Abstract
Objective
Experimental evidence supports an antineoplastic activity of marine omega-3 polyunsaturated fatty acids (ω-3 PUFAs; including eicosapentaenoic acid, docosahexaenoic acid, and docosapentaenoic acid). However, the influence of ω-3 PUFAs on colorectal cancer (CRC) survival is unknown.
Design
Within the Nurses’ Health Study and Health Professionals Follow-up Study, we prospectively studied CRC-specific and overall mortality in a cohort of 1,659 CRC patients according to intake of marine ω-3 PUFAs and its change after diagnosis.
Results
Higher intake of marine ω-3 PUFAs after CRC diagnosis was associated with lower risk of CRC-specific mortality (P for trend=0.03). Compared with patients who consumed less than 0.10 g/day of marine ω-3 PUFAs, those consuming at least 0.30 g/day had an adjusted hazard ratio for CRC-specific mortality of 0.59 (95% confidence interval [CI], 0.35 to 1.01). Patients who increased their marine ω-3 PUFA intake by at least 0.15 g/day after diagnosis had a hazard ratio of 0.30 (95% CI, 0.14 to 0.64, P for trend<0.001) for CRC deaths, compared with those who did not change or changed their intake by less than 0.02 g/day. No association was found between post-diagnostic marine ω-3 PUFA intake and all-cause mortality (P for trend=0.47).
Conclusion
High marine ω-3 PUFA intake after CRC diagnosis is associated with lower risk of CRC-specific mortality. Increasing consumption of marine ω-3 PUFAs after diagnosis may confer additional benefits to patients with CRC.
Keywords: prognosis, fish oil, lifestyle, nutrition
INTRODUCTION
Despite appreciable advances in treatment, colorectal cancer (CRC) still represents the third leading cause of cancer death in the United States, with about 49,700 individuals dying of the disease in 2015.[1] Substantial evidence indicates that dietary and lifestyle factors influence the likelihood of developing CRC,[2] but whether these risk factors impact survival of CRC remains largely unknown.[3] Understanding the role of modifiable indicators for prognosis is crucial to inform clinical practice and counseling to improve survival outcomes after cancer diagnosis.[4]
Marine omega-3 polyunsaturated fatty acids (ω-3 PUFAs), namely eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA), have been shown in laboratory studies to suppress tumor growth and angiogenesis, possibly through modulation of prostaglandin-endoperoxide synthase (PTGS) activity, alteration of cell surface receptor function, and regulation of gene expression.[5] Supplementation of ω-3 PUFAs has been reported to enhance antitumor effects of chemotherapeutic agents in CRC.[6, 7] Substantial, albeit inconsistent, evidence also suggests that ω-3 PUFAs can inhibit cancer-related cachexia by improving food intake, delaying the onset of anorexia, and preventing body weight loss.[8, 9] Therefore, it is plausible that intake of marine ω-3 PUFAs could provide an opportunity to improve survival among CRC patients.
Despite these data, to our knowledge no study has yet examined the association between intake of marine ω-3 PUFAs and survival of CRC patients. Therefore, we used data from two large prospective cohorts, the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS), to assess whether high intake of marine ω-3 PUFAs after CRC diagnosis was associated with lower mortality.
METHODS
Study population
Details about the NHS and HPFS have been described elsewhere.[10, 11] In brief, the NHS enrolled 121 700 US registered female nurses who were aged 30–55 years in 1976. The HPFS enrolled 51 529 US male health professionals who were aged 40–75 years in 1986. Similar follow-up procedures have been used in the two cohorts. Participants completed a mailed questionnaire inquiring about their medical history and lifestyle factors at baseline, and every two years thereafter. Dietary data were collected and updated using the food frequency questionnaires (FFQs) every four years. In the present analysis, we used 1984 for the NHS and 1986 for the HPFS as baseline, when we first collected detailed data on ω-3 PUFA intake. The follow-up rates have been 95.4% in the NHS and 95.9% in the HPFS for each of the questionnaires though 2010. This study was approved by the Institutional Review Board at the Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health.
Ascertainment of CRC cases
On each biennial follow-up questionnaire, participants were asked whether they had had a diagnosis of CRC during the previous 2 years. For participants who reported CRC diagnosis, we asked for their permission to acquire medical records and pathologic reports. Study physicians, blinded to exposure data, reviewed all medical records to confirm CRC diagnosis and to record the disease stage, histologic findings, and tumor location.[12] For nonresponders, we searched the National Death Index to identify deaths and to ascertain any CRC diagnosis that contributed to death or was a secondary diagnosis.[13] For CRC deaths, we requested permission from next-of-kin to review medical records. In this analysis, we included participants who were diagnosed with CRC throughout follow-up and completed the FFQ after diagnosis (N=994 in the NHS and 665 in the HPFS) (see the flow chart in Supplementary Figure 1).
Measurement of mortality
Most of the deaths were identified through family members or the postal system in response to the follow-up questionnaires. We also searched the names of persistent nonresponders in the National Death Index. The cause of death was assigned by study physicians blinded to exposure data. More than 96% of deaths have been identified using these methods.[13]
Assessment of marine ω-3 PUFA intake
Detailed description of ω-3 PUFA intake assessment has been reported previously,[14, 15] and provided in the Supplementary Material. In each FFQ, we asked participants how often, on average, they consumed each food of a standard portion size during the previous year. Nine response options were provided, ranging from “never or less than once per month” to “6 or more times per day”. We calculated the average daily intake for each nutrient by multiplying the reported frequency of consumption of each item by its nutrient content and then summing across from all foods. Use of fish oil supplement was also assessed and included in calculation of marine ω-3 PUFA intake, which was the sum of EPA, DHA and DPA consumption. We adjusted nutrient intake for total caloric intake using the nutrient residual method. FFQs have demonstrated good reproducibility and validity in assessing marine ω-3 PUFA intake,[16, 17] as described in the Supplementary Material.
Dietary intake reported on the first FFQ at least one year after diagnosis was used for post-diagnostic intake to avoid assessment during the period of active treatment. Categories of marine ω-3 PUFA intake (g/day) were predefined as <0.10, 0.10 to 0.19, 0.20 to 0.29, and 0.30 or more, consistent with prior analysis.[18] We also calculated the change of marine ω-3 PUFA intake by subtracting from the post-diagnostic intake the intake reported on the last FFQ before CRC diagnosis (pre-diagnosis intake).
Covariate assessments
We collected information on body height, weight, smoking status, and regular use of aspirin and non-steroidal anti-inflammatory drugs (NSAIDs) from each biennial questionnaire. We assessed mainly recreational or leisure-time physical activity using the validated questionnaire in 1980, 1982, 1986, 1988, 1992, and biennially thereafter in the NHS; and every two years in the HPFS. Physical activity was calculated by summing the products of time spent on a variety of activities with the average metabolic equivalent (MET) for that activity.[19]
Statistical analysis
We calculated person-time of follow-up from the return date of the FFQ that was used for post-diagnostic assessment to death, or the end of the study period (June 1, 2012 for the NHS, January 31, 2012 for the HPFS), whichever came first. In the main analysis, death from CRC was the primary end point, and deaths from other causes were censored. In secondary analyses, death from any cause was the end point.
We plotted the Kaplan-Meier curves and performed the log-rank tests across categories of marine ω-3 PUFA intake. Cox proportional hazards regression models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) of death, adjusted for marine ω-3 PUFA intake prior to CRC diagnosis and other potential predictors for cancer survival (see Table 1 and footnote of Table 2). We tested proportional hazards assumption by including the interaction term between marine ω-3 PUFA intake and time into the model, and did not find statistical evidence for violation of this assumption. We also stratified by lifestyle and clinicopathological factors, and calculated the HR of mortality per 1-standarad deviation increment (0.2 g/day) of marine ω-3 PUFA intake using the median intake of each category as a continuous variable. Test of interaction was performed using a likelihood ratio test by comparing the model with product terms between stratified covariate and marine ω-3 PUFAs to that without these terms. We used SAS 9.3 for all analyses (SAS Institute, Cary, NC). All statistical tests were two-sided and P<0.05 was considered statistically significant.
Table 1.
Basic characteristics of colorectal cancer patients at diagnosis according to post-diagnostic marine ω-3 polyunsaturated fatty acid intake*
| Variable | <0.10 g/day | 0.10–0.19 g/day | 0.20–0.29 g/day | ≥0.30 g/day |
|---|---|---|---|---|
| No. of participants (%) | 486 (29) | 358 (21) | 274 (17) | 541 (33) |
| Age, year | 69.7 | 68.7 | 66.9 | 68.7 |
| Height, inch | 66.8 | 66.8 | 66.9 | 66.9 |
| Body mass index, kg/m2 | 26.1 | 26.6 | 26.0 | 26.4 |
| Physical activity, MET-hours/week | 17.9 | 16.9 | 19.9 | 23.0 |
| Packyears of smoking | 18.1 | 18.2 | 16.2 | 15.9 |
| Current smokers, % | 7 | 7 | 6 | 5 |
| Multivitamin use, % | 58 | 56 | 58 | 67 |
| Fish oil use, % | 0 | 0 | 0 | 23 |
| Regular use of aspirin, %† | 38 | 43 | 43 | 39 |
| Menopausal hormone therapy, %‡ | 21 | 20 | 18 | 16 |
| Dietary consumption | ||||
| Alcohol, g/day | 7.3 | 8.2 | 8.4 | 8.5 |
| Total folate, µg/day | 653 | 658 | 653 | 773 |
| Calcium, mg/day | 1169 | 1107 | 1180 | 1244 |
| Vitamin D, IU/day | 475 | 487 | 541 | 677 |
| Total fiber, g/day | 20.5 | 20.6 | 21.8 | 22.7 |
| Processed red meat, serving/week | 1.9 | 1.9 | 1.9 | 1.6 |
| Poultry, serving/week | 2.2 | 2.7 | 3.1 | 3.1 |
| Total fish, serving/week | 0.5 | 1.3 | 1.9 | 2.8 |
| Dark fish, serving/week | 0.0 | 0.1 | 0.4 | 0.8 |
| Tuna, serving/week | 0.3 | 0.7 | 0.8 | 1.0 |
| Other fish, serving/week | 0.2 | 0.5 | 0.6 | 0.9 |
| Ω-6 polyunsaturated fatty acids, g/day | 10.8 | 10.7 | 10.6 | 11.3 |
| Cancer subsite, % | ||||
| Proximal colon | 42 | 43 | 40 | 45 |
| Distal colon | 29 | 32 | 31 | 31 |
| Rectum | 25 | 21 | 25 | 18 |
| Unspecified | 4 | 4 | 4 | 6 |
| Differentiation, % | ||||
| Well differentiated | 14 | 12 | 130 | 14 |
| Moderately differentiated | 62 | 59 | 61 | 55 |
| Poorly differentiated | 12 | 13 | 12 | 13 |
| Unspecified | 12 | 16 | 14 | 18 |
| Stage, % | ||||
| I | 35 | 33 | 31 | 34 |
| II | 30 | 30 | 26 | 31 |
| III | 21 | 23 | 26 | 20 |
| IV | 2 | 4 | 5 | 4 |
| Unspecified | 12 | 10 | 12 | 11 |
Abbreviations: MET, metabolic equivalent.
Means are calculated for continuous variables. All variables are age-standardized except age.
Regular users are defined as ≥2 standard (325-mg) tablets of aspirin per week.
Proportion of current postmenopausal hormone use is calculated among postmenopausal women only.
Table 2.
Post-diagnostic marine ω-3 polyunsaturated fatty acid intake and colorectal cancer-specific and all-cause mortality
| <0.10 g/day | 0.10–0.19 g/day | 0.20–0.29 g/day | ≥0.30 g/day | P for trend * | |
|---|---|---|---|---|---|
| Median intake (interquartile range) | 0.06 (0.04–0.08) | 0.14 (0.12–0.16) | 0.24 (0.22–0.27) | 0.49 (0.36–0.80) | |
| Person-years | 3,782 | 3,094 | 2,401 | 4,294 | |
| Colorectal cancer-specific mortality | |||||
| No. of deaths (n=169) | 55 | 47 | 25 | 42 | |
| Age-adjusted HR (95% CI) † | 1 (referent) | 0.99 (0.65–1.50) | 0.74 (0.44–1.24) | 0.65 (0.42–1.03) | 0.04 |
| Multivariable-adjusted HR (95% CI) ‡ | 1 (referent) | 0.98 (0.62–1.55) | 0.77 (0.44–1.37) | 0.59 (0.35–1.01) | 0.03 |
| All-cause mortality | |||||
| No. of deaths (n=561) | 164 | 135 | 85 | 177 | |
| Age-adjusted HR (95% CI) † | 1 (referent) | 1.09 (0.86–1.39) | 0.93 (0.71–1.23) | 0.96 (0.77–1.21) | 0.51 |
| Multivariable-adjusted HR (95% CI) ‡ | 1 (referent) | 1.12 (0.87–1.45) | 0.90 (0.67–1.21) | 0.95 (0.73–1.25) | 0.47 |
Abbreviation: CI, confidence interval; HR, hazard ratio.
P for trend was calculated using median intake for each category of marine ω-3 polyunsaturated fatty acid intake.
Cox proportional hazards regression model stratified by age groups at diagnosis (<60, 60–64, 65–69, 70–74, and ≥75 years), sex, and cancer stage (I, II, III, IV, and unspecified), with additional adjustment for age at diagnosis (continuous).
Further adjusted for pre-diagnostic intake of marine ω-3 polyunsaturated fatty acids (<0.10, 0.10–0.19, 0.20–0.29, and ≥0.3 g/day), grade of differentiation (1–3 and unspecified), subsite (proximal colon, distal colon, rectum and unspecified), pack-years of smoking (0, 1–15, 16–25, 26–45, >45), alcohol consumption (<0.15, 0.15–1.9, 2.0–7.4, ≥7.5 g/d), BMI (<23, 23–24.9, 25–27.4, 27.5–29.9, ≥30 kg/m2), physical activity (women: <5, 5–11.4, 11.5–21.9, ≥22 MET-hours/week; men: <7, 7–14.9, 15–24.9, ≥25 MET-hours/week), regular use of aspirin and NSAIDs (yes or no), postmenopausal hormone use (women only: never, current, past users), and intake of folate and vitamin D (in quartiles)
RESULTS
Basic characteristics of participants at diagnosis
Among 1,659 eligible participants with CRC, we documented 561 deaths, of which 169 were classified as CRC-specific deaths over a median of 10.4 years of follow-up. Other major causes of death included cardiovascular diseases (n=153) and other cancers than CRC (n=113). Participants with higher intake of marine ω-3 PUFAs were more likely to be physically active, to take multivitamins, to drink alcohol, and to consume more vitamin D and fiber, and were less likely to smoke (Table 1). Cancer subsite, differentiation, and stage did not differ across categories of marine ω-3 PUFA intake.
Marine ω-3 PUFA intake after diagnosis and survival
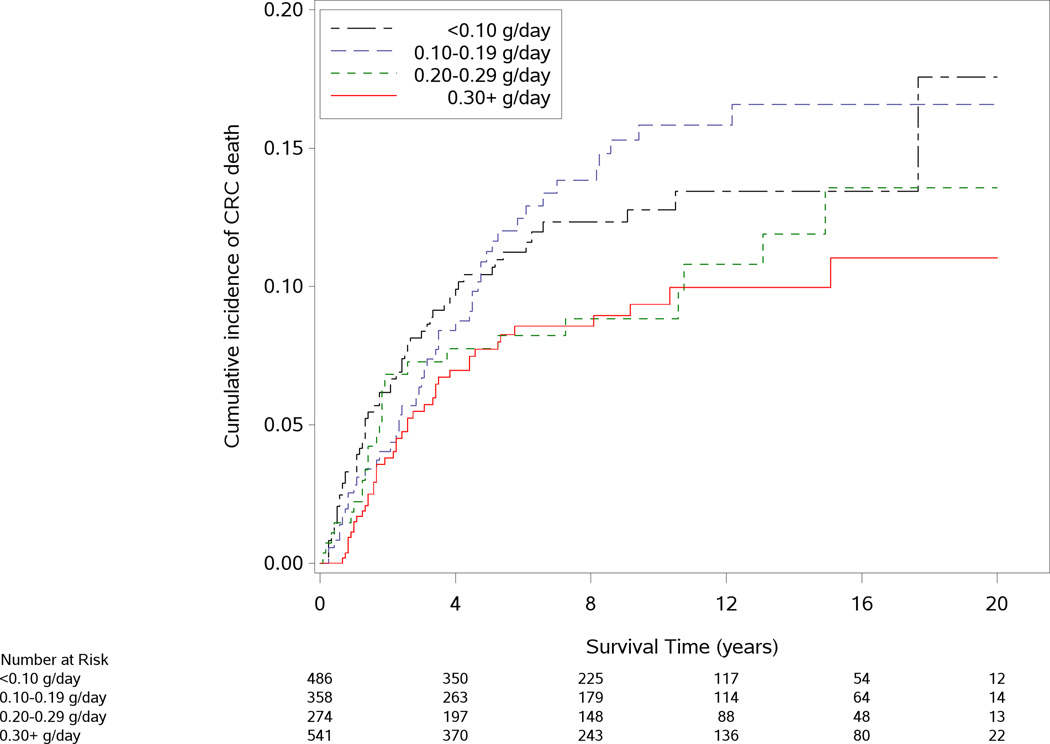
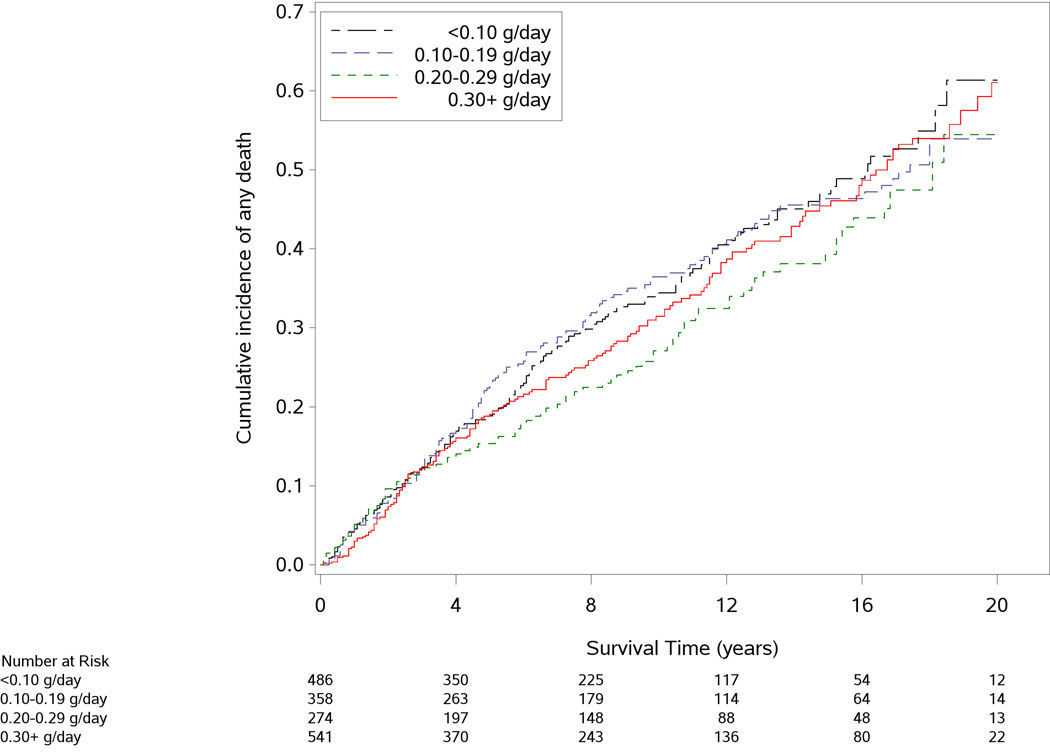
The median interval between CRC diagnosis and marine ω-3 PUFA assessment was 2.8 years (interquartile range: 2.0 to 3.9 years). As shown in Figure 1, participants who consumed higher amounts of marine ω-3 PUFAs after diagnosis tended to have a lower risk of CRC-specific mortality (P for log-rank test=0.02). In contrast, all-cause mortality did not appear to differ by categories of marine ω-3 PUFA intake (P=0.72).
Figure 1.
Cumulative incidence of colorectal cancer (CRC)-specific death (A) and all-cause death (B) according to post-diagnostic intake of marine ω-3 polyunsaturated fatty acids. P value for the log-rank test across categories of marine ω-3 polyunsaturated fatty acid intake was 0.02 for CRC-specific mortality and 0.72 for all-cause mortality.
Table 2 shows the HR estimates of mortality according to post-diagnostic intake of marine ω-3 PUFAs. Higher intake was associated with a dose-dependent reduction of CRC-specific mortality, even after adjusting for pre-diagnostic consumption and other potential determinants of survival (P for trend=0.03). Compared to patients who consumed less than 0.1 g/day, those who consumed at least 0.3 g/day of marine ω-3 PUFAs after CRC diagnosis had an HR for CRC-specific mortality of 0.59 (95% CI, 0.35–1.01). We did not find any statistically significant association with all-cause mortality (P for tend=0.47). We observed similar results between the NHS and HPFS cohorts (P for heterogeneity by cohort=0.23 for CRC-specific mortality and 0.30 for all-cause mortality; Supplementary Table 1).
When marine ω-3 PUFAs were assessed according to dietary sources, those derived from foods and supplements both showed an inverse association with CRC-specific mortality, although the statistical power was limited for the analysis of supplemental fish oil due to low prevalence of use (Supplementary Table 2). Participants who consumed marine ω-3 PUFAs of at least 0.3 g/day from foods had an HR of 0.60 (95% CI, 0.35–1.04) compared to those who consumed less than 0.10 g/day (P for trend=0.06). Fish oil users had an HR of 0.63 (95% CI, 0.24–1.71) compared to non-users.
To test the possibility that exclusion of patients who did not complete post-diagnostic FFQs due to early death, severe illness or CRC recurrence may have biased our results, we restricted our analysis to the 1,293 participants who completed their FFQs within 4 years after diagnosis. The results were similar with a HR for CRC-specific mortality of 0.65 (95% CI, 0.35–1.19, P for tend=0.08) comparing the highest to the lowest categories of marine ω-3 PUFA intake. In addition, to minimize bias associated with occult recurrences or other undiagnosed major illnesses which could influence dietary intake, we excluded 69 patients who died within one year after their post-diagnostic dietary assessment. Although statistical power was somewhat diminished, participants in the highest category of intake had an HR of 0.76 (95% CI, 0.41–1.40) for CRC-specific mortality compared to those with the lowest intake.
Marine ω-3 PUFA intake and survival within subgroups
In an exploratory analysis, we examined the influence of post-diagnostic marine ω-3 PUFA intake across strata of other predictors of cancer recurrence and mortality (Supplementary Figure 2). For CRC-specific mortality, we found a statistically significant interaction between marine ω-3 PUFAs and height (P=0.01), and the inverse association of marine ω-3 PUFA intake with mortality was stronger among tall participants. For all-cause mortality, the association with marine ω-3 PUFA intake differed by height, BMI, and regular use of aspirin (P for interaction=0.003, 0.01, and 0.06, respectively). There appeared to be an inverse association among participants who were tall, had a BMI of <25 kg/m2, or did not regularly take aspirin, with adjusted HRs of 0.85 (95% CI, 0.74–0.99), 0.90 (95% CI, 0.79–1.02) and 0.88 (95% CI, 0.76–1.03) per 0.2 g/day increment of marine ω-3 PUFA intake, respectively. However, given the limited sample size and multiple comparisons conducted, these results should be interpreted cautiously.
The association between marine ω-3 PUFA intake and mortality did not differ across tumor subsites, differentiation levels, and stages. We also performed a sensitivity analysis by excluding 56 patients with stage IV cancers. The results were essentially unchanged (data not shown).
Change in marine ω-3 PUFA intake and survival
The correlation between pre- and post-diagnostic intake of marine ω-3 PUFAs was modest (correlation coefficient, 0.50; P<0.001). We assessed whether changing marine ω-3 PUFA intake after diagnosis was associated with mortality (Table 3). Compared to participants who did not appreciably alter their intake (amount of change <0.02 g/day), those who increased intake by at least 0.15 g/day had an HR of 0.30 for CRC-specific mortality (95% CI, 0.14–0.64), whereas those who decreased their intake by the same amount had an HR of 1.10 (95% CI, 0.59–2.08) (P for trend<0.001). Similar pattern was found for all-cause mortality (P for trend=0.03), and the corresponding HRs were 0.87 (95% CI, 0.62–1.21) and 1.21 (95% CI, 0.86–1.69), respectively.
Table 3.
Change in marine ω-3 polyunsaturated fatty acid intake after diagnosis and colorectal cancer-specific and all-cause mortality
| Decrease of ≥0.15 g/day |
Decrease of 0.02– 0.14 g/day |
Change of <0.02 g/day |
Increase of 0.02– 0.14 g/day |
Increase of ≥0.15 g/day |
P for trend * |
|
|---|---|---|---|---|---|---|
| Median intake (interquartile range) | −0.25 (−0.41, −0.20) | −0.06 (−0.10, −0.03) | 0 (−0.01, 0.01) | 0.06 (0.04, 0.10) | 0.37 (0.20, 0.62) | |
| Person-years | 2,271 | 3,670 | 2,208 | 2,945 | 2,477 | |
| Colorectal cancer-specific mortality | ||||||
| No. of deaths (n=169) | 36 | 51 | 35 | 34 | 13 | |
| Age-adjusted HR (95% CI) † | 1.02 (0.55–1.89) | 1.02 (0.63–1.63) | 1 (referent) | 0.96 (0.57–1.61) | 0.28 (0.13–0.57) | <0.001 |
| Multivariable-adjusted HR (95% CI) ‡ | 1.10 (0.59–2.08) | 1.16 (0.71–1.91) | 1 (referent) | 1.10 (0.64–1.89) | 0.30 (0.14–0.64) | <0.001 |
| All-cause mortality | ||||||
| No. of deaths (n=561) | 108 | 161 | 88 | 127 | 77 | |
| Age-adjusted HR (95% CI) † | 1.26 (0.91–1.75) | 1.25 (0.95–1.65) | 1 (referent) | 1.24 (0.93–1.65) | 0.82 (0.59–1.13) | 0.005 |
| Multivariable-adjusted HR (95% CI) ‡ | 1.21 (0.86–1.69) | 1.23 (0.93–1.63) | 1 (referent) | 1.21 (0.90–1.62) | 0.87 (0.62–1.21) | 0.03 |
Abbreviation: CI, confidence interval; HR, hazard ratio.
P for trend was calculated using median intake for each category of marine ω-3 polyunsaturated fatty acid intake.
Cox proportional hazards regression model stratified by age groups at diagnosis (<60, 60–64, 65–69, 70–74, and ≥75 years), sex, and cancer stage (I, II, III, IV, and unspecified) with additional adjustment for age at diagnosis (continuous).
Further adjusted for pre-diagnostic intake of marine ω-3 polyunsaturated fatty acids (continuous), grade of differentiation (1–3 and unspecified), subsite (proximal colon, distal colon, rectum and unspecified), pack-years of smoking (0, 1–15, 16–25, 26–45, >45), alcohol consumption (<0.15, 0.15–1.9, 2.0–7.4, ≥7.5 g/d), BMI (<23, 23–24.9, 25–27.4, 27.5–29.9, ≥30 kg/m2), physical activity (women: <5, 5–11.4, 11.5–21.9, ≥22 MET–hours/week; men: <7, 7–14.9, 15–24.9, ≥25 MET-hours/week), regular use of aspirin and NSAIDs (yes or no), postmenopausal hormone use (women only: never, current, past users), and intake of folate and vitamin D (in quartiles).
DISCUSSION
Higher intake of marine ω-3 PUFAs after CRC diagnosis was associated with lower risk of CRC-specific mortality. CRC patients who increased their intake from their levels before diagnosis experienced a substantial reduction in CRC-specific mortality and a moderate reduction in all-cause mortality. Our findings provide novel evidence for the potential benefit of increasing marine ω-3 PUFA consumption among CRC patients.
Due to the high incidence rate as well as improved diagnosis and treatment, CRC represents the second most prevalent cancer in the US. More than 1.2 million Americans are living with a diagnosis of CRC, among whom 64.9% live more than 5 years and 58.3% live more than 10 years.[20] Many of these cancer survivors are highly motivated to seek information about lifestyle changes to improve their prognosis. However, the evidence is limited for the influence of modifiable lifestyle factors on CRC survival.
Marine ω-3 PUFAs have demonstrated anti-CRC activity in animal and in vitro studies.[5] EPA and DHA treatment has been shown to reduce cellular proliferation and increase apoptosis of human CRC cells. A consistent 40–60% reduction in size of xenograft CRC tumor has been observed in rodents supplemented with ω-3 PUFAs compared with controls.[21, 22] As an alternative substrate for PTGS2 (cyclooxygenase-2 [COX-2]), marine ω-3 PUFAs may compete with arachidonic acid and reduce the production of pro-tumorigenic prostaglandin (PG) E2.[23, 24] Furthermore, incorporation of ω-3 PUFAs into cell phospholipid membranes changes the fluidity, structure and function of lipid rafts, resulting in altered downstream signaling by cell surface receptors, such as epidermal growth factor receptor.[25, 26] Moreover, ω-3 PUFAs are highly peroxidizable and increase levels of intracellular reactive oxygen species (ROS), a byproduct of cell growth. Although moderately increased levels of ROS damage DNA and promote mutagenesis in cells, recent evidence indicates that high ROS levels exert an oxidative stress that can restrain tumor progression and metastasis by causing cell senescence or death.[27, 28]
Several lines of evidence also support a beneficial effect of marine ω-3 PUFAs on cancer survival. In a randomized controlled trial (RCT) of 60 patients, supplementation of marine ω-3 PUFAs restored the decreased ratio of T-helper cells to T-suppressor cells and prolonged the survival of cancer patients.[29] Recently, a Phase II RCT showed that oral administration of EPA as the free fatty acid 2 g daily prior to surgery resulted in an increased content of EPA in tumor tissue, and reduced vascularity and mortality among patients with CRC cell liver metastasis.[30] Moreover, ω-3 PUFAs have been shown to potentiate the cytotoxicity of anti-neoplastic agents by overcoming multiple drug resistance and promoting an oxidative environment toxic to highly proliferative tumor cells.[31] In addition, ω-3 PUFAs have been suggested to have effects on mitigating cancer cachexia, partly due to its suppressive activity against inflammation and proteolysis, although current evidence remains inconsistent.[32] A recent cohort study reported that an increase in fish oil supplementation over 24 months after diagnosis was associated with improved physical functioning among stage II CRC patients.[33]
Consistent with these data, we found that patients who consumed higher marine ω-3 PUFAs after diagnosis had substantially lower risk of death from CRC. Although post-diagnostic marine ω-3 PUFA intake was not associated with overall mortality, patients who increased their intake from the levels before diagnosis demonstrated a moderate reduction in all-cause mortality. Moreover, we noted a potential benefit of higher marine ω-3 PUFAs for overall mortality among individuals who were tall, had a BMI of <25 kg/m2, or did not regularly use aspirin, although the possibility for chance findings cannot be excluded. Fatty acid composition and concentration have been shown to regulate growth hormone secretion,[22] and genetic variations in ω-3 PUFA metabolism have been associated with body height and weight.[34] Previous studies have also reported that ω-3 PUFA may have a stronger anti-CRC effect among individuals who do not regularly use aspirin,[35] an anti-inflammatory agent that shares antitumor pathways with ω-3 PUFA and has been proposed as a promising chemopreventive agent for CRC.[36, 37] Given these preliminary data, further studies are needed to investigate whether marine ω-3 PUFAs interact with metabolic factors and aspirin to influence CRC development and progression.
Strengths of the current study include the prospective design, detailed collection of pre- and post-diagnostic data, comprehensive medical record review of both CRC diagnosis and death, and long-term follow-up. Some limitations are worth noting. First, data on cancer recurrence were unavailable. Nevertheless, because the median survival for metastatic CRC was approximately 10 to 12 months during much of the period of this study,[38] CRC-specific mortality should be a reasonable surrogate for cancer-specific outcomes. Second, treatment data are not collected in the cohorts. However, about 60% of patients had stage I or stage II disease, in which surgery alone would generally be the standard of care, and no interaction by disease stage was observed. Furthermore, although there are differences in the likelihood of use of adjuvant chemotherapy based on the factors such as socioeconomic status, the fairly homogenous nature of participants (health professionals) would likely increase the probability of at least standard therapy.[39, 40] Comorbidities and access to health care may also confound our findings; however, given the population studied, we would expect the latter to be diminished. Moreover, although comorbidities have may influence overall survival,[41, 42] such diseases are less likely to affect CRC-specific mortality,[43] the primary endpoint of this study. In addition, only a fraction of patients providing post-diagnosis data were included in this study. Therefore, both the statistical power and generalizability of our findings were limited. Further studies are needed in a larger population. Finally, we cannot exclude the possibility of residual confounding from other dietary or lifestyle factors. However, our results are robust to adjustment for multiple major risk factors of mortality.
In conclusion, marine ω-3 PUFA intake after diagnosis may lower the risk of CRC-specific mortality. Increasing consumption of marine ω-3 PUFAs after diagnosis may confer additional benefits to patients with CRC.
Supplementary Material
SUMMARY.
What is already known about this subject?
Marine omega-3 polyunsaturated fatty acids (ω-3 PUFAs) have been associated with lower risk of colorectal cancer (CRC).
Marine ω-3 PUFAs suppress tumor growth and angiogenesis.
Supplementation of ω-3 PUFAs enhances antitumor effects of chemotherapeutic agents in CRC and inhibits cancer-related cachexia.
What are the new findings?
Higher intake of marine ω-3 PUFAs after CRC diagnosis was associated with lower risk of CRC-specific mortality.
Patients who increased their marine ω-3 PUFA intake after diagnosis had a lower risk of death from CRC, compared with those who did not change.
How might it impact on clinical practice in the foreseeable future?
Our findings provide the first line of population-based evidence for the benefit of marine ω-3 PUFAs on CRC survival.
If replicated by other studies, our results support the clinical recommendation of increasing marine ω-3 PUFAs among patients with CRC.
Acknowledgments
FUNDING
This work was supported by U.S. National Institutes of Health (NIH) grants [P01 CA87969 to M.J. Stampfer; UM1 CA186107 to M.J. Stampfer; P01 CA55075 to W.C. Willett; UM1 CA167552 to W.C. Willett; P50 CA127003 to C.S.F.; K24 DK098311, R01 CA137178 to A.T.C.; R01 CA151993, R35 CA197735 to S.O.; R03 CA17671, K07 CA188126 to X.Z.]; and by grants from the Project P Fund for Colorectal Cancer Research, The Friends of the Dana-Farber Cancer Institute, Bennett Family Fund, and the Entertainment Industry Foundation through National Colorectal Cancer Research Alliance. The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Andrew T. Chan previously served as a consultant for Bayer Healthcare, Pozen Inc, and Pfizer Inc. for work unrelated to the topic of this manuscript. This study was not funded by Bayer Healthcare, Pozen Inc, or Pfizer Inc.
We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-up Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Abbreviations
- CI
confidence interval
- COX-2
cyclooxygenase-2
- CRC
colorectal cancer
- DHA
docosahexaenoic acid
- DPA
docosapentaenoic acid
- EPA
eicosapentaenoic acid
- FFQ
food frequency questionnaire
- HPFS
Health Professionals Follow-up Study
- HR
hazard ratio
- MET
metabolic equivalent
- NHS
Nurses’ Health Study
- NSAID
non-steroidal anti-inflammatory drug
- ω-3 PUFA
omega-3 polyunsaturated fatty acid
- PG
prostaglandin
- PTGS
prostaglandin-endoperoxide synthase
- RCT
randomized controlled trial
- ROS
reactive oxygen species
Footnotes
COMPETING INTERESTS
No other conflict of interest exists.
AUTHOR CONTRIBUTIONS
Drs Song and Chan have full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: M.S., X.Z., J.A.M., E.L.G., A.T.C.
Acquisition of data: M.S., X.Z., J.A.M., E.L.G., S.O., C.S.F., A.T.C.
Analysis and interpretation of data: M.S., J.A.M., E.L.G., C.S.F., A.T.C.
Drafting of the manuscript: M.S.
Critical revision of the manuscript for important intellectual content: M.S., X.Z., J.A.M., E.L.G., S.O., C.S.F., A.T.C.
Statistical analysis: M.S.
Obtained funding: X.Z., E.L.G., S.O., C.S.F., A.T.C.
Administrative, technical, or material support: E.L.G., S.O., C.S.F., A.T.C.
Study supervision: A.T.C.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148:1244–1260. e16. doi: 10.1053/j.gastro.2014.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Blarigan EL, Meyerhardt JA. Role of physical activity and diet after colorectal cancer diagnosis. J Clin Oncol. 2015;33:1825–1834. doi: 10.1200/JCO.2014.59.7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doyle C, Kushi LH, Byers T, Courneya KS, Demark-Wahnefried W, Grant B, et al. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J Clin. 2006;56:323–353. doi: 10.3322/canjclin.56.6.323. [DOI] [PubMed] [Google Scholar]
- 5.Cockbain AJ, Toogood GJ, Hull MA. Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut. 2012;61:135–149. doi: 10.1136/gut.2010.233718. [DOI] [PubMed] [Google Scholar]
- 6.Calviello G, Di Nicuolo F, Serini S, Piccioni E, Boninsegna A, Maggiano N, et al. Docosahexaenoic acid enhances the susceptibility of human colorectal cancer cells to 5-fluorouracil. Cancer chemotherapy and pharmacology. 2005;55:12–20. doi: 10.1007/s00280-004-0846-6. [DOI] [PubMed] [Google Scholar]
- 7.Gelsomino G, Corsetto PA, Campia I, Montorfano G, Kopecka J, Castella B, et al. Omega 3 fatty acids chemosensitize multidrug resistant colon cancer cells by down-regulating cholesterol synthesis and altering detergent resistant membranes composition. Mol Cancer. 2013;12:137. doi: 10.1186/1476-4598-12-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCowen KC, Bistrian BR. Immunonutrition: problematic or problem solving? Am J Clin Nutr. 2003;77:764–770. doi: 10.1093/ajcn/77.4.764. [DOI] [PubMed] [Google Scholar]
- 9.Colomer R, Moreno-Nogueira JM, Garcia-Luna PP, Garcia-Peris P, Garcia-de-Lorenzo A, Zarazaga A, et al. N-3 fatty acids, cancer and cachexia: a systematic review of the literature. Br J Nutr. 2007;97:823–831. doi: 10.1017/S000711450765795X. [DOI] [PubMed] [Google Scholar]
- 10.Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–468. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 11.Colditz GA, Manson JE, Hankinson SE. The Nurses' Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6:49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 12.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. Springer; 2010. [Google Scholar]
- 13.Stampfer MJ, Willett WC, Speizer FE, Dysert DC, Lipnick R, Rosner B, et al. Test of the National Death Index. Am J Epidemiol. 1984;119:837–839. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 14.Iso H, Rexrode KM, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, et al. Intake of fish and omega-3 fatty acids and risk of stroke in women. Jama. 2001;285:304–312. doi: 10.1001/jama.285.3.304. [DOI] [PubMed] [Google Scholar]
- 15.Song M, Chan AT, Fuchs CS, Ogino S, Hu FB, Mozaffarian D, et al. Dietary intake of fish, omega-3 and omega-6 fatty acids and risk of colorectal cancer: A prospective study in U.S. men and women. Int J Cancer. 2014 doi: 10.1002/ijc.28878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garland M, Sacks FM, Colditz GA, Rimm EB, Sampson LA, Willett WC, et al. The relation between dietary intake and adipose tissue composition of selected fatty acids in US women. Am J Clin Nutr. 1998;67:25–30. doi: 10.1093/ajcn/67.1.25. [DOI] [PubMed] [Google Scholar]
- 17.Hunter DJ, Rimm EB, Sacks FM, Stampfer MJ, Colditz GA, Litin LB, et al. Comparison of measures of fatty acid intake by subcutaneous fat aspirate, food frequency questionnaire, and diet records in a free-living population of US men. Am J Epidemiol. 1992;135:418–427. doi: 10.1093/oxfordjournals.aje.a116302. [DOI] [PubMed] [Google Scholar]
- 18.Song M, Nishihara R, Wu K, Qian ZR, Kim SA, Sukawa Y, et al. Marine omega-3 polyunsaturated fatty acids and risk of colorectal cancer according to microsatellite instability. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7:81–86. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 20.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 21.Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 22.Quabbe HJ, Bratzke HJ, Siegers U, Elban K. Studies on the relationship between plasma free fatty acids and growth hormone secretion in man. J Clin Invest. 1972;51:2388–2398. doi: 10.1172/JCI107051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogino S, Nosho K, Kirkner GJ, Kawasaki T, Meyerhardt JA, Loda M, et al. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58:90–96. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanamala J, Glagolenko A, Yang P, Carroll RJ, Murphy ME, Newman RA, et al. Dietary fish oil and pectin enhance colonocyte apoptosis in part through suppression of PPARdelta/PGE2 and elevation of PGE3. Carcinogenesis. 2008;29:790–796. doi: 10.1093/carcin/bgm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CDC National Center for Health Statistics. National Health and Nutrition Examination Survey. [Accessed November 3, 2015];2011 http://wwwn.cdc.gov/nchs/nhanes/search/nhanes11_12.aspx.
- 26.Yaqoob P. The nutritional significance of lipid rafts. Annu Rev Nutr. 2009;29:257–282. doi: 10.1146/annurev-nutr-080508-141205. [DOI] [PubMed] [Google Scholar]
- 27.Song M, Nishihara R, Wang M, Chan AT, Qian ZR, Inamura K, et al. Plasma 25-hydroxyvitamin D and colorectal cancer risk according to tumour immunity status. Gut. 2016;65:296–304. doi: 10.1136/gutjnl-2014-308852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenq RR, Ubeda C, Taur Y, Menezes CC, Khanin R, Dudakov JA, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012;209:903–911. doi: 10.1084/jem.20112408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gogos CA, Ginopoulos P, Salsa B, Apostolidou E, Zoumbos NC, Kalfarentzos F. Dietary omega-3 polyunsaturated fatty acids plus vitamin E restore immunodeficiency and prolong survival for severely ill patients with generalized malignancy: a randomized control trial. Cancer. 1998;82:395–402. doi: 10.1002/(sici)1097-0142(19980115)82:2<403::aid-cncr21>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 30.Cockbain AJ, Volpato M, Race AD, Munarini A, Fazio C, Belluzzi A, et al. Anticolorectal cancer activity of the omega-3 polyunsaturated fatty acid eicosapentaenoic acid. Gut. 2014;63:1760–1768. doi: 10.1136/gutjnl-2013-306445. [DOI] [PubMed] [Google Scholar]
- 31.Guarner F, Perdigon G, Corthier G, Salminen S, Koletzko B, Morelli L. Should yoghurt cultures be considered probiotic? Br J Nutr. 2005;93:783–786. doi: 10.1079/bjn20051428. [DOI] [PubMed] [Google Scholar]
- 32.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature. 2012;487:104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mujico JR, Baccan GC, Gheorghe A, Diaz LE, Marcos A. Changes in gut microbiota due to supplemented fatty acids in diet-induced obese mice. Br J Nutr. 2013;110:711–720. doi: 10.1017/S0007114512005612. [DOI] [PubMed] [Google Scholar]
- 35.Hall MN, Campos H, Li H, Sesso HD, Stampfer MJ, Willett WC, et al. Blood levels of long-chain polyunsaturated fatty acids, aspirin, and the risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:314–321. doi: 10.1158/1055-9965.EPI-06-0346. [DOI] [PubMed] [Google Scholar]
- 36.Bibbins-Domingo K. Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016 doi: 10.7326/M16-0577. [DOI] [PubMed] [Google Scholar]
- 37.Drew DA, Cao Y, Chan AT. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer. 2016;16:173–186. doi: 10.1038/nrc.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Sung CY, Lee N, Ni Y, Pihlajamaki J, Panagiotou G, et al. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1518189113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirata A, Kishino S, Park SB, Takeuchi M, Kitamura N, Ogawa J. A novel unsaturated fatty acid hydratase toward C16 to C22 fatty acids from Lactobacillus acidophilus. J Lipid Res. 2015;56:1340–1350. doi: 10.1194/jlr.M059444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kishino S, Takeuchi M, Park SB, Hirata A, Kitamura N, Kunisawa J, et al. Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. Proc Natl Acad Sci U S A. 2013;110:17808–17813. doi: 10.1073/pnas.1312937110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Druart C, Bindels LB, Schmaltz R, Neyrinck AM, Cani PD, Walter J, et al. Ability of the gut microbiota to produce PUFA-derived bacterial metabolites: Proof of concept in germ-free versus conventionalized mice. Mol Nutr Food Res. 2015;59:1603–1613. doi: 10.1002/mnfr.201500014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Druart C, Neyrinck AM, Vlaeminck B, Fievez V, Cani PD, Delzenne NM. Role of the lower and upper intestine in the production and absorption of gut microbiota-derived PUFA metabolites. PLoS One. 2014;9:e87560. doi: 10.1371/journal.pone.0087560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakurama H, Kishino S, Mihara K, Ando A, Kita K, Takahashi S, et al. Biohydrogenation of C20 polyunsaturated fatty acids by anaerobic bacteria. J Lipid Res. 2014;55:1855–1863. doi: 10.1194/jlr.M045450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.