Abstract
We studied whether default functionality of the human brain, as revealed by task-independent decreases in activity occurring during goal-directed behaviors, is functionally reorganized by blindness. Three groups of otherwise normal adults were studied: early blind, adventitiously blind, and normally sighted. They were imaged by using functional MRI during performance of a word association task (verb generation to nouns) administered by using auditory stimuli in all groups and Braille reading in blind participants. In sighted people, this task normally produces robust task-independent decreases relative to a baseline of quiet wakefulness with eyes closed. Our functional MRI results indicate that task-independent decreases are qualitatively similar across all participant groups in medial and dorsal prefrontal, lateral parietal, anterior precuneus, and posterior cingulate cortices. Similarities in task-independent decreases are consistent with the hypothesis that functional reorganization resulting from the absence of a particular sensory modality does not qualitatively affect default functionality as revealed by task-independent decreases. More generally, these results support the notion that the brain largely operates intrinsically, with sensory information modulating rather than determining system operations.
Much previous functional brain imaging work in normally sighted (NS) adult humans has documented activity decreases during performance of various goal-directed behaviors relative to a control state, such as quiet wakefulness with eyes closed, visual fixation, or a minimally demanding task (1-3). These activity decreases have a variable relationship to task-specific activity increases. Some decreases appear to represent activity attenuation in sensory systems irrelevant to the task; these task-dependent decreases can occur in sensory cortex both within and separate from the modality engaged by the task (4-7).
Other activity decreases appear to be independent of the performed task. With remarkable regularity, these task-independent decreases occur in medial and lateral parietal cortices, posterior cingulate cortex, dorsal and ventral medial prefrontal cortex, and the amygdalae (reviewed in ref. 8). We propose that task-independent decreases represent suppression of default brain functionality (9) and have cited detailed circulatory and metabolic evidence showing that such decreases do not correspond to “activations” in the resting state, as has been suggested (3). Rather, task-independent decreases occur in areas that are functionally active but not physiologically “activated.” Default brain activity suggests spontaneous functions that are attenuated only when we reallocate resources to temporarily engage in goal-directed behaviors. Hence our designation of “default” functions (8, 9).
Numerous studies show that blindness leads to cortical reorganization manifesting as increased visual cortex activity during performance of tasks involving tactile and auditory stimuli (reviewed in refs. 10 and 11). Of interest is whether cortical reorganization in blindness extends to default functions. Evaluating the functional architecture of such processes in blind people also addresses the more general issue of sensory contributions to default functionality. Our view is that default brain activity is largely concerned with the maintenance of a probabilistic model of anticipated events (12-14). This perspective is consistent with the view that brain function is largely intrinsic, with sensory information modulating rather than determining operations. In this context, then, we assessed whether default functionality, as revealed by task-independent decreases, is reorganized in blindness.
We compared functional MRI (fMRI) responses in three groups of otherwise normal adults: early blind (EB), late blind (LB) and NS. All participants performed a word association task known to produce robust task-independent decreases in sighted adults (generating appropriate verbs/action words for common English nouns) (1, 3). Our results indicate that default functionality is largely independent of visual integrity.
Materials and Methods
Behavioral Tasks and Experimental Design. The methods used to obtain the present data are extensively described in two previous articles focused exclusively on positive (activation) blood oxygen level-dependent (BOLD) responses (15, 16). Briefly, we acquired fMRI at 1.5 T by using an asymmetric spin echo, echo-planar imaging sequence sensitive to BOLD contrast (T2* evolution time 50 msec) and whole brain coverage (6- or 8-mm slices, 3.75-mm in-plane resolution). Transformation to Talairach atlas space (17) was accomplished on the basis of high resolution (1 × 1 × 1.25 mm) sagittal T1-weighted magnetization prepared-rapid gradient echo images.
We distinguish between EB (no sight at birth or by 5 years of age) and LB (sight lost after an average age of ≈11 years) individuals. All LB participants could read print before the onset of complete blindness. Table 1 lists demographic characteristics of the blind participants. The NS group was matched for age. All but one EB participant were right-handed. All participants had normal brain anatomy and reported no neurological abnormalities (apart from blindness). All gave informed consent according to the guidelines of the Human Studies Committee of Washington University and were paid for their participation.
Table 1. Blind participant characteristics.
| Braille
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Participant | Age, years | Sex | Dominant hand | Age onset, years | Years | Words per min | Cause of blindness | Braille* | Auditory† |
| Early blind | |||||||||
| Early 1 | 51 | F | R | 2 | 45 | 145.4 | ROP/RLF | X | X |
| Early 2 | 50 | M | R | 0 | 44 | 152 | ROP/RLF | X | X |
| Early 3 | 49 | F | R | 0 | 143.8 | Optic nerve hypoplasia | X | ||
| Early 4 | 34 | F | L | 1 | 26 | 76 | Leber's disease | X | X |
| Early 5 | 39 | F | R | 3 | 34 | 99.7 | Glaucoma | X | X |
| Early 6 | 23 | M | R | 0 | 18 | 76 | ROP/RLF | X | |
| Early 7 | 67 | M | R | 5 | 60 | 63.8 | Cataracts | X | |
| Early 8 | 51 | M | R | 0 | 25 | N/A | Optic nerve hypoplasia | X | |
| Early 9 | 45 | M | R | 2 | 39 | N/A | ROP/RLF | X | |
| Early 10 | 33 | M | R | 0 | 28 | 88.7 | ROP/RLF | X | |
| Early 11 | 24 | M | R | 0 | 18 | 76 | Leber's disease | X | |
| Late blind | |||||||||
| Late 1 | 37 | F | R | 7 | 30 | 81.8 | Rubella | X | X |
| Late 2 | 50 | M | R | 10 | 45 | 66.5 | Glaucoma | X | |
| Late 3 | 41 | M | R | 12 | 29 | 75 | Coats disease | X | X |
| Late 4 | 63 | M | R | 12 | 55 | 83.9 | Glaucoma | X | X |
| Late 5 | 67 | F | R | 21 | 48 | N/A | Retinitis pigmentosa | X | X |
| Late 6 | 48 | F | R | 27 | 18 | 125 | Retinitis pigmentosa | X | X |
| Late 7 | 44 | F | R | 12 | 14 | N/A | Optic nerve atrophy | X | |
| Late 8 | 47 | F | R | 36 | 10 | 32.6 | Stevens-Johnson syndrome | X | |
Participants covertly generated appropriate verbs in response to presented nouns (e.g., think “build” for “house”) (15, 16). The word lists for all studies were identical, and homonymous auditory stimuli (e.g., “son,” “sun”) were excluded. Word order was randomized for each subject. Because of known practice effects (18), items were not repeated for a given participant within a study. Some blind individuals participated in both the Braille and the subsequent auditory studies (Table 1, last column). However, because >1 year elapsed between experiments, we believe that item practice effects in the auditory study were negligible. Sighted volunteers had no previous experience with the task. Task compliance was assessed after each fMRI run by asking participants to overtly recall their covert responses for 10 (randomly selected) of the 60 nouns in the just-presented run (16). Mean rate of recall was 81.3%.
We used a “clustered volume acquisition” fMRI design (19), in which scanner pulses occupied only ≈2 sec of each frame, leaving an ≈3-sec quiet period for stimulus presentation. Three verb-generate frames (≈16 sec) regularly alternated with three control frames (≈16 sec). Each fMRI run included 60 words and took ≈9 min. Only data from individuals completing at least four fMRI runs (80 task/control cycles) are included in the current analyses.
In the Braille experiment (15) the mean length of the noun stimuli was six Braille cells. The control stimulus was six Braille symbols for the pound sign (“######”). In Braille, “#” normally indicates that following cells are interpretable as numbers. Our readers instantly identified control fields as such and recognized that no verb generation was required. Participants were instructed to exert the same haptic behavior (i.e., scan the entire Braille field) for all stimuli. This strategy controlled for the gross sensory and motor aspects of the task as indexed by little or no BOLD modulation in somatosensory or motor cortex (15).
In the auditory experiment, the control stimulus was an unintelligible, time-reversed word matched in intensity and duration to the noun stimuli (16). The control stimulus was constant within runs but varied between runs. Participants were instructed to not identify the time-reversed words. This strategy successfully balanced primitive auditory stimulus features as indexed by absence of task-related BOLD modulation in primary auditory cortex (16).
Analyses of fMRI Data. We preprocessed fMRI data to reduce artifacts and correct for head motion. Images were transformed to atlas space (12-parameter affine warp) and resampled to 2-mm cubic voxels. Task-related BOLD responses were analyzed in stages. First, we examined individual participant responses in each paradigm (Braille, auditory) by using the general linear model (20-22). Response strength was estimated by using a regressor obtained by convolution of the (six-frame) task plus control epochs with a standard hemodynamic response model (23). Statistical parametric maps were estimated as in spm (24-26). The t-statistic maps were converted to equally probable Z scores for display. We corrected for multiple comparisons by using thresholds computed on the basis of Monte Carlo simulations (27) at a multiple-comparisons-corrected false-detection rate of P = 0.05 (Z = -4.5) over at least three contiguous, face-connected voxels. The thresholded maps then were combined over individuals by using a fixed-effects analysis strategy (28) to generate Z-score maps for five groups: Braille-EB, Braille-LB, auditory-EB, auditory-LB, and auditory-NS (Fig. 1). Voxel-wise arithmetic averaging of Z-score maps (Braille-EB, Braille-LB, auditory-EB, and auditory-LB) generated a composite, blind Z-score map. The blind composite and the sighted map were projected onto smoothed and flattened canonical cortical surfaces (29, 30) (Fig. 2) to facilitate comparison of blind vs. sighted response topography.
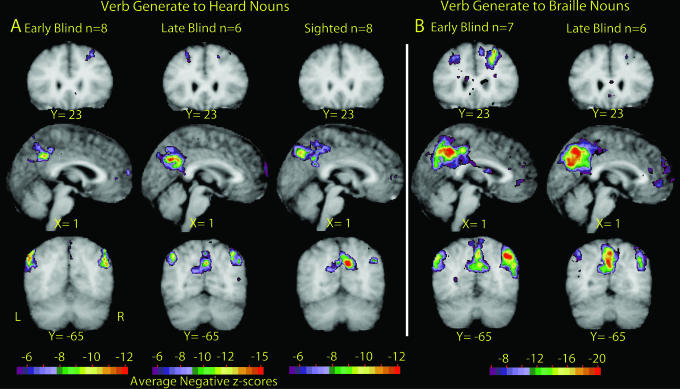
Fig. 1.
Selective sections show significant negative Z scores (negative BOLD responses). All data are shown in Talairach atlas space (17, 61). (A) Results obtained with verb generation to heard nouns (16) in EB, LB, and NS participants. (B) Results obtained with verb generation to Braille-read nouns (15) in EB and LB participants.
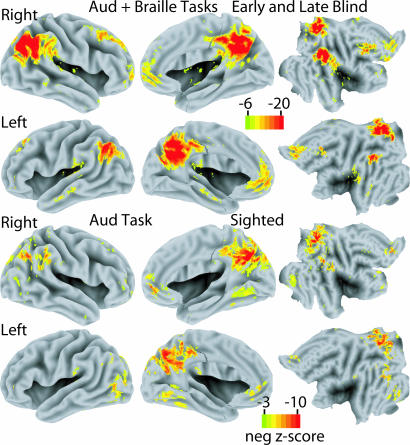
Fig. 2.
Average negative Z-score statistical parameter maps for all blind participants across both verb-generation experiments and all sighted participants for verb generation to heard nouns. Images are shown on standardized inflated 3D medial and lateral cortical hemispheres (left two columns) and transformed flat maps (right column) (29).
To explore group-dependent differences with greater power than afforded by voxel-wise comparisons, objectively defined regions of interest (ROIs) were determined as follows. A composite Z-score map was generated as described above except now including all groups. ROIs were objectively defined on the basis of voxel-wise Z-statistic maps computed in atlas space. A peak-search procedure first identified local extrema, thereby generating an initial list of foci. Then the algorithm consolidated, by coordinate averaging, foci closer than a selected minimum distance of 15 mm. ROIs were created by defining 12-mm spherical regions centered on the consolidated foci. Eight disjoint regions centered on significant peaks were selected (Table 2) based on proximity to coordinate locations previously identified by metaanalysis of positron-emission tomography data in sighted individuals (1). Group comparisons of response strength were computed on the basis of magnitudes estimated by correlation of regional responses against a waveform modeled by using an assumed hemodynamic response. t tests were computed for all possible comparisons (e.g., EB-Braille vs. LB-Braille, EB-Braille vs. NS-auditory, etc.). Only the P values for significant t test results are listed in Table 2. Table 2 also lists the Euclidean distance between the present foci and previously reported data.
Table 2. t test results by region and groups.
|
P*
|
||||||||
|---|---|---|---|---|---|---|---|---|
| ROI | BA | X,Y,Z | Auditory LB > NS | Braille EB > NS | Braille LB > NS | Braille EB + LB > NS | Braille EB > LB | Euclidean distance,† mm |
| 1 | 7 | -2, -46, 44 | - | - | - | 0.04 | - | 15.7 (31) |
| 2 | 7 | 04, -77, 42 | - | - | - | - | 0.03 | 5.1 (31) |
| 3 | 39 | -46, -69, 32 | - | 0.0001 | - | - | 0.0005 | 12.1 (31), 9.4 (2) |
| 4 | 39 | 45, -62, 34 | 0.02 | 0.0007 | - | 0.03 | - | 6.1 (3) |
| 5 | 8 | -23, 25, 44 | - | 0.005 | 0.003 | 0.0007 | - | 3.2 (31), 6.8 (2), 9.1 (3) |
| 6 | 8 | 27, 22, 46 | - | 0.0007 | 0.05 | 0.001 | 0.02 | |
| 7 | 32 | 01, 47, 03 | - | - | - | - | - | 15.5 (31), 11.8 (3) |
| 8 | 31 | 01, -60, 28 | 0.03 | - | - | 0.02 | - | 14.7 (31), 7.4 (3) |
Table lists P values only for significant differences (P ≤ 0.05) in response magnitudes. Unlisted group comparisons (e.g., auditory vs. Braille, EB) were not significant in any ROI
Distance between the present foci and data reported in the references indicated in parentheses
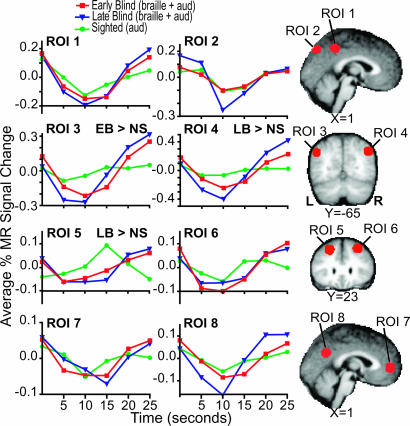
Finally, task-related BOLD modulation waveforms (percent magnetic resonance signal change) were computed for all eight ROIs without assuming a hemodynamic response model (20-22) (Fig. 3). Because the principal question here concerns the effects of blindness, we averaged response waveforms over input modality (Braille + auditory).
Fig. 3.
Regional BOLD time courses expressed as percent modulation for EB (red line), LB (blue line), and NS (green line) participants. The EB and LB data have been averaged over the Braille and auditory versions of the verb-generation task. The NS data are from verb generation to heard nouns only. ROI numbers are as in Table 2. Locations of defined regions are shown on selected sections that match those illustrated in Fig. 1.
Results
Distribution of Negative BOLD Responses. Both experiments revealed significant negative BOLD responses in posterior cingulate and adjacent medial parietal cortices (ROIs 1, 2, and 8; Table 2 and Figs. 1,2,3) as well as in the angular and supramarginal gyri bilaterally (ROIs 3 and 4). Negative BOLD responses were also observed in prefrontal cortex dorsally and bilaterally just lateral to the midline (ROIs 5 and 6; Table 2 and Figs. 1, 2, 3) and just to the left of the midline in the pregenual portion of Brodmann's area (BA) 10 (ROI 7; Table 2 and Figs. 1,2,3).
For blind participants, the distribution of negative responses was independent of input modality (Braille vs. auditory) (Fig. 1). Moreover, the observed response topography largely matched the distribution of negative responses seen in the sighted humans performing naive verb generation to heard nouns (Fig. 2). More generally, the coordinates of the present negative responses were close to those of task-independent decreases seen in previous studies (Table 2).
Dependence of Negative BOLD Responses on Input Modality and Age at Onset of Blindness. Differences between LB and EB negative BOLD responses were observed only in the Braille experiment (Table 2 and Fig. 1). Specifically, the spatial extent of ROIs 5 and 6 was greater for EB in comparison with LB (Fig. 1B, Y = 23). Also, response magnitudes were significantly greater for EB compared with LB in ROIs 2, 3, and 6 (Table 2). These differences reflect response strength rather than location.
Blind vs. Sighted Participants. Blind vs. sighted response strength differences were found in several areas. EB-Braille negative BOLD responses were greater than NS-auditory responses bilaterally in lateral parietal cortex. LB responses were greater than NS responses but only on the right and only for the auditory task (ROIs 3 and 4; Table 2 and Figs. 1 and 2). Also, in ROIs 3 and 4, NS showed no negative BOLD responses that passed our threshold settings (Fig. 3). In dorsal prefrontal cortex, both EB and LB (separately and combined) showed significantly larger responses than NS but only when comparing Braille data to auditory NS data (ROIs 5 and 6; Table 2).
Discussion
Blind participants performing covert verb generation showed negative BOLD responses that closely matched the typical topography of task-independent decreases seen in sighted persons (1-3, 8, 9). Thus, default brain functionality viewed from this perspective is similar in sighted and blind individuals. Some group differences, however, were noted and deserve comment.
Bilaterally in lateral parietal angular and supramarginal gyri, our blind, but not sighted participants exhibited typical task-independent decreases as reported in prior metaanalyses (1-3). McKiernan and colleagues consider variations in task difficulty to explain individual differences in activity decreases (31). Whereas they found significant decreases across various task manipulations in left angular gyrus, these did not vary with task difficulty manipulations. Also, areas exhibiting the greatest variations with task difficulty, such as the posterior cingulate and precuneus (31), showed minimal differences in our data for sighted and blind participants. Thus, our imaging data are inconsistent with the hypothesis that differences in difficulty experienced by blind vs. sighted participants contributed to findings in parietal cortex.
A complication in the comparisons of deactivation during auditory stimulation in sighted and Braille stimulation in blind participants is that differences may reflect either group (blind or sighted) or stimulus type (auditory or Braille). However, this limitation applies for this contrast only. Direct comparisons, based on the auditory task alone, confirm the minimal differences between blind and sighted groups without confounding of stimulus type.
Eventually, better understanding of these parietal regions may provide more specific explanations for these differences. Recent functional brain imaging data have provided relevant information about right parietal supramarginal and angular gyri (ROI 4; Fig. 2). On the basis of these data, it has been suggested that this region, in conjunction with the right ventral prefrontal cortex, acts as a “circuit breaker” that interrupts ongoing cognitive activity when novel or unexpected stimuli are detected (32). Reduced activity during task performance, then, might be viewed as restricting the range of stimuli influencing this area during specific goal-directed tasks. Why such a system should be more attenuated in blind than sighted participants cannot be stated with certainty. We can only speculate that our blind participants were better able than the sighted participants to reduce the expectation of encountering stimuli unrelated to the task at hand, possibly because of coping skills related to blindness. Such skills may be particularly relevant in a setting in which sighted subjects intentionally kept their eyes closed during scanning in contrast to the blind for whom this was automatic. Moreover, many of the blind participants in the auditory experiment had prior fMRI experience (15, 16) that may have further reduced the expectation of novel events. Studies of the type that led to the circuit breaker hypothesis (32) performed in blind participants would be most useful in resolving these speculations.
Bilateral lateral parietal cortices, again areas associated with task-independent decreases (ROIs 3 and 4; Fig. 2), along with the adjacent region of the superior temporal sulcus, have been suggested as having a role in social perception in both humans and monkeys (33). This work suggests that a contribution to social perception derives from self-other distinctions based on visual detection of biological motion. If, as our data suggest, blind individuals preserve task-independent decreases in lateral parietal cortex, our understanding of what nonvisual information contributes to self-other distinctions and the role that these areas play in such a process will need to be expanded. The recent work by Astafiev and colleagues (34) showing that these lateral parietal areas are also sensitive to endogenous signals concerned with action generation provides a suggestive insight. Again, a study of the type reported by Astafiev and colleagues repeated in blind individuals would be of interest.
We previously noted (8) that activity in the default mode may relate to visuospatial attention to the environment. Given that the lateral parietal regions (in addition to medial parietal regions) are implicated in such functions, the presence of greater deactivations in these lateral parietal areas in blind people raises some question about this earlier interpretation, because visuospatial functions are unlikely in blind people. Attention to the environment, however, involves more than just visual stimulation; blind people are especially attuned and orient toward tactile and auditory stimuli. Thus, a more general idea is that default environmental monitoring is served by these parietal regions, and, by necessity, this involves possible spatial processing of nonvisual stimuli in blind people.
In prefrontal cortex, covert performance of naive verb generation induced similar task-independent decreases in sighted and blind participants, except that response magnitudes in ROI 5 (Fig. 3) were greater in the blind reading Braille compared with sighted participants hearing words. These prefrontal cortex responses generally were considerably less impressive than task-independent decreases in other areas seen across a broad spectrum of cognitive tasks. Although a complete explanation is not presently available, it is useful to consider the rather complex relationship between task-independent decreases in this area and the familiarity subjects have with task stimuli (i.e., common English nouns).
Decreases are routinely observed in ventral medial (BA 24, 25, and 32) and dorsal medial (BA 8, 9, and 10) prefrontal cortices in group averages from large metaanalyses across many tasks (1-3). The metaanalysis by Shulman and colleagues (1) combined data from naive and practiced verb generation. Averaging in this way obscured differences that were later found in task-independent decreases resulting from practice (see figure 2 in ref. 35; see also ref. 36). Relevant here is that these medial task-independent decreases were much less prominent in the naive condition. In fact, little change was noted in dorsal medial prefrontal cortex during naive performance of the task. Practiced performance, however, was associated with prominent decreases in both dorsal and medial prefrontal cortex.
The failure to elicit prominent task-independent decreases in naive performance of the verb generation task was attributed to anxiety associated with a very demanding cognitive task (35). Supporting evidence for this assertion was provided in a companion study of anticipatory anxiety (37), where it was shown that activity decreases in ventral medial prefrontal cortex were inversely proportional to anxiety levels (i.e., less anxiety led to greater activity decreases). Dorsal medial prefrontal cortex did not follow this pattern, which possibly reflects this area being more related to some self-referential component of the task (38-40).
The above information may be interpreted by assuming some performance anxiety in our subjects (both blind and sighted) and some degree of self-reflection. Interpreting our results in this way additionally suggests that the greater decrease in dorsal left prefrontal cortex in EB was associated with greater task engagement and, hence, less self-reflection. However, we are cautious here, because our control tasks were not entirely passive and could also have elicited significant activity decreases in prefrontal cortex. As we noted elsewhere, the interpretation of activity decreases is clearest when using a control task of resting quietly with eyes closed (8, 9). Nevertheless, the activity decreases we observed are, most impressively, similar in blind and sighted people.
More generally, we believe these findings likely have important implications regarding the role of sensory systems in brain function. Accepting that task-independent decreases reflect attenuation of so-called default brain functions, our results suggest that these functions do not reorganize in blindness. These findings concur with the view that sensory information may contribute modestly to inherent brain functionality (13, 41-43). Consistent with this view is the idea that the brain maintains a probabilistic model of anticipated events and that the majority of ongoing neuronal activity is used to generate internal representations against which relatively impoverished sensory information is compared (13, 14). This model gains considerable force, in our view, when it is realized that the majority of the brain's energy budget likely supports intrinsic functionality rather than moment-to-moment variations in activity. A brief review of the allocation of the brain's energy budget is instructive.
In the average adult human, the brain represents ≈2% of total body weight but consumes ≈20% of total body energy (44), which is 10 times that predicted by brain weight alone. In relation to this very high rate of ongoing or “basal” metabolism, regional imaging signals are remarkably small. Thus, changes in absolute blood flow in areas typically affected by cognitive tasks are rarely >5-10% of the brain's resting blood flow. These modest modulations in ongoing circulatory activity often do not appreciably affect overall brain blood flows during even vigorous sensory and motor activity (45-47). For interesting exceptions related to more demanding cognitive tasks see refs. 48, 49, 50, 51.
Because activations correspond to increased glucose utilization not accompanied by a proportionate increase in oxygen consumption [i.e., an increase in aerobic glycolysis (51-53), it can be readily estimated that energy consumption increases may be as little as 10% of blood flow increases because of the much reduced ATP production resulting from glycolysis as compared with oxidative phosphorylation. If changes in brain activity, although important, account for such a small fraction of the brain's energy budget, what is the remainder used for? The answer comes from several important studies. Measurements of brain energy metabolism, using magnetic resonance spectroscopy (54-57) in a variety of experimental settings, have indicated that ≈80% of brain energy consumption is devoted to glutamate cycling and, hence, signaling. Complementary analyses arrived at similar conclusions by using extant anatomic, physiologic, and metabolic data to assess the cost of different components of gray matter excitatory signaling (58, 59).
In light of the above discussion, it is noteworthy that overall brain metabolic rates are similar in blind and sighted individuals (60). This latter study, which used positron-emission tomography to measure regional blood flow, oxygen consumption, and glucose utilization, reported that regional glucose utilization was ≈12% higher in primary visual cortex of blind individuals than in sighted controls. However, blood flow in the same area was ≈18% lower in blind people, whereas the metabolic rate for oxygen did not differ between the two groups. It is not immediately apparent how to interpret such somewhat incongruent findings. Because only three subjects contributed to each group in the study by De Volder and colleagues (60), additional work of this type with greater sample sizes will help clarify these results. Suffice it to say here that the presence of normal circulation and metabolism in the brains of blind individuals would be consistent with our finding that intrinsic default functionality of the brain, as assessed by task-independent decreases, is largely unaffected by blindness.
In conclusion, our data indicate that default functionality is predominantly not reorganized in blindness. This result stands in marked contrast to data showing extensive reorganization of functionality used to accomplish goal-directed tasks (reviewed in refs. 10 and 11). These findings are relevant to considerations of the dependence of brain function on sensory information. Our view is that intrinsic brain activity is largely concerned with maintenance of a probabilistic model of anticipated events and is, therefore, not heavily dependent on sensory information (12-14). According to this perspective, the brain operates intrinsically, with sensory information modulating rather than determining the operation of the system.
Acknowledgments
We thank V. Raja for processing image data, Dr. E. Akbudak for scanner pulse sequences, and Dr. M. McAvoy for statistical analysis. This work was supported by National Institutes of Health Grants NS37237 and NS06833.
Abbreviations: BA, Brodmann's area; BOLD, blood oxygen level-dependent; EB, early blind; fMRI, functional MRI; LB, late blind; NS, normally sighted; ROI, region of interest.
References
- 1.Shulman, G. L., Fiez, J. A., Corbetta, M., Buckner, R. L., Miezin, F. M., Raichle, M. E. & Petersen, S. E. (1997) J. Cognit. Neurosci. 9, 648-663. [DOI] [PubMed] [Google Scholar]
- 2.Binder, J. R., Frost, J. A., Hammeke, T. A., Bellgowan, P. S., Rao, S. M. & Cox, R. W. (1999) J. Cognit. Neurosci. 11, 80-95. [DOI] [PubMed] [Google Scholar]
- 3.Mazoyer, B., Zago, L., Mellet, E., Bricogne, S., Etard, O., Houde, O., Crivello, F., Joliot, M., Petit, L. & Tzourio-Mazoyer, N. (2001) Brain Res. Bull. 54, 287-298. [DOI] [PubMed] [Google Scholar]
- 4.Drevets, W. C., Burton, H., Videen, T. O., Snyder, A. Z., Simpson, J. R., Jr., & Raichle, M. E. (1995) Nature 373, 249-252. [DOI] [PubMed] [Google Scholar]
- 5.Ghatan, P. H., Hsieh, J. C., Petersson, K. M., Stone-Elander, S. & Ingvar, M. (1998) Neuroimage 7, 23-29. [DOI] [PubMed] [Google Scholar]
- 6.Smith, A. T., Singh, K. D. & Greenlee, M. W. (2000) NeuroReport 11, 271-277. [DOI] [PubMed] [Google Scholar]
- 7.Shmuel, A., Yacoub, E., Pfeuffer, J., Van de Moortele, P. F., Adriany, G., Hu, X. & Ugurbil, K. (2002) Neuron 36, 1195-1210. [DOI] [PubMed] [Google Scholar]
- 8.Gusnard, D. A. & Raichle, M. E. (2001) Nat. Rev. Neurosci. 2, 685-694. [DOI] [PubMed] [Google Scholar]
- 9.Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A. & Shulman, G. L. (2001) Proc. Natl. Acad. Sci. USA 98, 676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burton, H. (2003) J. Neurosci. 23, 4005-4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadato, N., Okada, T., Honda, M. & Yonekura, Y. (2002) Neuroimage 16, 389-400. [DOI] [PubMed] [Google Scholar]
- 12.Ingvar, D. H. (1985) Hum. Neurobiol. 4, 127-136. [PubMed] [Google Scholar]
- 13.Olshausen, B. A. (2003) in The Visual Neurosciences, eds. Chalupa, L. M. & Werner, J. S. (MIT Press, Cambridge, MA), pp. 1603-1615.
- 14.Kording, K. P. & Wolpert, D. M. (2004) Nature 427, 244-247. [DOI] [PubMed] [Google Scholar]
- 15.Burton, H., Snyder, A. Z., Conturo, T. E., Akbudak, E., Ollinger, J. M. & Raichle, M. E. (2002) J. Neurophysiol. 87, 589-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burton, H., Snyder, A. Z., Diamond, J. & Raichle, M. E. (2002) J. Neurophysiol. 88, 3359-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talairach, J. & Tournoux, P. (1988) Coplanar Stereotaxic Atlas of the Human Brain (Thieme, New York).
- 18.Raichle, M. E., Fiez, J. A., Videen, T. O., MacLeod, A. M., Pardo, J. V., Fox, P. T. & Petersen, S. E. (1994) Cereb. Cortex 4, 8-26. [DOI] [PubMed] [Google Scholar]
- 19.Bandettini, P. (2001) in Functional Magnetic Resonance Imaging: An Introduction to Methods, eds. Jezzard, P., Matthews, P. & Smith, S. (Oxford Univ. Press, Oxford), pp. 124-143.
- 20.Miezin, F. M., Maccotta, L., Ollinger, J. M., Petersen, S. E. & Buckner, R. L. (2000) Neuroimage 11, 735-759. [DOI] [PubMed] [Google Scholar]
- 21.Ollinger, J. M., Corbetta, M. & Shulman, G. L. (2001) Neuroimage 13, 210-217. [DOI] [PubMed] [Google Scholar]
- 22.Ollinger, J. M., Corbetta, M. & Shulman, G. L. (2001) Neuroimage 13, 218-229. [DOI] [PubMed] [Google Scholar]
- 23.Boynton, G. M., Engel, S. A., Glover, G. H. & Heeger, D. J. (1996) J. Neurosci. 16, 4207-4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friston, K., Holmes, A., Worsley, K., Poline, J., Frith, C. & Frackowiak, R. (1995) Hum. Brain Mapp. 2, 189-210. [DOI] [PubMed] [Google Scholar]
- 25.Friston, K., Holmes, A., Poline, J., Grasby, P., Williams, S., Frackowiak, R. & Turner, R. (1995) NeuroImage 2, 45-53. [DOI] [PubMed] [Google Scholar]
- 26.Friston, K. J., Fletcher, P., Josephs, O., Holmes, A., Rugg, M. D. & Turner, R. (1998) Neuroimage 7, 30-40. [DOI] [PubMed] [Google Scholar]
- 27.Forman, S. D., Cohen, J. D., Fitzgerald, M., Eddy, W. F., Mintun, M. A. & Noll, D. C. (1995) Magn. Reson. Med. 33, 636-647. [DOI] [PubMed] [Google Scholar]
- 28.Bosch, V. (2000) J. Magn. Reson. 11, 61-64. [DOI] [PubMed] [Google Scholar]
- 29.Van Essen, D. C. (2002) Curr. Opin. Neurobiol. 12, 574-579. [DOI] [PubMed] [Google Scholar]
- 30.Van Essen, D. C. (2004) in The Visual Neurosciences, eds. Chalupa, L. & Werner, J. (MIT Press, Cambridge, MA), pp. 507-521.
- 31.McKiernan, K. A., Kaufman, J. N., Kucera-Thompson, J. & Binder, J. R. (2003) J. Cogn. Neurosci. 15, 394-408. [DOI] [PubMed] [Google Scholar]
- 32.Corbetta, M. & Shulman, G. L. (2002) Nat. Rev. Neurosci. 3, 201-215. [DOI] [PubMed] [Google Scholar]
- 33.Allison, T., Puce, A. & McCarthy, G. (2000) Trends Cogn. Sci. 4, 267-278. [DOI] [PubMed] [Google Scholar]
- 34.Astafiev, S. V., Stanley, C. M., Shulman, G. L. & Corbetta, M. (2004) Nat. Neurosci. 7, 542-548. [DOI] [PubMed] [Google Scholar]
- 35.Simpson, J. R., Jr., Snyder, A. Z., Gusnard, D. A. & Raichle, M. E. (2001) Proc. Natl. Acad. Sci. USA 98, 683-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raichle, M. E. (1998) Philos. Trans. R. Soc. London B 353, 1889-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simpson, J. R., Jr., Drevets, W. C., Snyder, A. Z., Gusnard, D. A. & Raichle, M. E. (2001) Proc. Natl. Acad. Sci. USA 98, 688-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gusnard, D. A., Akbudak, E., Shulman, G. L. & Raichle, M. E. (2001) Proc. Natl. Acad. Sci. USA 98, 4259-4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelley, W. M., Macrae, C. N., Wyland, C. L., Caglar, S., Inati, S. & Heatherton, T. F. (2002) J. Cogn. Neurosci. 14, 785-794. [DOI] [PubMed] [Google Scholar]
- 40.Kerr, D. L., Gusnard, D. A., Snyder, A. Z. & Raichle, M. E. (2004) NeuroReport 15, 607-610. [DOI] [PubMed] [Google Scholar]
- 41.Brown, T. G. (1911) Proc. R. Soc. London B 84, 308-319. [Google Scholar]
- 42.Brown, T. G. (1915) J. Physiol. (London) 48, 18-46. [Google Scholar]
- 43.Llinas, R. & Pare, D. (1991) Neuroscience 44, 521-535. [DOI] [PubMed] [Google Scholar]
- 44.Clark, D. D. & Sokoloff, L. (1999) in Basic Neurochemistry: Molecular, Cellular and Medical Aspects, ed. Siegel, G. J., Agranoff, B. W., Albers, R. W., Fisher, S. K. & Uhler, M. D. (Lippincott-Raven, Philadelphia), pp. 637-670.
- 45.Sokoloff, L., Mangold, R., Wechsler, R. L. & Kety, S. S. (1955) J. Clin. Invest. 34, 1101-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fox, P. T., Fox, J. M., Raichle, M. E. & Burde, R. M. (1985) J. Neurophysiol. 54, 348-369. [DOI] [PubMed] [Google Scholar]
- 47.Fox, P. T., Burton, H. & Raichle, M. E. (1987) J. Neurosurg. 67, 34-43. [DOI] [PubMed] [Google Scholar]
- 48.Lennox, W. G. (1931) Arch. Neurol. Psychiatry 26, 725-730. [Google Scholar]
- 49.Roland, P. E., Eriksson, L., Stone-Elander, S. & Widen, L. (1987) J. Neurosci. 7, 2373-2389. [PMC free article] [PubMed] [Google Scholar]
- 50.Friston, K. J., Frith, C. D., Liddle, P. F., Dolan, R. J., Lammertsma, A. A. & Frackowiak, R. S. J. (1990) J. Cereb. Blood Flow Metab. 10, 458-466. [DOI] [PubMed] [Google Scholar]
- 51.Madsen, P. L., Hasselbalch, S. G., Hagemann, L. P., Olsen, K. S., Bulow, J., Holm, S., Wildschiodtz, G., Paulson, O. B. & Lassen, N. A. (1995) J. Cereb. Blood Flow Metab. 15, 485-491. [DOI] [PubMed] [Google Scholar]
- 52.Fox, P. T., Raichle, M. E., Mintun, M. A. & Dence, C. (1988) Science 241, 462-464. [DOI] [PubMed] [Google Scholar]
- 53.Blomqvist, G., Seitz, R. J., Sjogren, I., Halldin, C., Stone-Elander, S., Widen, L., Solin, O. & Haaparanta, M. (1994) Acta Physiol. Scand. 151, 29-43. [DOI] [PubMed] [Google Scholar]
- 54.Sibson, N. R., Dhankhar, A., Mason, G. F., Behar, K. L., Rothman, D. L. & Shulman, R. G. (1997) Proc. Natl. Acad. Sci. USA 94, 2699-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sibson, N. R., Dhankhar, A., Mason, G. F., Rothman, D. L., Behar, K. L. & Shulman, R. G. (1998) Proc. Natl. Acad. Sci. USA 95, 316-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shulman, R. G., Hyder, F. & Rothman, D. L. (2001) Proc. Natl. Acad. Sci. USA 98, 6417-6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hyder, F., Rothman, D. L. & Shulman, R. G. (2002) Proc. Natl. Acad. Sci. USA 99, 10771-10776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ames, A., III (2000) Brain Res. Brain Res. Rev. 34, 42-68. [DOI] [PubMed] [Google Scholar]
- 59.Attwell, D. & Laughlin, S. B. (2001) J. Cereb. Blood Flow Metab. 21, 1133-1145. [DOI] [PubMed] [Google Scholar]
- 60.De Volder, A. G., Bol, A., Blin, J., Robert, A., Arno, P., Grandin, C., Michel, C. & Veraart, C. (1997) Brain Res. 750, 235-244. [DOI] [PubMed] [Google Scholar]
- 61.Lancaster, J. L., Glass, T. G., Lankipalli, B. R., Downs, H., Mayberg, H. & Fox, P. T. (1995) Hum. Brain Mapp. 3, 209-223. [Google Scholar]