. 2014; Werner & Griebeler, 2014) that did not exert a similar selective pressure on optimal body design as endothermy. Heterogeneity might even have occurred on the level of metabolism between dinosaur lineages. Additionally, the respiratory system of diapsids with its heterogenous lung, pneumatized bones and space occupied by variable coelomic air sacs, and unidirectional air flow (O'Connor & Claessens, 2005; Perry et al. 2011; Farmer, 2015) may exert additional selective pressures on the shape of the torso (Claessens, 2015) that are not yet fully understood. A specific prediction about a difference in the body cavity volume between herbivorous non‐avian dinosaurs with and without adaptations for ingesta particle size reduction (Hummel & Clauss, 2011; Clauss et al. 2013) could also not be confirmed in the present study.
In contrast, the general concept of larger body cavity volumes that accommodate larger guts in herbivores is supported for mammals. Reasons for the distinct diet difference in mammals may be the large sample size, the large number of extant specimens (in which constructing correct skeletal mounts may be easier), and the fact that mounts of fossil forms can be more easily constructed with extant species as reference guidelines. Additionally, the high overall mammalian level of metabolism and efficient cursoriality, which might have led to an evolutionary arms race of predators and prey (Lovegrove, 2001) that represented a high level of selective pressure for an optimized torso volume, may be responsible for the clearer separation of diet types. Given that basal synapsids had relatively higher torso volumes than mammals, one could hypothesize an evolutionary optimization or ‘escalation’ (Vermeij, 1987, 2013) of the body shape in the synapsid lineage.
In developing evolutionary arms race scenarios, such as between predators and prey, the effects of differences in body shape with their effect on the center of gravity (Bates et al. 2009b, 2016), differences in the weight of digestive organ tissue (Schiek & Millar, 1985), and especially the effects of putative differences in the weight of digestive tract contents (Müller et al. 2013) should be considered, which may lead to different non‐muscle:muscle ratios in predators and prey. In the context of changes within lineages, such as changes in insular forms in the absence of predators, estimating body cavity dimensions from carefully reconstructed mounted skeletons may provide additional evidence to understand constraints of vertebrate bauplan evolution.
In our dataset, diapsids and synapsids shared the characteristic of an increasing divergence in the ‘free‐hull ratio’ with increasing body size. Some species had a high, and some had a low proportion of the body cavity delineated by soft tissue only. Such differences may be linked to differences in cursoriality (Bramble, 1987), where a more rigid torso (with a lower ‘free‐hull ratio’) may be a prerequisite for galloping. For example, considering the debate about the locomotion capabilities of Triceratops (Thulborn, 1982; Paul & Christiansen, 2000), the similarity of Triceratops to proboscideans (which do not gallop) with respect to an abdominal cavity with particularly little bony support might represent an additional argument against galloping in the former group. Differences in the ‘free‐hull ratio’ may also be related to the degree that the gut can accommodate increasing intake levels by distension without compromising digesta retention times (Clauss et al. 2007).
Examples such as the proboscideans and the proboscis monkey (Nasalis larvatus) in Fig. 2a emphasize a limitation of the convex hull method that may arguably even lead to an underestimation of the real difference between herbivores and carnivores: the part of the convex hull that is not supported by bony structures, and hence is estimated as a relatively straight line, might in reality be a bulging abdominal wall. Whereas in carnivores, the rib cage may usually represent the most ventral part of the torso contour, this lowest point is typically not marked by the rib cage in herbivores, but is positioned posterior to it and marked by the soft‐tissue abdominal wall (Starck, 1982). The reconstruction of this soft tissue border is particularly difficult from mounted skeletons (Bates et al. 2009b). In the proboscis monkey, with its typical bulging belly (Harding, 2015), it seems even as if a reduction in the extent of the rib cage facilitates the extreme expansion of the abdominal cavity – an effect not reflected in the convex hull estimate of the torso in this species. Correspondingly, in our dataset, the proboscis monkey represented an outlier as the mammalian herbivore with the smallest relative torso volume (Fig. 2a). For a more realistic approximation of the total body cavity volumes, more comprehensive studies that include 3D reconstructions of taxidermic specimens or live animals at various stages of food intake levels may be required. To our knowledge, no systematic investigations on these different bauplan strategies exist. In theory, animals could evolve a voluminous body cavity either by soft tissue expansion, by a deepening and broadening of their ribcage and corresponding pelvic structures, or by a combination of both.
In conclusion, differences in the body cavity volume that exist between herbivores and carnivores exist in mammals that most likely reflect differences in the digestive anatomy and physiology between these groups (Stevens & Hume, 1998). The apparent decrease in body cavity volume from basal synapsids to mammals possibly represents an example of evolutionary optimization. In the comparison of dinosaurs with mammals, in addition to questions about the reliability of skeletal reconstructions, our preliminary findings may hint at fundamental bauplan differences linked to the different lung anatomy between synapsids and diapsids, due to different levels of metabolism leading to differences in the distinction in digestive anatomy between trophic guilds, or other hitherto unknown factors.
Data accessibility
The Supporting Information contains images of all skeletons used in this study with their calculated convex hulls, all original measurements and species characteristics, three outliers not considered in the final dataset, and details on the construction of the phylogenetic tree. The Phylogenetic tree as Mesquite (nexus) file (129Tetrapods.nex) as well as all digital 3D skeleton reconstructions are deposited (full skeleton, isolated femur, isolated torso, torso convex hull, mirrored torso where applicable) in Morphobank Project P2404 (http://morphobank.org/permalink/?P2404). Download the .ply files and open in Meshlab (freely available at http://meshlab.sourceforge.net/); if you open the whole skeleton and the hull at the same time, you can see the reconstructed torso hull and the skeleton together.
Author contributions
MC, OH, PMS designed the study. IN, MC, BH acquired the data; HCG provided additional data; IN processed the data; IN, DF, JK, MC prepared the torsos; IN, DF, JK took the digital measurements; CM collated the phylogenetic tree; CM and MC analyzed the resulting measurements. MC, IN, CM prepared the first draft of the manuscript that then received input from all co‐authors.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
Fig. S1. 3D skeleton reconstructions of early synapsids (including indications of the convex hull of the body cavity).
Fig. S2. 3D skeleton reconstructions of mammals (including indications of the convex hull of the body cavity).
Fig. S3. 3D skeleton reconstructions of birds (including indications of the convex hull of the body cavity).
Fig. S4. 3D skeleton reconstructions of non‐avian dinosaurs (including indications of the convex hull of the body cavity).
Fig. S5. 3D skeleton reconstructions of reptiles and amphibia (including indications of the convex hull of the body cavity).
Fig. S6. Phylogenetic tree (also available as supplementary nexus file).
Fig. S7. Visual outlier inspection: exclusion of the flamingo (combination of very long femur and very small torso) and two marine mammals (combination of very short femurs and very voluminous torsos).
Table S1. Specimens used in this study, categories and measurements.
Table S2. Time of divergence provided for different nodes in the tree.
Table S3. Results of statistical analyses according to Torso volume = a (factor) Body massb (and the corresponding factor × body mass interaction) in OLS and PGLS for extant mammals, birds and reptiles (n = 63).
Table S4. Results of statistical analyses according to Body mass = a (factor) Femur lengthb (and the corresponding factor × femur length interaction) in OLS and PGLS for extant mammals, birds and reptiles (n = 63).
Acknowledgements
The authors thank Najat Aquesbi and Mohammed Rochdy (Ministry of Energy and Mines, Rabat), Thomas Bolliger (Sauriermuseum Aathal), Ken Carpenter (Utah State University Eastern Prehistoric Museum), Loic Costeur (Natural History Museum Basle), Rainer Hutterer (Alexander Koenig Research Museum Bonn), Chen‐sen Li (Beijing Museum of Natural History), Mark Norell and Carl Mehling (American Museum of Natural History), Barbara Oberholzer (Zoological Museum, University of Zurich), Daniela Schwarz (Natural History Museum Berlin), Paul Simoens and Marjan Doom (Department of Morphology, Ghent University), Ingmar Werneburg (University of Tübingen), and Ye Yong and Peng Guangzhao (Zigong Dinosaur Museum). Lucas Walstijn and Lida Fanara helped with digital processing. The authors thank two reviewers for insightful comments that improved the manuscript. MC thanks Deborah Sarah Peter for inspiring this work. This study was supported by DFG Grants CL 182/6‐1 and GU 414/3‐1, and is publication no. 172 of the DFG Research Unit 533 Biology of Sauropod Dinosaurs: The Evolution of Gigantism.
References
- Aiello LC, Wheeler P (1995) The expensive tissue hypothesis: the brain and the digestive system in human and primate evolution. Curr Anthropol 36, 199–221. [Google Scholar]
- Aldoma A, Marton ZC, Tombari F, et al. (2012) Tutorial: point cloud library: threedimensional object recognition and 6 DOF pose estimation. IEEE Robot Autom Mag 19, 80–91. [Google Scholar]
- Barry RE (1977) Length and absorptive surface area apportionment of segments of the hindgut for eight species of small mammals. J Mammal 58, 419–420. [PubMed] [Google Scholar]
- Bates KT, Falkingham PL, Breithaupt BH, et al. (2009a) How big was ‘Big Al’? Quantifying the effect of soft tissue and osteological unknowns on mass predictions for Allosaurus (Dinosauria:Theropoda). Palaeontol Electron 12, 14A. [Google Scholar]
- Bates KT, Manning PL, Hodgetts D, et al. (2009b) Estimating mass properties of dinosaurs using laser imaging and 3D computer modelling. PLoS One 4, e4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates KT, Mannion PD, Falkingham PL, et al. (2016) Temporal and phylogenetic evolution of the sauropod dinosaur body plan. R Soc Open Sci 3, 150 636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramble DM (1987) Cursorial specialization of the mammalian thorax. Am Zool 27, 87A. [Google Scholar]
- Brassey CA, Sellers WI (2014) Scaling of convex hull volume to body mass in modern primates, non‐primate mammals and birds. PLoS One 9, e91691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant J (1915) The carnivorous and herbivorous types in man: the possibility and utility of their recognition. I. Introduction and outline. Boston Med Surg J, i, 312–326. [Google Scholar]
- Calder WA (1996) Size, Function and Life History. Cambridge, MA: Harvard University Press. [Google Scholar]
- Campione NE, Evans DC (2012) A universal scaling relationship between body mass and proximal limb bone dimensions in quadrupedal terrestrial tetrapods. BMC Biol, 10, 60 (21 pages). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivers DJ, Hladik CM (1980) Morphology of the gastrointestinal tract in primates: comparisons with other mammals in relation to diet. J Morphol 166, 337–386. [DOI] [PubMed] [Google Scholar]
- Cignoni P, Corsini M, Ranzuglia G (2008) Meshlab: an open‐source 3d mesh processing system. ERCIM News 73, 45–46. [Google Scholar]
- Claessens L (2015) Anatomical transformations and respiratory innovations of the archosaur trunk In: Great Transformations in Vertebrate Evolution. (eds Dial KP, Shubin N, Brainerd EL.), pp. 91–106. Chicago: University of Chicago Press. [Google Scholar]
- Clauss M, Streich WJ, Schwarm A, et al. (2007) The relationship of food intake and ingesta passage predicts feeding ecology in two different megaherbivore groups. Oikos 116, 209–216. [Google Scholar]
- Clauss M, Nunn C, Fritz J, et al. (2009) Evidence for a tradeoff between retention time and chewing efficiency in large mammalian herbivores. Comp Biochem Physiol A 154, 376–382. [DOI] [PubMed] [Google Scholar]
- Clauss M, Steuer P, Müller DWH, et al. (2013) Herbivory and body size: allometries of diet quality and gastrointestinal physiology, and implications for herbivore ecology and dinosaur gigantism. PLoS One 8, e68714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvier G, Duméril AM (1838) Leçons D'anatomie Comparée. Tome Deuxième, Troisième Édition. Bruxelles: Société Typographique Belge. [Google Scholar]
- DeGolier TF, Mahoney SA, Duke GE (1999) Relationships of avian cecal lengths to food habits, taxonomic position, and intestinal lengths. Condor 101, 622–634. [Google Scholar]
- Farmer CG (2015) Similarity of crocodilian and avian lungs indicates unidirectional flow is ancestral for archosaurs. Integr Comp Biol 55, 962–971. [DOI] [PubMed] [Google Scholar]
- Freckleton RP, Harvey PH, Pagel M (2002) Phylogenetic analysis and comparative data: a test and review of evidence. Am Nat 160, 712–726. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Ponce J (2010) Accurate, dense, and robust multiview stereopsis. IEEE Trans Pattern Anal Mach Intell 32, 1362–1376. [DOI] [PubMed] [Google Scholar]
- Grady JM, Enquist BJ, Dettweiler‐Robinson E, et al. (2014) Evidence for mesothermy in dinosaurs. Science 344, 1268–1272. [DOI] [PubMed] [Google Scholar]
- Griffen BD, Mosblack H (2011) Predicting diet and consumption rate differences between and within species using gut ecomorphology. J Anim Ecol 80, 854–863. [DOI] [PubMed] [Google Scholar]
- Gunga H‐C, Kirsch K, Rittweger J, et al. (1999) Body size and body volume distribution in two sauropods from the Upper Jurassic of Tendaguru (Tanzania). Fossil Rec 2, 91–102. [Google Scholar]
- Gunga H‐C, Suthau T, Bellmann A, et al. (2007) Body mass estimations for Plateosaurus engelhardti using laser scanning and 3D reconstruction methods. Naturwissenschaften 94, 623–630. [DOI] [PubMed] [Google Scholar]
- Gunga H‐C, Suthau T, Bellmann A, et al. (2008) A new body mass estimation of Brachiosaurus brancai Janensch, 1914 mounted and exhibited at the Museum of Natural History (Berlin, Germany). Fossil Rec 11, 28–33. [Google Scholar]
- Harding LE (2015) Nasalis larvatus (Primates: Colobini). Mamm Species 47, 84–99. [Google Scholar]
- Hotton N III, Olson EC, Beerbower R (1997) Amniote origins and the discovery of herbivory In: Amniote Origins: Completing the Transition to Land. (eds Sumida SS, Martin KLM.), pp. 207–264. San Diego: Academic Press. [Google Scholar]
- Hummel J, Clauss M (2011) Feeding and digestive physiology In: Understanding the Life of Giants the Biology of the Sauropod Dinosaurs. (eds Klein N, Remes K, Gee CT, Sander M.), pp. 11–33. Bloomington: Indiana University Press. [Google Scholar]
- Hutchinson JR, Bates KT, Molnar J, et al. (2011) A computational analysis of limb and body dimensions in Tyrannosaurus rex with implications for locomotion, ontogeny, and growth. PLoS One 6, e26037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe I (2002) Principal Component Analysis. New York: Springer. [Google Scholar]
- Karachle PK, Stergiou KI (2010) Gut length for several marine fish: relationships with body length and trophic implications. Mar Biodivers Rec 3, e106. [Google Scholar]
- Lavin SR, Karasov WH, Ives AR, et al. (2008) Morphometrics of the avian small intestine compared with that of nonflying mammals: a phylogenetic approach. Physiol Biochem Zool 81, 526–550. [DOI] [PubMed] [Google Scholar]
- Losos JB, Greene HW (1988) Ecological and evolutionary implications of diet in monitor lizards. Biol J Linn Soc 35, 379–407. [Google Scholar]
- Lovegrove BG (2001) The evolution of body armor in mammals: plantigrade constraints of large body size. Evolution 55, 1464–1473. [DOI] [PubMed] [Google Scholar]
- Maddison DR, Schulz K‐S (2007) The tree of life web project. http://tolweb.org. last accessed 20 March 2016.
- Müller DWH, Codron D, Meloro C, et al. (2013) Assessing the Jarman‐Bell Principle: scaling of intake, digestibility, retention time and gut fill with body mass in mammalian herbivores. Comp Biochem Physiol A 164, 129–140. [DOI] [PubMed] [Google Scholar]
- O'Connor PM, Claessens LP (2005) Basic avian pulmonary design and flow‐through ventilation in non‐avian theropod dinosaurs. Nature 436, 253–256. [DOI] [PubMed] [Google Scholar]
- O'Grady SP, Morando M, Avila L, et al. (2005) Correlating diet and digestive tract specialization: examples from the lizard family Liolaemidae. Zoology 108, 201–210. [DOI] [PubMed] [Google Scholar]
- Orme D, Freckleton R, Thomas G, et al. (2010) Caper: comparative analyses of phylogenetics and evolution in R. R package version 04/r71 See http://caperr-forger-projectorg/.
- Orr RT (1976) Vertebrate Biology. Philadelphia: WB Saunders. [Google Scholar]
- Pagel M (1999) Inferring the historical patterns of biological evolution. Nature 401, 877–884. [DOI] [PubMed] [Google Scholar]
- Paul GS, Christiansen P (2000) Forelimb posture in neoceratopsian dinosaurs: implications for gait and locomotion. Paleobiology 26, 450–465. [Google Scholar]
- Perry SF, Breuer T, Pajor N (2011) Structure and function of the sauropod respiratory system In: Understanding the Life of Giants the Biology of the Sauropod Dinosaurs. (eds Klein N, Remes K, Gee CT, Sander M.), pp. 83–93. Bloomington: Indiana University Press. [Google Scholar]
- Peters RH (1983) The Ecological Implications of Body Size. Cambridge: Cambridge University Press. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, et al. R Development Core Team (2011) Nlme: linear and nonlinear mixed effects models. R package version 3 1–102 Available at https://cranr-projectorg/web/packages/nlme/.
- Potgieter KR, Davies‐Mostert HT (2012) A simple visual estimation of food consumption in carnivores. PLoS One 7, e34543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand AS, Dugan BA, Monteza H, et al. (1990) The diet of a generalized folivore: Iguana iguana in Panama. J Herpetol 24, 211–214. [Google Scholar]
- Reisz RR (2006) Origin of dental occlusion in tetrapods: signal for terrestrial vertebrate evolution? J Exp Zool 306B, 261–277. [DOI] [PubMed] [Google Scholar]
- Reisz RR, Sues HD (2000) Herbivory in late Paleozoic and Triassic terrestrial vertebrates In: Evolution of Herbivory in Terrestrial Vertebrates: Perspectives from the Fossil Record. (ed. Sues HD.), pp. 9–41. Cambridge: Cambridge University Press. [Google Scholar]
- Revell LJ (2010) Phylogenetic signal and linear regression on species data. Methods Ecol Evol 1, 319–329. [Google Scholar]
- Schiek JO, Millar JS (1985) Alimentary tract measurements as indicators of diets of small mammals. Mammalia 49, 93–104. [Google Scholar]
- Sellers WI, Hepworth‐Bell J, Falkingham PL, et al. (2012) Minimum convex hull mass estimations of complete mounted skeletons. Biol Lett 8, 842–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibly RM, Brown JH, Kodric‐Brown A (2012) Metabolic Ecology. A Scaling Approach. Chichester, UK: Wiley‐Blackwell. [Google Scholar]
- Snavely N, Seitz SM, Szeliski R (2006) Photo tourism: exploring photo collections in 3D. ACM Trans Graph 25, 835–846. [Google Scholar]
- Starck D (1982) Vergleichende Anatomie der Wirbeltiere auf Evolutionsbiologischer Grundlage. Band 3: Organe des Aktiven Bewegungsapparates, der Koordination, der Umweltbeziehung, des Stoffwechsels und der Fortpflanzung. Berlin: Springer. [Google Scholar]
- Stevens CE, Hume ID (1998) Contributions of microbes in vertebrate gastrointestinal tract to production and conservation of nutrients. Physiol Rev 78, 393–427. [DOI] [PubMed] [Google Scholar]
- Stoinski S, Suthau T, Gunga H‐C (2011) Reconstructing body volume and surface area of dinosaurs using laser scanning and photogrammetry In: Understanding the Life of Giants the Biology of the Sauropod Dinosaurs. (eds Klein N, Remes K, Gee CT, Sander M.), pp. 94–115. Bloomington: Indiana University Press. [Google Scholar]
- Sues HD, Reisz RR (1998) Origins and early evolution of herbivory in tetrapods. Trends Ecol Evol 13, 141–145. [DOI] [PubMed] [Google Scholar]
- Team RDC (2011) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria ISBN 3‐900051‐07‐0, URL http://wwwR-projectorg/.
- Thulborn RA (1982) Speeds and gaits of dinosaurs. Palaeogeogr Palaeoclimatol Palaeoecol 38, 227–256. [Google Scholar]
- Treves F (1886) Abstracts of six lectures on the intestinal canal and peritoneum in the mammalia. Br Med J i, 583–584, 638–640. [Google Scholar]
- Vermeij GJ (1987) Evolution and Escalation: an Ecological History of Life. Princeton, NJ: Princeton University Press. [Google Scholar]
- Vermeij GJ (2013) On escalation. Annu Rev Earth Planet Sci 41, 1–19. [Google Scholar]
- Wagner CE, McIntyre PB, Buels KS, et al. (2009) Diet predicts intestine length in Lake Tanganyika's cichlid fishes. Funct Ecol 23, 1122–1131. [Google Scholar]
- Walls GY (1981) Feeding ecology of the tuatara (Sphenodon punctatus) on Stephens Island, Cook Strait. N Z J Ecol 4, 89–97. [Google Scholar]
- Wang DH, Pei YX, Yang JC, et al. (2003) Digestive tract morphology and food habits in six species of rodents. Folia Zool 52, 51–56. [Google Scholar]
- Weishampel DB, Dodson P, Osmolska A (1990) The Dinosauria. Berkeley: University of California Press. [Google Scholar]
- Werner J, Griebeler EM (2014) Allometries of maximum growth rate versus body mass at maximum growth indicate that non‐avian dinosaurs had growth rates typical of fast growing ectothermic sauropsids. PLoS One 9, e88834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilman H, Belmaker J, Simpson J, et al. (2014) Elton traits 1.0: species‐level foraging attributes of the world's birds and mammals. Ecology, 95, 2027. [Google Scholar]
- Wings O, Sander PM (2007) No gastric mill in sauropod dinosaurs: new evidence from analysis of gastrolith mass and function in ostriches. Proc R Soc B 274, 635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C (2007) SiftGPU: a GPU implementation of scale invariant feature transform (SIFT). Available at: http://cs.unc.edu/~ccwu/siftgpu.
- Wu C (2012) VisualSFM: a Visual Structure from Motion System. Available at http://www.cs.washington.edu/home/ccwu/vsfm.
- Wu C, Agarwal S, Curless B, et al. (2011) Multicore bundle adjustment. Proc Conf Comput Vis Pattern Recognit 24, 3057–3064. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. 3D skeleton reconstructions of early synapsids (including indications of the convex hull of the body cavity).
Fig. S2. 3D skeleton reconstructions of mammals (including indications of the convex hull of the body cavity).
Fig. S3. 3D skeleton reconstructions of birds (including indications of the convex hull of the body cavity).
Fig. S4. 3D skeleton reconstructions of non‐avian dinosaurs (including indications of the convex hull of the body cavity).
Fig. S5. 3D skeleton reconstructions of reptiles and amphibia (including indications of the convex hull of the body cavity).
Fig. S6. Phylogenetic tree (also available as supplementary nexus file).
Fig. S7. Visual outlier inspection: exclusion of the flamingo (combination of very long femur and very small torso) and two marine mammals (combination of very short femurs and very voluminous torsos).
Table S1. Specimens used in this study, categories and measurements.
Table S2. Time of divergence provided for different nodes in the tree.
Table S3. Results of statistical analyses according to Torso volume = a (factor) Body massb (and the corresponding factor × body mass interaction) in OLS and PGLS for extant mammals, birds and reptiles (n = 63).
Table S4. Results of statistical analyses according to Body mass = a (factor) Femur lengthb (and the corresponding factor × femur length interaction) in OLS and PGLS for extant mammals, birds and reptiles (n = 63).
Data Availability Statement
The Supporting Information contains images of all skeletons used in this study with their calculated convex hulls, all original measurements and species characteristics, three outliers not considered in the final dataset, and details on the construction of the phylogenetic tree. The Phylogenetic tree as Mesquite (nexus) file (129Tetrapods.nex) as well as all digital 3D skeleton reconstructions are deposited (full skeleton, isolated femur, isolated torso, torso convex hull, mirrored torso where applicable) in Morphobank Project P2404 (http://morphobank.org/permalink/?P2404). Download the .ply files and open in Meshlab (freely available at http://meshlab.sourceforge.net/); if you open the whole skeleton and the hull at the same time, you can see the reconstructed torso hull and the skeleton together.


