Abstract
Reactive Oxygen Species (ROS) increase during aging, potentially affecting many tissues including brain, heart, and bone. ROS alter signaling pathways and constitute potential therapeutic targets to limit oxidative damaging effects in aging-associated diseases. Parathyroid hormone receptors (PTHR) are widely expressed and PTH is the only anabolic therapy for osteoporosis. The effects of oxidative stress on PTHR signaling and trafficking have not been elucidated. Here, we used Fluorescence Resonance Energy Transfer (FRET)-based cAMP, ERK, and calcium fluorescent biosensors to analyze the effects of ROS on PTHR signaling and trafficking by live-cell imaging. PTHR internalization and recycling were measured in HEK-293 cells stably transfected with HA-PTHR. PTH increased cAMP production, ERK phosphorylation, and elevated intracellular calcium. Pre-incubation with H2O2 reduced all PTH-dependent signaling pathways. These inhibitory effects were not a result of PTH oxidation since PTH incubated with H2O2 triggered similar responses. PTH promoted internalization and recycling of the PTHR. Both events were significantly reduced by H2O2 pre-incubation. These findings highlight the role of oxidation on PTHR signaling and trafficking, and suggest the relevance of ROS as a putative target in diseases associated with oxidative stress such as age-related osteoporosis.
Keywords: PTH, PTH receptor, hydrogen peroxide (PubChem CID:784), oxidative stress, signaling, trafficking
1. Introduction
Reactive oxygen species (ROS), including hydrogen peroxide (H2O2), are generated during mitochondrial oxidative metabolism and also produced by exogenous sources. Oxidative stress arises from an imbalance between an increase of ROS and the antioxidant cellular defensive mechanisms [1,2]. Oxidative stress causes nucleic acid, lipid and protein damage [2], and is involved in the pathogenesis of several diseases, including age-related bone loss in osteoporosis and some types of nephropathies [3–5]. During skeletal aging, accumulation of ROS affects several regulatory processes such as osteoblast and osteoclast apoptosis, osteoblastogenesis, and adipogenesis, involved in bone homeostasis. Disruption of these activities adversely affects bone mass and strength [6,7]. In addition, ROS play an essential role in the pathology of several renal diseases including ischemic acute renal failure, renal graft rejection, acute glomerulonephritis, toxic renal diseases [8], and diabetic nephropathy [5]. ROS-dependent oxidation of proteins results in structural modifications that may change their function. These alterations partially result from changes in signaling pathways that are increased or decreased by ROS. Identifying these pathways and targets represent potential therapeutic opportunities to limit the consequences of oxidative damage in ROS-associated diseases [1].
Parathyroid hormone (PTH) receptor type I (PTHR) is a seven transmembrane G-coupled protein receptor (GPCR) that is highly expressed in the kidney and bone, where it is a key component of bone development and mineral-ion balance, and is a target for current and proposed drugs to treat bone and mineral disorders [9]. The PTHR is a member of Family B GPCRs and signals through Gs and Gq, which in turn activate adenylyl cyclases/cAMP/PKA and phospholipase C/inositol phosphates (IP3)/calcium/PKC cascades, respectively [10]. ERK1/2 MAP kinase is another signaling pathway triggered by activated PTHR that regulates bone and renal functions [11,12]. The ligands for PTHR, PTH and PTH-related protein (PTHrP), are involved in the etiology of processes such as osteoporosis and hypercalcemia of malignancy. PTH(1-34) (teriparatide) is employed therapeutically for management of osteoporosis and PTHrP(1-34) (abaloparatide) is presently in clinical studies. After ligand binding and activation, most GPCRs are phosphorylated, bind β-arrestins, and are endocytosed. Desensitized receptors are subsequently recycled to the plasma membrane or trafficked to lysosomes for degradation [13].
Based on the pathogenic potential of ROS and given the essential role of PTHR signaling in bone and renal physiology we analyzed the effects of oxidative stress as found in osteoporosis and diabetic nephropathy on PTHR activity. To accomplish this, we characterized the actions of ROS, modeled with H2O2, on PTHR signaling and trafficking. Here, we show that PTH-dependent cAMP, ERK1/2, and calcium signaling, as well as PTHR endocytosis and recycling, are inhibited by H2O2.
2. Materials and Methods
2.1 Cell culture and transfection
HEK-293 cells were stably transfected with human influenza hemagglutinin (HA)-PTHR (HEK-293R) [14] and cultured in Dulbecco’s modified Eagle’s medium/F-12 medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 0.75 mg/ml geneticin. HEK-293R cells express 6.0 × 105 PTHR/cell, with an average Kd of 14.2 nM. Cells were maintained at 37 °C in a humidified atmosphere of 5% CO2, 95% air. Cells were pre-incubated for 15 min with different concentrations of H2O2 (1–500 μM) followed by stimulation with 10 nM–1 μM [Nle8,18,Tyr34]-PTH(1-34) (hereafter NNT-PTH) (Bachem, Torrance, CA), an oxidant-resistant PTH peptide.
For transient transfections, HEK-293R cells were grown on poly-D-lysine-coated glass 25-mm coverslips for 12 h prior to transfection with FuGENE 6 (Roche Applied Science) in complete medium. After 24 h, coverslips in HEPES/ bovine serum albumin buffer (pH=7.4) (HEPES buffer containing 0.1% (w/v) BSA) were transferred to an Attofluor chamber (Invitrogen, Carlsbad, CA) for live-cell imaging.
2.2 Fluorescence Resonance Energy Transfer (FRET)
HEK-293R cells were transiently transfected with the cAMP biosensor EPAC [15] or with the ERK-NES biosensor [16]. cAMP formation and ERK activation were measured in real time in live cells by fluorescence resonance energy transfer (FRET) as described [17]. Cells were observed using a 40× 1.30 NA oil immersion objective on a Nikon A1s confocal microscope attached to a Ti-E inverted base. FRET signals are reported as the normalized FRET ratio (nFRET) of the yellow fluorescent protein (YFP) and cyan fluorescent protein emission ratio (FYFP/FCFP).
2.3 Intracellular calcium
Intracellular calcium activity ([Ca2+]i) was measured with Fluo-4 AM (Invitrogen, Carlsbad, CA) following the manufacturer’s protocol. Briefly, HEK-293R cells were cultured on poly-D-lysine-coated glass 25-mm coverslips with 2 μM Fluo-4 AM in Hanks’ balanced salt solution (HBSS) (Invitrogen) at 22 °C for 45 min. Cells were washed three times with HBSS and further incubated at 22 °C for 30 min. Calcium measurements were performed with a Nikon A1s inverted fluorescence microscope. Fluorescence was recorded at 1-s intervals for up to 20 min. At least 30–40 cells were counted under each condition. Intracellular calcium activity was calculated from the following equation: [Ca2+]i = Kd × (F − Fmin)/(Fmax − F), where F is the measured fluorescence intensity; Fmax is the fluorescence measured after addition of 10 μM ionomycin (maximum [Ca2+]i increase); Fmin is the fluorescence measured after addition of 10 mM EGTA (minimum [Ca2+]i), and Kd is the dissociation constant of the dye-Ca2+ complex (520 nm) [17].
2.4 Receptor internalization
PTHR internalization was measured in HEK-293R cells seeded on poly-D-lysine-coated 24-well plates. Confluent cells were treated with NNT-PTH in the presence or absence of H2O2 and subsequently fixed with 3.7% paraformaldehyde at room temperature. After 3 washes with phosphate-buffered saline (PBS) [pH=7.4] cells were blocked with 1% BSA for 45 min and incubated with a rabbit polyclonal anti-HA antibody (Covance, Berkeley, CA; 1:1000) for 1 h at room temperature.
Cells were then washed with PBS, re-blocked with 1% BSA for 15 min, and incubated with an anti-rabbit IgG conjugated with alkaline phosphatase (Santa Cruz Biotechnology, Santa Cruz, CA, 1:1000). After washing, alkaline phosphatase substrate p-nitrophenyl phosphate was added for 30 min, 100 μL of the reaction mixture were transferred to a 96-well plate, and absorbance was measured at 405 nm [17].
2.5 Statistical analysis
Data are presented as the mean ± SEM from 3 independent experiments. Multiple comparisons were evaluated by nonparametric Kruskal-Wallis with post-hoc analysis using Dunn’s multiple comparison test (Prism, GraphPad). Differences greater than P ≤ 0.05 were assumed to be significant.
3. Results
3.1 PTH-dependent signaling is inhibited by H2O2
NNT-PTH promptly augmented intracellular cAMP as reflected by the rapid induction of EPAC (Fig. 1A) and ERK-NES (Fig. 1B) and augmented intracellular Ca2+ (Fig. 2A, 2B).
Fig. 1. H2O2 suppresses PTH-stimulated cAMP formation and ERK activation.
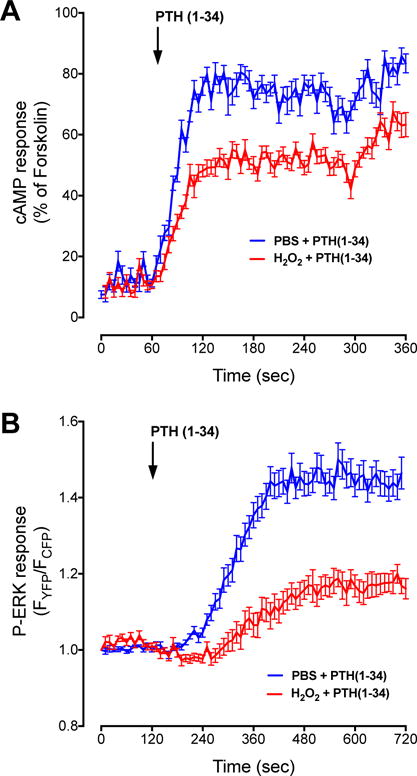
A) HEK-293R cells were transiently transfected with an EPAC FRET sensor to measure cAMP. After a 60-sec baseline recording 100 nM NNT-PTH in PBS was added and recording continued for an additional 5 min. Pretreatment with 100 μM H2O2 for 15 min decreased cAMP formation. Data are the mean ± SEM of N = 3 independent experiments with n = 20 (PTH[1-34] or 30 (H2O2 + PTH[1-34]). B) pERK1/2 activation was similarly measured using the ERK-NES FRET sensor. Here, a 2-min baseline recording was followed by introduction of 100 nM NNT-PTH. 100 μM H2O2 for 15 min decreased pERK1/2. Data are the mean ± SEM of N = 3 independent experiments with n = 22 (PTH[1-34] or 15 (H2O2 + PTH[1-34]).
Fig. 2. Intracellular calcium accumulation upon PTH stimulation is inhibited by preincubating cells with H2O2.
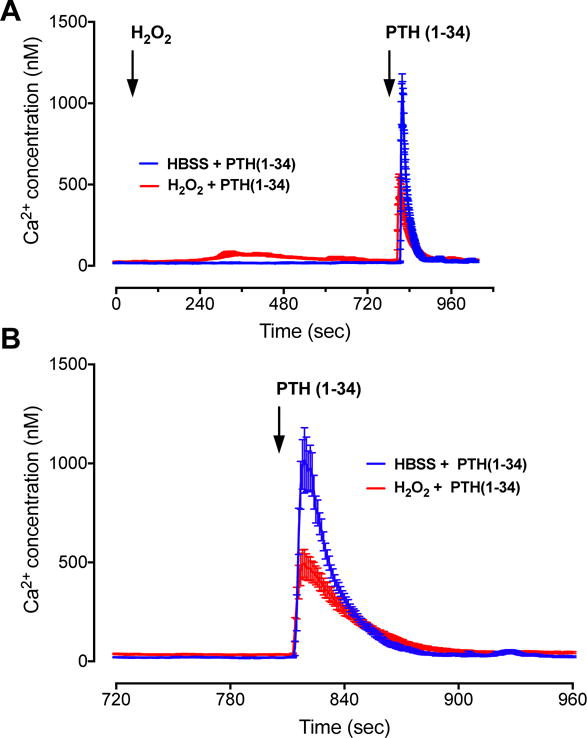
A) 100 nM NNT-PTH caused rapid increases in Fluo-4 AM fluorescence in HEK-293R cells, corresponding to an elevation of intracellular calcium activity. Pretreatment with 100 μM H2O2 for 15 min decreased calcium signaling triggered by NNT-PTH. B) Detailed changes of Fluo-4 AM after PTH stimulation. Data represent the mean ± SEM of N = 3 independent experiments; n = 17 for H2O2 + NNT-PTH and n = 20 for HBSS + NNT-PTH.
To define the effects of oxidative stress on PTHR signaling, we analyzed the response of exposure to 100 μM H2O2, which we previously demonstrated to be a maximally effective dose on ERK1/2, p38, JNK, p66Shc, and Akt kinase phosphorylation [18]. Pre-incubation of HEK-293R cells with 100 μM H2O2 significantly decreased NNT-PTH-dependent cAMP intracellular accumulation and ERK phosphorylation (Fig. 1A and B). Similarly, H2O2 dampened the magnitude of NNT-PTH stimulation cytoplasmic calcium activity (Fig. 2).
Oxidation of PTH results in loss of biological activity [19–22]. To determine whether the reduced PTHR signaling following H2O2 treatment was caused by PTH oxidation, we pre-incubated the NNT-PTH peptide with H2O2. No apparent differences of cAMP accumulation were observed after exposure of H2O2-pre-incubated NNT-PTH compared to NNT-PTH controls (HBSS) (Fig. 3).
Fig. 3. Ligand-dependent accumulation of cAMP is not inhibited by incubation of PTH with H2O2.
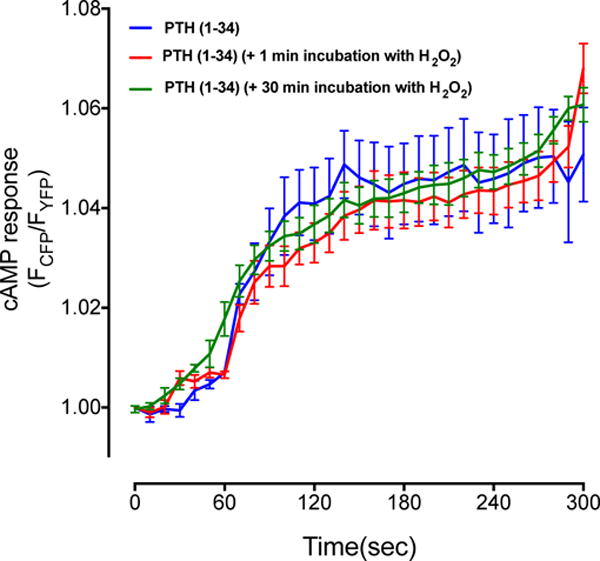
100 nM NNT-PTH increased cAMP accumulation in HEK-293R cells. No significant changes in cAMP responses were observed after preincubating NNT-PTH with 100 μM H2O2 for 5 or 30 min. Data represent the mean ± SEM of N = 3 independent experiments n = 10 cells for each condition.
3.2 H2O2 modifies PTH receptor trafficking
We next evaluated the effects of H2O2 on PTHR trafficking by measuring internalization and recycling to the plasma membrane. Under control conditions up to 30% of PTHR internalized upon NNT-PTH stimulation. Receptor endocytosis was greater at 100 nM and 1 μM compared to 10 nM NNT-PTH. In contrast, H2O2 pre-incubation of HEK-293 cells decreased receptor internalization in a concentration-dependent manner (Fig. 4A). At 10 nM NNT-PTH, H2O2 virtually abolished PTHR sequestration, while at 100 nM or 1 μM inhibition was 35–40%.
Fig. 4. PTHR internalization and recycling upon PTH stimulation are inhibited by H2O2.
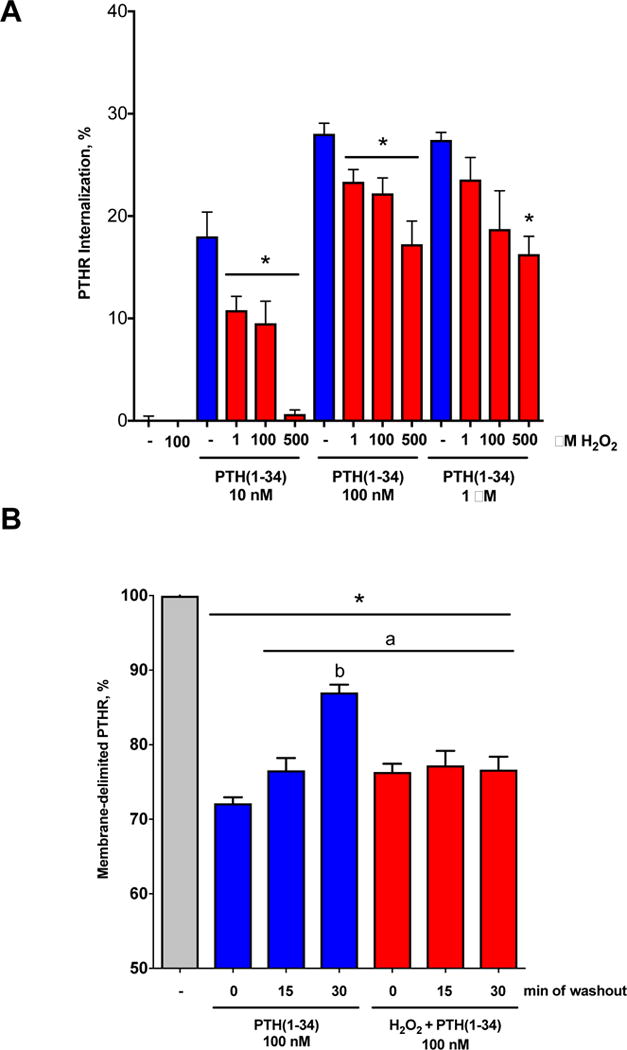
A) NNT-PTH at 10 nM, 100 nM or 1 μM concentrations induced PTHR internalization within 30 min. Pretreating cells with 1–500 μM H2O2 significantly inhibited PTHR endocytosis. B) PTHR internalized by 100 nM NNT-PTH recycles to the cell membrane in a time-dependent manner following peptide washout. PTHR recycling was annihilated by pretreating cells for 15 min with 100 μM H2O2. Data represent the mean ± SEM of N = 3 independent experiments. *P <0.05 vs. no PTH (−); aP <0.05 vs. NNT-PTH (0); bP <0.05 vs. corresponding H2O2.
Once internalized, resensitized receptors recycle to the plasma membrane, allowing cells to reuse receptors efficiently [13]. Under control conditions, i.e., in the absence of H2O2, PTHR recycling to the plasma membrane was essentially complete within 30 min after peptide removal. In striking contrast, pre-incubation with H2O2 abolished PTHR recycling (Fig. 4B).
4. Discussion
PTHR is expressed at high levels in bone and kidney and at lower levels in a number of tissues, including blood vessels, heart, liver, and nervous system mediating a large array of endocrine or paracrine/autocrine functions in response to systemic PTH or locally produced PTHrP, respectively [23]. Many diseases associated with oxidative stress affect organs where PTHR acts and, despite the key actions of this receptor in these organs, the effects of oxidative stress on PTHR signaling have not been studied. Here, we show that H2O2 decreases PTH-dependent PTHR signaling and trafficking, suggesting a new target for treatment in oxidative stress-mediated diseases involving PTHR signaling.
Patients with advanced stages of renal disease, involutional osteoporosis, cardiovascular diseases, among others, exhibit increased levels of systemic oxidative stress with the potential to oxidize hormones such as PTH. Several reports show that oxidized PTH has weakened biological activity as a consequence of diminished PTHR binding [20,24,25]. PTH has two methionine residues at positions 8 and 18 and studies by independent groups have shown that oxidation of PTH diminishes its interaction with PTHR, does not stimulate the PTH to generate cAMP, and triggers abnormal calcium, phosphorus and vitamin D biological responses [25–27]. The secondary structure of PTH is essential for its receptor binding, with methionine-8 critical for the folding and biological activity of the hormone [19,25]. Thus, oxidation of methionine-8 is implicated both in binding and in activation of adenylyl cyclase [25]. To avoid the loss of biological activity due to PTH oxidation, here we used a modified peptide, where methionines at positions 8 and 18 are replaced by norleucine [Nle8,18,Tyr34]-PTH(1-34) [28]. In addition, comparable cAMP responses obtained in experiments with or without H2O2 preincubation with NNT-PTH suggest that decreased PTHR signaling and trafficking following treatment with H2O2 are caused by oxidation of the receptor or downstream effectors and not to ligand oxidation.
PTH binding to PTHR stabilizes the receptor in an active conformation that leads to interaction of the intracellular loop regions of PTHR with Gs and Gq proteins, which results in the initiation of intracellular signaling [10]. Cysteines are thought to play a critical role in receptor function and conformation by modifying structure through the introduction of disulfide bonds or by acting as reactive enzyme centers, together with amino acids like histidine and asparagine [29,30]. The high predisposition of ROS to affect cysteine residues is especially relevant given the role of GPCR and G-protein cysteine residues in the formation of intra- and inter-molecular disulfide bridges and receptor complexes, formation of ligand binding domains, and stabilization of protein conformations through modifications such as palmitoylation and prenylation, which facilitate downstream signal transduction [31]. PTHR harbors eight extracellular that have been shown to be essential for receptor function [32], and five additional intracellular cysteines distributed in loops 1 and 3, transmembrane domain 7, and the C-terminal intracellular tail.Cystein-217 in intracellular loop 1 is necessary for adenylyl cyclase/cAMP signaling [33]. It is possible though, that oxidation of cysteines in PTHR changes conformation of the receptor or modifies binding domains resulting in reduced interactions with signaling partners. Supporting this notion, signaling from members of the tyrosine kinase receptor family such as the epidermal growth factor receptor (EGFR), has shown to be regulated by cysteine oxidation [34], and oxidative stress generated by H2O2 results in an aberrant pattern of receptor phosphorylation [35]. In these studies H2O2 functions as a secondary messenger to regulate intracellular signaling cascades, largely through the modification of specific cysteine residues such as Cys-797 in the EGFR active site within redox-sensitive protein targets [36]. Exogenous ROS also cause direct cysteine oxidation in GPCRs, including the β2 adrenergic receptor [37], in which cysteine residues are key regulators of adenylyl cyclase stimulation [38]. GPCR signaling can also be modified by ROS targeting of receptor protein partners. Oxidative stress induces G protein-coupled receptor kinases-2 (GRK2) translocation to the membrane, where GRK2 interacts with Gq/11α, uncoupling it from the dopamine D1 receptor and leading to reduced phospholipase C signaling [39]. Similar mechanisms to those described above may explain the decreased PTHR signaling in following H2O2 exposure.
Our data not only show decreased PTHR signaling but also diminished receptor internalization and recycling. In contrast to most receptors studied to date, in which signaling is rapidly extinguished by receptor endocytosis, PTHR signaling in response to PTH continues even after internalization of the activated receptor [40]. Prolonged PTHR intracellular signaling involves internalization and stabilization of an active [PTH-PTHR] complex in early endosomes [40,41]. Thus, inhibition of PTHR internalization by H2O2 as shown here may result in moderated signaling due to reduced formation of the endocytic PTHR complex. In this regard, several reports describe inhibition of endocytosis of a variety of molecules, including receptors, by pretreating cells with H2O2 [42–44]. Furthermore, PTHR endocytosis occurs in a clathrin-dependent manner [45] and clathrin-dependent endocytosis is disrupted under oxidative stress by a mechanism involving tyrosine phosphorylation of clathrin heavy chain [46]. Once the PTHR internalizes into early endosomes, it subsequently traffics to an actin-sorting nexin 27-retromer tubule complex (ASRT), a sorting platform on early endosomes that promotes recycling of surface receptors and has recently been described to mediate rapid PTHR recycling to the surface [41,47,48]. Interestingly, the retromer complex has been described as a target of oxidative stress. Oxidative stress switches the glucose transporter 4 (GLUT4) sorting direction to lysosomes through inhibition of retromer function, by a protein kinase CK2-dependent mechanism, instead of promoting translocation from intracellular compartments to the plasma membrane [49]. In this respect, oxidative stress may contribute to the altered receptor trafficking and the observed impaired cellular signaling.
Although further investigation will be required to elucidate the precise molecular mechanisms that mediate decreased PTHR signaling and internalization and recycling, the present study reveals novel responses to oxidative stress by the PTHR and suggests a novel mechanism of pathological PTHR dysfunction in oxidative stress-mediated diseases.
Acknowledgments
This work was supported by grants from the Spanish Instituto de Salud Carlos III (PI11/00449 and RETICEF RD12/0043/0008) to PE and by the National Institutes (NIH) (DK105811) to PAF.
References
- 1.Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 2.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jilka RL, Almeida M, Ambrogini E, Han L, Roberson PK, Weinstein RS, Manolagas SC. Decreased oxidative stress and greater bone anabolism in the aged, when compared to the young, murine skeleton with parathyroid hormone administration. Aging Cell. 2010;9:851–867. doi: 10.1111/j.1474-9726.2010.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31:266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–1454. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- 6.Almeida M. Aging and Oxidative Stress: A new look at old bone. IBMS BoneKEy. 2010;7:340–352. [Google Scholar]
- 7.Wauquier F, Leotoing L, Coxam V, Guicheux J, Wittrant Y. Oxidative stress in bone remodelling and disease. Trends Mol Med. 2009;15:468–477. doi: 10.1016/j.molmed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Baud L, Ardaillou R. Reactive oxygen species: production and role in the kidney. Am J Physiol. 1986;251:F765–776. doi: 10.1152/ajprenal.1986.251.5.F765. [DOI] [PubMed] [Google Scholar]
- 9.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 10.Abou-Samra AB, Juppner H, Force T, Freeman MW, Kong XF, Schipani E, Urena P, Richards J, Bonventre JV, Potts JT, Jr, et al. Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc Natl Acad Sci USA. 1992;89:2732–2736. doi: 10.1073/pnas.89.7.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, Lefkowitz RJ. Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem. 2006;281:10856–10864. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- 12.Mahalingam CD, Sampathi BR, Sharma S, Datta T, Das V, Abou-Samra AB, Datta NS. MKP1-dependent PTH modulation of bone matrix mineralization in female mice is osteoblast maturation stage specific and involves P-ERK and P-p38 MAPKs. J Endocrinol. 2013;216:315–329. doi: 10.1530/JOE-12-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanyaloglu AC, von ZM. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–568. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- 14.Wang B, Bisello A, Yang Y, Romero GG, Friedman PA. NHERF1 regulates parathyroid hormone receptor membrane retention without affecting recycling. J Biol Chem. 2007;282:36214–36222. doi: 10.1074/jbc.M707263200. [DOI] [PubMed] [Google Scholar]
- 15.Nikolaev VO, Bunemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem. 2004;279:37215–37218. doi: 10.1074/jbc.C400302200. [DOI] [PubMed] [Google Scholar]
- 16.Komatsu N, Aoki K, Yamada M, Yukinaga H, Fujita Y, Kamioka Y, Matsuda M. Development of an optimized backbone of FRET biosensors for kinases and GTPases. Mol Biol Cell. 2011;22:4647–4656. doi: 10.1091/mbc.E11-01-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alonso V, Ardura JA, Wang B, Sneddon WB, Friedman PA. A naturally occurring isoform inhibits parathyroid hormone receptor trafficking and signaling. J Bone Miner Res. 2011;26:143–155. doi: 10.1002/jbmr.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ardura JA, Portal-Núñez S, Castelbón-Calvo I, Martínez de Toda I, De la Fuente M, Esbrit P. Parathyroid hormone-related protein protects osteoblastic cells from oxidative stress by activation of MKP1 phosphatase. J Cell Physiol. 2016 doi: 10.1002/jcp.25473. [DOI] [PubMed] [Google Scholar]
- 19.O’Riordan JL, Woodhead JS, Hendy GN, Parsons JA, Robinson CJ, Keutmann HT, Dawson BF, Potts JT., Jr Effect of oxidation on biological and immunological activity of porcine parathyroid hormone. J Endocrinol. 1974;63:117–124. doi: 10.1677/joe.0.0630117. [DOI] [PubMed] [Google Scholar]
- 20.Frelinger AL, 3rd, Zull JE. Oxidized forms of parathyroid hormone with biological activity. Separation and characterization of hormone forms oxidized at methionine 8 and methionine 18. J Biol Chem. 1984;259:5507–5513. [PubMed] [Google Scholar]
- 21.Zull JE, Smith SK, Wiltshire R. Effect of methionine oxidation and deletion of amino-terminal residues on the conformation of parathyroid hormone. Circular dichroism studies. J Biol Chem. 1990;265:5671–5676. [PubMed] [Google Scholar]
- 22.Hocher B, Armbruster FP, Stoeva S, Reichetzeder C, Gron HJ, Lieker I, Khadzhynov D, Slowinski T, Roth HJ. Measuring parathyroid hormone (PTH) in patients with oxidative stress–do we need a fourth generation parathyroid hormone assay? PLoS One. 2012;7:e40242. doi: 10.1371/journal.pone.0040242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clemens TL, Cormier S, Eichinger A, Endlich K, Fiaschi-Taesch N, Fischer E, Friedman PA, Karaplis AC, Massfelder T, Rossert J, Schluter KD, Silve C, Stewart AF, Takane K, Helwig JJ. Parathyroid hormone-related protein and its receptors: nuclear functions and roles in the renal and cardiovascular systems, the placental trophoblasts and the pancreatic islets. Br J Pharmacol. 2001;134:1113–1136. doi: 10.1038/sj.bjp.0704378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frelinger AL, 3rd, Zull JE. The role of the methionine residues in the structure and function of parathyroid hormone. Arch Biochem Biophys. 1986;244:641–649. doi: 10.1016/0003-9861(86)90632-6. [DOI] [PubMed] [Google Scholar]
- 25.Zull JE, Smith SK, Wiltshire R. Effect of methionine oxidation and deletion of amino-terminal residues on the conformation of parathyroid hormone. Circular dichroism studies. J Biol Chem. 1990;265:5671–5676. [PubMed] [Google Scholar]
- 26.Galceran T, Lewis-Finch J, Martin KJ, Slatopolsky E. Absence of biological effects of oxidized parathyroid hormone-(1-34) in dogs and rats. Endocrinology. 1984;115:2375–2378. doi: 10.1210/endo-115-6-2375. [DOI] [PubMed] [Google Scholar]
- 27.Horiuchi N. Effects of oxidation of human parathyroid hormone on its biological activity in continuously infused, thyroparathyroidectomized rats. J Bone Miner Res. 1988;3:353–358. doi: 10.1002/jbmr.5650030316. [DOI] [PubMed] [Google Scholar]
- 28.Rosenblatt M, Goltzman D, Keutmann HT, Tregear GW, Potts JT., Jr Chemical and biological properties of synthetic, sulfur-free analogues of parathyroid hormone. J Biol Chem. 1976;251:159–164. [PubMed] [Google Scholar]
- 29.Betts MJ, Russell RB. Amino acid properties and consequences of substitutions. Wiley; Hoboken, NJ: 2003. [Google Scholar]
- 30.Lipton SA, Choi YB, Takahashi H, Zhang D, Li W, Godzik A, Bankston LA. Cysteine regulation of protein function–as exemplified by NMDA-receptor modulation. Trends Neurosci. 2002;25:474–480. doi: 10.1016/s0166-2236(02)02245-2. [DOI] [PubMed] [Google Scholar]
- 31.O’Dowd BF, Hnatowich M, Caron MG, Lefkowitz RJ, Bouvier M. Palmitoylation of the human beta 2-adrenergic receptor. Mutation of Cys341 in the carboxyl tail leads to an uncoupled nonpalmitoylated form of the receptor. J Biol Chem. 1989;264:7564–7569. [PubMed] [Google Scholar]
- 32.Lee C, Gardella TJ, Abou-Samra AB, Nussbaum SR, Segre GV, Potts JT, Jr, Kronenberg HM, Juppner H. Role of the extracellular regions of the parathyroid hormone (PTH)/PTH-related peptide receptor in hormone binding. Endocrinology. 1994;135:1488–1495. doi: 10.1210/endo.135.4.7523099. [DOI] [PubMed] [Google Scholar]
- 33.Thomas BE, Wittelsberger A, Woznica I, Hsieh MY, Monaghan P, Lee BK, Rosenblatt M. Cysteine at position 217 in the intracellular loop 1 plays a critical role in human PTH receptor type 1 membrane translocation and function. J Bone Miner Res. 2007;22:609–616. doi: 10.1359/jbmr.070101. [DOI] [PubMed] [Google Scholar]
- 34.Truong TH, Carroll KS. Redox regulation of epidermal growth factor receptor signaling through cysteine oxidation. Biochemistry. 2012;51:9954–9965. doi: 10.1021/bi301441e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan EM, Heidinger JM, Levy M, Lisanti MP, Ravid T, Goldkorn T. Epidermal growth factor receptor exposed to oxidative stress undergoes Src- and caveolin-1-dependent perinuclear trafficking. J Biol Chem. 2006;281:14486–14493. doi: 10.1074/jbc.M509332200. [DOI] [PubMed] [Google Scholar]
- 36.Paulsen CE, Truong TH, Garcia FJ, Homann A, Gupta V, Leonard SE, Carroll KS. Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nat Chem Biol. 2011;8:57–64. doi: 10.1038/nchembio.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burns RN, Moniri NH. Agonist- and hydrogen peroxide-mediated oxidation of the beta2 adrenergic receptor: evidence of receptor s-sulfenation as detected by a modified biotin-switch assay. J Pharmacol Exp Ther. 2011;339:914–921. doi: 10.1124/jpet.111.185975. [DOI] [PubMed] [Google Scholar]
- 38.Liggett SB, Bouvier M, O’Dowd BF, Caron MG, Lefkowitz RJ, DeBlasi A. Substitution of an extracellular cysteine in the beta 2-adrenergic receptor enhances agonist-promoted phosphorylation and receptor desensitization. Biochem Biophys Res Commun. 1989;165:257–263. doi: 10.1016/0006-291x(89)91063-2. [DOI] [PubMed] [Google Scholar]
- 39.Banday AA, Lokhandwala MF. Oxidative stress reduces renal dopamine D1 receptor-Gq/11alpha G protein-phospholipase C signaling involving G protein-coupled receptor kinase 2. Am J Physiol Renal Physiol. 2007;293:F306–315. doi: 10.1152/ajprenal.00108.2007. [DOI] [PubMed] [Google Scholar]
- 40.Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, Gardella TJ, Vilardaga JP. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol. 2009;5:734–742. doi: 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feinstein TN, Wehbi VL, Ardura JA, Wheeler DS, Ferrandon S, Gardella TJ, Vilardaga JP. Retromer terminates the generation of cAMP by internalized PTH receptors. Nat Chem Biol. 2011;7:278–284. doi: 10.1038/nchembio.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aguilar-Gaytan R, Mas-Oliva J. Oxidative stress impairs endocytosis of the scavenger receptor class A. Biochem Biophys Res Commun. 2003;305:510–517. doi: 10.1016/s0006-291x(03)00796-4. [DOI] [PubMed] [Google Scholar]
- 43.Cheng J, Vieira A. Oxidative stress disrupts internalization and endocytic trafficking of transferrin in a human malignant keratinocyte line. Cell Biochem Biophys. 2006;45:177–184. doi: 10.1385/CBB:45:2:177. [DOI] [PubMed] [Google Scholar]
- 44.Mougeolle A, Poussard S, Decossas M, Lamaze C, Lambert O, Dargelos E. Oxidative stress induces caveolin 1 degradation and impairs caveolae functions in skeletal muscle cells. PLoS One. 2015;10:e0122654. doi: 10.1371/journal.pone.0122654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sneddon WB, Magyar CE, Willick GE, Syme CA, Galbiati F, Bisello A, Friedman PA. Ligand-selective dissociation of activation and internalization of the parathyroid hormone (PTH) receptor: conditional efficacy of PTH peptide fragments. Endocrinology. 2004;145:2815–2823. doi: 10.1210/en.2003-1185. [DOI] [PubMed] [Google Scholar]
- 46.Ihara Y, Yasuoka C, Kageyama K, Wada Y, Kondo T. Tyrosine phosphorylation of clathrin heavy chain under oxidative stress. Biochem Biophys Res Commun. 2002;297:353–360. doi: 10.1016/s0006-291x(02)02195-2. [DOI] [PubMed] [Google Scholar]
- 47.McGarvey JC, Xiao K, Bowman SL, Mamonova T, Zhang Q, Bisello A, Sneddon WB, Ardura JA, Jean-Alphonse F, Vilardaga JP, Puthenveedu MA, Friedman PA. Actin-Sorting Nexin 27 (SNX27)-Retromer Complex Mediates Rapid Parathyroid Hormone Receptor Recycling. J Biol Chem. 2016;291:10986–11002. doi: 10.1074/jbc.M115.697045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pavlos NJ, Friedman PA. GPCR Signalling and Trafficking: The long and short of it. Trends in Endocrinology & Metabolism. 2016 doi: 10.1016/j.tem.2016.10.007. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma J, Nakagawa Y, Kojima I, Shibata H. Prolonged insulin stimulation down-regulates GLUT4 through oxidative stress-mediated retromer inhibition by a protein kinase CK2-dependent mechanism in 3T3-L1 adipocytes. J Biol Chem. 2014;289:133–142. doi: 10.1074/jbc.M113.533240. [DOI] [PMC free article] [PubMed] [Google Scholar]


