Abstract
Background & Aims
Proliferation, differentiation, and morphogenesis of the intestinal epithelium are tightly regulated by a number of molecular pathways. Coordinated action of intestine is achieved by gastrointestinal hormones, most of which exert these actions through G-protein–coupled receptors. We herein investigated the role of Gαq/11-mediated signaling in intestinal homeostasis.
Methods
Intestinal tissues from control (Gnaqflox/floxGna11+/+), Int-Gq knock-out (KO) (VilCre+/-Gnaqflox/floxGna11+/+), G11 KO (Gnaqflox/floxGna11-/-), and Int-Gq/G11 double knock-out (DKO) (VilCre+/-Gnaqflox/floxGna11-/-) mice were examined by microscopy, transmission electron microscopy, and immunohistochemistry. The effect of Gαq/11-mediated signaling was studied in the cell lineage, proliferation, and apoptosis. Dextran sodium sulfate (DSS) colitis was induced to study the role of Gαq/11 in colon.
Results
Paneth cells were enlarged, increased in number, and mislocalized in Int-Gq/G11 DKO small intestine. Paneth cells also reacted with PAS and Muc2 antibody, indicating an intermediate character of Paneth and goblet cells. The nuclear β-catenin, T-cell factor 1, and Sox9 expression were reduced severely in the crypt base of Int-Gq/G11 DKO intestine. Proliferation was activated in the crypt base and apoptosis was enhanced along the crypt. Int-Gq/G11 DKO mice were susceptible to DSS colitis. Proliferation was inhibited in the crypt of unaffected and regenerative areas. Cystic crypts, periodic acid–Schiff–positive cells, and Muc2-positive cells were unusually observed in the ulcerative region.
Conclusions
The Gαq/11-mediated pathway plays a pivotal role in the preservation of intestinal homeostasis, especially in Paneth cell maturation and positioning. Wnt/β-catenin signaling was reduced significantly in the crypt base in Gαq/G11-deficient mice, resulting in the defective maturation of Paneth cells, induction of differentiation toward goblet cells, and susceptibility to DSS colitis.
Keywords: Paneth Cell, Intermediate Cell, Wnt, Gnaq, Gna11
Abbreviations used in this paper: Atoh1, atonal homolog 1; BrdU, bromodeoxyuridine; Defa1, defensin α1; Dll1, delta-like 1; DSS, dextran sodium sulfate; FGF, fibroblast growth factor; Fzd, frizzled; Hes, hairy/enhancer of split; IEC, intestinal epithelial cell; Ihh, Indian hedgehog; mRNA, messenger RNA; NICD, Notch intracellular cytoplasmic domain; PAS, periodic acid–Schiff; PCR, polymerase chain reaction; PKC, protein kinase C; qPCR, quantitative real-time polymerase chain reaction; Tcf, T-cell factor; TEM, transmission electron micrograph; TUNEL, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling
Summary.
Gut hormones are important in coordinated actions of intestine and they exert actions through G-protein–coupled receptors. We show that Gαq/11-mediated signaling plays a pivotal role in the maturation and positioning of Paneth cells and in the maintenance of intestinal homeostasis.
The epithelium of small intestine is composed of 4 distinct cell lineages: absorptive enterocytes and 3 secreting cell types including mucus-secreting goblet cells, antimicrobial peptide-secreting Paneth cells, and hormone-secreting enteroendocrine cells. All of these cell types originate from multipotent stem cells residing in niches in the lower parts of the crypt. Cell renewal, lineage commitment, and cell differentiation in the intestinal epithelium are coupled to cell migration in a precise, spatially organized manner.1 Stem cells give rise to progenitor cells, which are amplified by constant division along the bottom two thirds of the crypts.2 These daughter cells migrate up as they proliferate. In the transit-amplifying zone near the top of the crypt, these cells terminally differentiate into the 4 main cell types. Then, absorptive enterocytes, goblet cells, and enteroendocrine cells migrate up the villi and Paneth cells migrate down to reside at the crypt base. The epithelium of large intestine lacks Paneth cells.
The digestive tract consists of a variety of tissues, each with a specific function necessary for the effective handling of a meal. The coordination of the complex functions of digestion, absorption, and excretion of a meal is achieved largely by molecules of neuroendocrine origin.3 Gastrointestinal hormones, including cholecystokinin, gastrin, secretin, histamine, glucose-dependent insulinotropic polypeptide, glucagon-like-peptide-1, and vasoactive intestinal peptide, regulate their target cells through guanine nucleotide-binding protein-coupled receptors (G-protein–coupled receptors). Another important group of modulators are neurotransmitters, such as acetylcholine, which are released from vagal nerve terminals and exert their roles through the muscarinic receptor subtype M3. Secretin, glucose-dependent insulinotropic polypeptide, and glucagon-like-peptide-1 exert their signals through the Gαs family of heterotrimeric G proteins.4 In contrast, cholecystokinin, gastrin, and acetylcholine exert their signals through the Gαq family of G proteins. Mammals express 4 Gαq class α-subunits, of which 2, Gαq and Gα11, are widely expressed5; their activation results in the stimulation of phospholipase C-β isoform and consequent inositol 1,4,5-triphosphate-mediated intracellular calcium mobilization and protein kinase C (PKC) activation.6 The expression of Gα14 and Gα15/16 is restricted to certain tissues, such as kidney and hematopoietic organs, respectively.7, 8
The gastrointestinal system is a rich source of neuroendocrine hormones that interact with at least 10 families of G-protein coupled receptors containing more than 30 known receptor subtypes.3 Although the physiological relevance in the regulation of intestinal homeostasis remains unclear, the sheer number of potential Gαq/11-coupled receptors suggests an importance of this G-protein family in the intestine.
Proliferation, differentiation, and morphogenesis in the intestinal epithelium are tightly regulated by a number of molecular pathways. Stem cells generate daughter cells that undergo lineage commitment and maturation through the combined action of the Wnt/β-catenin and Notch signaling pathways. Cells adopt either an absorptive or a secretory cell fate according to the balance between Wnt and Notch signaling.9 Both pathways also regulate transcription networks that further define the differentiation of intestinal epithelial cells (IECs).10
The most established effects of Wnt/β-catenin in IECs are those involved in cell proliferation, in particular by maintaining the proliferative state of progenitors.11 However, Wnt/β-catenin signaling is not confined to proliferating immature cells, but also is active in fully differentiated Paneth cells.11 Wnt/β-catenin signaling maintains the correct positioning of the Paneth cells by controlling the expression of genes encoding ephrin B2/B3 receptors and the ephrin B1 ligand1 and terminal maturation of Paneth cells.11, 12, 13, 14, 15
The Notch cascade mediates cell-to-cell signaling and has been shown to be essential for the maintenance of the proliferative crypt compartment, as well as for the formation of absorptive enterocytes.10 Notch signaling occurs when the transmembrane Notch receptor is bound by ligands expressed on adjacent cells. The intracellular cytoplasmic domain of the receptor is cleaved from the transmembrane domain by γ-secretase and translocates to the nucleus, where it associates with the transcriptional activator protein RBP-Jk (CSL/CBF/Su[H]/Lag-1) to stimulate the expression of target genes, such as Hairy/enhancer of split 1 (Hes1) and Hes5.16 Progenitor cells that express Hes1 will differentiate into absorptive enterocytes, whereas progenitors that express atonal homolog 1 (Atoh1, also known as Math1) are committed to the secretory lineage, differentiating into goblet, Paneth, or enteroendocrine cells. Goblet and Paneth cells continue to share similar characteristics, whereas the differentiation of secretory precursors into endocrine fate is regulated by neurogenin3.17
To study the role of the Gαq class of G proteins in intestinal homeostasis, we generated and examined mice with intestine-specific knockout of the genes encoding Gαq and Gα11. We herein show that the Gαq/11-mediated signaling pathway plays a pivotal role in Paneth cell maturation and positioning. It also plays a critical role in the maintenance of intestinal homeostasis.
Materials and Methods
Animals
C57BL/6 (B6) Gnaqflox/floxGna11-/- mice were kindly provided by Dr Stefan Offermanns of the Institute of Pharmacology, University of Heidelberg (Germany). To delete Gnaq in intestinal epithelial cells in adult mice, we crossed Gnaqflox/floxGna11-/- mice to Villin-Cre transgenic mice, which express Cre recombinase under the control of the villin promoter.18 The villin promoter drives stable and homogeneous expression of Cre recombinase in nearly all epithelial cells in the small intestine and, to a lesser extent, the large intestine.18
All mice were maintained in a specific pathogen-free animal facility with free access to food and water, except for during experiments on dextran sodium sulfate (DSS)-induced colitis. All experiments using mice were approved by the Institutional Animal Care and Use Committee of Akita University.
Polymerase Chain Reaction for Genotyping, Conventional Reverse-Transcription Polymerase Chain Reaction, and Quantitative Real-Time Polymerase Chain Reaction
The primers used in this study are listed in Supplementary Table 1. Genomic DNA was isolated from mouse tails and amplified by standard polymerase chain reaction (PCR). Total RNA was obtained from IECs using an RNeasy Mini kit (Qiagen, Valencia, CA), which were scraped off the intestinal tissue with a spatula. First-stranded complementary DNA was synthesized from total RNA using Superscript First-stranded Synthesis System for reverse-transcription PCR (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Quantitative real-time PCR (qPCR) was performed using ABI PRISM 7900HT (Applied Biosystems, Foster City, CA): denaturation at 94°C for 30 seconds, annealing at 60°C for 30 seconds, and extension at 72°C for 30 seconds.
Materials
The primary antibodies used in the studies are listed in Supplementary Table 2. Horseradish-peroxidase–conjugated donkey anti-mouse IgG, horseradish-peroxidase–conjugated donkey anti-rabbit IgG, horseradish-peroxidase–conjugated donkey anti-goat IgG, and Cy3-conjugated donkey anti-rabbit IgG were from Jackson Immuno Research (West Grove, PA). DSS (molecular weight, 36–50 kilodaltons) was purchased from MP Biomedicals (Solon, OH).
Immunohistochemistry
Eight- to 10-week-old mice were killed, and the intestinal tissues were excised. Formalin-fixed and paraffin-embedded samples or frozen sections were used for immunohistochemistry. Detection of β-catenin staining was performed using the Envision+ system (Dako, Glostrup, Denmark). Tyramide signal amplification (Molecular Probes, Waltham, MA) was used for the immunofluorescent detection of delta-like 1 (Dll1). Staining was visualized by secondary antibodies or tyramide substrates conjugated with Alexa-594 (Molecular Probes). Nuclei were counterstained with hematoxylin (Wako Chemicals, Doshoumachi, Osaka) or with 4′,6-diamidino-2-phenylindole (Invitrogen, Carlsbad, CA). Light microscopy and immunofluorescence microscopy were performed as described previously.19 For the electron microscopy, small tissue pieces from intestine were postfixed with 2.5% glutaraldehyde and 1% OsO4 and embedded in an epoxy resin. Semithin and ultrathin sections were stained with toluidine blue and uranyl acetate/lead citrate for observation under both a light microscope and an electron microscope, respectively. The ultrastructural analysis and quantification of intestinal crypts and villi were determined on random micrographs showing the full face of the structure, where the crypt and villi clearly were identified. Quantitative analyses were measured using ImageJ software (National Institutes of Health, Bethesda, MD).
Western Blotting
IECs, which had been scraped from intestinal tissue using a spatula, were homogenized in a lysis buffer (100 mmol/L NaCl, 20 mmol/L Tris/HCl, pH 7.5, and 1% Triton X-100; Sigma-Aldrich, St. Louis, MO). After centrifugation, the crude extracts were boiled in Laemmli 2× sample buffer. Twenty to 50 μg of protein was loaded onto each lane of 5%–15% sodium dodecyl sulfate-polyacrylamide gels and run at 200 V. The proteins then were transferred onto nitrocellulose membranes at 60 V for 4 hours. The membranes were incubated sequentially with Blocking Ace (Snow Brand Milk Products, Sapporo, Japan), primary antibodies and secondary antibodies, and then were detected using an enhanced chemiluminescence Western blot detection reagent (Amersham Biosciences, Piscataway, NJ) to visualize the secondary antibody. The densitometry analysis was performed using the ImageJ software program.
Terminal Deoxynucleotidyl Transferase-Mediated Deoxyuridine Triphosphate Nick-End Labeling Assay
Apoptotic cells were detected using an In Situ Cell Death Detection Kit, TMR red (Roche Applied Science, Mannheim, Germany) according to the manufacturer’s instructions. The nuclei were counterstained with 4',6-diamidino-2-phenylindole. The number of terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL)-positive cells was counted at a magnification of ×200 with an Olympus IX70 fluorescence microscope (Shinjuku, Tokyo).
Induction of DSS Colitis and Grading of Histologic Changes
DSS (molecular weight, 36–50 kilodaltons) was added to the drinking water of the mice at a final concentration of 3% (wt/vol) for 5 consecutive days (days 1–5), followed by distilled water.20 The whole body weights of mice were measured every day. Some of the mice were killed at the indicated times and colonic samples were subjected to H&E staining, histology, and immunohistochemistry.
Statistical Analysis
All data are presented as the means ± SD. The Student t test was used to compare the values between 2 mice lines and a 1-way analysis of variance, and post hoc tests were used for comparisons among the 4 mouse lines. P values less than .05 were considered to indicate statistical significance.
Results
The Generation of Intestinal Epithelial Cell–Specific Gαq/Gα11-Deficient Mice
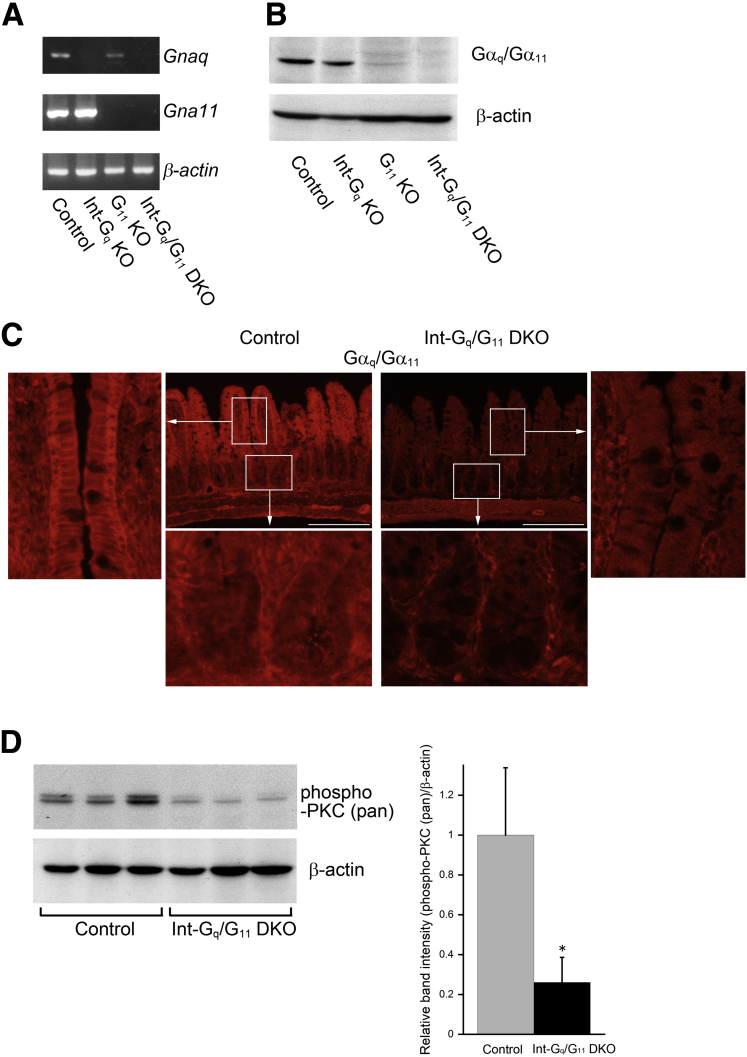
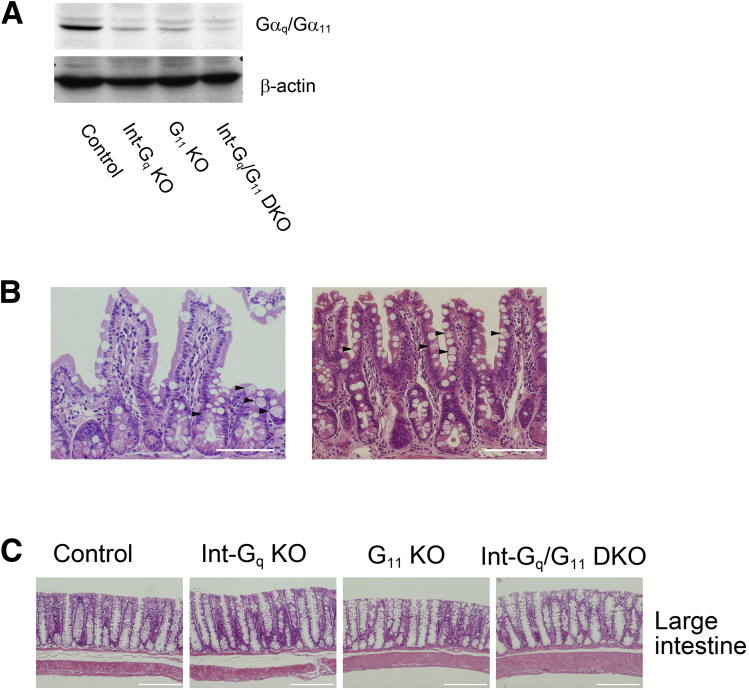
To generate intestinal epithelial cell–specific Gαq/Gα11-deficient mice, we crossed the VilCre mouse line18 with mice carrying floxed alleles of the gene coding for Gαq, Gnaq (Gnaqflox/flox),21 and constitutive Gα11-deficiency (Gna11-/-).22 In the VilCre mouse line, Cre recombinase is expressed specifically in the intestinal epithelium from 10.5 days postcoitum onward.18 We generated 4 mouse lines: control mice (Gnaqflox/floxGna11+/+, henceforth termed control), intestine-specific Gαq-deficient mice (VilCre+/-Gnaqflox/floxGna11+/+, termed Int-Gq knock-out), Gα11-deficient mice (Gnaqflox/floxGna11-/-, termed G11 KO), and intestine-specific Gαq/Gα11-double-deficienct mice (VilCre+/-Gnaqflox/floxGna11-/-, termed Int-Gq/G11 double knock-out). These mice were born at the expected numbers, were fertile, and showed no obvious abnormalities. Their body weights did not differ from that of C57BL/6 wild-type mice or VilCre mice (VilCre+/-Gnaq+/+Gna11+/+) (data not shown). PCR analysis and Western blot of intestinal mucosa showed a clear reduction in the expressions of Gαq and Gα11 in these mouse lines, particularly in Int-Gq/G11 DKO mice (Figure 1A and B). The band intensity of Gna11 in PCR analysis and Gα11 on Western blot was stronger than that of Gnaq and Gαq. This may be owing to the abundant expression of Gα11 in mouse small intestine compared with Gαq.5 In contrast, on Western blot of large intestine, the band intensity of Gαq/Gα11 in Int-Gq KO and G11 KO mice was reduced to almost the same level. This may imply that Gαq and Gα11 were expressed in nearly equal quantities in the large intestine (Supplementary Figure 1A). Immunoreactivity for Gαq/Gα11 was observed primarily in the basolateral membrane of the cells in the villi and crypts and in the cells in the lamia propria. It clearly disappeared in the IECs of Int-Gq/G11 DKO mice, but not in the cells in lamia propria (Figure 1C). The loss of Gαq/Gα11 in the intestinal mucosa resulted in a significant reduction in the PKC phosphorylation of several isoforms, whereas the messenger RNA (mRNA) levels of PKCα and PKCδ were not changed (Figure 1D and Supplementary Figure 2).
Figure 1.
The expression of Gαq and Gα11 in small intestinal epithelial cells in 4 mouse lines: control (Gnaqflox/floxGna11+/+), Int-Gq KO (VilCre+/-Gnaqflox/floxGna11+/+), G11 KO (Gnaqflox/floxGna11-/-), and Int-Gq/G11 DKO (VilCre+/-Gnaqflox/floxGna11-/-). (A) Reverse-transcription PCR analysis of mRNA expression for Gnaq and Gna11 in small intestine. (B) Western blot analysis of Gαq/Gα11 expression. (C) Immunohistochemical detection of Gαq/Gα11 expression in small intestine of control and Int-Gq/G11 DKO mice. Lower and side panels show the boxed areas with higher magnification. Scale bars: 200 μm. (D) Western blot analysis of the phosphorylation of PKC. This antibody detects endogenous levels of PKC α, βI, βII, δ, ε, η and θ isoforms when it is phosphorylated at a carboxyl terminal residue homologous to serine 660 of PKC βII. Right: ImageJ densitometry analysis. The values represent the means ± SD (n = 3). *P < .05.
Supplementary Figure 1.
(A) Western blot analysis of Gαq/Gα11 expression in 4 mouse lines: control (Gnaqflox/floxGna11+/+), Int-Gq KO (VilCre+/-Gnaqflox/floxGna11+/+), G11 KO (Gnaqflox/floxGna11-/-), and Int-Gq/G11 DKO (VilCre+/-Gnaqflox/floxGna11-/-). (B) H&E staining in small intestine of Int-Gq/G11 DKO mice. Enlarged Paneth cells were distributed along the crypt–villus axis. (C) HE staining in large intestine of 4 mouse lines. Morphologic changes were not detected. Scale bars: 200 μm.
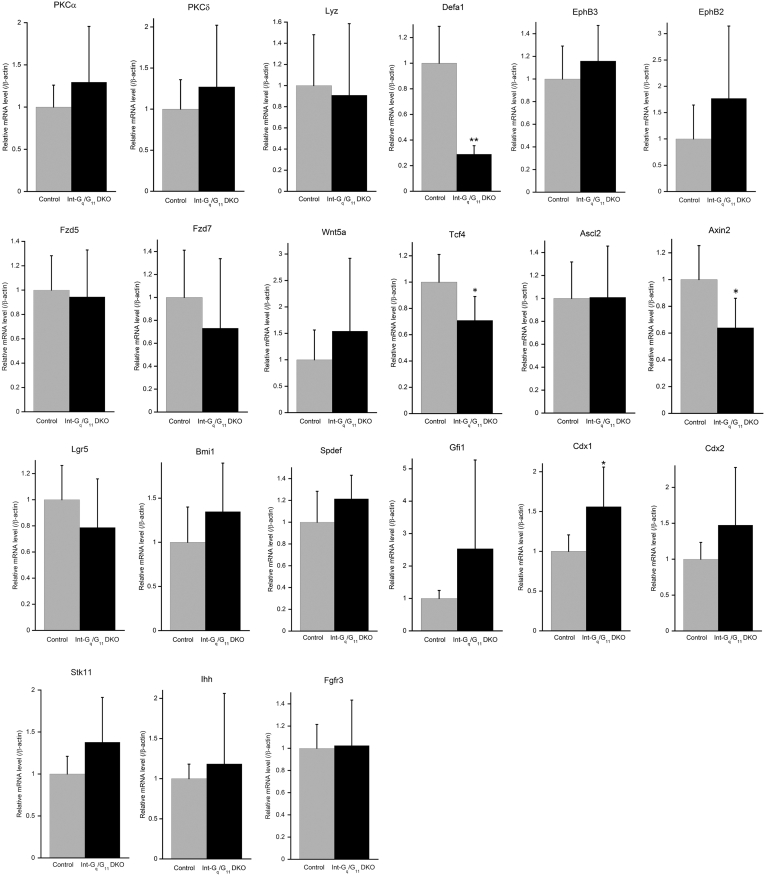
Supplementary Figure 2.
Relative expression of Paneth cell markers (Lyz, Defa1), stem cell markers (leucine-rich repeat-containing G-protein-coupled receptor 5 [Lgr5], Bmi1), transcription factors (Cdx1, Cdx2, spdef), Wnt/β-catenin signaling molecules (Fzd5, Fzd7, Wnt5a), Notch signaling molecules (Dll4, Notch1, Hes1, Atoh1), receptors (EphB3, EphB2, Fgfr3), and others (Stk11 [LKB1], Ihh) were measured by quantitative real-time PCR. Data represent the means ± SD of 3 mice per genotype from 2 independent experiments. *P < .05, **P < .01, according to analysis of variance.
Paneth Cells Are Enlarged, Increased in Number, and Mislocalized in Int-Gq/G11 DKO Intestine
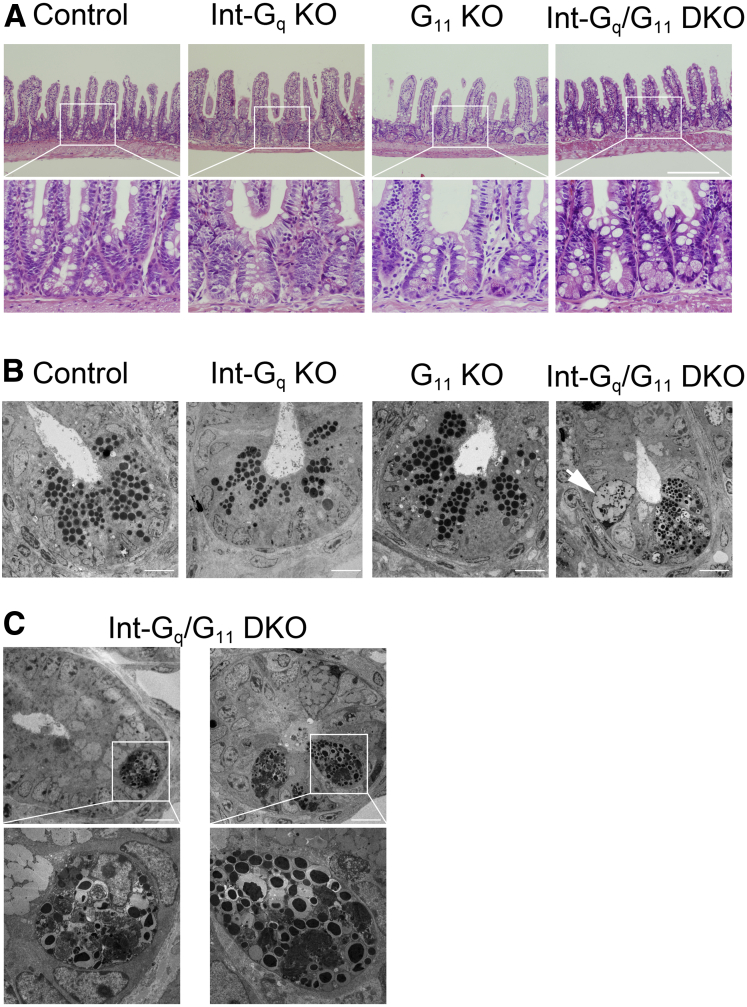
To investigate whether intestine-specific inactivation of Gαq/Gα11 affected the homeostasis of IECs, we performed histologic and immunohistochemical studies. The general morphology and architecture of the intestinal tract from 2- to 6-month-old adult mice were unaffected. However, H&E staining showed that Gαq/Gα11 deficiency led to the enlargement of Paneth cells with characteristic eosinophilic staining in Int-Gq/G11 DKO intestine (Figure 2A). Moreover, such cells were observed in the upper part of crypt and villi (Figure 2A and Supplementary Figure 1B, arrowheads). G11 KO Paneth cells showed a similar, but slight, alteration. In contrast, Paneth cells were localized to the crypt base and the alteration was not observed in Int-Gq KO mice (Figure 2A). No apparent macroscopic or microscopic phenotype was observed in large intestine in these 4 mouse lines (Supplementary Figure 1C).
Figure 2.
Morphologic studies of small intestine in 4 mouse lines. (A) H&E staining in small intestine. Scale bar: 200 μm. The enlargement of Paneth cells with characteristic eosinophilic staining was observed in the crypt of Int-Gq/G11 DKO mice. Slightly enlarged Paneth cells also were observed in G11 KO mice. In contrast, the alteration was not observed in Int-Gq KO mice. (B) Transmission electron micrographs in the crypt. Scale bars: 10 μm. Paneth cells in Gq/G11-deficient crypts showed a bipartite structure in their secretory granules: a smaller electron-dense core and a peripheral halo. An unusually large granulomucous cell also was observed (arrow). (C) Unhealthy-looking Paneth cells in Gq/G11-deficient crypts. Scale bars: 10 μm. Electron-dense materials might be degraded in a cell.
We next examined the transmission electron micrographs (TEM) of these mice. In Paneth cells of Int-Gq/G11 DKO mice, secretory granules showed a bipartite structure with a small, round central core of high electron density and a peripheral halo of lower density. The diameter of high electron density was much smaller than that in the other 3 types of mice. This feature resembles intermediate cells (intermediate between goblet cells and Paneth cells), which are considered to be rare cells in the transition between undifferentiated cells and more mature secretory cells.23 An unusually large granulomucous cell also was observed (Figure 2B, arrow). In addition, unhealthy-looking cells were observed in Int-Gαq/Gα11 DKO crypts. Electron-dense granules and intracellular materials appeared to be degraded in these cells (Figure 2C).
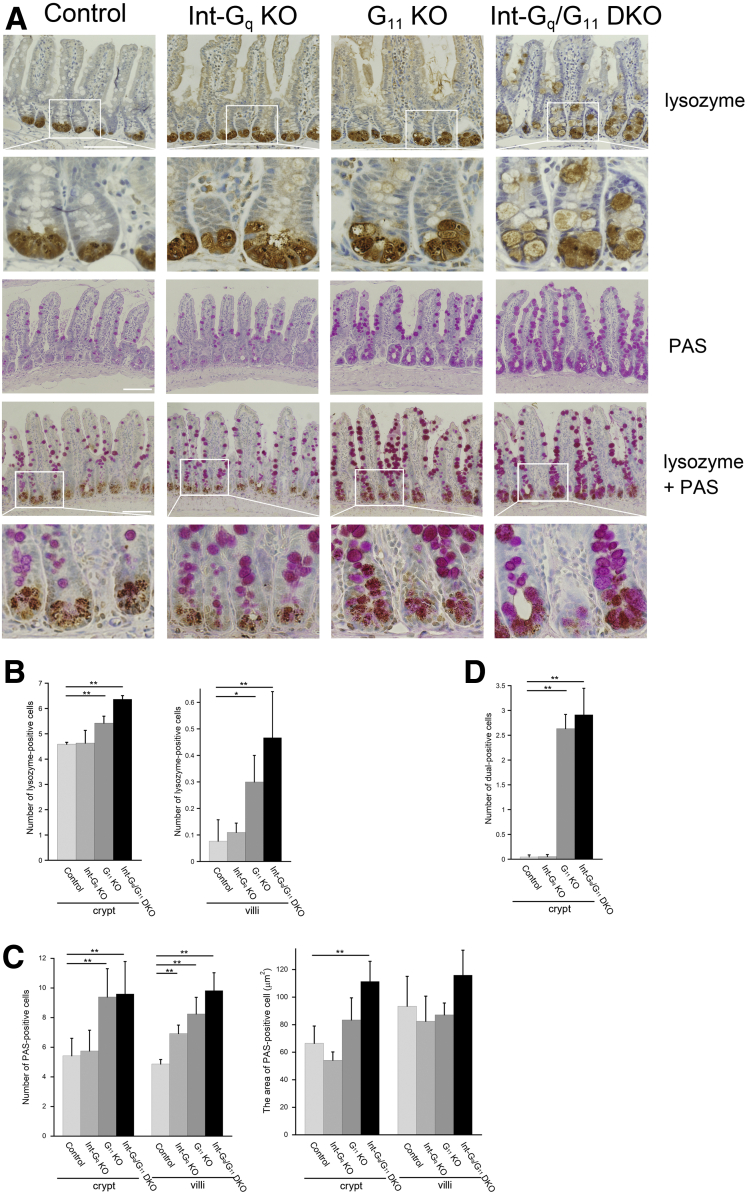
Next, we investigated whether Int-Gq/G11 DKO IECs showed correct differentiation along the 4 intestinal epithelial cell lineages. H&E staining showed an enlargement of Paneth cells in Int-Gq/G11 DKO crypt, whereas the electron-dense area in secretory granules was much smaller in TEM. Then, immunohistochemistry for lysozyme, an early marker of Paneth cell specification, was examined (Figure 3A). Lysozyme staining confirmed that the enlarged cells at the crypt base of Int-Gq/G11 DKO intestine expressed a key Paneth cell biomarker and lysozyme-positive cells were distributed abnormally above the crypt base. In the G11 KO mice, the lysozyme-positive cells were slightly enlarged. We quantified the number of lysozyme-positive cells in the crypt and villi. As shown in Figure 3B, the number of lysozyme-positive cells was increased both in crypt and villi in the G11 KO and Int-Gq/G11 DKO intestine. The Paneth cells showed an aberrant morphology because lysozyme-positive cells were enlarged in size, increased in number, and mislocalized in the crypt and villi on Gαq/11 deletion. The number of mispositioned Paneth cells was increased gradually toward the distal part of ileum (data not shown). This may coincide with the expression of Cre recombinase.18 Subsequent studies were performed using the distal part of ileum.
Figure 3.
Disturbance of Paneth cells and goblet cells in Gαq/11 deficiency. (A) Immunostaining showed that enlarged lysozyme-positive cells were mislocalized along crypt and villi in Int-Gq/G11 DKO intestine. G11 KO mice showed a similar but slight alteration. PAS staining indicated the expansion of mucin-containing cells both in the crypts and villi of G11 KO and Int-Gq/G11 DKO mice. Double-positive cells were observed in G11 KO and Int-Gq/G11 DKO crypts. Scale bars: 100 μm. (B) An analysis of the number of lysozyme-positive cells along crypt and villi. (C) An analysis of the number and area of PAS-stained cells along crypt and villi. (D) Quantification of dual-positive cells along the crypt. The cell number and size are graphed as the means ± SD (30 crypts and villi/mouse, n = 4 per genotype). Three independent experiments were performed with similar results. Representative figures are shown. *P < .05, **P < .01, according to an analysis of variance.
Expansion of Intermediate Cells in Crypt and Villi in Int-Gq/G11 DKO Intestine
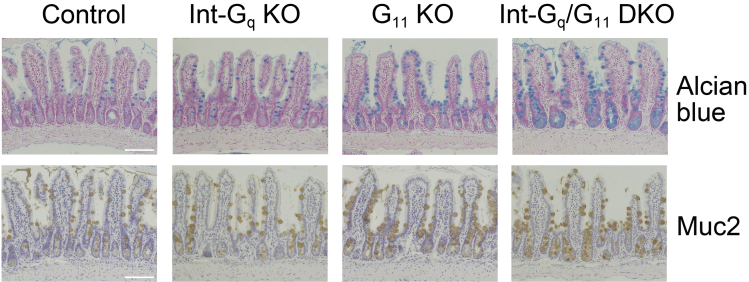
Next, we performed periodic acid–Schiff (PAS) staining, Alcian blue staining, and immunohistochemistry for Muc2 to examine the goblet cells (Figure 3A and Supplementary Figure 3). When compared with control, PAS reactivity clearly was increased in both the crypts and villi of G11 KO and Int-Gq/G11 DKO IECs (Figure 3A). For confirmation, we quantified the number of PAS-positive cells (Figure 3C). In the G11 KO and Int-Gq/G11 DKO small intestine, the number of PAS-positive cells was increased significantly in both the crypts and villi (Figure 3D). The number of PAS-positive cells also was increased in the villi of the Int-Gq KO small intestine. The size of PAS-positive cells was increased in Int-Gq/G11 DKO crypt (1.7-fold, in comparison with control). The quantification of Alcian blue–stained cells and Muc2-positive cells showed similar results (Supplementary Figure 3, data not shown).
Supplementary Figure 3.
Alcian blue staining and immunohistochemistry of Muc2 in Int-Gq/G11 DKO small intestine. Scale bars: 100 μm.
The increase in the number of lysozyme-positive cells and PAS-positive cells within crypt on Gαq/11 deficiency suggested that some of these cells were dual-positive, expressing both Paneth and goblet cell markers, which is referred to as intermediate cells in the literature.23, 24, 25 We then performed simultaneous lysozyme and PAS staining. As expected, dual-positive cells were increased significantly in number in the G11 KO and Int-Gq/G11 DKO crypts (Figure 3A and D). In the villus region, all lysozyme-positive cells were PAS positive, the right panel in Figure 3B indicates the number of dual-positive cells in the villi.
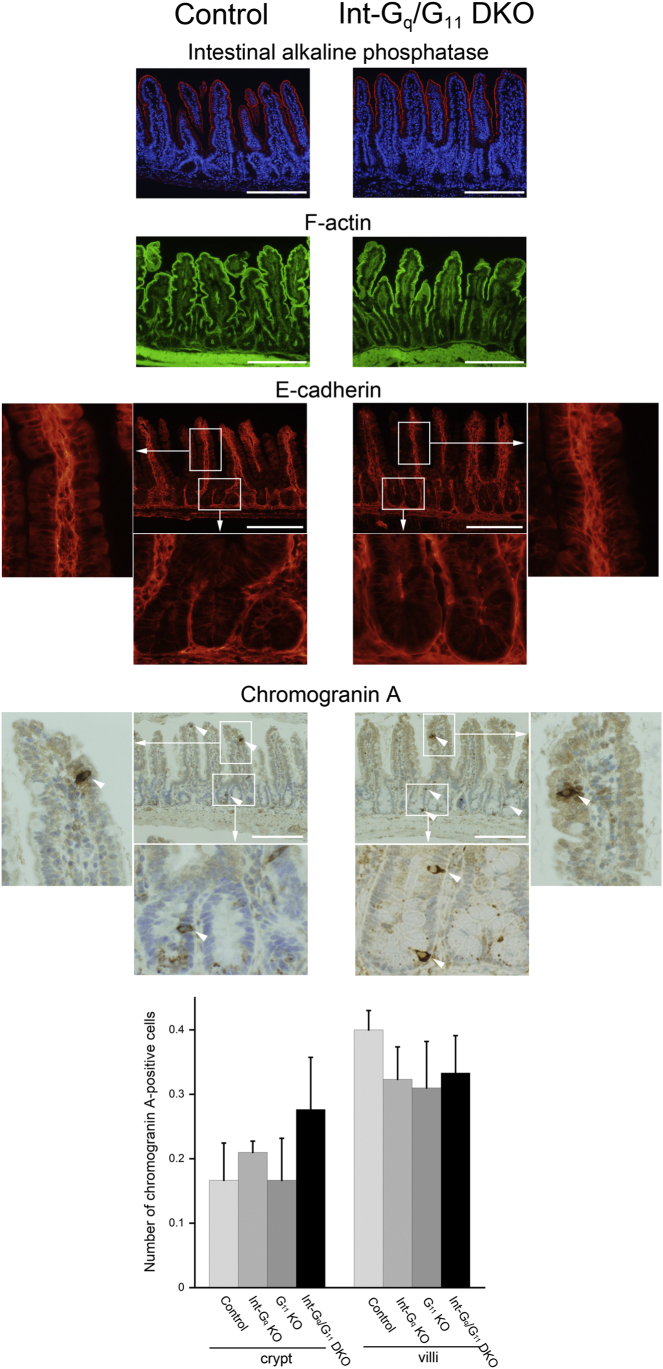
We next evaluated enterocytes and enteroendocrine cells by staining for intestinal alkaline phosphatase, a differentiation marker of enterocytes, and chromogranin A, a commonly used marker for neuroendocrine cells. We also examined the cell polarity by staining for F-actin, mainly localized in the subapical area, and E-cadherin, a component of adherence junction. As shown in Figure 4, no significant differences were detected in these staining series between the control and Int-Gq/G11 DKO mice. There were no differences also in Int-Gq KO and G11 KO mice (data not shown). The number of chromogranin A–positive cells in crypt and villi showed similar results in the 4 genotypes.
Figure 4.
Preservation of enterocytes and enteroendocrine cells and cell polarity in Gαq/11-deficient mice. Immunohistochemical studies were performed for intestinal alkaline phosphatase (enterocyte marker), chromogranin A (endocrine cell marker), F-actin (mainly localized in the subapical membrane), and E-cadherin (localized in the basolateral membrane). No differences were observed between control and Int-Gq/G11 DKO mice. Scale bars: 200 μm. Bottom: Analysis of the number of chromogranin A–positive cells (arrowheads) in crypt and villi in the 4 genotypes. Cell numbers are graphed as the means ± SD (30 crypts and villi/mouse, n = 4 per genotype).
These results indicate that the increase in lysozyme and PAS reactivity in Gαq/11-deficient intestine was a result of an increased number of intermediate cells in crypt and villi and an increased number of goblet cells in villi. We observed a decrease in diameter of high electron density in Paneth cell granules with a peripheral halo in TEM of Int-Gq/G11 DKO crypt (Figure 2B), suggesting that the defective maturation of Paneth cells may be involved in the unusual features. Quantitative real-time PCR was performed using specific primers for Paneth cell markers, lysozyme and defensin α1 (Defa1). Although lysozyme mRNA level remained unchanged, the Defa1 mRNA level was decreased significantly in Int-Gq/G11 DKO mice (Supplementary Figure 2). This may be owing to the fact that Defa1 is a target gene of Wnt signaling whereas lysozyme is not.16 The decrease in Defa1 mRNA level suggests that Paneth cells have not undergone full differentiation to the Paneth cell lineage in Int-Gq/G11 DKO mice.
Gαq/11-Deficiency Reduces Wnt/β-Catenin Signaling in IECs, Especially in Paneth Cells
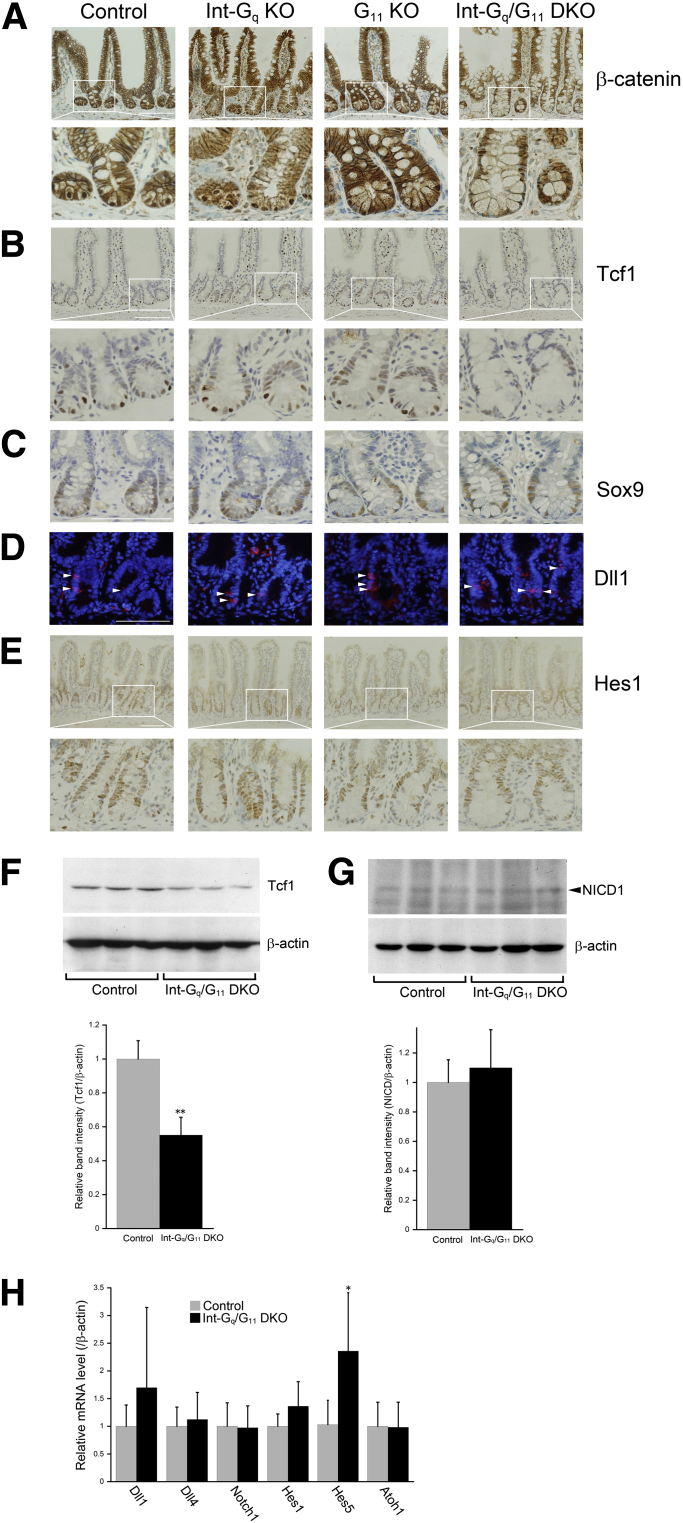
Paneth cell positioning in the normal adult intestinal epithelium is tightly regulated by canonical Wnt signaling and the consequent control over EphB3 expression.1, 27 Paneth cells located in the crypt base contain high levels of nuclear β-catenin.1 Immunostaining with a β-catenin antibody showed membrane-localized β-catenin along the crypt–villus axis, as well as nuclear β-catenin in cells occupying basal positions of the crypt in control and Int-Gq KO mice (Figure 5A). In contrast, the lining enlarged cells at Int-Gq/G11 DKO crypt base were devoid of nuclear β-catenin staining; those in the G11 KO crypt base showed a decreased number of nuclear β-catenin–positive cells. Immunohistochemical staining for T-cell factor 1 (Tcf1; also known as transcription factor 7) showed similar results and a Western blot analysis showed the reduced expression of Tcf1 in the small intestinal mucosa of Int-Gq/G11 DKO mice (Figure 5B and F). The transcription factor Sox9 is a Wnt/β-catenin transcriptional target that is required for Paneth cell specification.14, 27 Sox9 was expressed in all epithelial cells in the lower two thirds of the crypts, namely in the domains of transiently proliferating cells and of Paneth cells in control and Int-Gq KO mice.14, 27 In Int-Gq/G11 DKO mice, nuclear staining of Sox9 disappeared in the enlarged cells at the crypt base and was reduced in number in G11 KO mice (Figure 5C). The Wnt receptor frizzled5 (Fzd5) plays a critical role in Wnt-dependent Paneth cell maturation and binds Wnt5a as a ligand.15 There were no significant differences in the mRNA levels of Wnt5a, Fzd5, and Fzd7 in the control or Int-Gq/G11 DKO IECs, whereas the mRNA level of Tcf4 was reduced in Int-Gq/G11 DKO IECs (Supplementary Figure 2). A qPCR for canonical Wnt targets showed the decreased expression of Axin2, but not EphB3, EphB2, or Ascl2, in the Int-Gq/G11 DKO intestinal mucosa. These results suggest that Gαq/11 deficiency reduces Wnt signaling partially in IECs, but strongly in Paneth cells.
Figure 5.
Reduction in Wnt/β-catenin signaling and a subtle alteration in Notch signaling in Gαq/11-deficient IECs. Immunohistochemical studies were performed for (A) β-catenin, (B) Tcf1, (C) Sox9, (D) Dll1, and (E) Hes1. Immunoreactivities for nuclear β-catenin, Tcf1, and Sox9 were reduced in the crypt base of G11 KO and greatly in Int-Gq/G11 DKO mice compared with control and Int-Gq KO mice. Scale bars: 100 μm. Western blot was performed for the (F) Tcf1 and (G) NICD1. Lower panel: ImageJ densitometry analysis. (H) The relative mRNA expression in the Notch signaling molecules was evaluated by a quantitative real-time PCR. The data represent the means ± SD of 3 mice per genotype. Two independent experiments were performed with similar results (n = 3). *P < .05, **P < .01.
Gαq/11 Deficiency Results in the Subtle Alteration in Notch Signaling
Notch signaling has been shown to play a pivotal role in secretory cell lineages.16, 28, 29 Immunostaining of Dll1, one of the Notch signaling ligands, showed that there were no differences in the crypts of the 4 genotypes (Figure 5D). Immunostaining of Hes1, one of the important Notch signaling effectors, showed no significant changes (Figure 5E). A Western blot analysis to detect the Notch1 intracellular cytoplasmic domain (NICD1) showed no differences between control and Int-Gq/G11 DKO IECs (Figure 5G). A qPCR for Dll1, Dll4, Notch1, Hes1, and Atoh1 showed no changes, whereas Hes5 mRNA expression was increased in the Int-Gq/G11 DKO IECs (Figure 5H). This may coincide with the increased number of goblet cells in Int-Gq/G11 DKO IECs because Hes5 is expressed not only in the proliferating crypt, but also in the postmitotic cells in the lower villi,30 and Hes5 activation has been reported to increase goblet cell numbers by driving the differentiation of postmitotic cells into mature goblet cells.16
Because Paneth cells are located next to putative stem cells, the defective maturation of Paneth cells may affect stem homeostasis. Activation of Wnt/β-catenin signaling pathway has been shown to be important not only for Paneth cell lineage allocation, but also for the regulation and maintenance of intestinal stem cells.14, 27 A number of transcription factors and kinases also are known to regulate cell fate specification of intestinal progenitors, including SAM pointed domain-containing Ets transcription factor (Spdef), Gfi1, and Stk11 (Lkb1).31, 32, 33 We thus examined the expression levels of these genes and stem cell markers, leucine-rich repeat-containing G-protein-coupled receptor 5 and Bmi-1. Interestingly, the phenotype of mislocalized Paneth cells was indistinguishable from that of LKB1-/- mice.33 However, we did not observe any significant changes in mRNA level, except that Cdx1 mRNA level was increased slightly but significantly in Int-Gq/G11 DKO intestine (Supplementary Figure 2).
Proliferation in the Crypt Base and Apoptosis Are Increased in Gαq/11-Deficienct Mice
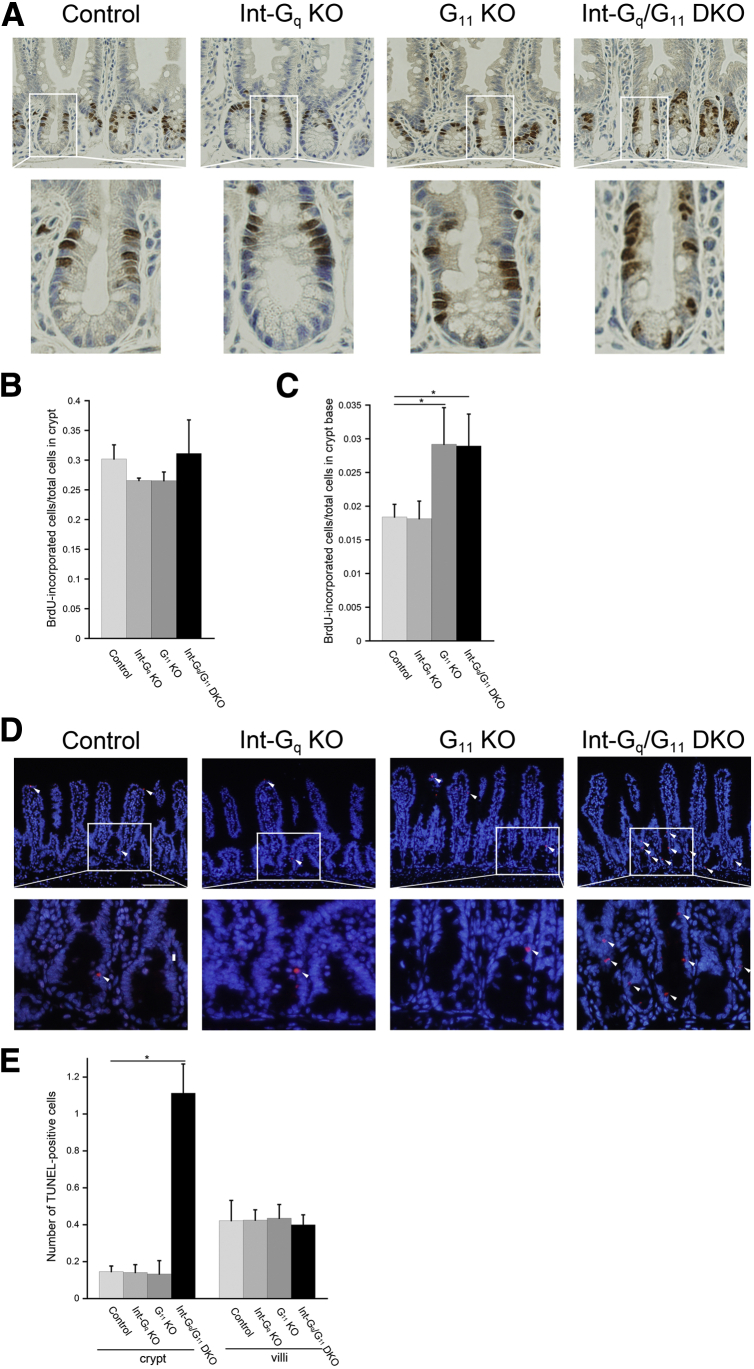
The increase in intermediate cell number may be related to the alteration of proliferation of IECs. To confirm this, a bromodeoxyuridine (BrdU) experiment was performed. Mice were injected with BrdU, euthanized 2 hours later, and the location and proportion of cells in the S phase were scored. Proliferative cells were restricted to the crypt compartment. The ratio of BrdU-labeled cells over the total number of cells along the crypt circumference was not altered in the 4 genotypes (Figure 6A and B). However, when we carefully counted BrdU-incorporated cells in the crypt base (the lower third of the crypt), there was a significant increase in G11 KO and Int-Gq/G11 DKO crypt (Figure 6A and C).
Figure 6.
Increase of proliferation in crypt base and apoptosis in crypt of the Gαq/11-deficient intestine. (A) Proliferation was examined by BrdU staining at 2 hours after the injection (scale bars: 100 microm). The number of BrdU-positive cells was counted in the (B) crypt and (C) crypt base (the lower third of the crypt). Cell numbers are graphed as the means ± SD (30 crypts/mouse; n = 3 per genotype). (D) Apoptotic cells were measured using a TUNEL assay (arrowheads; scale bars: 100 microm). (E) The number of TUNEL-positive cells was graphed as the means ± SD (30 crypts or villi/mouse; n = 3 per genotype). Three independent experiments were performed with similar results. Representative figures are shown. *P < .05.
We next investigated apoptotic cells using a TUNEL assay. In control, Int-Gq KO, and G11 KO mice, apoptotic cells were found in the crypt only occasionally, however, more apoptotic cells were observed in the Int-Gq/G11 DKO crypt (Figure 6D and E, arrowheads).
Int-Gq/G11 DKO Mice Are Susceptible to DSS-Induced Colitis
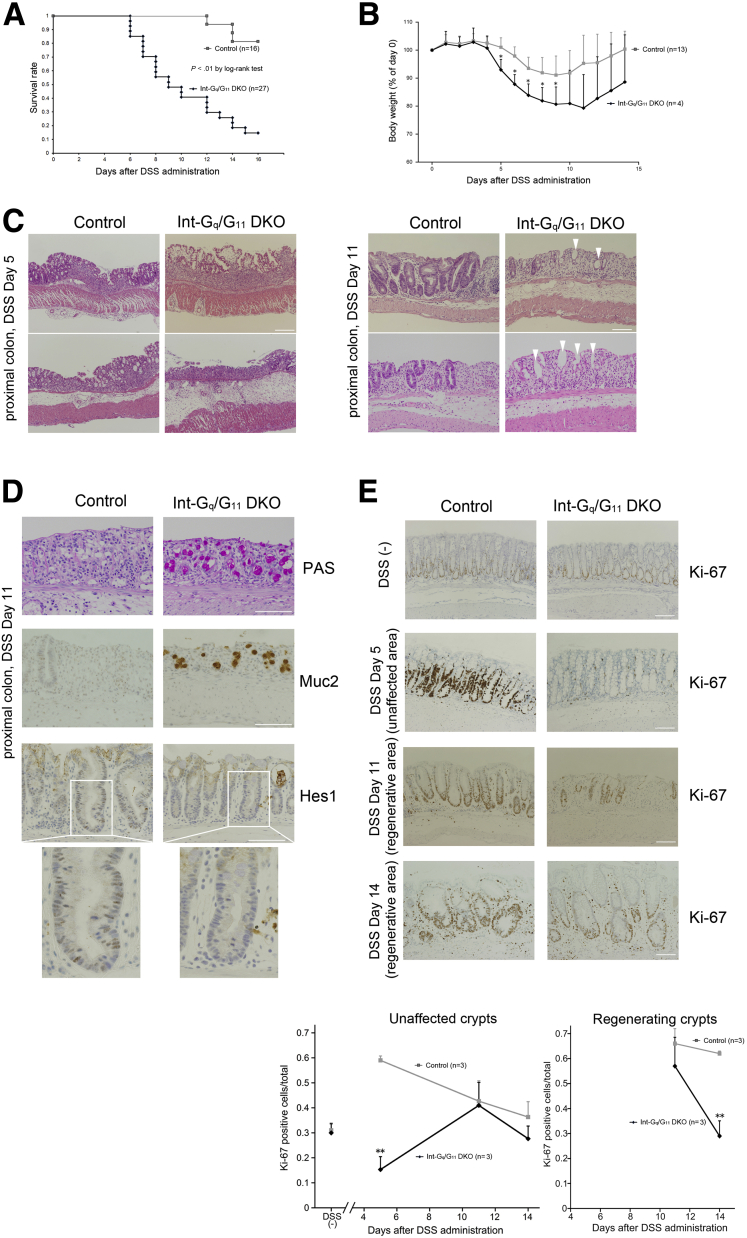
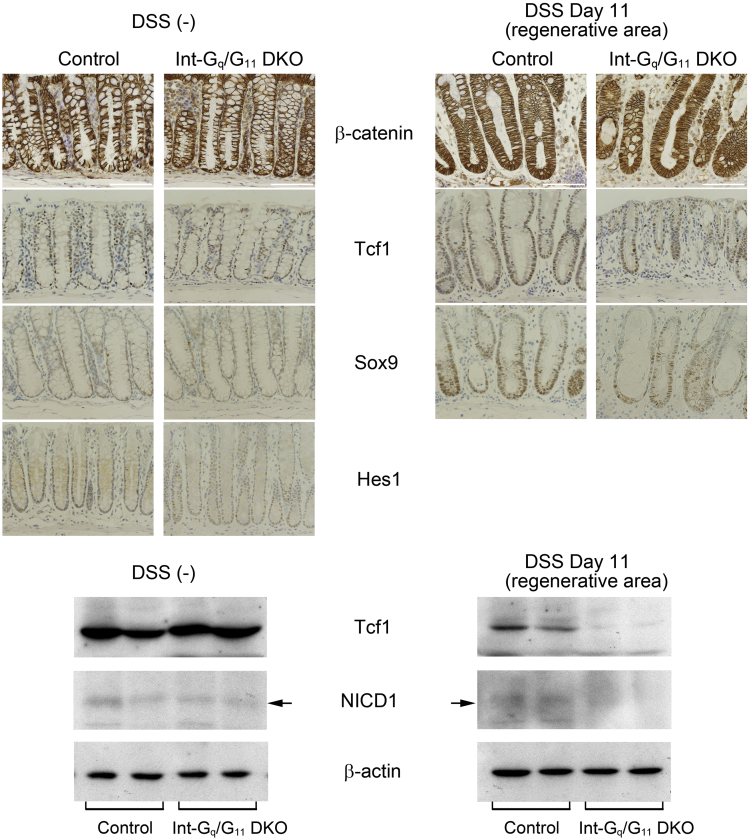
There were no abnormalities found in the physiological condition of the Gαq/11-deficient colonic epithelium (Supplementary Figure 1). Intermediate cells that we found in the Gαq/11-deficient small intestine were reported to be observed during intestinal infection and inflammation.34, 35 We then induced acute colitis in control and Int-Gq/G11 DKO mice by the continuous administration of DSS (3%) in the drinking water for 5 days. DSS administration gradually induced symptoms resembling ulcerative colitis. Most of the mice developed bloody diarrhea and rectal bleeding 4 days after the administration of DSS, and these symptoms gradually worsened. Int-Gq/G11 DKO mice showed severe mortality after the administration of DSS (Figure 7A). Int-Gq/G11 DKO mice that survived showed a severe weight loss compared with control (Figure 7B). We then compared the histologic changes in the acute colitis stage (day 5 after the start of DSS administration) and healing stage (day 11). Histologic analyses on day 5 showed larger areas of ulceration, crypt loss, thickening of the mucosa with abundant edema, and more massive inflammatory cell infiltration into the mucosa and submucosa in the Int-Gq/G11 DKO proximal colon (Figure 7C). In the healing stage, we observed regenerating crypts in the injured area of control mice. In Int-Gq/G11 DKO mice, regeneration was reduced and instead cystic crypts, lined with an epithelial layer that had lost the typical columnar cell appearance, were observed in the ulcerative area (Figure 7C, arrowheads). Such structures rarely were observed in the ulcerative region of the control mice. When we measured and added the length of the ulcerative region where the cystic crypts were observed, the region was found to be significantly longer in Int-Gq/G11 DKO mice (control, 0.019 ± 0.038 mm/mouse; Int-Gq/G11 DKO, 6.4 ± 3.6 mm/mouse; n = 4 per genotype; P = .04). The total length of colon did not change between the 2 genotypes (data not shown). We next performed PAS staining and immunohistochemistry for Muc2 using the healing stage proximal colon specimens. PAS-positive cells and Muc2-positive cells were observed in the ulcerative region of Int-Gq/G11 DKO, but not in control (Figure 7D). Immunoreactivity of lysozyme was not observed and that of chromogranin A remained unchanged (data not shown). Nuclear staining of Hes1 was observed in the regenerating crypts of control mice, but it was greatly reduced in Int-Gq/G11 DKO colon (Figure 7D). Under physiological conditions, no differences in nuclear staining for Hes1 were observed between the 2 genotypes (Supplementary Figure 4). Next, we examined the proliferation of epithelial cells in the proximal colon using anti–Ki-67 antibody. In untreated mice, the rates of Ki-67–stained nuclei/total nuclei in control mice and Int-Gq/G11 DKO mice were 0.31 ± 0.03 and 0.30 ± 0.04, respectively (P = .73). In the acute colitis stage (day 5), the proliferation in the unaffected crypts was enhanced greatly in the control mice, whereas it was decreased severely in the Int-Gq/G11 DKO colon (control, 0.59 ± 0.02; Int-Gq/G11 DKO, 0.15 ± 0.05; P < .01). In the regenerating crypts of the Int-Gq/G11 DKO colon, proliferation was decreased slightly on day 11 (control, 0.66 ± 0.06; Int-Gq/G11 DKO, 0.57 ± 0.11; P = .30), but it was decreased significantly on day 14 (control, 0.62 ± 0.01; Int-Gq/G11 DKO, 0.29 ± 0.06; P < .01). These results suggest that in DSS-induced colitis, proliferation was inhibited and the regenerative process was altered, with a tendency to differentiate toward goblet cells, not enterocytes, during the regeneration in Int-Gq/G11 DKO colon. Consequently, Gαq/11 deficiency resulted in a poor survival rate during colitis.
Figure 7.
Requirement of Gαq/11 signaling during regeneration of colonic epithelia in DSS colitis. (A) The survival rate was monitored until day 16 after the administration of DSS (n = 16 in control mice and n = 27 in Int-Gq/G11 DKO mice). (B) Body weight of the survivors (% of weight at day X/day 0) is graphed as the means ± SD (n = 13 in control mice and n = 4 in Int-Gq/G11 DKO mice). (C) H&E staining of a proximal colon specimen in the acute colitis stage (day 5 after the administration of DSS) and healing stage (day 11). Two representative images from independent mice are shown. Cystic crypts, lined with an epithelial layer that had lost the typical columnar cell appearance, were observed during the healing stage of Int-Gq/G11 DKO mice (arrowheads). (D) PAS-positive and Muc2-positive cells were observed in the ulcerative area of Int-Gq/G11 DKO proximal colon. Nuclear Hes1 immunoreactivity was decreased in the regenerating crypts of Int-Gq/G11 DKO colon. (E) Proliferation was examined in the proximal colon by immunohistochemistry for Ki-67. The rate of Ki-67–positive cells in the unaffected crypts (DSS (-), days 5, 11, and 14) and in the regenerating crypts (days 11 and 14) is graphed as the means ± SD (n = 3 per genotype). Three independent experiments were performed with similar results. Representative images and figures are shown. *P < .05, **P < .01 (scale bars: 100 μm).
Supplementary Figure 4.
Immunohistochemical studies were performed for β-catenin, Tcf1, Sox9, and Hes1 in control and Int-Gq/G11 DKO mice using DSS-untreated and DSS-treated colonic specimens. Scale bars: 100 μm. Western blot was performed for the Tcf1, NICD1, and β-actin using proximal colonic mucosa, which was scraped off of colon tissue with a spatula (n = 2).
Discussion
In this study, we used mice with a disruption of the Gnaq and Gna11 gene to investigate the role of this trimeric G protein in regulating intestinal homeostasis. Signaling through Gαq/11 is important in mediating the aspect of epithelial cell morphogenesis and plays a critical role in modulating the maturation of Paneth cells. It also plays a pivotal role in regeneration after DSS-induced colitis.
In light and electron microscopy, we noted a conspicuous population of cells in the Int-Gq/G11 DKO small intestine that was intermediate in appearance between Paneth and goblet cells (Figure 2). Such intermediate cells rarely are observed at crypt–villus junctions in wild-type small intestine and likely represent common progenitor cells of the Paneth and goblet cell lineages.23, 25 These cells have been reported to be observed during intestinal infection, inflammation, and the inhibition of Notch signaling.29, 34, 35 Classic intermediate cells contain apical eosinophilic granules smaller than those in mature Paneth cells and contain both electron-lucent granules, as seen in goblet cells, and electron-dense granules, as seen in Paneth cells. In TEMs of Int-Gq/G11 DKO mice, we observed that Paneth cells gained features, suggestive of intermediate cells, with larger mucin-filled vacuoles than observed in classic intermediate cells. The number of intermediate cells was increased dramatically in Int-Gq/G11 DKO mice, and these cells were distributed ectopically along the crypt–villus axis (Figures 2 and 3). Similar but slight changes were observed in the G11 KO crypts but not in the Int-Gq KO crypts. This may be owing to the abundant expression of Gα11 in comparison with Gαq in the mouse small intestine5 (Figure 1B). Enterocytes remained unaltered in morphology and their polarity was not disturbed in Int-Gq/G11 DKO intestine. Gαq/11 signaling might be dispensable in enterocytes or other Gαq/11 family genes may be expressed and thus partially may compensate for the loss of Gαq/11. However, signaling through Gαq/11 appears to play a crucial role in the segregation of the Paneth/goblet cell lineages and in the terminal differentiation and maintenance of mature Paneth cell phenotype.
Paneth and goblet cells are thought to be closely related and emerge from a common progenitor cell.36 As a consequence of losing the maturation of Paneth cells, we might observe an increase in goblet cell number in the crypts and villi of G11 KO and Int-Gq/G11 DKO mice (Figure 3). The control of intestinal stem cell fate, maintenance, and maturation depends on the correct dosing of Wnt/β-catenin signals along the crypt. Wnt/β-catenin signals promote a number of critical processes in the intestinal epithelium.11 The key event in this signaling pathway is the stabilization of β-catenin and its interaction with Tcf transcription factors within the nucleus.1 Interference with the Wnt pathway results in depletion of the Paneth cell lineage26; conversely, a loss of the key negative regulator of this pathway, Apc, results in an expansion of the Paneth cell lineage, indicating that proper development of the Paneth cell lineage requires fine tuning of β-catenin activation.11, 15, 37 Because nuclear-localized β-catenin typically is observed only in Paneth cells, Paneth cell differentiation appears to require the highest levels of Wnt/β-catenin signal.11, 15 We found that nuclear β-catenin and Tcf1 was absent in Paneth cells of Int-Gq/G11 DKO crypt base, suggesting that signaling through Gαq/11 modulates Wnt/β-catenin signaling in some way. The Wnt pathways include canonical Wnt signaling, which is referred to as Wnt/β-catenin pathway and noncanonical Wnt signaling, which is β-catenin–independent and subdivided into 2 general categories: the Wnt/Ca2+ and Wnt/c-Jun-N-terminal kinase pathways. Fzd is a 7-pass transmembrane receptor and is associated with heterotrimeric G protein in the Wnt/Ca2+ pathway, which activates phospholipase C-β and thereby the intracellular release of calcium. Induced calcium flux activates PKC and calcium-calmodulin–dependent kinase II, which can antagonize the Wnt/β-catenin pathway.38 Mouse Fzd3, Fzd4, and Fzd6 activate PKC and calcium-calmodulin–dependent kinase II, but Fzd7 and Fzd8 do not affect their activity.39 If this pathway plays a role, Wnt/β-catenin signaling is expected to be up-regulated under the condition of Gαq/11-deficiency. Thus, in the mouse model of the present study, signaling through Gαq/11 modulated Wnt/β-catenin signaling in another fashion.
Recent studies have suggested that Paneth cell lineage allocation and differentiation/maturation are regulated by distinct, but interacting, pathways. Specification of cells to the goblet and Paneth cell lineages involves numerous transcription factors, including Sox9, Atoh1, and SAM pointed domain-containing Ets transcription factor. Sox9, a high-mobility group box transcription factor expressed in intestinal crypt cells including Paneth cells, has been identified as a regulator of Paneth and goblet cell lineage specification in the intestine and colon.14, 27 Sox9, a direct transcriptional target of Wnt/β-catenin signaling, is needed for Paneth cell specification and Sox9-null mice lack Paneth cells in small intestine.14, 27 In fact, nuclear localization of Sox9 greatly was reduced in the crypt base of Int-Gq/G11 DKO intestine (Figure 5C). The sorting process of epithelial cells along the crypt–villus axis is dependent on the Wnt pathway via the modulation of ephrin-eph–receptor interactions.1 EphB3 homozygous null mice show striking defects in the localization of Paneth cells, which are distributed randomly throughout the crypt.1 However, in Int-Gq/G11 DKO mice, mRNA levels of EphB3 and EphB2 were not altered (Supplementary Figure 2). Rab8a knock-out mice have been reported recently.40 Wnt secretion is regulated partly by Rab8a-mediated anterograde transport. The deletion of Rab8a weakened the secretion of multiple Wnts, resulting in reduced Wnt signaling, defective Paneth cell maturation, and the expansion of leucine-rich repeat-containing G-protein-coupled receptor 5–positive cells, which resembles the phenotype of Int-Gq/G11 DKO mice. However, intermediate cells, the mislocalization of Paneth cells, and an increased number of goblet cells are not reported in Rab8a KO mice, implying that other signals also are involved in the disturbance of Int-Gq/G11 DKO IECs.
Notch signaling in intestine also plays an important role as a gatekeeper of progenitor cells and a regulator to the absorptive/secretory lineage.9 Inhibition of Notch signaling in the intestinal epithelium using conditional gene targeting of RBP-J or by pharmacologic γ-secretase inhibitors, which block the release of NICD, results in the loss of the proliferative crypt compartment and conversion of crypt progenitors into secretory cells.9 The proliferative crypt cells express Notch1 and Notch2, whereas Dll1 and Dll4 function redundantly as Notch ligands.28 Hes1 and Hes5 require Notch activation for transcription. Progenitor cells that express Hes1 will differentiate into absorptive enterocytes and those expressing Atoh1 are committed to the secretory lineage. Immunostaining of Dll1 and Hes1, Western blot of NICD1, and a qPCR of Dll1, Dll4, Notch1, Hes1, and Atoh1 did not show any alterations in the Int-Gq/G11 DKO IECs, other than an increase in the Hes5 mRNA level, suggesting that there was only a subtle alteration in the Notch signaling of the Int-Gq/G11 DKO IECs (Figure 5). The interaction of Notch signaling with the heterotrimeric G protein has not yet been reported.
Epithelial cells in small intestine communicate with the underlying mesenchymal components, including blood vessels, myofibroblasts, and immune cells, which secrete paracrine signals and collectively form the lamina propria.41 Numerous trophic factors, including Wnts, fibroblast growth factors (FGFs), serotonin, and Indian hedgehog (Ihh) are known to influence the number of Paneth cells residing at the base of intestinal crypts.15, 42, 43 In adult small intestine, mature Paneth cells may play a role in specification by steering progenitor cells away from the Paneth cell lineage through Ihh signaling, thereby controlling Paneth cell number.44 Conversely, conditional knockout of Ihh in mouse small intestine results in an increased number of Paneth cells.45, 46 Recent evidence also suggests that signaling through FGF receptor 3 (Fgfr3) plays a critical role in Paneth cell maturation.43 However, mRNA levels of Wnt5a, Ihh, and Fgfr3 remained unchanged in Int-Gq/G11 DKO intestine (Supplementary Figure 2).
Signaling through Wnt/β-catenin and the Tcf family transcription factors also plays a central role in proliferation during intestinal development.13 We performed an in vivo BrdU incorporation assay to monitor proliferating cells among IECs. The total number of BrdU-positive cells did not differ between the 4 genotypes, however, when we focused on the crypt base (lower third of the crypt), the number of BrdU-positive cells was increased in G11 KO and Int-Gq/G11 DKO crypts (Figure 6A–C). A subsequent TUNEL assay showed that there were more apoptotic cells in only Int-Gq/G11 DKO crypts (Figure 6D and E) and unhealthy-looking cells occasionally were observed in TEM of Int-Gq/G11 DKO crypts (Figure 2C). These results suggest that proliferation at the crypt base might be activated to compensate for the loss of mature Paneth cells and apoptosis was induced only when the Gαq and Gα11 both were deficient.
The colonic epithelium lacks Paneth cells and the morphologic architecture was preserved in Int-GqKO, G11 KO, and Int-Gq/G11 DKO mice (Supplementary Figure 1C). We induced DSS colitis in control and Int-Gq/G11 DKO mice and examined the role of Gαq/11 signaling in colon (Figure 7). Gαq/11 deficiency significantly exacerbated the clinical course of DSS-induced colitis. Severe colitis was observed at day 5 and mucosal regeneration was disturbed in the regenerating stage compared with control mice. Cystic crypts were observed in the ulcerative area of the Int-Gq/G11 DKO colon, where PAS-positive and Muc2-positive cells were detected. Most of the cells in cystic crypts were Ki-67 negative (data not shown). Nuclear Hes1 immunoreactivity was decreased in the regenerating crypts of the Int-Gq/G11 DKO colon. The rate of Ki-67–positive cells showed that proliferation was reduced severely in the unaffected area during the acute colitis stage and in the regenerating area. It previously was reported that inhibition of Notch activation using a γ-secretase inhibitor resulted in a severe exacerbation of colitis, and activation of Notch promoted proliferation and regeneration by suppressing goblet cell differentiation.47 As for Wnt signaling, no significant differences were observed in the regenerating crypts for the immunostaining of β-catenin, Tcf1, and Sox9. The band intensity of Tcf1 in Western blotting was reduced in the Int-Gq/G11 DKO colon, but this phenomenon may have been owing to the decreased regeneration of crypts in the Int-Gq/G11 DKO mice (Supplementary Figure 4). These results suggest that Gαq/11 deficiency inhibited Notch signaling, decreased proliferation, and induced differentiation toward goblet cells in injured and regenerating areas, resulting in a higher mortality rate.
Taken together, the Gαq/11-mediated signaling pathway plays a pivotal role in the maintenance of intestinal homeostasis, especially in Paneth cell maturation and positioning. Gαq/11 deficiency has a significant effect on Wnt/β-catenin signaling and a slight effect on Notch signaling, which results in the altered differentiation of IECs. Gαq/11 signaling also is indispensable for the regeneration of injured colonic epithelium. These new insights into signaling through Gαq/11 may broaden our understanding of intestinal homeostasis and may lead us to a new therapeutic approach for enterocolitis, including inflammatory bowel disease.
Acknowledgment
The authors thank Chihiro Taira, Hitoshi Watanabe, Sachiko Fujita, Keiko Iwamoto, and Shinsuke Chida for their excellent technical assistance.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Supplementary Material
Supplementary Table 1.
The Primers Used in the Present study
| Gene name | Accession no | Primer sequence | Size | Annealing temperature | |||
|---|---|---|---|---|---|---|---|
| Genotyping | |||||||
| Gna11+ | F | GCCCCTTGTACAGATGGCAG | 820 bp | 60°C | |||
| R | AGCATGCTGTAAGACCGTAG | ||||||
| Gna11- | F | CAGGGGTAGGTGATGATTGTGC | 450 bp | 60°C | |||
| R | GACTAGTGAGACGTGCTACTTCC | ||||||
| Gnaq+ | F | AGCTTAGTCTGGTGACAGAAGC | 600 bp | 60°C | |||
| Gnaqflox | R | GCATGCGTGTCCTTTATGTGAG | 700 bp | ||||
| Cre recombinase | F | CAATTTACTGACCGTACAC | 1026 bp | 60°C | |||
| R | TAATGCCATCTTCCAGCAG | ||||||
| Reverse-transcription PCR | |||||||
| Gnaq | NM_008139 | F | TGGACCGTGTAGCCGACCCT | 270 bp | 60°C | ||
| R | GTGCTTTGCTCTCCTCCATGCGG | ||||||
| Gna11 | NM_010301 | F | ACGAGGTGAAGGAGTCGAAGC | 573 bp | 60°C | ||
| R | CCATCCTGAAGATGATGTTCTCC | ||||||
| Actb | NM_007393 | F | TGAGAGGGAAATCGTGCGTG | 460 bp | 60°C | ||
| R | GATCCACATCTGCTGGAAGGTG | ||||||
| Real-time PCR | |||||||
| 1 | PKCα | NM_011101 | F | GCTTCCAGTGCCAAGTTTGC | 76 bp | 60°C | |
| R | GCACCCGGACAAGAGAACGTAA | ||||||
| 2 | PKCδ | NM_011103 | F | TATCAACTGGTCCCTCCTGG | 105 bp | 60°C | |
| R | CATTCAGGAACTCTGGGTCA | ||||||
| 3 | Lyz | NM_013590 | F | GGTCTACAATCGTTGTGAGTTGG | 387 bp | 65°C | |
| R | CTCCGCAGTTCCGAATATACT | ||||||
| 4 | Defa1 | NM_010031 | F | TCAAGAGGCTGCAAAGGAAGAGAAC | 94 bp | 60°C | |
| R | TGGTCTCCATGTTCAGCGACAGC | ||||||
| 5 | EphB3 | NM_010143 | F | CCATAGCCTATCGGAAGTTTACGT | 87 bp | 60°C | |
| R | TCGCTCTCCGTAGCTCATGAC | ||||||
| 6 | EphB2 | NM_001290753 | F | CTCTACTGTAACGGGGACGG | 119 bp | 60°C | |
| R | TTGAAGGTTCCTGATGGACA | ||||||
| 7 | Fzd5 | NM_001042659 | F | GTGCTTCATCTCCACGTCCA | 103 bp | 60°C | |
| R | CAGGTAGCACGCAGACAAGA | ||||||
| 8 | Fzd7 | NM_008057 | F | ACCCTACTGCTCCCTACCTG | 84 bp | 60°C | |
| R | AGAAGGGGAAAGACAAGCGG | ||||||
| 9 | Wnt5a | NM_009524 | F | ACAGGCATCAAGGAATGCCA | 104 bp | 60°C | +DMSO |
| R | CGGCTGCCTATTTGCATCAC | ||||||
| 10 | Tcf4 | NM_013685 | F | AGCCCGTCCAGGAACTATG | 101 bp | 60°C | |
| R | TGGAATTGACAAAAGGTGGA | ||||||
| 11 | Ascl2 | NM_008554 | F | TCCAGTTGGTTAGGGGGCTA | 107 bp | 60°C | |
| R | GCATAGGCCCAGGTTTCTTG | ||||||
| 12 | Axin2 | NM_015732 | F | TGAGATCCACGGAAACAGC | 104 bp | 60°C | |
| R | GTGGCTGGTGCAAAGACAT | ||||||
| 13 | Dll1 | NM_007865 | F | GGAGAAGATGTGCGACCCT | 103 bp | 60°C | |
| R | CTCCCCTGGTTTGTCACAGT | ||||||
| 14 | Dll4 | NM_019454 | F | ATGGGGAGGTCTGTTTTGTG | 101 bp | 60°C | |
| R | TATAACCCTTTGGCCCACTG | ||||||
| 15 | Notch1 | NM_008714 | F | CAAGAGGCTTGAGATGCTCC | 132 bp | 65°C | |
| R | AAGGATTGGAGTCCTGGCAT | ||||||
| 16 | Hes1 | NM_008235 | F | CTGGTGCTGATAACAGCGGA | 80 bp | 60°C | |
| R | AGGGCTACTTAGTGATCGGT | ||||||
| 17 | Hes5 | NM_010419 | F | CAAGGAGAAAAACCGACTGCG | 188 bp | 60°C | +DMSO |
| R | CGAAGGCTTTGCTGTGTTTCA | ||||||
| 18 | Atoh1 | NM_007500 | F | GTGCGATCTCCGAGTGAGAG | 108 bp | 60°C | |
| R | GGGATAAGCCCCGAACAACA | ||||||
| 19 | Lgr5 | NM_010195 | F | CAACCTCAGCGTCTTCACCT | 96 bp | 60°C | +DMSO |
| R | TCTTCTAGGAAGCAGAGGCG | ||||||
| 20 | Bmi1 | NM_007552 | F | CTTTCATTGTCTTTTCCGCC | 90 bp | 60°C | |
| R | TGGTTGTTCGATGCATTTCT | ||||||
| 21 | Spdef | NM_013891 | F | CACGTTGGATGAGCACTCGCTA | 142 bp | 60°C | |
| R | AGCCACTTCTGCACGTTACCAG | ||||||
| 22 | Gfi1 | NM_010278 | F | GGCAAAAGATTCCACCAGAA | 110 bp | 60°C | |
| R | TTGGAGCTCTGACTGAAGGC | ||||||
| 23 | Cdx1 | NM_009880 | F | GACGCCCTACGAATGGATGC | 80 bp | 60°C | |
| R | ACTTGTCCTTGGTTCGGGTC | ||||||
| 24 | Cdx2 | NM_007673 | F | CCTACCCACGAACAGCATCTACT | 69 bp | 60°C | |
| R | CCTGAGGTCCATAATTCCACTCA | ||||||
| 25 | Stk11 (Lkb1) | NM_001301853 | F | CTACTCCGAGGGATGTTGGA | 115 bp | 60°C | |
| R | GATAGGTACGAGCGCCTCAG | ||||||
| 26 | Ihh | NM_010544 | F | GACTCATTGCCTCCCAGAACTG | 150 bp | 60°C | |
| R | CCAGGTAGTAGGGTCACATTGC | ||||||
| 27 | Fgfr3 | NM_001163215 | F | TCGTGGCTGGAGCTACTTC | 95 bp | 60°C | |
| R | CTCCTGCTGGCTAGGTTCAG | ||||||
| 28 | Actb | NM_007393 | F | CTTCCTCCCTGGAGAAGAGC | 101 bp | 60°C | |
| R | AAGGAAGGCTGGAAAAGAGC | ||||||
DMSO, dimethyl sulfoxide; F, forward; Lgr5, leucine-rich repeat-containing G-protein-coupled receptor 5; R, reverse; Spdef, SAM pointed domain-containing Ets transcription factor.
Supplementary Table 2.
The Primary Antibodies Used in the Present Study
| Company | Catalog no | Dilution | |
|---|---|---|---|
| Alkaline phosphatase | Rockland, Limerick, PA | 200-4135 | 1:2000 |
| β-actin | Santa Cruz, Dallas, TX | sc-1616 | 1:1000 |
| β-catenin | BD Biosciences, San Jose, CA | 610154 | 1:50 |
| BrdU | Sigma-Aldrich, St. Louis, MO | B2531 | 1:1000 |
| Chromogranin A | Abcam, Cambridge, United Kingdom | ab15160 | 1:100 |
| Dll1 | R&D Systems, Minneapolis, MN | AF5026 | 1:500 |
| E-cadherin | BD Biosciences, San Jose, CA | c20820-050 | 1:200 |
| FITC-phalloidin | Sigma-Aldrich, St. Louis, MO | P5282 | 1:1000 |
| Gnaq/Gna11 | Abcam, Cambridge, United Kingdom | ab79337 | 1:200 |
| Hes-1 | Santa Cruz, Dallas, TX | sc-25392 | 1:150 |
| Hes-5 | Millipore, Darmstadt, Germany | AB5708 | 1:200 |
| Ki-67 | Cell Signaling, Danvers, MA | 12202 | 1:800 |
| Lysozyme | Dako, Glostrup, Denmark | A0099U2 | 1:1000 |
| Muc2 | Santa Cruz, Dallas, TX | sc-15334 | 1:200 |
| Notch1 | Abcam, Cambridge, United Kingdom | ab27526 | 1:200 |
| Phospho-PKC (pan) | Cell Signaling, Danvers, MA | 9371 | 1:1000 |
| Sox9 | Millipore, Darmstadt, Germany | AB5535 | 1:200 |
| Tcf1 | Cell Signaling, Danvers, MA | 2203 | 1:100 |
FITC, fluorescein isothiocyanate.
References
- 1.Batlle E., Henderson J.T., Beghtel H. β-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/EphrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 2.Barker N., Clevers H. Tracking down the stem cells of the intestine: strategies to identify adult stem cells. Gastroenterology. 2007;133:1755–1760. doi: 10.1053/j.gastro.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Wank S.A. I. CCK receptors: an exemplary family. Am J Physiol Gastrointest Liver Physiol. 1998;274:G607–G613. doi: 10.1152/ajpgi.1998.274.4.g607. [DOI] [PubMed] [Google Scholar]
- 4.Robertson R.P., Seaquist E.R., Walseth T.F. G proteins and modulation of insulin secretion. Diabetes. 1991;40:1–6. doi: 10.2337/diab.40.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Strathmann M., Simon M.I. G protein diversity: a distinct class of alpha subunits is present in vertebrates and invertebrates. Proc Natl Acad Sci U S A. 1990;87:9113–9117. doi: 10.1073/pnas.87.23.9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Exton J.H. Regulation of phosphoinositide phospholipases by hormones, neurotransmitters, and other agonists linked to G proteins. Annu Rev Pharmacol Toxicol. 1996;36:481–509. doi: 10.1146/annurev.pa.36.040196.002405. [DOI] [PubMed] [Google Scholar]
- 7.Amatruda T.T., Steele D.A., Slepak V.Z. G alpha 16, a G protein alpha subunit specifically expressed in hematopoietic cells. Proc Natl Acad Sci U S A. 1991;88:5587–5591. doi: 10.1073/pnas.88.13.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkie T.M., Scherle P.A., Strathmann M.P. Characterization of G-protein alpha subunits in the Gq class: expression in murine tissues and in stromal and hematopoietic cell lines. Proc Natl Acad Sci U S A. 1991;88:10049–10053. doi: 10.1073/pnas.88.22.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Es J.H., van Gijn M.E., Riccio O. Notch/[gamma]-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 10.van der Flier L.G., Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 11.Andreu P., Peignon G., Slomianny C. A genetic study of the role of the Wnt/β-catenin signalling in Paneth cell differentiation. Dev Biol. 2008;324:288–296. doi: 10.1016/j.ydbio.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 12.Fevr T., Robine S., Louvard D. Wnt/β-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol. 2007;27:7551–7559. doi: 10.1128/MCB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korinek V., Barker N., Moerer P. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 14.Mori–Akiyama Y., van den Born M., van Es J.H. SOX9 Is required for the differentiation of Paneth cells in the intestinal epithelium. Gastroenterology. 2007;133:539–546. doi: 10.1053/j.gastro.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 15.van Es J.H., Jay P., Gregorieff A. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol. 2005;7:381–386. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- 16.Zecchini V., Domaschenz R., Winton D. Notch signaling regulates the differentiation of post-mitotic intestinal epithelial cells. Genes Dev. 2005;19:1686–1691. doi: 10.1101/gad.341705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Q., Bermingham N.A., Finegold M.J. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 18.El Marjou F., Janssen K.-P., Hung-Junn Chang B. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39:186–193. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 19.Mashima H., Sato T., Horie Y. Interferon regulatory factor-2 regulates exocytosis mechanisms mediated by SNAREs in pancreatic acinar cells. Gastroenterology. 2011;141:1102–1113.e8. doi: 10.1053/j.gastro.2011.05.051. [DOI] [PubMed] [Google Scholar]
- 20.Okayasu I., Hatakeyama S., Yamada M. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 21.Wettschureck N., Rutten H., Zywietz A. Absence of pressure overload induced myocardial hypertrophy after conditional inactivation of G[alpha]q/G[alpha]11 in cardiomyocytes. Nat Med. 2001;7:1236–1240. doi: 10.1038/nm1101-1236. [DOI] [PubMed] [Google Scholar]
- 22.Offermanns S., Zhao L.-P., Gohla A. Embryonic cardiomyocyte hypoplasia and craniofacial defects in Gαq. Gα11-mutant mice. EMBO J. 1998;17:4304–4312. doi: 10.1093/emboj/17.15.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Troughton W.D., Trier J.S. Paneth and goblet cell renewal in mouse duodenal crypts. J Cell Biol. 1969;41:251–268. doi: 10.1083/jcb.41.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calvert R., Bordeleau G., Grondin G. On the presence of intermediate cells in the small intestine. Anat Rec. 1988;220:291–295. doi: 10.1002/ar.1092200310. [DOI] [PubMed] [Google Scholar]
- 25.Kamal M., Wakelin D., Ouellette A.J. Mucosal T cells regulate Paneth and intermediate cell numbers in the small intestine of T. spiralis-infected mice. Clin Exp Immunol. 2001;126:117–125. doi: 10.1046/j.1365-2249.2001.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinto D., Gregorieff A., Begthel H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bastide P., Darido C., Pannequin J. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol. 2007;178:635–648. doi: 10.1083/jcb.200704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pellegrinet L., Rodilla V., Liu Z. Dll1- and Dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology. 2011;140:1230–1240.e7. doi: 10.1053/j.gastro.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.VanDussen K.L., Carulli A.J., Keeley T.M. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development. 2012;139:488–497. doi: 10.1242/dev.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schröder N., Gossler A. Expression of Notch pathway components in fetal and adult mouse small intestine. Gene Expr Patterns. 2002;2:247–250. doi: 10.1016/s1567-133x(02)00060-1. [DOI] [PubMed] [Google Scholar]
- 31.Bjerknes M., Cheng H. Cell Lineage metastability in Gfi1-deficient mouse intestinal epithelium. Dev Biol. 2010;345:49–63. doi: 10.1016/j.ydbio.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 32.Gregorieff A., Stange D.E., Kujala P. The Ets-domain transcription factor Spdef Promotes maturation of goblet and Paneth cells in the intestinal epithelium. Gastroenterology. 2009;137:1333–1345.e3. doi: 10.1053/j.gastro.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 33.Shorning B.Y., Zabkiewicz J., McCarthy A. Lkb1 deficiency alters goblet and Paneth cell differentiation in the small intestine. PLoS One. 2009;4:e4264. doi: 10.1371/journal.pone.0004264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vidrich A., Buzan J.M., Barnes S. Altered epithelial cell lineage allocation and global expansion of the crypt epithelial stem cell population are associated with ileitis in SAMP1/YitFc mice. Am J Pathol. 2005;166:1055–1067. doi: 10.1016/S0002-9440(10)62326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walsh R., Seth R., Behnke J. Epithelial stem cell-related alterations in Trichinella spiralis-infected small intestine. Cell Prolif. 2009;42:394–403. doi: 10.1111/j.1365-2184.2009.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crosnier C., Stamataki D., Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- 37.Sansom O.J., Reed K.R., Hayes A.J. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lien W.-H., Fuchs E. Wnt some lose some: transcriptional governance of stem cells by Wnt/β-catenin signaling. Genes Dev. 2014;28:1517–1532. doi: 10.1101/gad.244772.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kohn A.D., Moon R.T. Wnt and calcium signaling: β-catenin-independent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 40.Das S., Yu S., Sakamori R. Rab8a vesicles regulate Wnt ligand delivery and Paneth cell maturation at the intestinal stem cell niche. Development. 2015;142:2147–2162. doi: 10.1242/dev.121046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Powell D.W., Pinchuk I.V., Saada J.I. Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol. 2011;73:213–237. doi: 10.1146/annurev.physiol.70.113006.100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brodrick B., Vidrich A., Porter E. Fibroblast growth factor receptor-3 (FGFR-3) regulates expression of paneth cell lineage-specific genes in intestinal epithelial cells through both TCF4/β-catenin-dependent and -independent signaling pathways. J Biol Chem. 2011;286:18515–18525. doi: 10.1074/jbc.M111.229252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vidrich A., Buzan J.M., Brodrick B. Fibroblast growth factor receptor-3 regulates Paneth cell lineage allocation and accrual of epithelial stem cells during murine intestinal development. Am J Physiol Gastrointest Liver Physiol. 2009;297:G168–G178. doi: 10.1152/ajpgi.90589.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varnat F., Heggeler B.B.T., Grisel P. PPARβ/δ regulates paneth cell differentiation via controlling the hedgehog signaling pathway. Gastroenterology. 2006;131:538–553. doi: 10.1053/j.gastro.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Kosinski C., Stange D.E., Xu C. Indian hedgehog regulates intestinal stem cell fate through epithelial−mesenchymal interactions during development. Gastroenterology. 2010;139:893–903. doi: 10.1053/j.gastro.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Dop W.A., Heijmans J., Büller N.V.J.A. Loss of Indian hedgehog activates multiple aspects of a wound healing response in the mouse intestine. Gastroenterology. 2010;139:1665–1676.e10. doi: 10.1053/j.gastro.2010.07.045. [DOI] [PubMed] [Google Scholar]
- 47.Okamoto R., Tsuchiya K., Nemoto Y. Requirement of Notch activation during regeneration of the intestinal epithelia. Am J Physiol Gastrointest Liver Physiol. 2009;296:G23–G35. doi: 10.1152/ajpgi.90225.2008. [DOI] [PubMed] [Google Scholar]