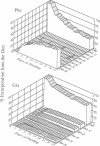
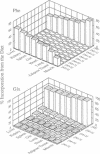
Abstract
The edible alga Spirulina platensis was uniformly labeled with 13C by growth in an atmosphere of pure 13CO2. The labeled biomass was then incorporated into the diet of a laying hen for 27 days. The isotopic enrichment of individual amino acids in egg white and yolk proteins, as well as in various tissues of the hen at the end of the feeding period, was analyzed by negative chemical ionization gas chromatography/mass spectrometry. The amino acids of successive eggs showed one of two exclusive enrichment patterns: complete preservation of the intact carbon skeleton or extensive degradation and resynthesis. The same observation was made in tissue proteins. These patterns were cleanly divided according to known nutritional amino acid essentiality/nonessentiality but revealed differences in labeling among the nonessential amino acids: most notable was that proline accretion was derived entirely from the diet. Feeding uniformly 13C-labeled algal protein and recovering and analyzing de novo-synthesized protein provides a useful method to examine amino acid metabolism and determine conditional amino acid essentially in vivo.
Full text
PDF

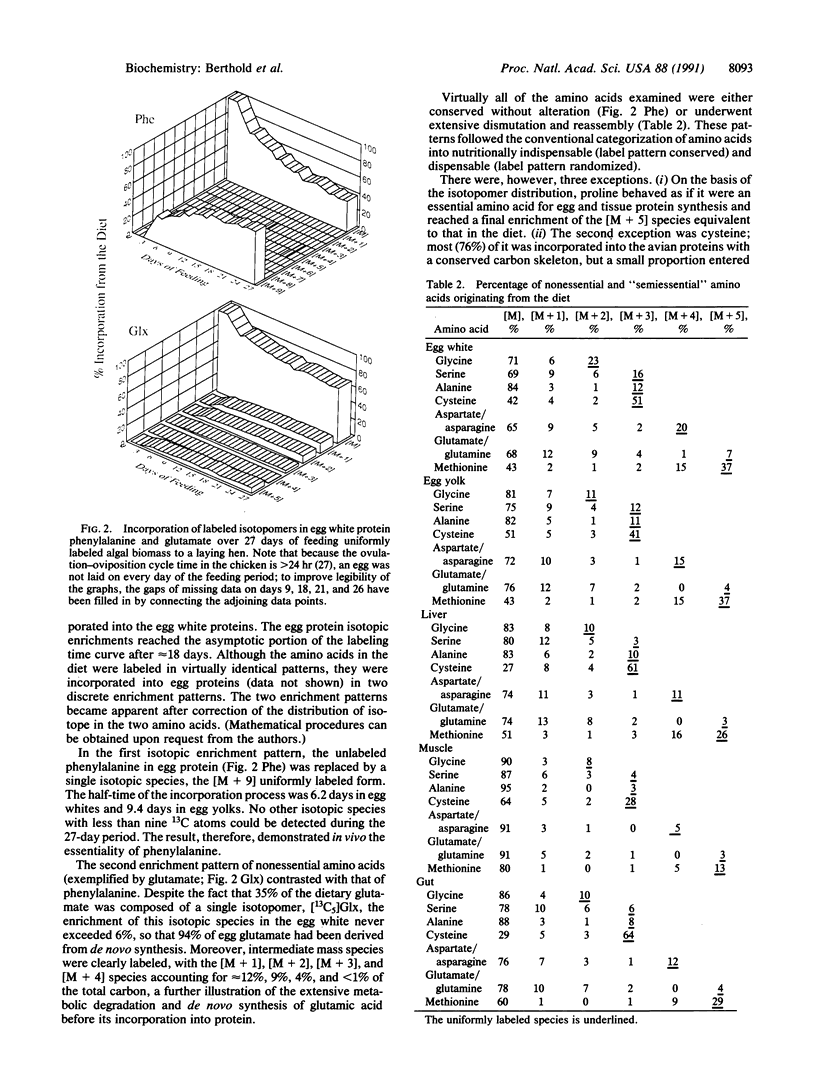

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clément G., Giddey C., Menzi R. Amino acid composition and nutritive valve of the alga Spirulina maxima. J Sci Food Agric. 1967 Nov;18(11):497–501. doi: 10.1002/jsfa.2740181101. [DOI] [PubMed] [Google Scholar]
- Des Rosiers C., Landau B. R., Brunengraber H. Interpretation of isotopomer patterns in tracing glycogen synthesis and glucose recycling using [13C6]glucose. Am J Physiol. 1990 Nov;259(5 Pt 1):E757–E762. doi: 10.1152/ajpendo.1990.259.5.E757. [DOI] [PubMed] [Google Scholar]
- Edwards C. H., Wade W. D., Freeburne M. M., Jones E. G., Stacey R. E., Sherman L., Seo C. W., Edwards G. A. Formation of methionine from alpha-amino-n-butyric acid and 5'-methylthioadenosine in the rat. J Nutr. 1977 Oct;107(10):1927–1936. doi: 10.1093/jn/107.10.1927. [DOI] [PubMed] [Google Scholar]
- FISHER H., JOHNSON D., Jr The amino acid requirement of the laying hen. II. Classification of the essential amino acids required for egg production. J Nutr. 1956 Oct 10;60(2):275–282. doi: 10.1093/jn/60.2.275. [DOI] [PubMed] [Google Scholar]
- Graber G., Baker D. H. The essential nature of glycine and proline for growing chickens. Poult Sci. 1973 May;52(3):892–896. doi: 10.3382/ps.0520892. [DOI] [PubMed] [Google Scholar]
- Hutchens T. W., Henry J. F., Yip T. T., Hachey D. L., Schanler R. J., Motil K. J., Garza C. Origin of intact lactoferrin and its DNA-binding fragments found in the urine of human milk-fed preterm infants. Evaluation by stable isotopic enrichment. Pediatr Res. 1991 Mar;29(3):243–250. doi: 10.1203/00006450-199103000-00005. [DOI] [PubMed] [Google Scholar]
- Irving C. S., Cooney C. L., Brown L. T., Gold D., Gordon J., Klein P. D. Microbial fermentative preparation of L-[15N2]lysine and its tracer: application to serum amino acid kinetic studies. Anal Biochem. 1983 May;131(1):93–98. doi: 10.1016/0003-2697(83)90139-2. [DOI] [PubMed] [Google Scholar]
- Irving C. S., Malphus E. W., Thomas M. R., Marks L., Klein P. D. Infused and ingested labeled lysines: appearance in human-milk proteins. Am J Clin Nutr. 1988 Jan;47(1):49–52. doi: 10.1093/ajcn/47.1.49. [DOI] [PubMed] [Google Scholar]
- Irving C. S., Thomas M. R., Malphus E. W., Marks L., Wong W. W., Boutton T. W., Klein P. D. Lysine and protein metabolism in young women. Subdivision based on the novel use of multiple stable isotopic labels. J Clin Invest. 1986 Apr;77(4):1321–1331. doi: 10.1172/JCI112437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON D., Jr, FISHER H. The amino-acid requirement of laying hens. 3. Minimal requirement levels of essential amino-acids: techniques and development of diet. Br J Nutr. 1958;12(3):276–285. doi: 10.1079/bjn19580039. [DOI] [PubMed] [Google Scholar]
- Jackson A. A., Shaw J. C., Barber A., Golden M. H. Nitrogen metabolism in preterm infants fed human donor breast milk: the possible essentiality of glycine. Pediatr Res. 1981 Nov;15(11):1454–1461. doi: 10.1203/00006450-198111000-00014. [DOI] [PubMed] [Google Scholar]
- Kalderon B., Gopher A., Lapidot A. Metabolic pathways leading to liver glycogen repletion in vivo, studied by GC-MS and NMR. FEBS Lett. 1986 Aug 11;204(1):29–32. doi: 10.1016/0014-5793(86)81381-3. [DOI] [PubMed] [Google Scholar]
- Katz J., Lee W. N., Wals P. A., Bergner E. A. Studies of glycogen synthesis and the Krebs cycle by mass isotopomer analysis with [U-13C]glucose in rats. J Biol Chem. 1989 Aug 5;264(22):12994–13004. [PubMed] [Google Scholar]
- Laidlaw S. A., Kopple J. D. Newer concepts of the indispensable amino acids. Am J Clin Nutr. 1987 Oct;46(4):593–605. doi: 10.1093/ajcn/46.4.593. [DOI] [PubMed] [Google Scholar]
- Lichtenstein A. H., Cohn J. S., Hachey D. L., Millar J. S., Ordovas J. M., Schaefer E. J. Comparison of deuterated leucine, valine, and lysine in the measurement of human apolipoprotein A-I and B-100 kinetics. J Lipid Res. 1990 Sep;31(9):1693–1701. [PubMed] [Google Scholar]
- Lifschitz C. H., Wolin M. J., Reeds P. J. Characterization of carbohydrate fermentation in feces of formula-fed and breast-fed infants. Pediatr Res. 1990 Feb;27(2):165–169. doi: 10.1203/00006450-199002000-00016. [DOI] [PubMed] [Google Scholar]
- Meguid M. M., Matthews D. E., Bier D. M., Meredith C. N., Young V. R. Valine kinetics at graded valine intakes in young men. Am J Clin Nutr. 1986 May;43(5):781–786. doi: 10.1093/ajcn/43.5.781. [DOI] [PubMed] [Google Scholar]
- Meredith C. N., Wen Z. M., Bier D. M., Matthews D. E., Young V. R. Lysine kinetics at graded lysine intakes in young men. Am J Clin Nutr. 1986 May;43(5):787–794. doi: 10.1093/ajcn/43.5.787. [DOI] [PubMed] [Google Scholar]
- Millward D. J., Rivers J. P. The nutritional role of indispensable amino acids and the metabolic basis for their requirements. Eur J Clin Nutr. 1988 May;42(5):367–393. [PubMed] [Google Scholar]
- Rannels D. E., Wartell S. A., Watkins C. A. The measurement of protein synthesis in biological systems. Life Sci. 1982 May 17;30(20):1679–1690. doi: 10.1016/0024-3205(82)90300-9. [DOI] [PubMed] [Google Scholar]
- Reeds P. J. Amino acid needs and protein scoring patterns. Proc Nutr Soc. 1990 Oct;49(3):489–497. doi: 10.1079/pns19900057. [DOI] [PubMed] [Google Scholar]
- Shulman R. J., Boutton T. W., Klein P. D. Impact of dietary cereal on nutrient absorption and fecal nitrogen loss in formula-fed infants. J Pediatr. 1991 Jan;118(1):39–43. doi: 10.1016/s0022-3476(05)81841-4. [DOI] [PubMed] [Google Scholar]
- Simpson R. J., Neuberger M. R., Liu T. Y. Complete amino acid analysis of proteins from a single hydrolysate. J Biol Chem. 1976 Apr 10;251(7):1936–1940. [PubMed] [Google Scholar]
- Waterlow J. C. Protein turnover with special reference to man. Q J Exp Physiol. 1984 Jul;69(3):409–438. doi: 10.1113/expphysiol.1984.sp002829. [DOI] [PubMed] [Google Scholar]
- Young V. R. 1987 McCollum award lecture. Kinetics of human amino acid metabolism: nutritional implications and some lessons. Am J Clin Nutr. 1987 Nov;46(5):709–725. doi: 10.1093/ajcn/46.5.709. [DOI] [PubMed] [Google Scholar]
- Zhao X. H., Wen Z. M., Meredith C. N., Matthews D. E., Bier D. M., Young V. R. Threonine kinetics at graded threonine intakes in young men. Am J Clin Nutr. 1986 May;43(5):795–802. doi: 10.1093/ajcn/43.5.795. [DOI] [PubMed] [Google Scholar]