Abstract
Protein-nucleic acid interactions involved in the assembly process of the Escherichia coli 30S ribosomal subunit were quantitatively analyzed by high-resolution scanning transmission electron microscopy. The in vitro reconstituted ribonucleoprotein (core) particles were characterized by their morphology, mass, and radii of gyration. During the assembly of the 30S subunit, the 16S rRNA underwent significant conformational changes that were governed by the cooperative interactions of the ribosomal proteins. The sequential association of the first 12 proteins with the 16S rRNA resulted in the formation of core particles containing up to three mass centers at distinct stages of the assembly process. These globular mass centers may correspond to the three major domains (5', central, and 3') of the 16S rRNA. Through the subsequent interactions of the late assembly proteins with the 16S rRNA, two of the three domains merge, yielding the basic structural traits of the native 30S subunit. The fine morphological features of the native 30S subunit became distinctly resolved only after the addition of the full complement of proteins. The fully reconstituted 30S subunits are active in polyphenylalanine synthesis assays. Visualization of the assembly mechanism of the E. coli 30S ribosomal subunit revealed domain-specific folding of the 16S rRNA through the formation of distinct intermediate core particles hitherto not observed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boublik M., Oostergetel G. T., Mandiyan V., Hainfeld J. F., Wall J. S. Structural analysis of ribosomes by scanning transmission electron microscopy. Methods Enzymol. 1988;164:49–63. doi: 10.1016/s0076-6879(88)64034-1. [DOI] [PubMed] [Google Scholar]
- Brimacombe R., Atmadja J., Stiege W., Schüler D. A detailed model of the three-dimensional structure of Escherichia coli 16 S ribosomal RNA in situ in the 30 S subunit. J Mol Biol. 1988 Jan 5;199(1):115–136. doi: 10.1016/0022-2836(88)90383-x. [DOI] [PubMed] [Google Scholar]
- Brimacombe R. The emerging three-dimensional structure and function of 16S ribosomal RNA. Biochemistry. 1988 Jun 14;27(12):4207–4214. doi: 10.1021/bi00412a001. [DOI] [PubMed] [Google Scholar]
- Buck M. A., Cooperman B. S. Single protein omission reconstitution studies of tetracycline binding to the 30S subunit of Escherichia coli ribosomes. Biochemistry. 1990 Jun 5;29(22):5374–5379. doi: 10.1021/bi00474a024. [DOI] [PubMed] [Google Scholar]
- Capel M. S., Kjeldgaard M., Engelman D. M., Moore P. B. Positions of S2, S13, S16, S17, S19 and S21 in the 30 S ribosomal subunit of Escherichia coli. J Mol Biol. 1988 Mar 5;200(1):65–87. doi: 10.1016/0022-2836(88)90334-8. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E. The functional role of ribosomal RNA in protein synthesis. Cell. 1989 May 19;57(4):525–529. doi: 10.1016/0092-8674(89)90122-0. [DOI] [PubMed] [Google Scholar]
- Ehresmann C., Ehresmann B., Millon R., Ebel J. P., Nurse K., Ofengand J. Cross-linking of the anticodon of Escherichia coli and Bacillus subtilis acetylvalyl-tRNA to the ribosomal P site. Characterization of a unique site in both E. coli 16S and yeast 18S ribosomal RNA. Biochemistry. 1984 Jan 31;23(3):429–437. doi: 10.1021/bi00298a006. [DOI] [PubMed] [Google Scholar]
- Ehresmann C., Ofengand J. Two-dimensional gel electrophoresis technique for determination of the cross-linked nucleotides in cleavable covalent RNA-RNA complexes. Application to Escherichia coli and Bacillus subtilis acetylvalyl-tRNA covalently linked to E. coli 16S and yeast 18S ribosomal RNA. Biochemistry. 1984 Jan 31;23(3):438–445. doi: 10.1021/bi00298a007. [DOI] [PubMed] [Google Scholar]
- Girshovich A. S., Bochkareva E. S., Ovchinnikov Y. A. Elongation factor G and protein S12 are the nearest neighbours in the Escherichia coli ribosome. J Mol Biol. 1981 Sep 15;151(2):229–243. doi: 10.1016/0022-2836(81)90513-1. [DOI] [PubMed] [Google Scholar]
- Gregory R. J., Zeller M. L., Thurlow D. L., Gourse R. L., Stark M. J., Dahlberg A. E., Zimmermann R. A. Interaction of ribosomal proteins S6, S8, S15 and S18 with the central domain of 16 S ribosomal RNA from Escherichia coli. J Mol Biol. 1984 Sep 15;178(2):287–302. doi: 10.1016/0022-2836(84)90145-1. [DOI] [PubMed] [Google Scholar]
- Gualerzi C. O., Pon C. L. Initiation of mRNA translation in prokaryotes. Biochemistry. 1990 Jun 26;29(25):5881–5889. doi: 10.1021/bi00477a001. [DOI] [PubMed] [Google Scholar]
- Held W. A., Ballou B., Mizushima S., Nomura M. Assembly mapping of 30 S ribosomal proteins from Escherichia coli. Further studies. J Biol Chem. 1974 May 25;249(10):3103–3111. [PubMed] [Google Scholar]
- Held W. A., Nomura M. Escherichia coli 30 S ribosomal proteins uniquely required for assembly. J Biol Chem. 1975 Apr 25;250(8):3179–3184. [PubMed] [Google Scholar]
- Huang K. H., Fairclough R. H., Cantor C. R. Singlet energy transfer studies of the arrangement of proteins in the 30 S Escherichia coli ribosome. J Mol Biol. 1975 Oct 5;97(4):443–470. doi: 10.1016/s0022-2836(75)80053-2. [DOI] [PubMed] [Google Scholar]
- Kerlavage A. R., Hasan T., Cooperman B. S. Reverse phase high performance liquid chromatography of Escherichia coli ribosomal proteins: standardization of 70 S, 50 S, and 30 S protein chromatograms. J Biol Chem. 1983 May 25;258(10):6313–6318. [PubMed] [Google Scholar]
- Krzyzosiak W., Denman R., Nurse K., Hellmann W., Boublik M., Gehrke C. W., Agris P. F., Ofengand J. In vitro synthesis of 16S ribosomal RNA containing single base changes and assembly into a functional 30S ribosome. Biochemistry. 1987 Apr 21;26(8):2353–2364. doi: 10.1021/bi00382a042. [DOI] [PubMed] [Google Scholar]
- Lambert J. M., Boileau G., Cover J. A., Traut R. R. Cross-links between ribosomal proteins of 30S subunits in 70S tight couples and in 30S subunits. Biochemistry. 1983 Aug 2;22(16):3913–3920. doi: 10.1021/bi00285a029. [DOI] [PubMed] [Google Scholar]
- Langer J. A., Lake J. A. Elongation factor Tu localized on the exterior surface of the small ribosomal subunit. J Mol Biol. 1986 Feb 20;187(4):617–621. doi: 10.1016/0022-2836(86)90339-6. [DOI] [PubMed] [Google Scholar]
- Malhotra A., Tan R. K., Harvey S. C. Prediction of the three-dimensional structure of Escherichia coli 30S ribosomal subunit: a molecular mechanics approach. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1950–1954. doi: 10.1073/pnas.87.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandiyan V., Tumminia S., Wall J. S., Hainfeld J. F., Boublik M. Protein-induced conformational changes in 16 S ribosomal RNA during the initial assembly steps of the Escherichia coli 30 S ribosomal subunit. J Mol Biol. 1989 Nov 20;210(2):323–336. doi: 10.1016/0022-2836(89)90334-3. [DOI] [PubMed] [Google Scholar]
- Mizushima S., Nomura M. Assembly mapping of 30S ribosomal proteins from E. coli. Nature. 1970 Jun 27;226(5252):1214–1214. doi: 10.1038/2261214a0. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Binding of tRNA to the ribosomal A and P sites protects two distinct sets of nucleotides in 16 S rRNA. J Mol Biol. 1990 Jan 5;211(1):135–145. doi: 10.1016/0022-2836(90)90016-F. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature. 1987 Jun 4;327(6121):389–394. doi: 10.1038/327389a0. [DOI] [PubMed] [Google Scholar]
- Nierhaus K. H. The allosteric three-site model for the ribosomal elongation cycle: features and future. Biochemistry. 1990 May 29;29(21):4997–5008. doi: 10.1021/bi00473a001. [DOI] [PubMed] [Google Scholar]
- Nomura M., Mizushima S., Ozaki M., Traub P., Lowry C. V. Structure and function of ribosomes and their molecular components. Cold Spring Harb Symp Quant Biol. 1969;34:49–61. doi: 10.1101/sqb.1969.034.01.009. [DOI] [PubMed] [Google Scholar]
- Nomura M., Traub P., Guthrie C., Nashimoto H. The assembly of ribosomes. J Cell Physiol. 1969 Oct;74(2 Suppl):241+–241+. doi: 10.1002/jcp.1040740428. [DOI] [PubMed] [Google Scholar]
- Nowotny V., Nierhaus K. H. Assembly of the 30S subunit from Escherichia coli ribosomes occurs via two assembly domains which are initiated by S4 and S7. Biochemistry. 1988 Sep 6;27(18):7051–7055. doi: 10.1021/bi00418a057. [DOI] [PubMed] [Google Scholar]
- Pleij C. W., Rietveld K., Bosch L. A new principle of RNA folding based on pseudoknotting. Nucleic Acids Res. 1985 Mar 11;13(5):1717–1731. doi: 10.1093/nar/13.5.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers T., Changchien L. M., Craven G. R., Noller H. F. Probing the assembly of the 3' major domain of 16 S ribosomal RNA. Quaternary interactions involving ribosomal proteins S7, S9 and S19. J Mol Biol. 1988 Mar 20;200(2):309–319. doi: 10.1016/0022-2836(88)90243-4. [DOI] [PubMed] [Google Scholar]
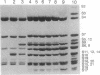
- Powers T., Stern S., Changchien L. M., Noller H. F. Probing the assembly of the 3' major domain of 16 S rRNA. Interactions involving ribosomal proteins S2, S3, S10, S13 and S14. J Mol Biol. 1988 Jun 20;201(4):697–716. doi: 10.1016/0022-2836(88)90468-8. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J., Craven G. R. Evidence that E. coli ribosomal protein S13 has two separable functional domains involved in 16S RNA recognition and protein S19 binding. Nucleic Acids Res. 1985 Sep 25;13(18):6767–6786. doi: 10.1093/nar/13.18.6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüler D., Brimacombe R. The Escherichia coli 30S ribosomal subunit; an optimized three-dimensional fit between the ribosomal proteins and the 16S RNA. EMBO J. 1988 May;7(5):1509–1513. doi: 10.1002/j.1460-2075.1988.tb02970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern S., Powers T., Changchien L. M., Noller H. F. Interaction of ribosomal proteins S5, S6, S11, S12, S18 and S21 with 16 S rRNA. J Mol Biol. 1988 Jun 20;201(4):683–695. doi: 10.1016/0022-2836(88)90467-6. [DOI] [PubMed] [Google Scholar]
- Stern S., Powers T., Changchien L. M., Noller H. F. RNA-protein interactions in 30S ribosomal subunits: folding and function of 16S rRNA. Science. 1989 May 19;244(4906):783–790. doi: 10.1126/science.2658053. [DOI] [PubMed] [Google Scholar]
- Stern S., Weiser B., Noller H. F. Model for the three-dimensional folding of 16 S ribosomal RNA. J Mol Biol. 1988 Nov 20;204(2):447–481. doi: 10.1016/0022-2836(88)90588-8. [DOI] [PubMed] [Google Scholar]
- Svensson P., Changchien L. M., Craven G. R., Noller H. F. Interaction of ribosomal proteins, S6, S8, S15 and S18 with the central domain of 16 S ribosomal RNA. J Mol Biol. 1988 Mar 20;200(2):301–308. doi: 10.1016/0022-2836(88)90242-2. [DOI] [PubMed] [Google Scholar]
- Wiener L., Brimacombe R. Protein binding sites on Escherichia coli 16S RNA; RNA regions that are protected by proteins S7, S14 and S19 in the presence or absence of protein S9. Nucleic Acids Res. 1987 May 11;15(9):3653–3670. doi: 10.1093/nar/15.9.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R. A. Protein-RNA interactions in the bacterial ribosome. Methods Enzymol. 1979;59:551–583. doi: 10.1016/0076-6879(79)59113-7. [DOI] [PubMed] [Google Scholar]