Abstract
Purpose
This pilot feasibility clinical trial evaluated the co-administration of vemurafenib, a small molecule antagonist of BRAFV600 mutations, and tumor infiltrating lymphocytes (TIL) for the treatment of metastatic melanoma.
Experimental Design
A metastatic tumor was resected for growth of TIL and patients were treated with vemurafenib for 2 weeks followed by resection of a second lesion. Patients then received a non-myeloablative pre-conditioning regimen, infusion of autologous TIL and high-dose interleukin-2 administration. Vemurafenib was restarted at the time of TIL infusion and was continued for 2 years or until disease progression. Clinical responses were evaluated by RECIST 1.0. Metastases resected prior to and after two weeks of vemurafenib were compared using TCRB deep sequencing, immunohistochemistry, proliferation and recognition of autologous tumor.
Results
The treatment was well tolerated and had a safety profile similar to that of TIL or vemurafenib alone. Seven of 11 patients (64%) experienced an objective clinical response and 2 patients (18%) had a complete response for 3 years (one response is ongoing at 46 months). Proliferation and viability of infusion bag TIL and peripheral blood T cells were inhibited in vitro by vemurafenib (PLX4032) when approaching the maximum serum concentration of vemurafenib. TCRB repertoire (clonotypes numbers, clonality and frequency) did not significantly change between pre- and post-vemurafenib lesions. Recognition of autologous tumor by T cells was similar between TIL grown from pre- and post-vemurafenib metastases.
Conclusions
Co-administration of vemurafenib and TIL was safe, feasible and generated objective clinical responses in this small pilot clinical trial.
INTRODUCTION
Metastatic melanoma accounts for ~10,000 annual deaths in the United States (1). Tumor infiltrating lymphocytes (TIL) display specificity for autologous tumor cells in vitro via tumor cell lysis and secretion of pro-inflammatory cytokines, e.g., interferon-γ (IFNγ), following TIL and tumor cell co-culture. TIL obtained from deposits of metastatic melanoma can be expanded ex vivo to large numbers and infused back into the autologous patients as adoptive T cell therapy (ACT) (2–4). The adoptive transfer of TIL with high-dose interleukin-2 (IL-2) following a non-myeloablative (NMA) chemotherapy pre-conditioning regimen resulted in an overall objective response rate of ~55% with ~20% complete remission (CR) rate (5–12). To attempt to improve these clinical results, we sought to evaluate, in a pilot clinical trial, the safety and feasibility of adding other agents to use in combination with TIL therapy.
A candidate for this application was vemurafenib, a specific inhibitor of somatic mutations at the V600 codon of the BRAF oncogene (13). About half of metastatic melanoma patients express BRAFV600E or BRAFV600K mutations, which are not present in normal tissues. These BRAF mutations result in constitutive MAP kinase signaling and uncontrolled proliferation (14), and vemurafenib directs the senescence and apoptosis of BRAFV600E/K cancer cells (15, 16). Metastatic melanoma patients treated with vemurafenib experienced a 53% and 48% overall response and 6% and 1% CR rate in phase II and III clinical trials, respectively (17–20). Vemurafenib was reported to increase the density of lymphocyte infiltrates in melanoma metastases, alter the intratumor T cell repertoire and not significantly affect the proliferation or viability of peripheral blood T cells at concentrations of vemurafenib ≤50 μM (21–23). Furthermore, tumor expression of melanocyte differentiation antigens, e.g., gp100, MART-1, tyrosinase and TRP1/2, were reported to increase following vemurafenib treatment (21, 24). Thus, a clinical trial evaluating the combination of vemurafenib and TIL was a rational approach to evaluate therapeutic additivity or synergy with different classes of treatment modalities.
A pilot clinical trial was initiated at the Surgery Branch, National Cancer Institute (NCI), to assess safety and feasibility of this combination therapy. To evaluate whether vemurafenib impacted on TIL, we chose to resect metastatic melanoma deposits prior to and after two weeks of vemurafenib treatment. The pre-vemurafenib TILs were used for therapy. Following administration of the non-myeloablative (NMA) chemotherapeutic preparative regimen (cyclophosphamide and fludarabine), TILs were infused with high-dose IL-2 and vemurafenib was restarted at the time of cell infusion. Patient peripheral blood T cells and infusion bag TILs were assayed for proliferation and viability in vitro when incubated with PLX4032 (research-grade vemurafenib) to assess the possible impact of vemurafenib on T cells in vivo. TIL fragments expanded from pre- and post-vemurafenib lesions were compared for (i) lymphocyte infiltration by immunohistochemistry, (ii) TCRB deep sequencing to evaluate TCR repertoire and (iii) recognition of autologous tumor cells. This pilot clinical trial directly evaluated the combination of a kinase inhibitor targeted therapy with adoptive T cell therapy.
MATERIALS AND METHODS
Ethics
Written, informed consent was granted from all study participants. This study was approved by the Investigational Review Board (IRB) at the NCI and was registered at https://clinicaltrials.gov under NCT01585415.
Trial Design
Eligible patients were between 18 and 66 years of age with a life expectancy >3 months, an ECOG ≤ 1 and measurable metastatic melanoma that expressed BRAFV600E or BRAFV600K mutations as assessed by a CLIA certified laboratory. Patients were not eligible if they had received prior vemurafenib, were receiving systemic steroid therapy, had mean QTc interval >450 msec, < 45% left ventricular ejection fraction, or were experiencing active systemic infections, coagulation disorders, active major medical illnesses of the cardiovascular, respiratory or immune system, primary immunodeficiency, opportunistic infections or severe immediate hypersensitivity to agents used in this study. More than 4 weeks must have elapsed since any prior systemic therapy and toxicities recovered to grade 1 or less (except for alopecia or vitiligo). More than 6 weeks must have elapsed since any antibody therapy, including anti-CTLA4 antibody. Patients with 3 or fewer brain metastases (<1 cm) were eligible if asymptomatic or had lesions treated with stereotactic radiosurgery and were clinically stable for 1 month after treatment or surgical resection.
Treatment schedule
A tumor was resected in order to grow TIL according to standard operating protocols (5, 8). Two weeks prior to initiation of the preparative regimen, vemurafenib was administered at 960 mg twice per day. Another metastatic lesion was resected after the 2 weeks of vemurafenib for in vitro comparisons to the lesion resected for TIL growth. Vemurafenib was then halted for the non-myeloablative preparative regimen with two days of cyclophosphamide 60 mg/kg/day followed by five days of fludarabine 25 mg/m2/day. Then TILs were infused and high-dose IL-2 (720,000 IU/kg; Aldesleukin; Novartis, PA) was administered every 8 hours until a maximum of 15 doses or to tolerance. Vemurafenib was restarted at the time of cell infusion and was continued until the patient was taken off study, had disease progression, or was a complete responder more than 2 years after TIL administration. Clinical responses were evaluated by Response Evaluation Criteria in Solid Tumors (RECIST) 1.0 from interval scans.
TIL expansion and administration
Standard TIL expansion was performed as previously described (5, 7). Briefly, 24 tumor fragments were cultured in individual wells of 24 well plates for 2–3 weeks in RPMI-1640 with L-Glutamine (Gibco, Fisher Scientific; Grand Island, NY), 10% heat-inactivated Human AB serum (NCI Surgery Branch), and 6000 IU/mL IL-2. Selected fragments were expanded in rapid expansion protocol with irradiated peripheral blood lymphocyte (PBL) feeder cells, 30 ng/mL OKT3 antibody (Miltenyi Biotec; Germany) and 3000 IU/mL IL-2 then pooled and administered to patients.
Tumor and TIL co-culture
Co-culture assays were performed in 50/50 media: 50% Aim-V media, 45% RPMI-1640 media with L-Glutamine (Gibco), 5% Human AB serum (NCI Surgery Branch), fungizone, penicillin/streptomycin (Gibco) and gentamicin (Lonza; Basel, Switzerland). TIL fragments were tested directly after expansion to select TIL for treatment. Correlative studies were performed from frozen stocks, which were rested overnight in 50/50 media with 3000 IU/mL IL-2 prior to co-culture. Autologous tumor cell digests were thawed and pre-incubated with either pan-MHC Class-I blocking antibody (clone W6/32) or PBS (negative control). Media (T cells only) and OKT3 served as negative and positive controls, respectively. After 24 hours of co-culture, secretion of IFNγ into co-culture supernatants was evaluated by ELISA (25).
In vitro proliferation and cell viability assays
Serial dilutions of PLX4032 (Fisher Scientific) were made in 50/50 media in parallel to serial dilutions of DMSO (vehicle). PBL were stimulated with OKT3 [50 ng/mL] and IL-2 [300 IU/mL] at a density of 2x106 cells/mL, fed 2 days later with fresh media supplemented with IL-2 and assayed 3 days later. Thawed infusion bag TILs were rested overnight in 50/50 media with 3000 IU/mL IL-2. T cells were mixed with 50/50 media supplemented with IL-2 (PBL: 600 IU/mL; TIL: 6000 IU/mL) and were added to an equal volume of diluted PLX4032 or DMSO. T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) fluorescent dye according to manufacturer’s instructions (BD Biosciences; San Jose, CA) prior to addition to the plates for proliferation assays. Baseline measurements of CFSE staining were acquired from each donor. T cells were cultured for 3 days with PLX4032 or DMSO at 37°C and stained for CD3, propidium iodide (PI) and AnnexinV (viability assays only), acquired on BD FACSCanto II (BD Biosciences) and analyzed by FlowJo (v.10.0.8). Data shown for proliferation assays were gated on lymphocytes and then live T cells (CD3+ PI−). Data shown for viability assays were gated on lymphocytes.
Deep sequencing of TCRB alleles
Genomic DNA was isolated from snap frozen tumor fragments with DNAeasy kit according to manufacturer’s instructions (Qiagen; Valencia, CA). TCRB alleles were sequenced at 200,000 reads by Adaptive Biotechnologies (Seattle, WA). Productive TCRB sequences, i.e., those that could be translated to open reading frames, were reported.
RESULTS
Combination of ACT and vemurafenib was well tolerated
The primary objective of this clinical trial was to determine the safety and feasibility of combining vemurafenib with adoptive T cell therapy. A total of 11 patients were enrolled, resected of a metastatic tumor and treated on this pilot clinical trial. Lymphocytopenia, neutropenia and thrombocytopenia were observed in all 11 patients as a direct result of the NMA chemotherapy. All patients had at least one transient and reversible grade 3 toxicity (Table 1), which included hyperbilirubinemia (n=1), hypocalcemia (n=1), hypermagnesemia (n=1), hypophosphatemia (n=1), hypokalemia (n=1), hyperkalemia (n=1), prolonged QTc interval (n=1), hypoxia (n=1), altered partial thromboplastin time (n=1), oliguria (n=1), rash (n=1), thrombus (n=1), dyspnea (n=1), pericoronitis (n=1), cellulitis (n=1), increased creatinine (n=2), febrile neutropenia (n=4), infection (n=5) and anemia (n=7). Two patients had reversible grade 4 toxicities, which were increased creatinine (n=1) and dyspnea (n=1). The treatment cycle was well tolerated overall and toxicity was similar to that seen with vemurafenib or standard TIL therapy alone.
Table 1. Patient demographics, treatment, and response.
Eleven patients were enrolled on the vemurafenib and ACT clinical trial and clinical data are detailed below. Abbreviations: Pt, patient; TIL, tumor infiltrating lymphocytes; IL-2, interleukin-2; VEM, vemurafenib; M, male; F, female; SQ, subcutaneous; LND, lymph node dissection; IFN, interferon; HD IL-2, high-dose interleukin-2; mets, metastases; LN, lymph node; RP LN, retroperitoneal lymph node; WLE, wide local excision; SLNB, sentinel lymph node biopsy; NMA, nonmyeloablative chemotherapy; TBI, total body irradiation; GM-CSF, granulocyte macrophage-colony stimulating factor; XRT, radiation; Ipi, ipilimumab; ALND, axillary lymph node dissection; PTT, partial thromboplastin time; Hb, hemoglobin; CR, complete response; PR, partial response; NR, non-response; PD, progression of disease; NCI, National Cancer Institute.
| Pt | Age/gender | Sites of Disease | Prior Treatment | Toxicity (Grade) | Infusion TIL number x109 | IL-2 doses | Decrease target tumors after VEM and before TIL (%) | Overall Response (months); greatest decrease tumor (%); PD Reason |
|---|---|---|---|---|---|---|---|---|
| 1 | 30M | Lung, SQ, skin | LND, IFN, HD IL-2 (x7) | PTT (3) Rash (3) Staphylococcus Epidermidis (3) |
92.7 | 6 | ↓5 | CR (46+); ↓100% |
| 2 | 41M | Brain, lung, liver, med LN, spleen | None | ↓Hb (3) ↓K+ (3) Febrile neutropenia (3) Micrococcus Luteus (3) |
33.9 | 7 | ↓21 | NR; 26%; New & increasing brain mets |
| 3 | 22M | Lung, liver, mediastinal LN, bone, muscle | WLE, SLNB, IFN | Hypoxia (3) Pericoronitis (3) Rhinovirus (3) Staphylococcus Bacteremia (3) |
36.3 | 5 | ↓58 | PR (6); ↓96%; New brain met |
| 4 | 24M | Skin, spleen, brain, gallbladder | NMA arm of 1200TBI vs NMA protocol at NCI (141.9x109 TIL at day −181) | ↓Hb (3) Febrile neutropenia (3) |
44.9 | 7 | ↓15 | NR; ↓23%; Increasing cutaneous mets |
| 5 | 53M | Skin | WLE, SLNB, and LN resection, HD IL-2 (x16) | ↑Creatinine (3) ↑Bilirubin (3) Infection (3) Cellulitis (3) |
86.4 | 5 | ↓39 | CR (36); ↓100%;Recurrent melanoma (thigh) |
| 6 | 50M | Skin, muscle, mesenteric LN | WLE, IFN, GM-CSF, HD IL-2 (x23), XRT, Ipi | ↓Ca++ (3) ↓PO4− (3) ↓Hb (3) Febrile neutropenia (3) ↑Creatinine (4) |
117.4 | 5 | ↓14 | PR (3); ↓37%; New brain mets |
| 7 | 27M | Liver, pancreas, abdominal LN, RP LN, skin, lung, med LN | ALND, WLE, SLNB, XRT, IFN, HD IL-2 (x1) | ↓Hb (3) Dyspnea (3) |
92.4 | 4 | ↓10 | PR (6); ↓62%; New SQ mets |
| 8 | 66M | Cervical LN, muscle, med LN, skin, abdominal LN, RP LN, mesenteric LN, lung, axillary LN | ALND, IFN, HD IL-2 (x14) | ↓Hb (3) Oliguria (3) Klebsiella Pneumoniae (3) |
58.5 | 4 | ↓29 | PR (5); ↓68%; New mesenteric met |
| 9 | 65M | Cervical LN, liver, brain | None | ↓Hb (3) Prolonged QTc interval (3) |
95.0 | 5 | ↓30 | NR; ↓30%; New brain metastases |
| 10 | 41F | Lung, adrenal, skin | Multiple excisions, WLE, HD IL-2 (x12), Ipi | ↓Hb (3) | 95.4 | 2 | ↓54 | PR (7); ↓58%; Increasing lung, cutaneous mets |
| 11 | 51M | Skin | WLE | ↑Creatinine (3) ↑K+ (3) ↑Mg++ (3) Febrile neutropenia (3) Thrombus (3) Dyspnea (4) |
104 | 4 | ↓17 | NR; ↓50%; New brain mets |
TIL and vemurafenib combination therapy mediated objective clinical responses
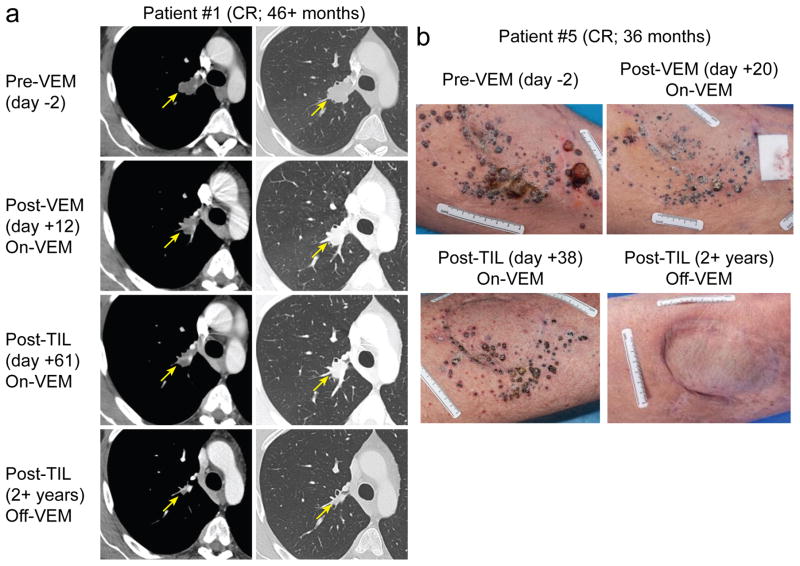
A secondary objective of this pilot clinical trial was to gain preliminary information concerning the ability of the combination therapy to mediate clinical tumor regressions in patients with metastatic melanoma. The median patient age was 41 years old (range: 22 – 66) and 10 of 11 (91%) of patients were male (Table 1). Metastases were most commonly in the skin, lymph nodes and lungs. Nine of 11 (82%) patients had prior therapy, including immunomodulatory agents such as high-dose IL-2 or interferon. One patient (#4) had prior TIL therapy. The median number of infused TIL was 9.24x1010 (range: 3.39x1010 – 1.17x1011) and patients received a median 5 doses of IL-2 (range: 2 – 7). Target lesions often decreased after 2 weeks of vemurafenib (median: 21%; range: 5% – 58%). Seven of 11 patients (64%) experienced an overall objective clinical response, including 2 patients (#1 and #5) with complete responses (18%) (Fig. 1). Patient #1 received 9.27x1010 TIL and 6 doses of IL-2 and his target lesion in the hilum slightly decreased in size following initial vemurafenib treatment, showed significant reduction in size after TIL therapy at 2 months, completely regressed by 26 months and remains absent ongoing at 46 months (Fig. 1a). Patient #5 received 8.64x1010 TIL and 5 doses of IL-2 and multiple subcutaneous masses on the left thigh dramatically decreased after initial vemurafenib therapy. Metastases continued to shrink after TIL therapy and were completely resolved by 12 months (Fig. 1b). Three years after initial treatment, patient #5 had progressive disease from recurrent melanoma in the thigh. The initial decrease in tumors after vemurafenib did not correlate to long-term response (Table 1), which was exemplified by patient #1 who had only 5% reduction post-vemurafenib but had a durable, complete tumor regression. We compared patient responses from this pilot trial to responses in patients with known BRAFV600 mutations who were treated during the same time interval on a separate clinical trial at the NCI Surgery Branch with TIL, IL-2 and NMA preparative regimen but did not receive vemurafenib (NCT00513604). These patients did not have a second lesion available for biopsy and thus were not included in the vemurafenib trial. Of these 15 patients, 9 experienced an objective clinical response (60%) and 3 had complete responses (20%), which were durable and are ongoing. In the comparison of these two small pilot trials there was no suggestion of a difference in anti-tumor response with the addition of vemurafenib to ACT (p=1.0; Fisher’s exact test).
Figure 1. Tumors of patients achieving complete tumor regression following treatment with TIL and vemurafenib.
(a) Computer tomographic images of tumors (indicated by arrows) before and after administration of TIL and vemurafenib (VEM). (b) Photographs of tumors before and after VEM and TIL administration.
PLX4032 inhibited growth and decreased viability of peripheral blood T cells and infusion bag TIL in vitro only at high concentrations approaching the vemurafenib serum CMAX
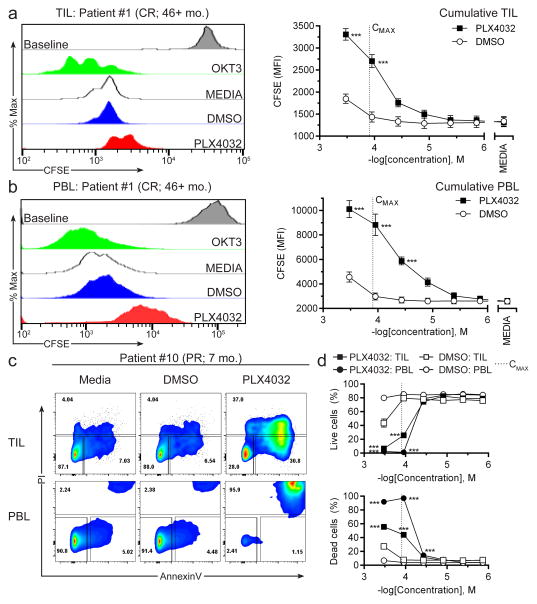
Previous studies reported that PLX4032 (vemurafenib) and PLX4072 (PLX4032 progenitor), did not impact the viability or proliferation of peripheral blood T cells at concentrations up to 50 μM in vitro (23, 26, 27). We performed similar testing on patient peripheral blood T cells to corroborate these findings and also tested the TIL used for treatment, which has not been evaluated to date. The assay stringency was increased by testing higher PLX4032 concentrations close to or above the maximum serum concentration (CMAX) of vemurafenib (125 μM) when given twice per day at 960 mg (28) as was done in this trial. PBL were stimulated with OKT3 and IL-2 then used to assay actively growing peripheral blood T cells in assays on day +5 of stimulation. Infusion bag TIL from stocks cryopreserved on the day of TIL infusion (day +14 of rapid expansion protocol) were evaluated. All patient’s infusion bag TIL and 4 representative PBLs from patients achieving each type of clinical response (#1, CR; #8, PR; #10, PR; #9, NR) were tested. Proliferation was evaluated by CFSE dilution assay, which measures the 2-fold reduction in CFSE fluorescence during each cell division. Viability assays were initiated as was done for proliferation assays, except that cells were not labeled with CFSE, and cell death was evaluated by staining for PI (dead cells) and AnnexinV (apoptotic/dying cells). T cells were mixed with IL-2 and either PLX4032 or DMSO (vehicle) in decreasing concentrations. After 3 days of culture, the proliferation of both infusion bag TIL (Fig. 2a) and peripheral blood T cells (Fig. 2b) were significantly inhibited at concentrations near the reported vemurafenib CMAX (125 μM), but proliferative capacity was maintained at concentrations below 10 μM. Some proliferation was measured in each sample indicating that even high PLX4032 concentrations did not completely abrogate T cell growth. Most of the T cells were alive (AnnexinVneg PIneg) in both TIL and PBL groups in mock treated (Media) and DMSO conditions at concentrations near the vemurafenib CMAX or below (Fig. 2c). In contrast, the PLX4032-treated TIL had high frequencies of dying (AnnexinV+ PIneg) and dead (AnnexinV+ PI+) cells when approaching the vemurafenib CMAX (Fig. 2c, top right). Almost all peripheral blood T cells were dead when treated with PLX4032 close to the vemurafenib CMAX (Fig. 2c, bottom right). The viability of TIL and peripheral T cells was high at PLX4032 concentration < 37 μM, which is consistent with previous reports in the literature (Fig. 2d) (23, 26, 27). These phenomena were observed in all 11 patients and in the four PBL donors tested indicating that it was not based on T cell source (PBL or TIL), clinical response or capacity to achieve clinical benefit. Thus, T cells infused into the bloodstream of patients receiving 960 mg vemurafenib twice per day may have experienced decreases in viability and proliferation from the vemurafenib.
Figure 2. Proliferation and cell viability of peripheral blood T cells and infused TIL following culture with vemurafenib (PLX4032).
PBL was mixed with OKT3 and IL-2 and after 5 days the actively dividing peripheral blood T cells were assayed. Infusion bag TIL, which were actively growing the rapid expansion protocol, were assayed from thawed vials of stocks frozen on the day of TIL infusion. T cells were mixed with IL-2 and added to either DMSO (vehicle) or PLX4032 then cultured for 3 days at 37°C. T cells were labeled prior to culture with CFSE to measure proliferation or were left unlabeled to assess viability following staining for AnnexinV and PI to quantify frequencies of live (AnnexinVneg PIneg), dying (AnnexinV+ PIneg) or dead (AnnexinV+ PI+) cells. Proliferation of (a) TIL or (b) peripheral blood T cells cultured with (left) OKT3 (positive control; green), media (negative control; white), DMSO (vehicle control; 111 μM; blue) or PLX4032 (111 μM; red) in one representative donor (patient #1; CR; 46+ mo.) or (right) across a range of concentrations of DMSO and PLX4032. Baseline levels of CFSE staining from day 0 of the experiments are displayed in gray histograms. (c) Viability of TIL (top) or peripheral blood T cells (bottom) from one representative donor (patient #10; PR; 7 mo.) when cultured with Media, DMSO or PLX4032 at 111 μM. (d) Frequencies of (top) live or (bottom) dead cells in cultures across a range of DMSO and PLX4032 concentrations. The reported vemurafenib CMAX [125 μM] is indicated by a dotted line on graphs. TIL data are compiled from all patients enrolled on the trial and are displayed as mean ± SEM (n=11). PBL data are compiled from patient’s PBL representing each clinical response group (#1, CR; #8, PR; #10, PR; #9, NR) and are displayed as mean ± SEM (n=4). Data are representative of at least two independent experiments. Two-way ANOVA with Bonferroni’s post-tests was used for statistical analysis between PLX4032 and DMSO groups. ***p<0.001
Equivalent or improved TIL fragment growth and lymphocyte infiltrate in vemurafenib-primed tumors relative to vemurafenib-naïve tumors
Another secondary objective of this study was to study the immunologic impact of vemurafenib administration on the lymphoid infiltrate in melanoma deposits. We chose to compare separate, independent metastases to evaluate global effects of vemurafenib on metastases and minimize the effects of sampling the same tumor multiple times. The first tumor was resected before vemurafenib and another tumor was resected after two weeks of vemurafenib treatment. We were able to obtain post-vemurafenib tumors from 9 of 11 (82%) of the patients (Table 2). At the time of resection, the tumors were dissected into fragments (n=24 if possible) and expanded in high-dose IL-2 in order to compare TIL fragment proliferation and functional responses as a function of vemurafenib administration. The frequency of fragments that expanded past the single well stage (approximately 106 cells/well) was similar between pre- and post-vemurafenib tumors. It should be noted that vemurafenib often resulted in shrinkage of each patient’s tumor, so the post-vemurafenib metastases were relatively smaller and fewer than 24 fragment cultures could be initiated for 5 of 9 patients (#4, #5, #6, #7 and #11). Tumors were stained for CD3, CD4, CD8 and CD20 (B-cell marker) and evaluated by immunohistochemistry to determine the relative lymphocyte infiltrate before and after vemurafenib. Increased T cell staining intensity, as measured by CD3 expression, was clearly observed in 6 of 8 patients evaluated (75%; #1, #5, #8, #9, #10 and #11). Helper T cells, as measured by CD4 expression, were more frequent in 5 of 9 patients assayed (56%; #1, #4, #5, #8 and #11). Cytotoxic T cells, as measured by CD8 expression, were more frequent in 7 of 9 patients assayed (78%; #1, #4, #5, #8, #9, #10 and #11). An associated increased staining for B cells (CD20+) was not observed in most of these samples (#4, #5, #8 and #11) suggesting that the increased T cell infiltrate was unlikely to be a result of increased lymphocytes in general. It should also be noted that one of the patients (#6) who did not have increased lymphocyte infiltrate already contained high levels of T cells in the pre-vemurafenib tumor. Moreover, the two patients who experienced complete regressions of all metastatic disease (#1 and #5) had minimal lymphocytic infiltration prior to vemurafenib and had a large increase in the staining for CD3, CD4 and CD8 T cells in post-vemurafenib tumors. Thus, vemurafenib resulted in an increased presence of T cells in separate melanoma metastases which may have resulted from new lymphocyte infiltration or from lymphocyte concentration due to tumor shrinkage.
Table 2. Patient biopsies and lymphocyte infiltration into tumors before and after vemurafenib treatment.
The day reported is relative to start of vemurafenib. Infused TILs are designated by the bolded tumor ID for each patient where the number of TIL infused (x109) is denoted to the right. TIL fragments were considered to have grown if they achieved 106 cells/well in a 24 well plate. The numbers of total TIL fragments initiated are included in the denominators. Immunohistochemistry of tumors stained for CD3, CD4, CD8, and CD20 was determined by a single dedicated pathologist who was blinded to the status of VEM treatment. Grading was as follows: 0 = no staining; 1 = staining <5% of tumor; 2 = staining 5%–50% of tumor; 3 = staining >50% of tumor. Abbreviations: TIL, tumor infiltrating lymphocytes; R, right; L, left; n/a, not available; Bx, biopsy; SQ, subcutaneous.
| Patient | Biopsy Site | Day | Tumor ID | (TIL infused x109) | #TIL fragments grown | CD3 | CD4 | CD8 | CD20 |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 1 | R-shoulder mass | −15 | 3745 | 92.7 | 17/24 | 1+ | 0–1 | 1+ | 0 |
| L-cervical LN | +13 | 3760 | 24/24 | 3+ | 3+ | 3+ | 3+ | ||
|
| |||||||||
| 2 | R-axilla mass | −46 | 3744 | 33.9 | 18/24 | n/a | n/a | n/a | n/a |
|
| |||||||||
| 3 | R-thoracic mass | −15 | 3764 | 36.3 | 19/24 | n/a | n/a | n/a | n/a |
|
| |||||||||
| 4 | Cutaneous lesions | −23 | 3762 | 44.9 | 11/24 | n/a | 0 | 0–1 | 0 |
| Abdominal and chest lesions (3) | +13 | 3776 | 9/18 | 2 | 2 | 2 | 0 | ||
| R-forearm punch Bx | +25 | 3782 | n/a | n/a | n/a | n/a | n/a | ||
| Abdominal skin Bx | +56 | 3798 | n/a | n/a | n/a | n/a | n/a | ||
|
| |||||||||
| 5 | L-groin LN and intransit region | −18 | 3780 | 86.4 | 14/24 | 0 | 0 | 0 | 0 |
| L-thigh punch Bx | −2 | 3785 | n/a | n/a | n/a | n/a | n/a | ||
| L-thigh cutaneous | +10 | 3790 | n/a | 3+ | 3+ | 3+ | 0 | ||
| L-thigh skin Bx | +20 | 3797 | n/a | 3 | 3 | 3 | 0 | ||
| L-thigh skin Bx | +38 | 3804 | n/a | 3 | 3 | 3 | 0 | ||
| L-thigh keratinic acanthoma | +140 | 3845 | 5/5 | n/a | n/a | n/a | n/a | ||
|
| |||||||||
| 6 | R-epitrochlear LN | −21 | 3831 | 117.4 | 24/24 | 3+ | 3+ | 3+ | n/a |
| L-abdominal wall mass | +15 | 3841 | 6/6 | 2+ | 2+ | 2+ | n/a | ||
|
| |||||||||
| 7 | Back SQ masses | −21 | 3896 | 92.4 | 23/24 | 1–2 | 1+ | 1–2 | 0–1 |
| L-inguinal SQ mass | +14 | 3904 | 10/12 | 0 | 0–1+ | 0–1+ | 0 | ||
|
| |||||||||
| 8 | R-SQ back/neck mass | −65 | 3885 | 58.5 | 23/24 | 0 | 0 | 0 | 0 |
| L-cervical LN | +13 | 3918 | 18/24 | 2+ | 2+ | 2+ | 0 | ||
|
| |||||||||
| 9 | L-axillary LN | −36 | 3922 | 95.0 | 15/24 | 0–1+ | 1+ | 1+ | 0 |
| L-posterior neck mass | −1 | 3933 | 4/24 | 0–1+ | 0–1+ | 0–1+ | 0 | ||
| L-lateral neck mass | +13 | 3938 | 24/24 | 2+ | 1+ | 2+ | 1+ | ||
|
| |||||||||
| 10 | L-chest wall lesion | −30 | 3945 | 95.4 | 24/24 | 0–1+ | 1+ | 0–1+ | 0 |
| L-abdominal LN | +13 | 3952 | 15/24 | 2 | 1 | 1–2 | 0–1 | ||
|
| |||||||||
| 11 | R-axilla mass | −22 | 3957 | 104 | 15/24 | 1 | 0–1 | 0–1 | 0–1 |
| R-upper chest wall mass | +13 | 3964 | 15/18 | 3+ | 2+ | 1+ | 0 | ||
Clinical response was independent of TCR repertoire before and after vemurafenib
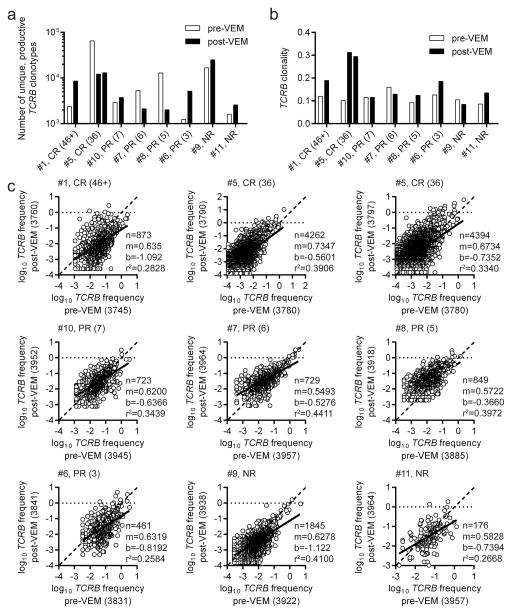
To evaluate whether this increased lymphocyte frequency represented a change in the T cell repertoire, we performed TCRB deep sequencing to measure productive TCR clonotypes in autologous tumors. Eight patients were evaluated in these assays because they had a vemurafenib-naïve lesion and a lesion resected following 2 weeks of vemurafenib treatment. Two post-vemurafenib lesions were resected for patient #5 and both tumors were included in analyses. Three of eight patients (#5, #7, and #8) had increased numbers of TCRB clonotypes in the pre-vemurafenib tumor relative to the post-vemurafenib deposit. Conversely, the other 5 patients (#1, #6, #9, #10 and #11) had greater numbers of TCRB clonotypes in the post-vemurafenib tumor relative to the pre-vemurafenib lesion (Fig. 3a). Differences in total numbers of TCRB clonotypes was not statistically different (p=0.431) between the two vemurafenib groups. Total numbers of TCRB clonotypes did not correlate with clinical outcome, which is exemplified by the two patients achieving CR who had opposite trends as one had more TCRB clonotypes in the post-vemurafenib tumor relative to the pre-vemurafenib tumor (#1) and vice versa for the other patient (#5). Clonality was measured because it normalized the productive TCRB clonotype reads for each sample and thus quantified TCR diversity (22, 29, 30) in each vemurafenib group. TCRB clonality increased in post-vemurafenib lesions compared to pre-vemurafenib tumors most dramatically in both patients achieving CR (#1 and #5) and one patient (#6) with a 3-month PR, and to a lesser extent in patients #8 (PR; 5 mo.) and #11 (NR) (Fig. 3b). Small decreases in TCRB clonality in post-vemurafenib tumors relative to pre-vemurafenib lesions were detected in patients #7 (PR; 6 mo.) and #9 (NR) and there was no change in TCRB clonality for patient #10 (PR; 10 mo.). Numbers of TCRB clonotypes and TCRB clonality had similar trends in all patients except for patient #5, who had decreased TCRB clonotypes and increased clonality in post-vemurafenib tumors relative to pre-vemurafenib lesions, and patient #8, who had a log10-fold decrease in TCRB clonotypes but only a slight increase in TCRB clonality in the post-vemurafenib tumor relative to pre-vemurafenib lesion. Overall, there was not a significant difference (p=0.130) in TCRB clonality between pre- and post-vemurafenib tumors and an increase or decrease in TCRB clonality could not be used to predict clinical response. Relative frequencies of unique, productive TCRB clonotypes present in both pre-vemurafenib (x-axes) and post-vemurafenib (y-axes) tumors were compared in linear regression analysis (Fig. 3c). The line of best-fit (solid line) and associated slope (m), y-intercept (b), correlation coefficient (r2) and number of shared TCRB clonotypes (n) were calculated for each patient. None of these parameters could be used to predict clinical response. Each slope was <1 suggesting that shared TCRB clonotypes were more frequent in the pre-vemurafenib tumor relative to the post-vemurafenib lesion. Autologous tumor pairs had sufficient differences in shared TCRB clonotype frequencies as evidenced by r2 values <0.5, i.e., a 1:1 frequency ratio was not observed. Hatched lines were drawn as reference to a 1:1 frequency ratio (m=1, b=0) on each graph. The top-ranking clonotypes present in the upper right of graphs did not display a dominant pattern of increased or decreased frequency in pre- or post-vemurafenib tumors. Moreover, high-ranking TCRB clonotypes in one tumor were typically high-ranking in the other tumor. These results suggested that vemurafenib treatment for two weeks did not unidirectionally alter the TCRB repertoire in independent metastases. Therefore, the increased lymphocyte infiltration post-vemurafenib could not be easily and directly correlated to the TCRB repertoire.
Figure 3. Number of unique, productive TCRB clonotypes, TCRB clonality and shared TCRB clonotype frequencies within tumors before and after vemurafenib treatment.
Fragments of resected tumors before or after vemurafenib (VEM) treatment were snap frozen and assayed for TCRB diversity by deep sequencing. The infusion bag TILs were derived from the pre-VEM samples. (a) Numbers of unique, productive TCRB clonotypes. (b) TCRB clonality (measure of TCR diversity). Patient #5 resections 3790 and 3797 are shown from left to right, respectively. (c) Frequencies of shared, unique, productive TCRB clonotypes in pre-vemurafenib (x-axes) or post-vemurafenib (y-axes) tumors. Linear regression analysis with line of best fit (solid line), number of shared sequences (n), slope of best-fit line (m), y-intercept of best-fit line (b) and correlation coefficient (r2) are next to each graph. Hatched lines represent m=1 and b=0, i.e., 1:1 frequency ratio.
Pre- and post-vemurafenib TIL fragments responded similarly to autologous tumor cells
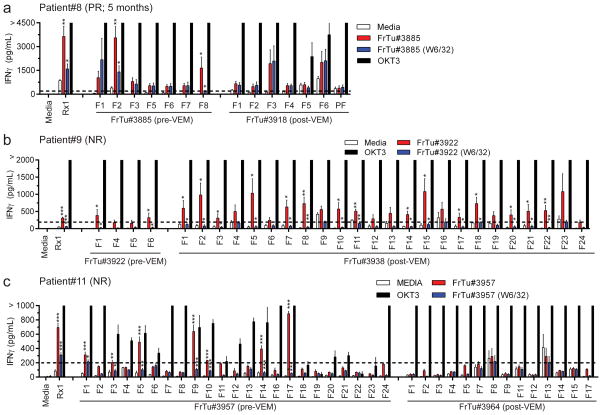
Another measure of whether there was a change in the T cell repertoire following vemurafenib was to examine the functionality of TIL fragments grown from the metastases before and after vemurafenib treatment. Although 9 of 11 patients had post-vemurafenib resections, TIL fragments were only expanded from 8 of the 9 post-vemurafenib lesions (TIL from patient #5 was from keratinic acanthoma rather than melanoma) and only 3 of the 8 patients with post-vemurafenib TIL had autologous tumor available for testing. Post-vemurafenib lesions were small presumably because vemurafenib mediated transient tumor regression, which inhibited our capability to test more patient’s TIL. We were also restricted to testing against the pre-vemurafenib tumors because the post-vemurafenib tumors did not yield enough cells from the enzymatic digestion for in vitro analysis. Thus, fragments available for evaluation in patients #8, #9 and #11 were co-cultured with autologous pre-vemurafenib single cell tumor digests (fresh tumor; FrTu) overnight either in the presence of MHC Class-I blocking antibody (clone W6/32; blue bars) or blocking antibody vehicle (PBS; red bars) and secretion of IFNγ was assessed by ELISA (Fig. 4). Media (T cells only; open bars) and OKT3 (agonistic anti-CD3 antibody; black bars) were negative and positive controls for co-cultures, respectively. A fragment was considered reactive if IFNγ >200 pg/mL and reached statistical significance compared to both no target (Media) and MHC Class-I blocking antibody with >50% inhibition of the unblocked response. Infusion bag TIL (Rx1) for patient #8 responded to the autologous tumor, which was inhibited by MHC Class-I blocking antibody (Fig. 4a, left). This patient had 2 of 7 (29%) reactive pre-vemurafenib (FrTu#3885) fragments and 0 of 7 reactive post-vemurafenib (FrTu#3918) fragments (Fig. 4a). Infusion bag TIL for patient #9 was reactive to autologous tumor but secreted the least IFNγ of the infused products tested (Fig. 4b, left). Two of four (50%) pre-vemurafenib (FrTu#3922) fragments and 15 of 24 (63%) post-vemurafenib (FrTu#3938) fragments were reactive to autologous tumor for patient #9 (Fig. 4b). Patient #11 also had infusion bag TIL with significant autologous tumor recognition (Fig. 4c, left). Seven of 23 (30%) pre-vemurafenib (FrTu#3957) fragments and 0 of 14 post-vemurafenib (FrTu#3964) fragments demonstrated significant reactivity to autologous tumor (Fig. 4c). In this small pilot sample there was no consistent change in autologous tumor reactivity of TIL obtained before or after vemurafenib administration. In sum, it appeared that vemurafenib added no significant benefit to ACT as evidenced by (i) similar clinical responses to ACT alone, (ii) inhibition of proliferation and increased cell death of both TIL and peripheral blood T cells at concentrations near the CMAX of vemurafenib at therapeutic doses, (iii) lack of consistent changes in TCRB repertoire in tumors before and after in vivo vemurafenib treatment and (iv) similar anti-tumor reactivity of TIL either naïve or primed with vemurafenib.
Figure 4. Tumor responses of TIL fragments from tumors before or after in vivo vemurafenib sensitization.
Results shown for (a) patient #8, (b) patient #9 and (c) patient #11. A tumor was resected for growth of TIL fragments and generation of the infused TIL product, and this sample was designated the pre-vemurafenib (pre-VEM) sample. Another tumor was resected after 2 weeks of vemurafenib treatment, but prior to TIL infusion, and TIL fragments were grown for comparison as the post-vemurafenib (post-VEM) sample. Cryopreserved TIL fragments (pre- and post-vemurafenib) were thawed and co-cultured with autologous pre-vemurafenib single cell tumor digestion at a 1:1 ratio overnight at 37°C. Tumor cells were pre-incubated with pan-MHC Class-I blocking antibody (W6/32) or vehicle (PBS) prior to co-culture to assess CD8-specific responses to the tumors. Target only (Media on x-axes) was included to quantify the baseline secretion of IFNγ into the co-cultures by TIL in the autologous tumor digest. Supernatants from co-cultures were evaluated for interferon-γ (IFNγ) secretion by ELISA. T cells only (Media; open bars) was a negative control and OKT3 (agonistic CD3 antibody; black bars) was a positive control for TIL samples. Infused TIL (Rx1) was included for reference to clinical response. Fragments are indicated by an “F” and “PF” represents a pool of multiple fragments. Results are pooled from two independent experiments and data are mean ± SEM (n = 4 technical replicates). Dotted line represents 200 pg/mL IFNγ, which was the cutoff for calling a fragment reactive. Student’s two-tailed t-tests were performed for statistical analysis between no target (Media) and tumor digest (* above red bars) or between tumor digest treated with PBS (mock; vehicle) or pan-MHC Class-I blocking antibody (* above blue bars). *p<0.05, **p<0.01, ***p<0.001
DISCUSSION
This pilot clinical trial evaluated the impact of targeted therapy combined with adoptive T cell therapy for patients with metastatic melanoma. The primary endpoint of the study was to evaluate the safety and feasibility of combining TIL therapy with vemurafenib. The combination treatment was well tolerated overall and toxicity was consistent with either agent as monotherapy. No unexpected, serious adverse events were observed. Additivity of toxicity may have occurred but was not a limiting factor in treatment and/or objective clinical response. Seven of 11 patients achieved clinical response and 2 patients had complete regressions of metastatic melanoma, one for 36 months and the other is ongoing after 46 months (Fig. 1). When compared to patients with BRAFV600E/K mutations who were treated with a similar TIL therapy without vemurafenib during the same time interval, the combination of vemurafenib and TIL was similar to TIL therapy. The numbers of patients used for these analyses (TIL alone, n=15; TIL and vemurafenib, n=11) were not powered to detect small differences. A larger, randomized clinical trial powered to detect responses between these two cohorts could further evaluate differences in response rates.
Future clinical trials could also use post-vemurafenib TIL for therapy. We chose vemurafenib-naïve tumors to generate TIL therapy because it was unknown how vemurafenib would impact TIL in the ACT setting. A post-vemurafenib TIL strategy could have particular importance for patients who are ineligible for ACT due to heavy disease burden. Vemurafenib would debulk their existing tumors, bridge them to surgery for TIL harvest and make them eligible for ACT. Vemurafenib could be administered before, during and after T cell transfer similarly to our clinical trial with the expectation of a similar safety profile. A murine model demonstrated that halting vemurafenib after adoptive T cell transfer abrogated the anti-tumor response of pmel-1 T cells towards vemurafenib-primed melanoma (31), suggesting that continuing vemurafenib following T cell transfer is warranted. Post-vemurafenib TILs were able to recognize pre-vemurafenib tumor cells in vitro, albeit without significant improvement in tumor-reactivity compared to pre-vemurafenib TIL (Fig. 4). We did not recover enough tumor cells to co-culture TIL with autologous pre-treatment tumor cells treated in vitro with or without vemurafenib. Future experiments could generate TIL fragments in the presence or absence of vemurafenib ex vivo and evaluate their autologous tumor recognition as a function of vemurafenib. Given that pre- and post-vemurafenib TILs were also similar in TIL fragment growth (Table 2) and TCRB repertoire (Fig. 3), it is plausible that post-vemurafenib TIL could be used for treatment in a manner comparable to conventional TIL therapy.
Some of the early benefits of vemurafenib on TIL may have been limited by our trial design. We observed higher staining for T cells infiltrating tumors after vemurafenib (Table 2), corroborating previous studies (21, 32), but the NMA chemotherapy preparative regimen likely eliminated these T cells prior to ACT. When melanoma lesions were serially sampled during vemurafenib treatment, the frequency of shared TCRB clonotypes increased (22). In our study, two independent tumors were compared by TCRB deep sequencing to evaluate global changes in the TCR repertoire as a function of vemurafenib. If the same TCRB clonotype was present in both tumors and was highly frequent in one tumor, then it was typically highly prevalent in the other tumor as well (Fig. 3c). Thus, selection of a pre- or post-vemurafenib tumor for generation of TIL treatment would not generally yield higher frequencies of the top shared TCRB clonotypes. These data also suggest that there were common antigens expressed by both tumors which were targets of shared TCRB clonotypes. Expression of melanocyte differentiation antigens, which are common antigens recognized by melanoma TIL, increased after treatment with vemurafenib and led to enhanced tumor recognition by TCR-transduced human T cells and murine pmel-1 T cells (23, 32). Similar increases in antigen expression were likely maintained after the NMA regimen and could have served as enhanced antigenic targets for infused TIL. It is possible that some of the TCRB clonotypes lost during the NMA regimen made it into the infusion bag TIL given the high similarity between pre- and post-vemurafenib tumors in shared TCRB clonotypes. If so, then an improvement in their clinical benefit is plausible given that these T cells were removed from the immunosuppressive tumor microenvironment and given in large quantities to the patient.
We established that vemurafenib (PLX4032) had a negative impact on the viability and proliferation of peripheral blood T cells and treatment TIL at concentrations approaching the vemurafenib serum CMAX of 125 μM (Fig. 2). Our results were unexpected given that studies performed prior to the onset of this pilot clinical trial established that peripheral blood T cells were unharmed by PLX4032 and PLX4072 (early-stage vemurafenib) but these experiments only evaluated concentrations ≤ 50 μM (23, 27). Our data recapitulate the reported data at these lower concentrations but add new information in regards to the impact of PLX4032 on T cells at higher therapeutic levels. This is an important consideration for future vemurafenib clinical trials because emerging immunity could be compromised by vemurafenib at sufficient concentrations. Upcoming clinical trials could monitor serum vemurafenib levels and adjust the vemurafenib dosing such that antitumor effects are maintained and damage to the peripheral and intratumoral T cell repertoires is minimized. In ACT trials, vemurafenib administration could be stopped for a few days after T cell transfer to allow for TIL trafficking out of the bloodstream into the tumor microenvironment. Vemurafenib could then be started or re-started to achieve therapeutic additivity or synergy in the absence of deleterious effects on T cells at high vemurafenib serum concentrations.
It may be worthwhile to focus future trials on combining other agents with TIL or adding more agents to the combination of vemurafenib and TIL. Resistance to vemurafenib can occur through downstream activation of MEK, and combinations of MEK inhibitors, e.g., trametinib or cobimetinib, with vemurafenib have demonstrated improved clinical response albeit with few durable, complete responses (33–37). Dabrafenib, another BRAFV600E inhibitor, has been given in combination with trametinib with objective clinical activity but the combination impacted function of peripheral blood T cells (38–40). MEK inhibitors alone were shown to inhibit T cells which may limit enthusiasm for combination of T cell therapy with both MEK and BRAFV600E/K targeted therapy (21, 41). T cell immunotherapy could also be combined directly with checkpoint inhibitor neutralizing antibodies, e.g., pembrolizumab or nivolumab (anti-PD-1), atezolizumab (anti-PD-L1) or ipilimumab (anti-CTLA-4) either with or without kinase inhibitors. The antibody would release inhibition of infused TIL as they encountered the negative regulatory molecule in the tumor microenvironment (42). This trial established that targeted therapy and ACT can be safely administered to patients and resulted in objective clinical responses which opens opportunities for testing other agents or combinations in clinical trials.
TRANSLATIONAL RELEVANCE.
One way to potentially improve adoptive T cell therapy is to combine it with the administration of small molecule inhibitors. Vemurafenib specifically inhibits mutated BRAFV600E/K molecules and directs cell death of BRAFV600E/K expressing tumors. Vemurafenib has been reported to increase tumor infiltration of lymphocytes and increase expression of melanoma/melanocyte T cell antigens. This pilot clinical trial evaluated the combination of vemurafenib with T cell transfer. Objective clinical responses were observed in 7 of 11 patients (64%), including 2 of 11 patients (18%) with complete regressions of metastatic disease. Comparisons of metastases before and after vemurafenib treatment revealed an increased presence of tumor infiltrating T cells but similar recognition of autologous tumor. This pilot trial showed the safety and feasibility of administering a kinase inhibitor with T cell transfer.
Acknowledgments
We thank the members of the TIL lab (NCI Surgery Branch) for processing and expanding TIL for therapies.
FINANCIAL SUPPORT
This research was supported through an award to Steven A. Rosenberg by the Intramural Research Program of the NIH at the National Cancer Institute.
Footnotes
Conflict of interest statement: The authors declare no competing financial interests.
References
- 1.DeVita VT, Lawrence TS, Rosenberg SA. Devita, Hellman, and Rosenberg’s cancer : principles & practice of oncology. 10. Philadelphia: Wolters Kluwer; 2015. [Google Scholar]
- 2.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–8. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwong ML, Neyns B, Yang JC. Adoptive T-cell transfer therapy and oncogene-targeted therapy for melanoma: the search for synergy. Clin Cancer Res. 2013;19:5292–9. doi: 10.1158/1078-0432.CCR-13-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lizee G, Overwijk WW, Radvanyi L, Gao J, Sharma P, Hwu P. Harnessing the power of the immune system to target cancer. Annu Rev Med. 2013;64:71–90. doi: 10.1146/annurev-med-112311-083918. [DOI] [PubMed] [Google Scholar]
- 5.Dudley ME, Gross CA, Somerville RP, Hong Y, Schaub NP, Rosati SF, et al. Randomized selection design trial evaluating CD8+-enriched versus unselected tumor-infiltrating lymphocytes for adoptive cell therapy for patients with melanoma. J Clin Oncol. 2013;31:2152–9. doi: 10.1200/JCO.2012.46.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phan GQ, Rosenberg SA. Adoptive cell transfer for patients with metastatic melanoma: the potential and promise of cancer immunotherapy. Cancer Control. 2013;20:289–97. doi: 10.1177/107327481302000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radvanyi LG, Bernatchez C, Zhang M, Fox PS, Miller P, Chacon J, et al. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2012;18:6758–70. doi: 10.1158/1078-0432.CCR-12-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besser MJ, Shapira-Frommer R, Itzhaki O, Treves AJ, Zippel DB, Levy D, et al. Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin Cancer Res. 2013;19:4792–800. doi: 10.1158/1078-0432.CCR-13-0380. [DOI] [PubMed] [Google Scholar]
- 11.Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16:2646–55. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 12.Goff SL, Dudley ME, Citrin DE, Somerville RP, Wunderlich JR, Danforth DN, et al. Randomized, Prospective Evaluation Comparing Intensity of Lymphodepletion Before Adoptive Transfer of Tumor-Infiltrating Lymphocytes for Patients With Metastatic Melanoma. J Clin Oncol. 2016;34:2389–97. doi: 10.1200/JCO.2016.66.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu-Lieskovan S, Robert L, Homet Moreno B, Ribas A. Combining targeted therapy with immunotherapy in BRAF-mutant melanoma: promise and challenges. J Clin Oncol. 2014;32:2248–54. doi: 10.1200/JCO.2013.52.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swaika A, Crozier JA, Joseph RW. Vemurafenib: an evidence-based review of its clinical utility in the treatment of metastatic melanoma. Drug Des Devel Ther. 2014;8:775–87. doi: 10.2147/DDDT.S31143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haferkamp S, Borst A, Adam C, Becker TM, Motschenbacher S, Windhovel S, et al. Vemurafenib induces senescence features in melanoma cells. J Invest Dermatol. 2013;133:1601–9. doi: 10.1038/jid.2013.6. [DOI] [PubMed] [Google Scholar]
- 16.Vultur A, Villanueva J, Herlyn M. Targeting BRAF in advanced melanoma: a first step toward manageable disease. Clin Cancer Res. 2011;17:1658–63. doi: 10.1158/1078-0432.CCR-10-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–14. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravnan MC, Matalka MS. Vemurafenib in patients with BRAF V600E mutation-positive advanced melanoma. Clin Ther. 2012;34:1474–86. doi: 10.1016/j.clinthera.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puzanov I, Amaravadi RK, McArthur GA, Flaherty KT, Chapman PB, Sosman JA, et al. Long-term outcome in BRAF(V600E) melanoma patients treated with vemurafenib: Patterns of disease progression and clinical management of limited progression. Eur J Cancer. 2015;51:1435–43. doi: 10.1016/j.ejca.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013;19:1225–31. doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper ZA, Frederick DT, Juneja VR, Sullivan RJ, Lawrence DP, Piris A, et al. BRAF inhibition is associated with increased clonality in tumor-infiltrating lymphocytes. Oncoimmunology. 2013;2:e26615. doi: 10.4161/onci.26615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70:5213–9. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 24.George AL, Suriano R, Rajoria S, Osso MC, Tuli N, Hanly E, et al. PLX4032 Mediated Melanoma Associated Antigen Potentiation in Patient Derived Primary Melanoma Cells. J Cancer. 2015;6:1320–30. doi: 10.7150/jca.11126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deniger DC, Pasetto A, Tran E, Parkhurst MR, Cohen CJ, Robbins PF, et al. Stable, Nonviral Expression of Mutated Tumor Neoantigen-specific T-cell Receptors Using the Sleeping Beauty Transposon/Transposase System. Mol Ther. 2016;24:1078–89. doi: 10.1038/mt.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JT, Li L, Brafford PA, van den Eijnden M, Halloran MB, Sproesser K, et al. PLX4032, a potent inhibitor of the B-Raf V600E oncogene, selectively inhibits V600E-positive melanomas. Pigment Cell Melanoma Res. 2010;23:820–7. doi: 10.1111/j.1755-148X.2010.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Comin-Anduix B, Chodon T, Sazegar H, Matsunaga D, Mock S, Jalil J, et al. The oncogenic BRAF kinase inhibitor PLX4032/RG7204 does not affect the viability or function of human lymphocytes across a wide range of concentrations. Clin Cancer Res. 2010;16:6040–8. doi: 10.1158/1078-0432.CCR-10-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grippo JF, Zhang W, Heinzmann D, Yang KH, Wong J, Joe AK, et al. A phase I, randomized, open-label study of the multiple-dose pharmacokinetics of vemurafenib in patients with BRAF V600E mutation-positive metastatic melanoma. Cancer Chemother Pharmacol. 2014;73:103–11. doi: 10.1007/s00280-013-2324-5. [DOI] [PubMed] [Google Scholar]
- 29.Stewart JJ, Lee CY, Ibrahim S, Watts P, Shlomchik M, Weigert M, et al. A Shannon entropy analysis of immunoglobulin and T cell receptor. Mol Immunol. 1997;34:1067–82. doi: 10.1016/s0161-5890(97)00130-2. [DOI] [PubMed] [Google Scholar]
- 30.Six A, Mariotti-Ferrandiz ME, Chaara W, Magadan S, Pham HP, Lefranc MP, et al. The past, present, and future of immune repertoire biology - the rise of next-generation repertoire analysis. Front Immunol. 2013;4:413. doi: 10.3389/fimmu.2013.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Acquavella N, Clever D, Yu Z, Roelke-Parker M, Palmer DC, Xi L, et al. Type I cytokines synergize with oncogene inhibition to induce tumor growth arrest. Cancer Immunol Res. 2015;3:37–47. doi: 10.1158/2326-6066.CIR-14-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu C, Peng W, Xu C, Lou Y, Zhang M, Wargo JA, et al. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clin Cancer Res. 2013;19:393–403. doi: 10.1158/1078-0432.CCR-12-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson DB, Menzies AM, Zimmer L, Eroglu Z, Ye F, Zhao S, et al. Acquired BRAF inhibitor resistance: A multicenter meta-analysis of the spectrum and frequencies, clinical behaviour, and phenotypic associations of resistance mechanisms. Eur J Cancer. 2015;51:2792–9. doi: 10.1016/j.ejca.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim KB, Kefford R, Pavlick AC, Infante JR, Ribas A, Sosman JA, et al. Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol. 2013;31:482–9. doi: 10.1200/JCO.2012.43.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong DJ, Ribas A. Targeted Therapy for Melanoma. Cancer Treat Res. 2016;167:251–62. doi: 10.1007/978-3-319-22539-5_10. [DOI] [PubMed] [Google Scholar]
- 36.Ribas A, Gonzalez R, Pavlick A, Hamid O, Gajewski TF, Daud A, et al. Combination of vemurafenib and cobimetinib in patients with advanced BRAF(V600)-mutated melanoma: a phase 1b study. Lancet Oncol. 2014;15:954–65. doi: 10.1016/S1470-2045(14)70301-8. [DOI] [PubMed] [Google Scholar]
- 37.Larkin J, Ascierto PA, Dreno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867–76. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 38.Liu L, Mayes PA, Eastman S, Shi H, Yadavilli S, Zhang T, et al. The BRAF and MEK Inhibitors Dabrafenib and Trametinib: Effects on Immune Function and in Combination with Immunomodulatory Antibodies Targeting PD-1, PD-L1, and CTLA-4. Clin Cancer Res. 2015;21:1639–51. doi: 10.1158/1078-0432.CCR-14-2339. [DOI] [PubMed] [Google Scholar]
- 39.Schadendorf D, Amonkar MM, Stroyakovskiy D, Levchenko E, Gogas H, de Braud F, et al. Health-related quality of life impact in a randomised phase III study of the combination of dabrafenib and trametinib versus dabrafenib monotherapy in patients with BRAF V600 metastatic melanoma. Eur J Cancer. 2015;51:833–40. doi: 10.1016/j.ejca.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–9. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 41.Vella LJ, Pasam A, Dimopoulos N, Andrews M, Knights A, Puaux AL, et al. MEK inhibition, alone or in combination with BRAF inhibition, affects multiple functions of isolated normal human lymphocytes and dendritic cells. Cancer Immunol Res. 2014;2:351–60. doi: 10.1158/2326-6066.CIR-13-0181. [DOI] [PubMed] [Google Scholar]
- 42.Mahoney KM, Freeman GJ, McDermott DF. The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma. Clin Ther. 2015;37:764–82. doi: 10.1016/j.clinthera.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]