Abstract
Increased expression of vascular endothelial growth factor (VEGF) contributes to the growth of many tumors by increasing angiogenesis. Although hypoxia is a potent inducer of VEGF, we previously showed that epidermal growth factor receptor amplification and loss of PTEN, both of which can increase phosphatidylinositol-3-kinase (PI3K) activity, increase VEGF expression. Using both adenoviral vectors and a cell line permanently expressing constitutively active myristoylated Akt (myrAkt), we show that activation of Akt, which is downstream of PI3K, increases VEGF expression in vitro and increases angiogenesis in a Matrigel plug assay. Transient transfection experiments using reporter constructs containing the VEGF promoter showed that up-regulation of VEGF by Akt is mediated through Sp1 binding sites located in the proximal promoter. Small interfering RNA directed against Sp1 prevented the induction of VEGF mRNA in response to myrAkt but not to hypoxia. Expression of myrAkt is associated with increased phosphorylation of Sp1 and its increased binding to a probe corresponding to the -88/-66 promoter region. In conclusion, our results indicate that Sp1 is required for transactivation of the VEGF by Akt. Others have proposed that the PI3K/Akt pathway can increase VEGF expression via the hypoxia-inducible factor 1 (HIF-1); however, our results suggest an alternative mechanism can also operate.
INTRODUCTION
Vascular endothelial growth factor (VEGF), which is an important mediator of angiogenesis in a variety of settings (Ferrara, 2002), is often overexpressed in cancers and may be important for their continued growth beyond a certain size. This is underscored by the fact that strategies to inhibit VEGF expression and function, including antibodies, kinase inhibitors, and soluble VEGF receptors, efficiently inhibit tumor growth in animal models of gliomas and other tumors (Manley et al., 2002; Bogler and Mikkelsen, 2003). Therefore, defining the mechanisms that regulate VEGF expression in cancer cells may have important implications for understanding tumor biology.
VEGF is strongly induced by hypoxia, and undoubtedly this is an important mechanism of induction in tumors (Shweiki et al., 1992). However, when grown in standard tissue culture conditions under ambient oxygen conditions, many cell lines express high levels of VEGF expression, indicating that other factors can also play a role in regulating VEGF (Feldkamp et al., 1999). Our previous work suggested that genetic changes found in many cancers, specifically activation of the epidermal growth factor receptor (EGFR) and mutation of the tumor suppressor gene PTEN, may lead to up-regulation of VEGF (Maity et al., 2000; Pore et al., 2003).
PTEN (phosphatase and tensin homolog deleted on chromosome ten), also known as MMAC-1 or TEP-1, functions primarily as a lipid phosphatase to dephosphorylate the D-3 position of phosphoinositide phosphates such as PI(3,4,5)P3 to convert them to PI(4,5)P2 (Vivanco and Sawyers, 2002). The PTEN tumor suppressor gene product therefore acts as an antagonist of phosphoinositide 3-kinase signaling, and its loss leads to increased Akt activity (Haas-Kogan et al., 1998; Wu et al., 1998; Cantley and Neel, 1999). PTEN is one of the most frequently mutated tumor suppressors and appears to play particularly important roles in regulating human cancer growth, adhesion, migration, invasion and apoptosis (Yamada and Araki, 2001). There is now accumulating evidence that loss of PTEN also plays a role in angiogenesis (Wen et al., 2001; Abe et al., 2003). Activation of Akt has also been implicated in tumorigenesis (Testa and Bellacosa, 2001) and angiogenesis (Jiang et al., 2000). Our results offer a mechanistic explanation as to how loss of PTEN and activation of Akt can lead to increased angiogenesis via up-regulation of VEGF expression.
Previously we showed that introduction of wild-type PTEN into cell lines deficient in PTEN decreased VEGF expression (Pore et al., 2003) Furthermore, this effect was mediated at the level of transcription since PTEN could down-regulate activity of a reporter construct containing the proximal VEGF promoter. Conversely, Akt could increase activity of this promoter construct. In the current study we examine the regulation of VEGF expression by Akt in greater detail. The promoter fragment previously found by us to be regulated by PTEN and Akt spans -88 to +54 and contains two Sp1 binding sites and one AP-2 binding site. This fragment of the VEGF promoter is far downstream of the hypoxia-inducible factor 1 (HIF-1) binding site. Using a variety of techniques including transient transfection of VEGF promoter reporter constructs, gel shift studies, and orthophosphate labeling, we examine the importance of these binding sites in the response of the VEGF promoter to Akt. Therefore, although others have proposed that the PI3K/Akt pathway can increase VEGF expression via the hypoxia-inducible factor 1 (HIF-1; Jiang et al., 2000; Zhong et al., 2000; Zundel et al., 2000; Laughner et al., 2001; Woods et al., 2002; Brugarolas et al., 2003), our results provide evidence of a HIF-1–independent pathway.
MATERIALS AND METHODS
Tissue Culture and Reagents
U87 MG, U251 MG, SF188 PC3, DU145, and astrocytes were cultured in DMEM (4500 mg/l glucose, Life Technologies, Rockville, MD) containing 10% fetal bovine serum (Atlanta Biologicals, Norcross, GA) and grown in an incubator with 5% carbon dioxide and 21% oxygen or under hypoxic conditions as described below. SF188, U87MG, U251MG, and LN229 are all glioblastoma cell lines, whereas PC3 and DU145 are prostate carcinoma cell lines. Initially, normal human astrocytes (NHAs) were obtained commercially (Clonetics, Cambrex Bioscience, Walkersville, MD) and passaged according to manufacturer's recommendations. The MuLV-based expression vectors, pBABEneo and pBABEhygro, containing genes encoding large T antigen (LT; and neomycin resistance gene product) and hTERT (and hygromycin resistance gene product), respectively (kindly provided by R. Weinberg; MIT), were sequentially introduced into NHAs in a manner similar to that described previously (Hahn et al., 1999). These defective retroviral vectors were used to sequentially infect the NHAs by standard procedures. After selection in G418 (400–600 μg/ml) and hygromycin (100 μg/ml), drug-resistant cell lines were generated that expressed LT (confirmed by immunohistochemistry) and hTERT (confirmed by TRAP assay). After immortalization, NHAs were able to grow well in DMEM with 10% fetal bovine serum. A modified retroviral expression vector was generated where the antibiotic resistance gene was replaced with green fluorescence protein (pBABEgfp). This vector was used to introduce myristoylated Akt into the previously immortalized NHAs. Cells that fluoresced green were isolated by fluorescent-activated cell sorting and the resultant cell pool expressing myrAkt was labeled NHA/Akt. Mithramycin was purchased from Sigma Chemicals (St. Louis, MO). LY29400 was purchased from Cell Signaling (Beverly, MA). Matrigel was purchased from BD Biosciences (San Diego, CA).
Northern Blot Analysis
Total RNA was isolated with RNazol (Life Technologies), using the manufacturer's directions. Ten to 15 μg of RNA was denatured with formaldehyde and formamide and run on a 0.9% agarose gel containing formaldehyde. RNA was transferred by capillary action in 20× SSC (1× SSC is 0.15 M NaCl, 0.15 M sodium citrate, pH 7) to a Duralon-UV membrane (Stratagene, La Jolla, CA) and UV cross-linked before hybridization. Labeling of radioactive probes was performed using 32P-dCTP and a Prime-It kit (Stratagene) using the manu facturer's directions. Hybridization was carried out at 65°C, after which the membranes were washed with 0.1× SSC, 0.1% SDS at 65°C. Autoradiography was carried out at -80°C with intensifying screens. A 200-base pair VEGF cDNA fragment excised with EcoRI from the pGEMh204 plasmid was used to make radioactive probes for hybridization. To verify equal loading between lanes, all gels were stained with ethidium bromide and the membranes were probed with a DNA fragment of the 18S rRNA.
Protein Extraction and Western Blot Analysis
For protein isolation, cells were trypsinized and washed once in PBS. The pellets were then solubilized in 0.3–0.5 ml of 1× sample lysis buffer (1% Triton X-100, 20 mM Tris, pH 7.6, 150 mM NaCl, 2 mM EDTA, 10% glycerol, 1 mM dithiothreitol (DTT), 1 mM orthovanadate supplemented with Complete protease inhibitors [Roche, Indianapolis, IN]), boiled for 5 min, and passed repeatedly through a 26-gauge needle. Samples were centrifuged at 10,000 × g, and the supernatants were retained. Protein concentrations were determined using a BCA Protein Assay kit (Pierce, Rockford, IL).
For Western blotting, equal amounts of total protein were run in each lane of an SDS-PAGE gel (12% acrylamide). Each protein sample was mixed with an equal volume of 2× Laemmli buffer and boiled at 95°C for 5 min before loading onto the gel. After completion of gel electrophoresis, protein was transferred to a Hybond nitrocellulose membrane (Amersham, Piscataway, NJ) over 1 h using a blotting apparatus. For detection of the phosphorylated form of Akt protein, we used a monoclonal antiphospho Akt antibody (New England Biolabs, Beverly, MA) followed by a goat anti-mouse antibody (Bio-Rad, Cambridge, MA). For detection of Sp1 protein, we used a polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) followed by a goat anti-rabbit antibody (Bio-Rad). For detection of Sp3 protein, we used a polyclonal antibody (Upstate Biotechnology, Lake Placid, NY; catalogue no. 07–107) followed by a goat anti-rabbit antibody (Bio-Rad). As a loading control, blots were reprobed with an anti-β-actin antibody (Sigma) at a 1:1000 dilution followed by a goat anti-mouse antibody (Bio-Rad) at a dilution of 1:500.
Reverse Transcription-Polymerase Chain Reaction
Total cellular RNA was isolated using Trizol regent (Life Technologies). cDNA synthesis was performed using the Titan One Tube reverse transcription-polymerase chain reaction (RT-PCR) System (Roche). The primers for polymerase chain reaction (PCR) of human VEGF were as follows: forward primer, 5′-CCA TGA ACT TTC TGC TGT CTT-3′; and reverse primer, 5′-ATC GCA TCA GGG GCA CAC AG-3′. This primer sequence amplifies a 249-base pair sequence. The PCR conditions were as follows: 30 min at 50°C, then hot start at 94°C for 2 min followed by 25 amplification cycles, each consisting of 94°C for 10 s, 55.3°C for 30 s, 68°C for 45 s. This was followed by a final extension step at 68°C for 2 min. The primers for PCR of human 18S were as follows: forward primer, 5′-TAT GGT TCC TTT GGT CGC TC-3′ and reverse primer, 5′-CTT GGA TGT GGT AGC CGT TT-3′. This primer sequence amplifies a 357-base pair sequence. The PCR conditions were as follows: 30 min at 50°C, then hot start at 94°C for 2 min followed by 22 amplification cycles, each consisting of 94°C for 10 s, 55.3°C for 30 s, 61°C for 45 s;. This was followed by a final extension step at 68°C for 2 min. After completion of the reaction, PCR products were separated on 2% agarose gels and visualized by staining with ethidium bromide. Preliminary experiments were done to determine PCR conditions and starting amount of RNA so that there was a linear relationship between amount of input RNA and density of band on ethidium bromide–stained gel.
Plasmid Constructs and Transient Transfections
The construction of the plasmids pGL3–1.5-kb VEGFprom has been described previously (Maity et al., 2000). An upstream primer, 5′-TAGCTCGAGCCGGGCG GCCGGG GC3′, engineered with an XhoI restriction site (underlined), and a downstream primer, 5′-TACAAGCTTCTAGCCCCAGCGCCACGA3′, engineered with a HindIII restriction site (underlined), were used to PCR amplify the fragment of the VEGF promoter from -88 to +54. This fragment was excised with XhoI and HindIII and subcloned into the plasmid pGL3-Basic to make pGL3(-88/+54)VEGFprom. To make the construct pGL3(-70/+54)VEGFprom, the same downstream primer was used, but the upstream primer was 5′-TAGCTCGAGGGGTCCCGGCGGGGCGGA-3′ engineered to contain an XhoI site (underlined). After PCR amplification, the fragment was excised with XhoI and HindIII and subcloned into these sites in pGL3-Basic. To make the -88/+54 mutant constructs, we used the AP-2, Sp1, and AP-2/Sp1 mutants in pGL2 and excised the -88/+54 base pair fragments using XmaI and HindIII and subcloned them into the sites in the pGL3 vector.
To make the 1.4-kB wt VEGF construct, we used pGL3–1.5-kb VEGFprom as a template to PCR amplify a 20-base pair fragment, which included the HIF-1 binding site using as the upstream primer 5′-CATGGTACCCCACAGTGCATACGTGGGCTCC-3′ (HIF-1 binding site underlined) and the downstream primer 5′-CATAGATCTCTGGCCTGCAGACATC-3′. The amplified fragment was cut with KpnI and PstI, and this fragment was subcloned back into pGL3–1.5-kb VEGFprom cut with KpnI/PstI. 1.4-kB wtVEGF was identical to pGL3–1.5-kb VEGFprom except that the latter contained ∼200 base pairs more of the VEGF promoter upstream of the HIF-1 binding site. The mut1 construct was made in a similar way except that the upstream primer was 5′-CATGGTACCCCACAGTGCATAAAAAGGCTCC-3′ (HIF-1 binding site underlined; mutation bold-faced). The mut1 construct was identical to 1.4-kB wt VEGF, except for the 4-base pair mutation in the HIF-1 binding site. Mutation of these sites has been previously shown to eliminate the ability of the element to bind HIF-1 and to activate transcription in response to hypoxia (Forsythe et al., 1996). To make the mut2 construct, we first excised a region in the proximal promoter from 1.4-kB wt VEGF using SmaI and HindIII. The proximal promoter region was then reconstituted by cutting the pGL3/-/+54/Sp1/AP-2mut construct with SmaI/HindII, isolating the 150-base pair insert and subcloning it back into 1.4-kB wt VEGF cut with SmaI/HindIII. The mut3 construct was made in the same way as the mut2 construct except that, instead of 1.4-kB wt VEGF, the starting vector was the mut1 construct, which contains the 4-base pair mutation in the HIF-1 binding site.
pCMV6/Akt K179M and pCMV6/myr Akt were obtained from Phil Tsichlis (Tufts University School of Medicine). pCMV4/Sp1 and pCMV4/Sp3 were obtained from Jonathan Horowitz (North Carolina State University; Udvadia et al., 1993, 1995). pSG5-AP-2α, pSG5-AP-2β, and pSG5-AP-2γ were obtained from Dr. Michael Tainsky (Karmanos Cancer Institute, Wayne State University School of Medicine; Wankhade et al., 2000). Transfections were performed using Fugene (Boehringer Mannheim, Indianapolis, IN) according to the manufacturer's instructions. Briefly, cells were split into 60-mm dishes so that 24 h later they were ∼50% confluent. At this time each dish was transfected using 6 μl of Fugene and 2 μg of the reporter plasmid and, in order to control for transfection efficiency, 1 μg of pSV-β-galactosidase (Promega, Madison, WI). Cells were harvested by removing the media, washing twice with PBS and directly adding 100 μl of lysis buffer per dish. Of this lysate, 80 μl was used for luciferase determinations and 10 μl for β-galactosidase assays. These determinations were performed using the LucLite kit (Packard Instrument Company, Downers Grove, IL) and the β-galactosidase Enzyme Assay System (Promega). Luciferase readings were performed on a TopCount Microplate Scintillation and Luminescence Counter (Packard Instrument Company).
Small interfering RNA (RNAi) were prepared by Dharmacon Research (Lafayette, CO). The targeted sequences used to silence transcription Sp-1 and Sp-3 were 5′-AAAGCGCUUCAUGAGGAGUGA-3′and 5′-AAGCGGCAGGUGGAGCCUUCACU-3′, respectively. The RNAi targeting sequence for green fluorescent protein (5′-GGC TAC GTC CAG GAG CGC ACC-3′) mRNA was used as a negative control. RNAi were transfected into SF188 cells by Oligofectamine (Invitrogen, Carlsbad, CA) according to manufacturer's instruction. Twenty-four hours after transfection, medium was replaced and further transfected with plasmid expressing myrAkt using Fugene 6 as described previously.
Adenovirus
Adenovirus expressing PTEN or phosphatase dead PTEN, dominant negative Akt or myrAkt capable of replicating in the “packaging” 293 cell line were made using the pAd-Easy protocol (He et al., 1998). The virus was stored in single-use aliquots at -80°C. Cells were infected at a multiplicity of infection (MOI) of 5–10, and cells were harvested 48 h postinfection.
Electrophoretic Mobility Shift Assay
Nuclear proteins were extracted as described previously (Dignam et al., 1983). The oligonucleotides corresponding to -88 base pairs and -65 base pairs of the human VEGF/VPF promoter were synthesized. The complementary sequences 5′-CCGGGGCGGGCCGGGGGCGGGGGTAT-3′ and 5′ ACCCCGCCCCCGGCCCGCCCCGG-3′ were labeled with [γ-32P]ATP and T4 polynucleotide kinase. Unincorporated [γ-32P]ATP were removed by centrifugation through G-25 Sephadex column (Boehringer Mannheim) according to manufacturer's recommendations. The DNA-binding reaction was performed for 30 min at room temperature in a volume of 20 μl, containing 5 μg of nuclear protein extract, 2.5 mg/ml bovine serum albumin, 105 cpm, 0.1 mg/ml poly[dI:dC] (Sigma), 5 μl of 4× binding buffer (1× buffer: 10 mM Tris-Cl, pH 7.8, 100 mM KCl, 5 mM MgCl2, 1 mM EDTA, 10% [vol/vol] glycerol, 1 mM DTT) with or without excess of unlabeled competitor, Sp1, AP2 consensus-oligonucleotide (Promega), Sp1, Sp3 (Santa Cruz Biotechnology) or AP2 antibody (Geneka Biotechnology, Carlsbad, CA). Samples were subjected to electrophoresis on a native 5% polyacrylamide gel run in 0.5× TGE (50 mM Tris-HCl, 400 mM glycine, 2 mM EDTA) for 2.5 h at 120 V.
Treatment with Alkaline Phosphatase
Whole cell extracts (5 μg) were equilibrated for 5 min at room temperature in 1× DNA binding buffer as described above. Calf intestinal alkaline phosphatase (CIP; 0.5 U; Boehringer Mannheim) was added on ice, and the mixture was incubated for 15 min. The reaction was stopped by the addition of an equal volume of phosphatase inhibitor (20 mM NaF, 20 mM sodium vanadate) and then the radioactive probe (105 cpm). Incubation was continued for 20 min at room temperature, and samples were subjected to electrophoresis as described above.
Orthophosphate Labeling
After 30 h of infection with respective adenovirus, SF188 cells were incubated in phosphate-free DMEM (Life Technologies-BRL) for 1 h, labeled in media containing 1 mCi [32P]orthophosphate (Amersham Pharmacia) for 8 h and harvested with the same buffer as described above for Western blotting. The protein solution was precleared with Agarose A (Invitrogen) and incubated with either an anti-Sp1 Antibody (Sigma) or an anti-Sp3 antibody (Santa Cruz Biotechnology) at 4°C for overnight. Immunoprecipitates were isolated with protein A, and the beads were washed four times with buffer. Finally beads were resuspended in 50 μl of 1× SDS-PAGE loading buffer (0.06 M Tris-HCl, pH 8.0, 1.71% SDS, 6% glycerol, 0.1 M DTT, 0.002% bromophenol blue) and boiled at 95°C. The released proteins were separated on 12% SDS-PAGE gel. To confirm the phosphorylation, beads were incubated with 20 mU of CIP (New England Biolabs) for 1 h at room temperature followed by boiling in 1× SDS-PAGE loading buffer. Separated proteins were transferred to a Hybond nitrocellulose membrane (Amersham) and autoradiographed.
In Vivo Study of Angiogenesis Using Matrigel Plug Assay
This assay was performed as described previously (Juarez et al., 2002). Angiogenesis was measured in Matrigel (Collaborative Biomedical Products, Bedford, MD). Matrigel (500 μl) containing 2 × 106 cells of each cell line was injected subcutaneously into 4–8-wk-old female BALB/c nude mice at sites lateral to the abdominal midline, two injections per mouse. As a negative control Matrigel with 100 μl PBS was injected in similar manner. All measurements were made at least in triplicate. Animals were sacrificed 5 d after Matrigel injection. The Matrigel plugs were recovered and photographed immediately. Plugs were then dissolved in PBS and incubated at 4°C overnight. Hemoglobin levels were determined using Drabkin's solution (Sigma) according to manufacturer's instructions.
Densitometry
Gels were scanned on an Epson 2450 Perfection Photoscanner (Long Beach, CA) using Adobe Photoshop 4.0.1 (San Jose, CA). Bands on the gels were quantitated using NIH Image 1.63 software.
RESULTS
Akt Regulates VEGF mRNA and Protein Expression
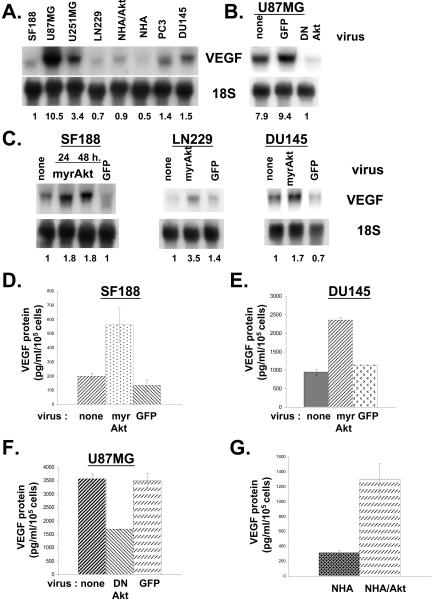
As shown in Figure 1A, there is tremendous heterogeneity in VEGF mRNA expression with some cell lines such as U87MG and U251MG expressing 3- to 10-fold higher levels than others such as SF188. Previously, we had shown that SF188 cells contained much lower levels of phosphorylated Akt than did either U87MG or U251MG cells (Pore et al., 2003). NHA/Akt, normal human astrocytes expressing myrAkt, contain approximately twice as much VEGF mRNA as the parental NHA astrocytes, suggesting that Akt can modulate VEGF expression. To confirm this, U87MG cells, which contain high levels of activated Akt secondary to PTEN mutation, were transduced with adenovirus expressing dominant negative Akt. Expression of dominant negative Akt markedly decreased VEGF mRNA expression (Figure 1B). Conversely, transduction of SF188, LN229, or DU145, all cell lines containing wild-type PTEN, with adenovirus expressing myrAkt led to a twoto threefold increase in VEGF mRNA expression.
Figure 1.
Akt modulates expression of VEGF in different cell lines. (A–C) Northern blots; 10 μg of total RNA were harvested from cells and run on gel. Membranes were probed for VEGF and ribosomal 18S (loading control). (A) VEGF mRNA levels from various cell lines grown under normal tissue culture conditions. (B) U87MG cells were infected with adenovirus expressing either dominant negative Akt or GFP at a multiplicity of infection (MOI) of 10. Cells were harvested 48 h later for RNA isolation. (C) SF188, LN229, or DU145 cells were infected with adenovirus expressing either myrAkt or GFP (MOI of 10). Cells were harvested 24 or 48 h later for RNA isolation. Numbers at bottom of gels in A–C represent relative VEGF levels (the ratio of VEGF band intensity to 18S band intensity). (D–G) VEGF protein levels. VEGF protein levels were determined by ELISA and normalized to the number of cells in each dish. (D and E) Media were sampled 24 h after infecting SF188 or DU145 cells with virus expressing either myrAkt or GFP or no virus. (F) Medium was sampled 24 h after infecting U87MG cells with virus expressing either dominant negative Akt or GFP or no virus. (G) Medium was taken from NHA and NHAAkt cells 24 h after plating.
In concordance with the mRNA data in Figure 1C, expression of myrAkt in SF188 cells and DU145 cells led to a greater than twofold increase in secreted VEGF expression (Figure 1, D and E). Conversely, expression of dominant negative Akt in U87MG cells led to a 50% decrease in secreted VEGF expression (Figure 1F). Furthermore, NHA/Akt cells secreted approximately threefold more VEGF protein than NHA cells (Figure 1G).
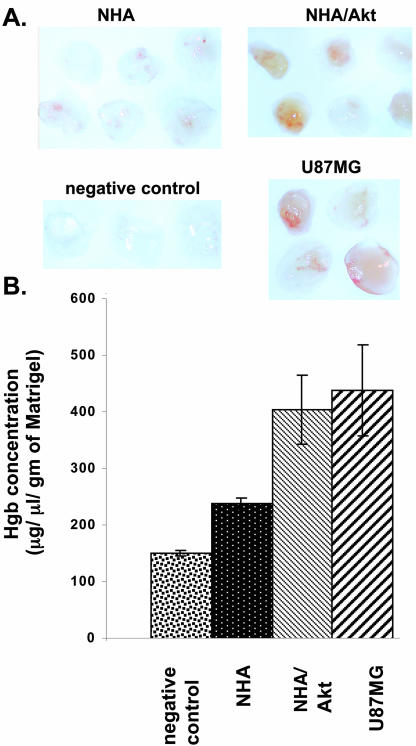
Increased VEGF Expression in NHA/Akt Cells Leads to Increased Angiogenesis
To determine whether the increased VEGF secretion seen in cells expressing Akt had functional consequences, we performed in vivo Matrigel assays. Either NHA or NHA/Akt cells were placed into Matrigel plugs, which were formed by subcutaneous injection into nude mice. NHA/Akt cells induced significantly more angiogenesis that did NHA cells, as determined by hemoglobin measurement (Figures 2, A and B). U87MG cells (positive control), which expressed high basal levels of VEGF expression, displayed significant angiogenesis, whereas plugs without cells (negative control) displayed no angiogenesis.
Figure 2.
Akt increases angiogenesis in vivo. Matrigel mixture containing U87MG, NHA, or NHA-Akt cells was injected subcutaneously. into nude mice at sites lateral to the abdominal midline. As a negative control, Matrigel containing 100 μl PBS was injected in similar way. Animals were sacrificed 5 d after injection. The mouse skin was detached along the abdominal midline, and the Matrigel plug was recovered and photographed immediately (A). (B) The relative level of hemoglobin present in each plug was determined using commercially available kit (Sigma). The amount of hemoglobin was calculated from standard hemoglobin curve. The amount of hemoglobin normalized to the weight of each Matrigel plug is plotted on y-axis.
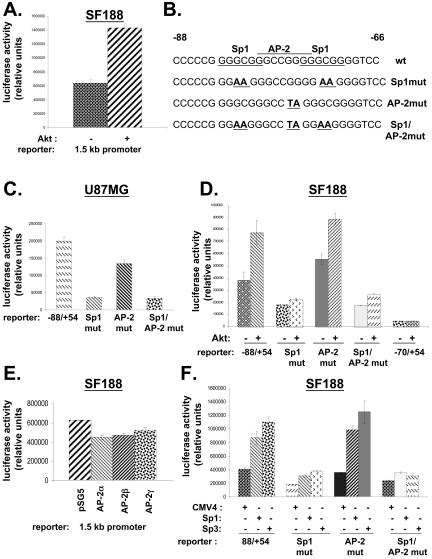
Sp1 Binding Sites in the Proximal VEGF Promoter Are Essential for Transactivation by Akt
Previously we showed that myrAkt led to a twofold increase in the activity of the -88/+54 VEGF promoter reporter in transient transfections in SF188 cells (Pore et al., 2003). MyrAkt had a similar effect on a reporter containing a 1.5-kbp segment of the promoter (Figure 3A). The -88/+54 region contains two Sp1 binding sites and one AP-2 binding site. To investigate the importance of these binding sites, we used -88/+54 promoter reporter constructs containing mutations in the two Sp1 binding sites, the AP-2 binding sites, or all three sites (Figure 3B). Mutation of the two Sp1 binding sites led to a significant decrease in promoter activity compared with the wild-type -88/+54 promoter in U87MG cells; whereas mutation of the AP-2 binding site had a more modest effect, suggesting that the AP-2 binding site is less important for basal expression of the promoter in these cells than the Sp1 binding sites (Figure 3C). Consistent with this, mutation of the AP-2 binding site in addition to the Sp1 binding sites (Sp1/AP-2mut) did not further decrease promoter activity compared with mutation of only the Sp1 binding sites (Sp1mut). The Sp1 binding sites were also important in the regulation of the promoter by Akt. The Sp1mut construct was not inducible by myrAkt in SF188 cells, whereas both the AP-2mut and the wild-type -88/+54 construct were inducible (Figure 3D). The -70/+54 construct, in which both Sp1 sites and the AP-2 site are truncated, showed very little promoter activity and was also not inducible by Akt. To assess the role of the AP-2 binding sites in the promoter, we performed transient transfection experiments with vectors expressing AP-2α, β, or γ, all three of which failed to up-regulate activity of the -88/+54 promoter (Figure 3E). In contrast, vectors expressing Sp1 or Sp3, two members of the Sp1 family of transcription factors, increased promoter activity (Figure 3F). As expected, this was not seen when the -88/+54 Sp1mut promoter construct, which contains mutations in the two Sp1/Sp3 binding sites, was used as the reporter. However, the up-regulation still occurred when the AP-2 binding site was mutated (AP-2 mut; Figure 3F).
Figure 3.
Role of Akt and Sp1 in VEGF promoter activation. In all panels, pSV-β-galactosidase was cotransfected as a control for transfection efficiency along with the indicated plasmids. Cells were harvested 48 h after transfection, and lysates were harvested for determination of luciferase and β-galactosidase activity. The ratio of luciferase to β-galactosidase is plotted on the y-axis. (A) SF188 cells were cotransfected with the 1.5-kb VEGF promoter luciferase reporter (2 μg) and 0.2 μg of either pCMV6/myr-Akt or filler control DNA (pCMV6). (B) Sequences of -88/-66 human VEGF promoter region. In the top sequence labeled wt, the AP-2 binding site is overlined, and Sp1 binding sites are underlined. The nucleotides in the Sp1 or AP-2 binding sites that were mutated in the three mutant promoter constructs (Sp1mut, AP-2mut, Sp1/AP-2mut) are boldfaced. (C) The four different VEGF promoter constructs (wt and three mutant constructs) were cotransfected separately into U87MG cells. (D) pCMV6/myrAkt (0.2 μg) and one of four VEGF promoter constructs (2 μg) was cotransfected into SF188 cells. (E) The 1.5-kb VEGF promoter luciferase reporter was cotransfected along with pSG5 vectors expressing one of the AP-2 isoforms (α, β, or γ; 0.2 μg each) into SF188 cells. (F) Vectors expressing Sp1 or Sp3 (0.2 μg each) were cotransfected with one of four VEGF promoter reporter constructs (2 μg) into SF188 cells. The results shown in this figure are representative of at least two independent experiments.
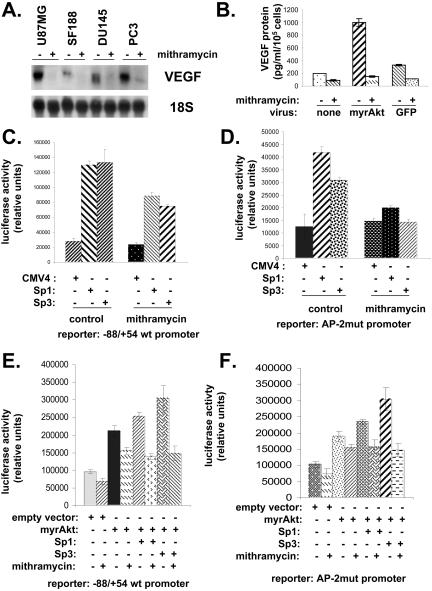
Mithramycin Interferes with Akt-mediated Induction of VEGF Expression
The results of the luciferase assays using mutant reporter constructs suggested that Sp1 family members were important for the regulation of the VEGF promoter by Akt. Mithramycin is a drug that has been used to block activity of Sp1 family members by binding to GC-rich regions (Blume et al., 1991; Nehls et al., 1993). Treatment of a variety of cell lines with the drug led to decreased VEGF mRNA expression (Figure 4A). Treatment of SF188 cells with the drug blocked the ability of myrAkt to increase VEGF expression (Figure 4B). Mithramycin also blunted the ability of Sp1 or Sp3 to transactivate the wild-type -88/+54 VEGF promoter in these cells (Figure 4C). The same result was obtained using the AP-2mut promoter (Figure 4D). Sp1 and myrAkt had an additive effect on the wild-type -88/+54 VEGF reporter with the two together leading to a slightly higher promoter activity than myrAkt by itself (Figure 4E). Likewise, Sp3 had an additive effect along with myrAkt in transactivating the -88/+54 VEGF promoter reporter. As shown in this figure, the effect of myrAkt on promoter activity, either by itself or in combination with Sp1 or Sp3, was blunted by mithramycin. Similar effects of myrAkt ± Sp 1 or Sp3 with or without mithramycin were seen when the AP-2 mutated promoter was used instead of the wild-type -88/+54 promoter (Figure 4F).
Figure 4.
Mithramycin interferes with Akt-mediated induction of VEGF expression and VEGF promoter transactivation. (A) U87 MG, SF188, DU145, and PC3 cells were treated with 100 nM mithramycin. After 24 h RNA was harvested from cells. Northern blotting was performed, and membranes were probed for VEGF and ribosomal 18S (loading control). (B) SF188 cells were infected with adenovirus expressing myrAkt or GFP or no virus 24 h after infection cells were treated with 100 nM mithramycin for another 24 h. Aliquots were taken, and VEGF protein was measured by ELISA. (C) Sp1 or Sp3 expression vectors (0.2 μg) were cotransfected along with pSV-β-galactosidase (0.1 μg) and the wt -88/+54 VEGF promoter reporter (2 μg) into SF188 cells. Panel D is identical to C except that the luciferase reporter used was the AP-2mut promoter rather than the wt -88/+54 reporter. (E) Sp1 or Sp3 expression vectors were cotransfected with pSV-β-galactosidase and the wt -88/+54 VEGF promoter reporter and in some cases with pCMV/myrAkt (0.2 μg) into SF188 cells. In some cases cells were exposed to mithramycin (100 nM). Panel F is identical to E except that the luciferase reporter used was the AP-2mut promoter rather than the wt -88/+54 reporter. The results shown in this figure are representative of at least two independent experiments.
Sp1 RNAi Abolishes VEGF mRNA Induction by Akt But Not by Hypoxia
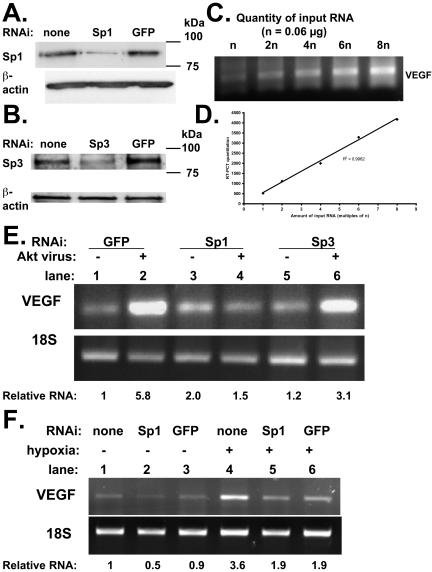
Mithramycin could potentially have nonspecific effects; therefore, we used RNAi to more selectively target Sp1 and Sp3. We designed oligonucleotides to degrade Sp1 and Sp3 (Materials and Methods) and showed by Western blotting that these were effective in decreasing the respective protein levels (Figures 5, A and B). We then performed RT-PCR with RNA from SF188 cells using a range of starting quantities (0.06–0.48 μg) to show that under the selected PCR conditions there was a linear correlation between the amount of input RNA and the amount of output (amplified) DNA (Figure 5, C and D). We then assessed the effect of manipulating Akt and Sp1/Sp3 levels on VEGF mRNA levels in SF188 cells. A 5.8-fold increase in VEGF mRNA expression was seen with Akt adenovirus when cells were treated with control (GFP) oligonucleotides (Figure 5C; compare lanes 1 and 2). In marked contrast, Sp1 RNAi oligonucleotides completely blocked the ability of Akt to up-regulate VEGF mRNA expression (Figure 5E; compare lanes 3 and 4), whereas Sp3 RNAi oligonucleotides had at most a modest effect (Figure 5C; lanes 5 and 6). Experiments were also conducted examining the effect of Sp1 RNAi on the induction of VEGF mRNA by hypoxia. There was a 3.8-fold induction in the level of VEGF mRNA with hypoxia in the presence of Sp1 RNAi (Figure 5F; compare lanes 2 and 5), similar to the level of induction seen with control GFP oligonucleotides (2.1-fold; compare lanes 3 and 6) or without any oligonucleotides (3.6-fold; compare lanes 1 and 4). Therefore, inhibition of Sp1 with RNAi prevents induction of VEGF by Akt but not by hypoxia.
Figure 5.
Sp1 RNAi abolishes Akt-mediated induction of VEGF mRNA expression. (A) Cells were transfected without RNAi, with Sp1 RNAi (600 pmol/well), or with control GFP RNAi. Forty-eight hours later, cells were harvested. (B) Cells were transfected without RNAi, with Sp3 RNAi (600 pmol/well) or with control GFP RNAi. Forty-eight hours later, cells were harvested. For both A and B, protein lysates were separated on SDS-PAGE gel and transferred to nitrocellulose membrane. Membrane was probed for Sp1 (A) and Sp3 (B). In both A and B, membranes were subsequently reprobed for β-actin. (C) RNA was extracted from SF188 cells. RT-PCR was performed using a range of starting quantities of RNA (0.06, 0.12, 0.24, 0.36, 0.48 μg). DNA was run on a gel that was stained with ethidium bromide. (D) DNA quantitation from RT-PCR was plotted vs. quantity of starting RNA. (E) SF188 cells were transfected with RNAi targeting Sp1, Sp3, or GFP. After 24 h, cells were transduced with adenovirus expressing myristoylated Akt or GFP (control). Forty hours after viral transduction, cells were harvested for RNA. (F) SF188 cells were transfected with RNAi targeting Sp1 or GFP. After 40 h, cells were exposed to 0.2% oxygen and then harvested for RNA after 8 h. (E and F) Results of RT-PCR performed for VEGF and 18S using 0.12 μg of total RNA.
Akt Increases Binding of Factors to the Proximal VEGF Promoter
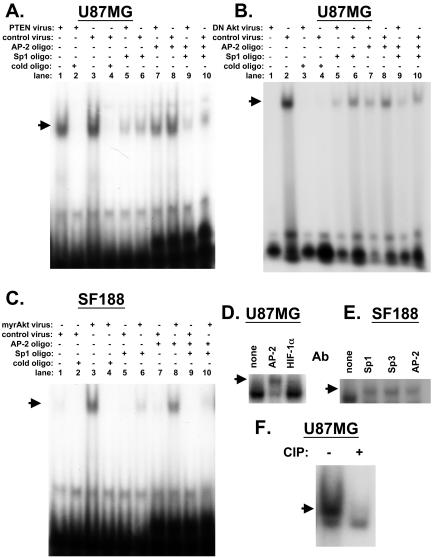
To provide further biochemical evidence of the importance of the Sp1 in the regulation of the VEGF promoter by Akt, we performed gel shift assays using an oligonucleotide corresponding to the -88/-66 region of the VEGF promoter. Before manipulating Akt expression, we tested the effect of introducing wild-type PTEN into U87MG cells, which have mutant PTEN. Previously, we had demonstrated that wild-type PTEN down-regulates VEGF expression and promoter activity in these cells (Pore et al., 2003). We found by gel shift assays that binding to the target probe was also decreased with wild-type PTEN (Figure 6A; lanes 1 vs. 3; lanes 5 vs. 6; lanes 7 vs. 8). In these cells, it appears that both Sp1/Sp3 and AP-2 bind to the promoter fragment because competition with both Sp1 and AP-2 binding cold oligonucleotides decreased the shifted band to a greater extent than either by itself (Figure 6A; compare lanes 6, 8, and 10).
Figure 6.
Gel shift assays performed with nuclear extracts from cells transduced with PTEN or Akt expressing adenovirus. (A–C) Oligonucleotides corresponding to -88 to -66 base pairs of the human VEGF promoter were labeled with [γ-32P]ATP. Gel shift assays were performed using nuclear extract from cells transduced with adenovirus expressing dead PTEN, wild-type PTEN, dominant negative Akt, myrAkt, or GFP as indicated in the headings above the figures. The DNA-binding reaction was performed with 100-fold molar excess of unlabeled competitor, Sp1, AP-2 consensus oligonucleotide as indicated. Cell line (SF188 or U87 MG) is indicated at top of gel. (A–C) Arrows point to shifted band. (D) Supershift gel shifts were performed using nuclear extract (5 μg) and 0.2 μg of AP-2 or HIF-1α (control) antibody. Arrow indicates position of supershifted complex. (E) Supershift gel shifts were performed using nuclear extract (5 μg) and 0.2 μg of Sp1, Sp3, or AP2 antibody. The position of supershifted complexes is indicated by arrow. (F) Lysates from U87MG cells were incubated with alkaline phosphatase for 15 min and then treated with phosphatase inhibitors in order to inactivate the enzyme. Then the radioactive probe was added, and gel shift assay was performed. Arrow points to shifted band.
Presumably wild-type PTEN decreases VEGF expression by suppressing Akt activity in these cells because dominant negative Akt also decreases VEGF expression (Figure 1, B and G). Gel shift studies confirmed that dominant negative Akt led to decreased binding to the -88/-66 base pair VEGF promoter fragment in U87MG cells (Figure 6B; lane 1 vs. lane 2). The binding of a complex to the -88/-66 probe could be completely abolished using an excess of cold oligonucleotides and partially competed by using an excess of either Sp1 or AP-2 consensus oligonucleotides, confirming the findings in Figure 6A.
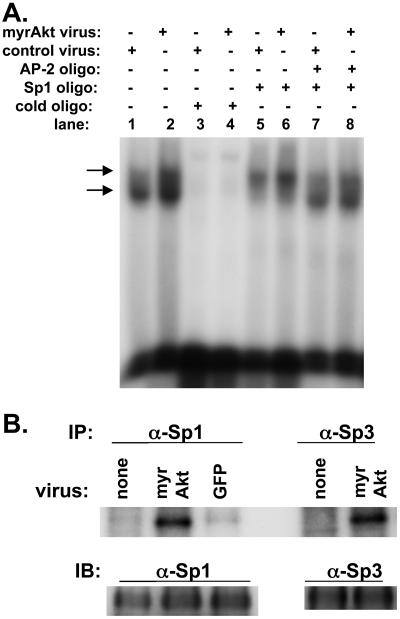
Because myrAkt increases both VEGF expression and promoter activity in SF188 cells, it should increase binding of factors to the VEGF promoter. As predicted, although transduction with a control adenovirus resulted in a barely visible gel-shifted band, transduction of cells with myrAkt led to a significant increase in oligonucleotide binding in these cells (Figure 6C; lane 1 vs. lane 3). This binding could be completely abolished using competing cold oligonucleotides (Figure 6C; lane 4). Competition with the AP-2 consensus oligonucleotides led to a slight decrease in the intensity of the shifted band in myr Akt-transduced cells (Figure 6C; lane 8 vs. lane 3). Competition with consensus Sp1 oligonucleotides led to a more obvious decrease in the intensity of the shifted band in myrAkt-transduced cells (Figure 6C; lane 6 vs. lane 3). The combination of both AP-2 and Sp1 consensus oligonucleotides led to almost complete disappearance of the gel-shifted band (Figure 6C, lane 10). These results suggest that most of the binding to the oligonucleotide under myrAkt transduced conditions in SF188 cell is due to Sp1 rather than AP-2. Gel shift studies performed with antibodies directed against Sp1, Sp3, or AP-2 showed that all three led to a supershift, confirming the presence of all three proteins in the complex (Figure 6, D and E). As a negative control, an antibody directed against HIF-1α did not lead to a supershift.
Akt Is Associated with Increased Phosphorylation of Sp1 Family Members
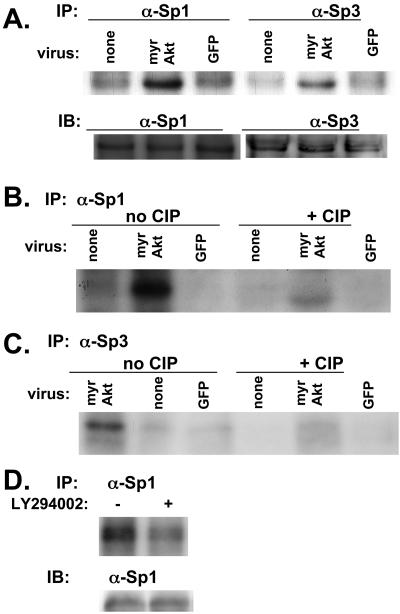
Akt appears to regulate the -88/+54 VEGF promoter via Sp1. Because Akt is a kinase and because Sp1 is known to be regulated by phosphorylation, we examined its phosphorylation status in response to Akt using a kinase assay. SF188 cells were metabolically labeled with [32P]orthophosphate, and then lysates were immunoprecipitated with antibodies directed against Sp1. This showed an increase in phosphorylated Sp1 protein in cells transduced with myrAkt adenovirus (Figure 7A; top gel labeled IP). Performing similar experiments with an anti-Sp3 antibody, we found that Akt also increased phosphorylation of this protein. To show that lysates used to load the lanes contained equal amounts of total protein, a separate gel was loaded with identical aliquots of the lysates used for Figure 7A. Western blotting for either Sp1 or Sp3 was performed with this membrane, which showed that equal amounts of total protein were present in the different lanes (Figure 7A; lower gel labeled IB). The bands seen in Figure 7A from the cells transduced with myrAkt adenovirus represented a phosphorylated form of Sp1 or Sp3 because the intensity of the bands decreased greatly when the lysates were incubated with calf intestinal alkaline phosphatase (Figure 7, B and C). To show that modulation of the PI3K/Akt pathway could alter Sp1 phosphorylation in another cell line and by another manipulation, we tested U87MG cells with the PI3K inhibitor LY294002. Treatment with the drug led to a decrease in Sp1 phosphorylation (Figure 7D). Therefore, Sp1 shows increased phosphorylation under conditions of Akt activation and decreased phosphorylation with inhibition of the PI3K/Akt pathway.
Figure 7.
myrAkt is associated with phosphorylation of Sp1 and Sp3 in SF188 cells. SF188 cells were infected with adenovirus expressing myrAkt or GFP. Thirty-six hours after infection, cells were in vivo–labeled with orthophosphate. In the top part of A labeled IP, in vivo–labeled proteins were immunoprecipitated with either Sp1 or Sp3 antibody as indicated. Immunoprecipitated complexes were separated on 10% SDS-PAGE gel and transferred to nitrocellulose membrane and autoradiographed. In the bottom part of A labeled IB (immunoblot), these same lysates were separated on 10% SDS-PAGE gel, transferred to nitrocellulose membrane, and probed with either Sp1 or Sp3 antibody to serve as a loading control. (B) The same procedure used in A was followed, but after immunoprecipitation with anti-Sp1 antibody, each protein extract was incubated with or without 10 mU of calf intestinal phosphatase (CIP) for 30 min at room temperature before running on an SDS-PAGE gel. The identical procedure was followed for C as in B, except lysates were immunoprecipitated with an anti-Sp3 antibody. (D) U87MG cells were treated with LY294002 (20 μM) or DMSO (control) for 16 h. At this time, cells were lysed and the procedure described for A was followed.
If phosphorylation of Sp1 results in its increased binding to the -88/-66 promoter region, then treatment of nuclear lysates with calf intestinal alkaline phosphatase should lead to deceased binding in a gel shift assay. This was performed as described in Materials and Methods, and as expected, gel shift assay showed that phosphatase treatment led to a significant decrease in the intensity of the shifted band (Figure 6F).
Intact Hypoxia Responsive Element in VEGF Promoter Is Not Required for Induction by Akt
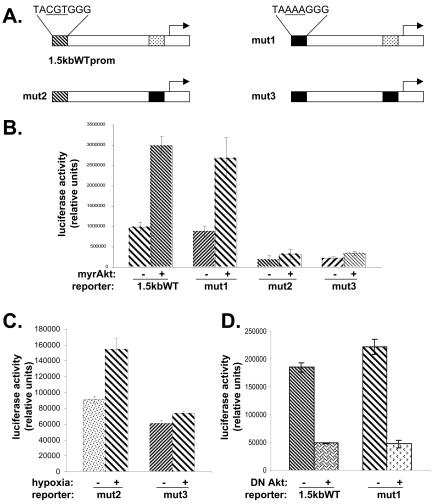
The previous results suggest that Akt can regulate the VEGF promoter via Sp1 binding sites found in the region between -88 and -66. However, this leaves unanswered the question whether the hypoxia response element (HRE), which is located ∼1 kbp upstream of the transcription start site, is also required for transactivation by Akt. To address this question, we made reporter constructs containing a mutation within the HRE (mut1), within the proximal Sp1 sites (mut2), or within both of these regions (mut3; Figure 8A). SF188 cells were cotransfected with one of the four constructs shown in Figure 8A along with either a vector expressing myrAkt or a control vector. Figure 8B shows that mutation of the HRE (mut1 construct) had no effect on inducibility by Akt compared with the wild-type construct. Both the mut2 and mut3 constructs had lower basal activity, and neither was inducible by Akt. The mut2 construct but not the mut3 construct showed inducibility with hypoxia, indicating that the HRE mutation was effective in knocking out transcription of the promoter under hypoxia (Figure 8C). The 1.5-kb WTprom and mut1 constructs were cotransfected into U87MG cells along with a vector expressing DN Akt. There are two points to note in Figure 8D. First, the basal activity of the mut1 construct compared with the wild-type construct was not decreased in these cells containing activated Akt, suggesting that HIF-1 plays a minimal role in regulating the promoter under normoxic conditions. Second, dominant negative Akt was able to down-regulate activity of both constructs. Therefore, an intact HIF-1 binding site is not required for regulation of the VEGF promoter by Akt.
Figure 8.
Mutation of HIF-1 binding site in VEGF promoter does not prevent its regulation by Akt. (A) Schematic of VEGF promoter constructs. The mut1 construct contains a mutation in the HIF-1 binding site of CGT to AAA as indicated (first box in solid black). The mut2 construct contains mutations in Sp1/AP-2 binding sites in the proximal promoter (second box in solid black). The mut3 construct contains mutations in both the HIF-1 binding site and Sp1/AP-2 region (fist and second boxes in solid black). (B) SF188 cells were cotransfected with (a) 0.2 μg of pCMV/myrAkt or pCMV6 (empty vector), (b) 2 μg of 1.5-kB wt VEGF, mut1, mut2, or mut3 vector, and (c) pSV-β-galactosidase (0.1 μg). Forty-eight hours later, dishes were harvested for both luciferase and β-galactosidase determination. (C) U87MG cells were cotransfected with (i) 2 μg of mut2 or mut3 vector and (ii) pSV-β-galactosidase (0.1 μg). Twenty-four hours later they were subjected to 1% O2. Samples were harvested after 8 h in hypoxia. (D) U87MG cells were cotransfected with (a) pCMV6/Akt K179M (0.2 μg), which is a plasmid expressing dominant negative Akt, (b) 2 μg of either a 1.4-kB wt VEGF reporter or mut1, and (c) pSV-β-galactosidase (0.1 μg). Forty-eight hours later, dishes were harvested.
Akt Is Associated with Increased Sp1 Binding and Phosphorylation in DU145 Prostate Carcinoma Cells
To show that the results we obtained regarding Sp1 binding and phosphorylation were not specific to a single cell type, we repeated some of these experiments with DU145, a prostate carcinoma cell line. Expression of myrAkt in this cell line increased the level of VEGF mRNA (Figure 1C), and treatment with mithramycin decreased the expression of VEGF mRNA (Figure 4A). As with SF188 glioblastoma cells, infection of DU145 cells with myrAkt expressing adenovirus led to increased i) binding of factors to a probe from the proximal VEGF promoter containing Sp1 binding sites (Figure 9A) and ii) phosphorylation of both Sp1 and Sp3 proteins (Figure 9B).
Figure 9.
Effect of myrAkt in DU145 prostate carcinoma cells. (A) Oligonucleotides corresponding to -88 to -66 base pairs of the human VEGF promoter were labeled with [γ-32P]ATP. Gel shift assay was performed using nuclear extract from cells transduced with adenovirus expressing myAkt or GFP (control). The DNA-binding reaction was performed with 100-fold molar excess of unlabeled competitor, Sp1, AP-2 consensus oligonucleotide as indicated. (B) DU145 cells were infected with adenovirus expressing myrAkt or GFP. Thirty-six hours after infection, cells were in vivo–labeled with orthophosphate. In the top part of B labeled IP, in vivo–labeled proteins were immunoprecipitated with either Sp1 or Sp3 antibody as indicated. Immunoprecipitated complexes were separated on 10% SDS-PAGE gel and transferred to nitrocellulose membrane and autoradiographed. In the bottom part of B labeled IB (immunoblot), these same lysates were separated on 10% SDS-PAGE gel, transferred to nitrocellulose membrane, and probed with either Sp1 or Sp3 antibody to serve as a loading control.
DISCUSSION
Angiogenesis is critical for tumor growth (Bergers and Benjamin, 2003). One of the most important angiogenic growth factors is VEGF, which is overexpressed in many human cancers. VEGF expression in tumors can be induced by more than one mechanism. Hypoxia, which is found in most tumors, has long been known to be a potent inducer of VEGF (Shweiki et al., 1992). There is also accumulating evidence that genetic changes found in tumors, particularly those that lead to Ras activation, also increase VEGF expression, an effect that is additive with hypoxia (Mazure et al., 1996; Feldkamp et al., 1999; Rak et al., 2002). Loss of PTEN has also been implicated in increased VEGF expression (Koul et al., 2002; Abe et al., 2003; Gomez-Manzano et al., 2003; Pore et al., 2003). The PI3K/PTEN/Akt pathway is a critical mediator of oncogenic signaling (Paez and Sellers, 2003). PTEN expression is lost in many human cancers including glioblastomas, melanomas, and cancers of the prostate, lung, breast, and endometrium (Knobbe et al., 2002; Vivanco and Sawyers, 2002). Loss of PTEN leads to activation of the PI3K/Akt pathway, but this can occur by alternate means such as amplification of the PI3K subunits or Akt overexpression, as seen in ovarian and breast cancers (Vivanco and Sawyers, 2002).
We previously showed that introduction of wild-type PTEN into cells lacking PTEN down-regulated VEGF expression and that this occurred at the transcriptional level (Pore et al., 2003). In the current report, we show that Akt can up-regulate VEGF expression at the mRNA and protein levels. Furthermore, this increase in Akt and VEGF expression in immortalized human astrocytes has the functional consequence of increasing angiogenesis. Akt induction of VEGF expression is mediated by increased transcription from the VEGF promoter, which appears to be dependent on Sp1 binding sites located between -88 and -70. This conclusion is based on three pieces of evidence. First, the basal level of the -88/+54 promoter activity is very low when both the Sp1 binding sites are mutated, and these mutations prevent Akt from transactivating the promoter in transient transfection experiments. Second, treatment of cells with mithramycin, an antibiotic that blocks Sp1 family members from binding to the promoter, leads to decreased inducibility of VEGF protein expression and promoter activity by Akt. Finally, RNAi directed against Sp1 abolishes induction of the VEGF mRNA by Akt. This effect was not due to a generalized effect of Sp1 RNAi on transcription in response to all stimuli because induction of VEGF mRNA was still seen in response to hypoxia.
A number of other groups have previously made the association between PI3K activation and VEGF up-regulation (Arbiser et al., 1997; Mazure et al., 1997; Jiang et al., 2000; Rak et al., 2000; Zhong et al., 2000; Zundel et al., 2000; Laughner et al., 2001; Treins et al., 2002; Woods et al., 2002; Gomez-Manzano et al., 2003). Some of these have implicated Akt as the downstream effector (Mazure et al., 1997; Jiang et al., 2000; Zhong et al., 2000; Zundel et al., 2000). Loss of function of the tuberous sclerosis complex proteins Tsc1 and Tsc2, which lie in a signaling pathway downstream of Akt and upstream of mTOR (mammalian target of rapamycin), leads to increased VEGF expression (Brugarolas et al., 2003; El-Hashemite et al., 2003). Studies that have investigated signaling events further downstream of Akt in the regulation of VEGF expression have focused on HIF-1, a heterodimeric transcription factor involved in the regulation of numerous genes in response to hypoxia (Jiang et al., 2000; Zhong et al., 2000; Zundel et al., 2000; Laughner et al., 2001; Woods et al., 2002; Brugarolas et al., 2003). The HIF-1 binding site in the VEGF promoter is located ∼1000 base pairs upstream of the transcription start site. Most of our transient transfection experiments were performed with a much shorter piece of the promoter that lacks a HIF-1α binding site, but which remains responsive to Akt. Furthermore, we found that selective mutation of the HIF-1 binding site in the context of the longer VEGF promoter did not abolish the up-regulation by myrAkt or the down-regulation by dominant negative Akt. Therefore, in contrast to most of the existing literature, our results indicate that Akt can transactivate the VEGF promoter and lead to increased VEGF expression independent of HIF-1. A recent report showed that expression of activated Akt in a hepatoma cell line lacking HIF-1 led to a dramatic increase in tumor size with increased VEGF secretion and tumor vascularization (Arsham et al., 2004). This study also suggests a dissociation between Akt-dependent and HIF-1–dependent VEGF up-regulation, as do our results. The specific mechanism was not elucidated by Arsham et al.; however, our findings offer a potential explanation.
Our results show that Sp1 is required for Akt-mediated induction of VEGF. Although both Sp1 and Sp3 can transactivate the VEGF promoter, based on the RNAi data, it appears that the Sp1 is the predominant family member that is required for induction of VEGF by Akt. Sp1 sites are found in many “housekeeping” genes, suggesting that Sp1 may have important roles in the basal transcription of these constitutively expressed genes. However, more recently Sp1 sites have been found to mediate transcription in response to diverse stimuli including oncogenes such as Ras and growth factors and cytokines including EGF, neuregulin, and TNF-α (Black et al., 2001). Numerous studies have shown that the Sp1 sites are important in regulation of the VEGF promoter(Ryuto et al., 1996; Finkenzeller et al., 1997; Gille et al., 1997, 1998; Milanini et al., 1998; Shi et al., 2001; Milanini-Mongiat et al., 2002; Sen et al., 2002; Schafer et al., 2003). However, the current study is the first to show that Sp1 is involved in the regulation of VEGF expression by the PI3K/Akt pathway. We speculate that this might occur via phosphorylation of Sp1 leading to its increased binding to and transactivation of the VEGF promoter. These observations are particularly relevant to cancers that sustain genetic changes that lead to activation of Akt such as loss of PTEN, receptor tyrosine kinase activation, or Akt mutation.
Acknowledgments
This work was supported by Public Health Service grants R01 CA093638-01 (A.M.), R01 CA73820-01 (E.J.B.), R01 CA90586-01 (to D.M.O.) from the National Cancer Institute, P50 AT000428 (E.J.B., A.M.) from the National Center for Complementary and Alternative Medicine, and by grants from the Brain Tumor Society (H-K. S., D.M.O.), American Brain Tumor Association (HK. S.), and Veterans Administration Review Program (D.M.O.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04–05–0374. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–05–0374.
References
- Abe, T., Terada, K., Wakimoto, H., Inoue, R., Tyminski, E., Bookstein, R., Basilion, J.P., and Chiocca, E.A. (2003). PTEN decreases in vivo vascularization of experimental gliomas in spite of proangiogenic stimuli. Cancer Res. 63, 2300-2305. [PubMed] [Google Scholar]
- Arbiser, J.L. et al. (1997). Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc. Natl. Acad. Sci. USA 94, 861-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsham, A.M., Plas, D.R., Thompson, C.B., and Simon, M.C. (2004). Akt and hypoxia-inducible factor-1 independently enhance tumor growth and angiogenesis. Cancer Res. 64, 3500-3507. [DOI] [PubMed] [Google Scholar]
- Bergers, G., and Benjamin, L.E. (2003). Angiogenesis: tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 3, 401-410. [DOI] [PubMed] [Google Scholar]
- Black, A.R., Black, J.D., and Azizkhan-Clifford, J. (2001). Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. J. Cell. Physiol. 188, 143-160. [DOI] [PubMed] [Google Scholar]
- Blume, S.W., Snyder, R.C., Ray, R., Thomas, S., Koller, C.A., and Miller, D.M. (1991). Mithramycin inhibits SP1 binding and selectively inhibits transcriptional activity of the dihydrofolate reductase gene in vitro and in vivo. J. Clin. Invest. 88, 1613-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogler, O., and Mikkelsen, T. (2003). Angiogenesis in glioma: molecular mechanisms and roadblocks to translation. Cancer J. 9, 205-213. [DOI] [PubMed] [Google Scholar]
- Brugarolas, J.B., Vazquez, F., Reddy, A., Sellers, W.R., and Kaelin, W.G., Jr. (2003). TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell 4, 147-158. [DOI] [PubMed] [Google Scholar]
- Cantley, L.C., and Neel, B.G. (1999). New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl. Acad. Sci. USA 96, 4240-4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam, J.D., Lebovitz, R.M., and Roeder, R.G. (1983). Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11, 1475-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hashemite, N., Walker, V., Zhang, H., and Kwiatkowski, D.J. (2003). Loss of Tsc1 or Tsc2 induces vascular endothelial growth factor production through mammalian target of rapamycin. Cancer Res. 63, 5173-5177. [PubMed] [Google Scholar]
- Feldkamp, M.M., Lau, N., Rak, J., Kerbel, R.S., and Guha, A. (1999). Normoxic and hypoxic regulation of vascular endothelial growth factor (VEGF) by astrocytoma cells is mediated by Ras. Int. J. Cancer 81, 118-124. [DOI] [PubMed] [Google Scholar]
- Ferrara, N. (2002). Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin. Oncol. 29, 10-14. [DOI] [PubMed] [Google Scholar]
- Finkenzeller, G., Sparacio, A., Technau, A., Marme, D., and Siemeister, G. (1997). Sp1 recognition sites in the proximal promoter of the human vascular endothelial growth factor gene are essential for platelet-derived growth factor-induced gene expression. Oncogene 15, 669-676. [DOI] [PubMed] [Google Scholar]
- Forsythe, J.A., Jiang, B.H., Iyer, N.V., Agani, F., Leung, S.W., Koos, R.D., and Semenza, G.L. (1996). Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 16, 4604-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille, J., Khalik, M., Konig, V., and Kaufmann, R. (1998). Hepatocyte growth factor/scatter factor (HGF/SF) induces vascular permeability factor (VPF/VEGF) expression by cultured keratinocytes. J. Invest. Dermatol. 111, 1160-1165. [DOI] [PubMed] [Google Scholar]
- Gille, J., Swerlick, R.A., and Caughman, S.W. (1997). Transforming growth factor-alpha-induced transcriptional activation of the vascular permeability factor (VPF/VEGF) gene requires AP-2-dependent DNA binding and transactivation. EMBO J. 16, 750-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Manzano, C. et al. (2003). Mechanisms underlying PTEN regulation of vascular endothelial growth factor and angiogenesis. Ann. Neurol. 53, 109-117. [DOI] [PubMed] [Google Scholar]
- Haas-Kogan, D., Shalev, N., Wong, M., Mills, G., Yount, G., and Stokoe, D. (1998). Protein kinase B (PKB/Akt) activity is elevated in glioblastoma cells due to mutation of the tumor suppressor PTEN/MMAC. Curr. Biol. 8, 1195-1198. [DOI] [PubMed] [Google Scholar]
- Hahn, W.C., Counter, C.M., Lundberg, A.S., Beijersbergen, R.L., Brooks, M.W., and Weinberg, R.A. (1999). Creation of human tumour cells with defined genetic elements. Nature 400, 464-468. [DOI] [PubMed] [Google Scholar]
- He, T.C., Zhou, S., da Costa, L.T., Yu, J., Kinzler, K.W., and Vogelstein, B. (1998). A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95, 2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, B.H., Zheng, J.Z., Aoki, M., and Vogt, P.K. (2000). Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc. Natl. Acad. Sci. USA 97, 1749-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez, J.C. et al. (2002). Histidine-proline-rich glycoprotein has potent antiangiogenic activity mediated through the histidine-proline-rich domain. Cancer Res. 62, 5344-5350. [PubMed] [Google Scholar]
- Knobbe, C.B., Merlo, A., and Reifenberger, G. (2002). Pten signaling in gliomas. Neuro-oncology 4, 196-211. [PMC free article] [PubMed] [Google Scholar]
- Koul, D., Shen, R., Garyali, A., Ke, L.D., Liu, T.J., and Yung, W.K. (2002). MMAC/PTEN tumor suppressor gene regulates vascular endothelial growth factor-mediated angiogenesis in prostate cancer. Int. J. Oncol. 21, 469-475. [PubMed] [Google Scholar]
- Laughner, E., Taghavi, P., Chiles, K., Mahon, P.C., and Semenza, G.L. (2001). HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol. Cell. Biol. 21, 3995-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity, A., Pore, N., Lee, J., Solomon, D., and O'Rourke, D.M. (2000). Epidermal growth factor receptor (EGFR) transcriptionally upregulates VEGF expression in human glioblastoma cells via a pathway involving PI(3) kinase and distinct from that induced by hypoxia. Cancer Res. 60, 5879-5886. [PubMed] [Google Scholar]
- Manley, P.W., Martiny-Baron, G., Schlaeppi, J.M., and Wood, J.M. (2002). Therapies directed at vascular endothelial growth factor. Expert Opin. Investig. Drugs 11, 1715-1736. [DOI] [PubMed] [Google Scholar]
- Mazure, N.M., Chen, E.Y., Laderoute, K.R., and Giaccia, A.J. (1997). Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood 90, 3322-3331. [PubMed] [Google Scholar]
- Mazure, N.M., Chen, E.Y., Yeh, P., Laderoute, K.R., and Giaccia, A.J. (1996). Oncogenic transformation and hypoxia synergistically act to modulate vascular endothelial growth factor expression. Cancer Res. 56, 3436-3440. [PubMed] [Google Scholar]
- Milanini, J., Vinals, F., Pouyssegur, J., and Pages, G. (1998). p42/p44 MAP kinase module plays a key role in the transcriptional regulation of the vascular endothelial growth factor gene in fibroblasts. J. Biol. Chem. 273, 18165-18172. [DOI] [PubMed] [Google Scholar]
- Milanini-Mongiat, J., Pouyssegur, J., and Pages, G. (2002). Identification of two Sp1 phosphorylation sites for p42/p44 mitogen-activated protein kinases: their implication in vascular endothelial growth factor gene transcription. J. Biol. Chem. 277, 20631-20639. [DOI] [PubMed] [Google Scholar]
- Nehls, M.C., Brenner, D.A., Gruss, H.J., Dierbach, H., Mertelsmann, R., and Herrmann, F. (1993). Mithramycin selectively inhibits collagen-alpha 1(I) gene expression in human fibroblast. J. Clin. Invest. 92, 2916-2921. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Paez, J., and Sellers, W.R. (2003). PI3K/PTEN/AKT pathway. A critical mediator of oncogenic signaling. Cancer Treat. Res. 115, 145-167. [PubMed] [Google Scholar]
- Pore, N., Liu, S., Haas-Kogan, D.A., O'Rourke, D.M., and Maity, A. (2003). PTEN mutation and epidermal growth factor receptor activation regulate vascular endothelial growth factor (VEGF) mRNA expression in human glioblastoma cells by transactivating the proximal VEGF promoter. Cancer Res. 63, 236-241. [PubMed] [Google Scholar]
- Rak, J., Mitsuhashi, Y., Sheehan, C., Tamir, A., Viloria-Petit, A., Filmus, J., Mansour, S.J., Ahn, N.G., and Kerbel, R.S. (2000). Oncogenes and tumor angiogenesis: differential modes of vascular endothelial growth factor up-regulation in ras-transformed epithelial cells and fibroblasts. Cancer Res. 60, 490-498. [PubMed] [Google Scholar]
- Rak, J., Yu, J.L., Kerbel, R.S., and Coomber, B.L. (2002). What do oncogenic mutations have to do with angiogenesis/vascular dependence of tumors? Cancer Res. 62, 1931-1934. [PubMed] [Google Scholar]
- Ryuto, M., Ono, M., Izumi, H., Yoshida, S., Weich, H.A., Kohno, K., and Kuwano, M. (1996). Induction of vascular endothelial growth factor by tumor necrosis factor alpha in human glioma cells. Possible roles of SP-1. J. Biol. Chem. 271, 28220-28228. [DOI] [PubMed] [Google Scholar]
- Schafer, G., Cramer, T., Suske, G., Kemmner, W., Wiedenmann, B., and Hocker, M. (2003). Oxidative stress regulates vascular endothelial growth factor-A gene transcription through Sp1- and Sp3-dependent activation of two proximal GC-rich promoter elements. J. Biol. Chem. 278, 8190-8198. [DOI] [PubMed] [Google Scholar]
- Sen, C.K., Khanna, S., Babior, B.M., Hunt, T.K., Ellison, E.C., and Roy, S. (2002). Oxidant-induced vascular endothelial growth factor expression in human keratinocytes and cutaneous wound healing. J. Biol. Chem. 277, 33284-33290. [DOI] [PubMed] [Google Scholar]
- Shi, Q. et al. (2001). Constitutive Sp1 activity is essential for differential constitutive expression of vascular endothelial growth factor in human pancreatic adenocarcinoma. Cancer Res. 61, 4143-4154. [PubMed] [Google Scholar]
- Shweiki, D., Itin, A., Soffer, D., and Keshet, E. (1992). Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359, 843-845. [DOI] [PubMed] [Google Scholar]
- Testa, J.R., and Bellacosa, A. (2001). AKT plays a central role in tumorigenesis. Proc. Natl. Acad. Sci. USA 98, 10983-10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treins, C., Giorgetti-Peraldi, S., Murdaca, J., Semenza, G.L., and Van Obberghen, E. (2002). Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J. Biol. Chem. 277, 27975-27981. [DOI] [PubMed] [Google Scholar]
- Udvadia, A.J., Rogers, K.T., Higgins, P.D., Murata, Y., Martin, K.H., Humphrey, P.A., and Horowitz, J.M. (1993). Sp-1 binds promoter elements regulated by the RB protein and Sp-1-mediated transcription is stimulated by RB coexpression. Proc. Natl. Acad. Sci. USA 90, 3265-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udvadia, A.J., Templeton, D.J., and Horowitz, J.M. (1995). Functional interactions between the retinoblastoma (Rb) protein and Sp-family members: superactivation by Rb requires amino acids necessary for growth suppression. Proc. Natl. Acad. Sci. USA 92, 3953-3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanco, I., and Sawyers, C.L. (2002). The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat. Rev. Cancer 2, 489-501. [DOI] [PubMed] [Google Scholar]
- Wankhade, S., Yu, Y., Weinberg, J., Tainsky, M.A., and Kannan, P. (2000). Characterization of the activation domains of AP-2 family transcription factors. J. Biol. Chem. 275, 29701-29708. [DOI] [PubMed] [Google Scholar]
- Wen, S., Stolarov, J., Myers, M.P., Su, J.D., Wigler, M.H., Tonks, N.K., and Durden, D.L. (2001). PTEN controls tumor-induced angiogenesis. Proc. Natl. Acad. Sci. USA 98, 4622-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, S.A., McGlade, C.J., and Guha, A. (2002). Phosphatidylinositol 3′-kinase and MAPK/ERK kinase 1/2 differentially regulate expression of vascular endothelial growth factor in human malignant astrocytoma cells. Neuro-oncology 4, 242-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X., Senechal, K., Neshat, M.S., Whang, Y.E., and Sawyers, C.L. (1998). The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc. Natl. Acad. Sci. USA 95, 15587-15591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, K.M., and Araki, M. (2001). Tumor suppressor PTEN: modulator of cell signaling, growth, migration and apoptosis. J. Cell Sci. 114, 2375-2382. [DOI] [PubMed] [Google Scholar]
- Zhong, H., Chiles, K., Feldser, D., Laughner, E., Hanrahan, C., Georgescu, M.M., Simons, J.W., and Semenza, G.L. (2000). Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 60, 1541-1545. [PubMed] [Google Scholar]
- Zundel, W. et al. (2000). Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 14, 391-396. [PMC free article] [PubMed] [Google Scholar]