Abstract
Eutrophication and climate change are increasing the incidence of severe hypoxia in fish nursery habitats, yet the programming effects of hypoxia on stress responsiveness in later life are poorly understood. In this study, to investigate whether early hypoxia alters the developmental trajectory of the stress response, zebrafish embryos were exposed to 4 h of anoxia at 36 h post-fertilization and reared to adults when the responses to secondary stressors were assessed. While embryonic anoxia did not affect basal cortisol levels or the cortisol response to hypoxia in later life, it had a marked effect on the responses to a social stressor. In dyadic social interactions, adults derived from embryonic anoxia initiated more chases, bit more often, entered fewer freezes and had lower cortisol levels. Adults derived from embryonic anoxia also performed more bites towards their mirror image, had lower gonadal aromatase gene expression and had higher testosterone levels. We conclude that acute embryonic anoxia has long-lasting consequences for the hormonal and behavioural responses to social interactions in zebrafish. Specifically, we demonstrate that acute embryonic anoxia favours the development of a dominant and aggressive phenotype, and that a disruption in sex steroid production may contribute to the programming effects of environmental hypoxia.
Keywords: developmental programming, hypoxia, stress response, aggression, dyadic interactions, zebrafish
1. Introduction
Developmental plasticity or fetal programming is the phenomenon where the developmental trajectory of an organism is altered by their early environment. If a stressor occurs during a critical window of sensitivity in development, then it can have lifelong impacts on the behaviour and physiology of an organism. For example, prenatal stress can alter the function of the hypothalamic–pituitary–adrenal (HPA) or –interrenal (HPI) axis [1]. This neuroendocrine system is responsible for the synthesis and release of glucocorticoids, which mobilize energy stores and restore homeostasis during stress [2]. Prenatal stress can change baseline glucocorticoid hormone and brain-receptor levels, resulting in altered stress sensitivity later in life [3–5]. However, the magnitude and direction of these secondary responses are highly variable and depend greatly on the timing of the initial stressor [4]. There is also evidence from various animal models that early stressors can impact anxiety, aggression and social behaviours [6]. While both functional HPA/I axis and behavioural changes often occur downstream of prenatal stress, in general the neurobiological mechanisms that mediate such effects and whether these behavioural changes are due to underlying alterations in stress-hormone synthesis remain largely unknown.
Hypoxia has become an increasingly relevant stressor for aquatic organisms worldwide [7]. Incidents of hypoxia and total anoxia are increasing in aquatic ecosystems, with young developing organisms being particularly sensitive to these stressors as they are confined within a chorion or egg mass [8]. In fish, early O2 deprivation can drastically alter adult phenotype, and has been linked to differential morphology, physiology and behaviour [9,10]. For example, zebrafish (Danio rerio) reared in chronic hypoxia are less aggressive than their normoxia-reared counterparts when tested in normoxic conditions [11]. The majority of these studies have focused exclusively on the impacts of chronic hypoxia exposure throughout development. However, in many ecosystems, fish embryos are more likely to encounter fluctuating levels of dissolved O2 (DO), and thus experience acute bouts of hypoxia or anoxia [7]. These periods of limited mobility also correspond to the development of the endocrine stress axis as many fish species cannot synthesize cortisol in response to environmental stressors until after hatching [12]. It is currently unknown if early O2 deprivation can impact the formation of the HPI axis, particularly if it occurs before fish can mount a glucocorticoid-mediated stress response.
We have isolated a critical developmental window of hypoxia and anoxia sensitivity in zebrafish embryos. Exposure to just 4 h of anoxia at 36 h post-fertilization (hpf) increases the larval hypoxia tolerance of zebrafish and leads to male-biased populations of adult fish (approx. 70% male) [13]. These exposures occur well before the HPI axis is stress responsive at 3–4 days post-fertilization (dpf) [12]. In rainbow trout embryos, acute temperature and handling stress prior to the development of the HPI axis results in a muted cortisol response to secondary stressors at five months of age [3]. Therefore, we tested the hypothesis that acute anoxia exposure at 36 hpf would alter the developmental trajectory of the stress response in zebrafish embryos. To test this hypothesis, zebrafish embryos were exposed to 4 h of anoxia at 36 hpf, then reared in normoxic conditions to sexually mature adults when their cortisol response to an environmental (hypoxia) or social (dyadic interaction) secondary stressor was assessed. We further hypothesized that these underlying changes in the endocrine stress response would impact the stress coping and aggression behaviour of adult fish.
2. Material and methods
(a). Animals
Zebrafish were raised in the Hagen Aqualab (University of Guelph) on a 14 L : 10 D photoperiod. For each experiment, fertilized eggs from four to five breeding baskets were pooled to increase genetic diversity and fish raised to adulthood originated from 10 separate breeding events. Embryos were raised at 28.5°C in egg water up to 5 dpf. After 5 dpf, fry were transferred into 2 l tanks, maintained at 27°C and fed fry powder (Argent, Redmond, WA, USA) twice daily until one month of age. Fish were then fed twice daily with brine shrimp and flake food. At four months of age, like-treated fish were condensed into 4 l tanks to ensure even density between tanks and to prevent within tank hierarchy formation. Densities were 40 ± 10 and 43 ± 11 fish per tank for the anoxia- and normoxia-exposed fish, respectively.
(b). Embryonic anoxia exposure
Embryos at 36 hpf were exposed to one of two O2 regimes for 4 h: a normoxic treatment (95% DO or 7.2 mg l−1) and an anoxic treatment (less than 0.5% DO or 0.04 mg l−1). DO was controlled by regulating delivery of N2 gas or air through ceramic air stones into 20 l tanks and monitored using an O2 electrode (liquid-dissolved O2 field probe, Hach, Loveland, CO, USA). Both treatments were allowed to recover in normoxia and raised to sexual maturity (six months post-fertilization). Survival was closely monitored at a regular interval and did not differ between treatments at any time point (p = 0.963).
(c). Adult hypoxia exposure
We determined the effects of embryonic anoxia exposure on subsequent basal and hypoxia-induced whole-body cortisol levels in adult zebrafish. Briefly, 56 adult zebrafish of either sex (half from the normoxia and anoxia embryonic treatments) were acclimated in groups for 3 days to 4 l tanks (eight tanks total; n = 7 per tank) that contained an air stone and a flow-through barrier placed 3 cm below the water surface to prevent aquatic surface respiration. All fish were fed twice daily until the day of exposure. Half of the fish from each embryonic treatment were exposed to hypoxia, and the other half were left undisturbed until all animals were euthanized. In the hypoxia groups, a plastic sheet was placed on the water surface, N2 gas was bubbled through the air stone to achieve 5% DO in 24 ± 1 min, and the hypoxic conditions were maintained for an additional 10 min. All fish were euthanized by rapid cooling in ice water, snap frozen on dry ice and stored at −80°C until determination of whole-body cortisol levels.
(d). Adult dyadic social interactions
We determined the effects of embryonic anoxia exposure on the behavioural and cortisol responses to dyadic social interactions in adult zebrafish. Briefly, sex- and weight-matched (less than or equal to 5% wet mass) pairs of adult zebrafish (10 pairs in total; n = 20), consisting of one fish each from the normoxia and anoxia embryonic treatments, were placed on either side of an opaque barrier in a flow-through spawning basket (20.5 × 9.5 × 10 cm) suspended in a 10 l aquarium. Fish were acclimated to these conditions for 3 days and fed twice daily until experimentation. On trial day, the barrier was removed and the fish were allowed to interact for 1 h. The social interactions were video recorded to allow behavioural assessment. The videos were viewed at half speed, allowing each fish to be tracked and scored manually for number of bites, chases initiated and freezes entered. Within a pair, the fish that received fewer bites, initiated more chases and froze less often was considered dominant [14]. After 1 h of dyadic interactions, each fish was euthanized and stored as above. In parallel, a separate group of control adult fish from the normoxia embryonic treatment (n = 7) were euthanized as above to quantify whole-body cortisol levels in the absence of dyadic social interactions.
(e). Adult mirror aggression test
A mirror aggression test [15,16] was used to explore possible differences in agonistic behaviour between adults derived from anoxia- and normoxia-treated embryos. Briefly, 36 adult fish of either sex (half each from the normoxia and anoxia embryonic treatments) were acclimated overnight individually in tanks (25.4 × 7.7 × 17.2 cm) with 1 l of water. This acclimation period aimed to remove isolation stress as a confounding factor. Fish were then placed into the back half of a flow-through spawning basket (as above). The spawning basket contained a mirror (9.5 × 10 cm) in the front half that was blocked by an opaque divider at 10.25 cm. Fish were then left to acclimate for 1 h in the mirror-less half of the container before the barrier was removed. Mirror interactions were video recorded for 25 min. The videos were viewed at half speed and scored manually for number of bites and parallel inspections of the mirror [16,17]. Ethovision XT software (v. 8.0.516) was used to measure duration (s) spent in front of the mirror (1 cm distance from the mirror), in the intermediate zone (2–10.25 cm) and in the back (10.25–20.5 cm) of the spawning basket [16], as well as the amount of time (s) moving and not moving.
(f). Adult light/dark tank test
A light/dark tank was used to test for anxiety differences between adults derived from anoxia- and normoxia-treated embryos [18]. Briefly, 54 fish of either sex (half each from the normoxia and anoxia embryonic treatments) were first acclimated overnight individually in tanks (25.4 × 7.7 × 17.2 cm) as above. Fish were then netted and placed into a 250 ml beaker for 1 h to remove handling stress as a confounding factor. Finally, the beaker was emptied into a tank (30 × 15 × 23 cm) that was equally divided into two zones, one black and one white, and contained 4 l of water. Fish were always released into the black zone and once released were video recorded for 8 min. Video analysis was conducted using Ethovision XT software to determine latency to enter the light zone, duration in light zone and number of zone transitions (dark to light). After each trial the light/dark tank was rinsed to remove any scents from the previous fish. To ensure that density and hierarchy would not confound observations, fish used in this test were separated into groups of nine 2 weeks prior to testing.
(g). Whole-body testosterone and aromatase gene expression
We determined whether differences in dominant and aggressive behaviours between adults derived from anoxia- and normoxia-treated embryos are associated with differences in whole-body testosterone levels and changes in gonadal aromatase gene expression. Briefly, 24 adult zebrafish of either sex (half each from the normoxia and anoxia embryonic treatments) were euthanized by rapid cooling in ice water. The gonads were immediately isolated, snap frozen on dry ice and stored at −80°C until total RNA extraction and quantification of gene expression. The remainder of each fish was snap frozen on dry ice and stored at −80°C until determination of whole-body testosterone levels. Finally, to determine the effects of embryonic anoxia exposure on the testosterone response to social interactions, we quantified whole-body testosterone levels in the fish from the dyadic social interactions experiment.
(h). Steroid extraction and quantification
Whole-body steroids were extracted following Fuzzen et al. [19]. Fish removed from −80°C storage were thawed on ice, blotted dry, weighed, sexed and placed in 6 ml of PBS with 1 mM EDTA. Fish were then homogenized for 30 s using a Euro Turrax T 20b homogenizer (IKA, Wilmington, NC). Homogenate (250 µl) was extracted three times with 1 ml of methanol. The soluble fractions were combined, dried under N2 at room temperature and reconstituted in 300 µl of 50 mM sodium acetate (pH 4.0). The samples were further purified using C18 solid phase extraction (100 mg C18 Cleanert SPE column, Bonna-Agela, Wilmington, DE, USA). Steroids eluted from the columns were collected in glass vials, dried under N2 gas at room temperature, reconstituted in assay buffer and stored at −20°C until analysis. Whole-body cortisol levels were measured in triplicate by radioimmunoassay (RIA) as previously described [19]. Whole-body testosterone levels were determined in triplicate using a testosterone RIA kit (MP Biomedicals, Costa Mesa, CA, USA). A serial dilution of whole-fish extract gave displacement curves that were parallel to the standard curves for each RIA. Intra- and interassay variances were 4.6% (n = 6) and 7.5% (n = 6) in the cortisol assay, and 3.6% (n = 6) and 8.1% (n = 3) in the testosterone assay. Whole-body cortisol and testosterone values are presented as ng g−1 body weight and are corrected for extraction efficiency.
(i). Gene expression quantification
Total RNA was extracted from adult testis and ovary samples using QIAzol lysis reagent (Qiagen, Toronto, ON, Canada). The concentration of total RNA was quantified, using a NanoDrop 8000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA), and RNA quality was assessed via electrophoresis using the 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Following extraction, 1 µg of total RNA was DNase treated (Ambion DNase I, Life Technologies, Burlington, ON, Canada) and reverse transcribed to cDNA using Superscript II RNaseH− Reverse Transcriptase (Invitrogen, Life Technologies). Gonad mRNA levels of cyp19a were assessed using quantitative real-time PCR as previously described [19]. Gene expression was normalized to the housekeeping gene ef1-α and quantified as previously described [19]. All primer sets had amplification efficiencies of more than 90% (electronic supplementary material, table S1).
(j). Statistical analysis
A two-way repeated measures ANOVA was used to compare percentage survival over time between anoxia- and normoxia-exposed fish. Comparison of whole-body cortisol between treatments in the adult hypoxia exposure experiment was conducted using a two-way ANOVA followed by a Tukey's post hoc test. For the dyadic social interactions experiment, a chi-squared test was used to compare observed dominant and subordinate outcomes for adults derived from each embryonic treatment against an expected equal proportion of outcomes. Whole-body cortisol levels, and counts for total bites given, chases initiated and freezes entered, were analysed using Welch t-tests. To determine if social subordination was a significant source of stress, whole-body cortisol levels of control, dominant and subordinate fish were compared using a one-way ANOVA followed by a Tukey's post hoc test. For the mirror aggression test, all comparisons between treatments were analysed using Welch t-tests. To compare between treatments in the light/dark anxiety test, a Welch t-test was conducted on the number of zone transitions (dark to light), whereas latency to enter the light zone and duration in the light zone were analysed using Mann–Whitney U-tests. For the cyp19a expression data, values were log transformed to pass assumptions and standardized to ef1α values. Gene expression was analysed, using Welch t-tests to determine differences between treatments within each sex. For whole-body testosterone, Welch t-tests were conducted independently on baseline and dyadic interaction fish to determine if embryonic treatment was a significant effect, and to determine differences between embryonic treatments within sex. All data were analysed for normality using a Shapiro–Wilk test, and all statistical tests were conducted using R statistical software (v. 2.14.2, 2012) with a significance level of p < 0.05.
3. Results
(a). Adult hypoxia exposure
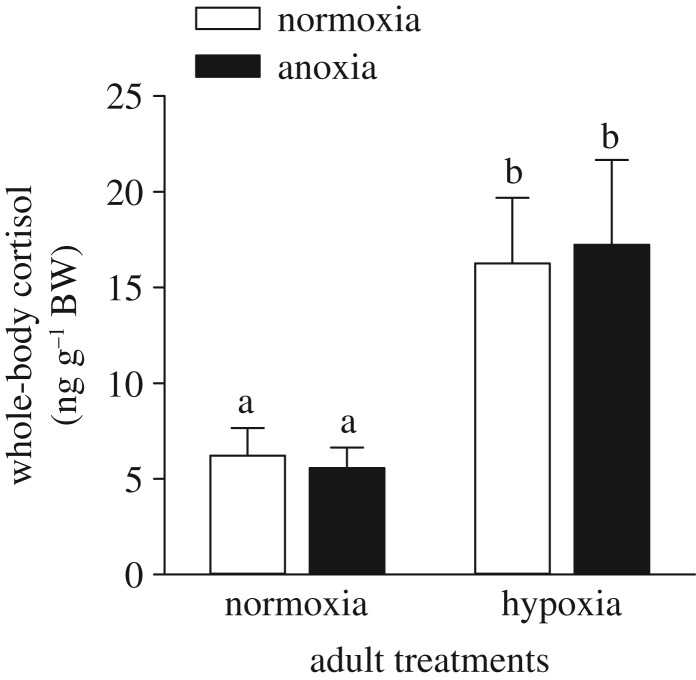
While hypoxia exposure in adult zebrafish increased whole-body cortisol levels threefold (p < 0.001; figure 1), neither basal cortisol levels (p = 0.895) nor the cortisol response to hypoxia exposure (p = 0.785) differed between adults derived from anoxia- and normoxia-exposed embryos. Across all fish, whole-body cortisol levels did not differ between sexes (p > 0.05; electronic supplementary material, table S2).
Figure 1.
Effects of exposing 36 hpf embryos to either 4 h of normoxia or anoxia on subsequent basal (normoxia) and hypoxia-induced whole-body cortisol levels in adult zebrafish. In the adult hypoxia treatment, fish were exposed to 5% DO for 10 min. Values are mean + s.e.m. (n = 13). Significant differences are indicated by dissimilar letters (two-way ANOVA and Tukey's post hoc test, p < 0.05).
(b). Adult dyadic social interactions
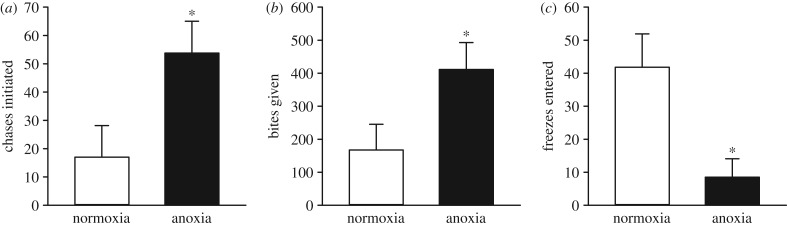
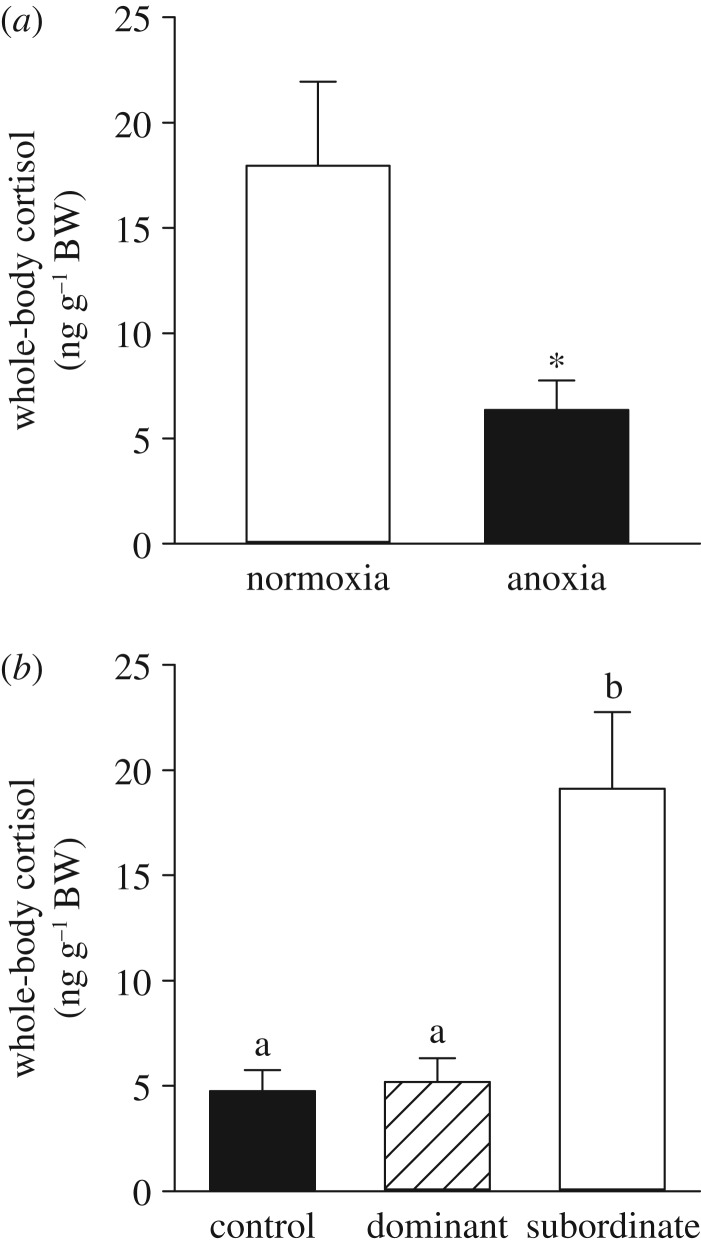
In the dyadic social interactions, all pairs developed clear dominant–subordinate relationships, and the adults derived from anoxia-exposed embryos were dominant in 90% of the contests (p = 0.002). Over the duration of the trials, the number of chases initiated (p = 0.016; figure 2a) and bites given (p = 0.022; figure 2b) were higher in anoxia- than normoxia-derived adults. In contrast, normoxia-derived adults entered more freezes than anoxia-derived adults (p = 0.006; figure 2c). In both anoxia- and normoxia-derived adults, no differences between sex were observed for the above behavioural measurements (electronic supplementary material, table S3). After 1 h of dyadic interactions, whole-body cortisol was lower in anoxia- than normoxia-derived adults (p = 0.005; figure 3a). Whole-body cortisol levels also differed between control, dominant and subordinate fish (p < 0.001; figure 3b). Subordinate fish were found to have a threefold increase in cortisol levels compared with control and dominant fish (p = 0.002 and p = 0.001, respectively), whereas control and dominant fish had similar whole-body cortisol values (p = 0.992). Male and female whole-body cortisol levels did not differ in any of the above comparisons (p > 0.05 for all; electronic supplementary material, table S2).
Figure 2.
Effects of exposing 36 hpf embryos to 4 h of either normoxia or anoxia on subsequent behavioural responses to dyadic social interactions in adult zebrafish. Pairs of adult zebrafish consisting of one fish each from the normoxia and anoxia embryonic treatments were allowed to interact for 1 h. The dyadic social interactions were scored for (a) chases initiated, (b) bites given and (c) freezes entered. Values are mean + s.e.m. (n = 10). A significant difference is indicated by an asterisk (Welch two-sample t-test, p < 0.05).
Figure 3.
Effects of exposing 36 hpf embryos to 4 h of either normoxia or anoxia on subsequent whole-body cortisol response to dyadic social interactions in adult zebrafish. (a) Pairs of adult zebrafish consisting of one fish each from the normoxia and anoxia embryonic treatments were allowed to interact for 1 h (n = 10). A significant difference is indicated by an asterisk (Welch two-sample t-test, p < 0.05). (b) Based on a behavioural assessment of the dyadic social interactions, each fish within a pair was assigned a dominant or subordinate status. A separate group of control adult fish from the normoxia embryonic treatment (n = 7) was used to quantify whole-body cortisol levels in the absence of dyadic social interactions. Values are mean + s.e.m. Significant differences are indicated by dissimilar letters (one-way ANOVA and Tukey's post hoc test, p < 0.05).
(c). Adult mirror aggression test
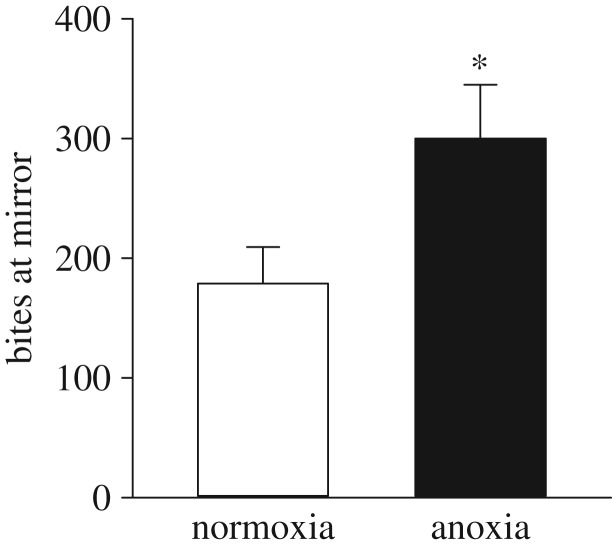
The number of bites at mirror over the 25 min test was higher in anoxia- than normoxia-derived adults (p = 0.019; figure 4), whereas the amount of parallel mirror inspections did not differ between treatments (p = 0.589). Adults derived from the two embryonic treatments were also found to spend a similar amount of time in the mirror, intermediate and back zones (p = 0.156, p = 0.219 and p = 0.473, respectively) of the spawning basket. Lastly, the amount of time spent moving and not moving did not differ between the two embryonic treatments (p = 0.468 and p = 0.300, respectively), and no differences between sex were observed for all behavioural measurements (p > 0.05 for all; electronic supplementary material, table S3).
Figure 4.
Effects of exposing 36 hpf embryos to 4 h of either normoxia or anoxia on subsequent number of bites at mirror in adult zebrafish during a 25 min mirror aggression test. Values are mean + s.e.m. (n = 11–12). A significant difference is indicated by an asterisk (Welch two-sample t-test, p < 0.05).
(d). Adult light/dark tank test
Adults derived from anoxia- and normoxia-exposed embryos did not differ in their response to the light/dark test. Analysis of the number of zone transitions, latency to enter the light zone and duration in the light zone between the two embryonic treatments did not differ (p = 0.914, p = 0.859 and p = 0.749, respectively). No difference between sex was observed for all behavioural measurements (p > 0.05 for all; electronic supplementary material, table S3).
(e). Gonadal aromatase gene expression and whole-body testosterone levels
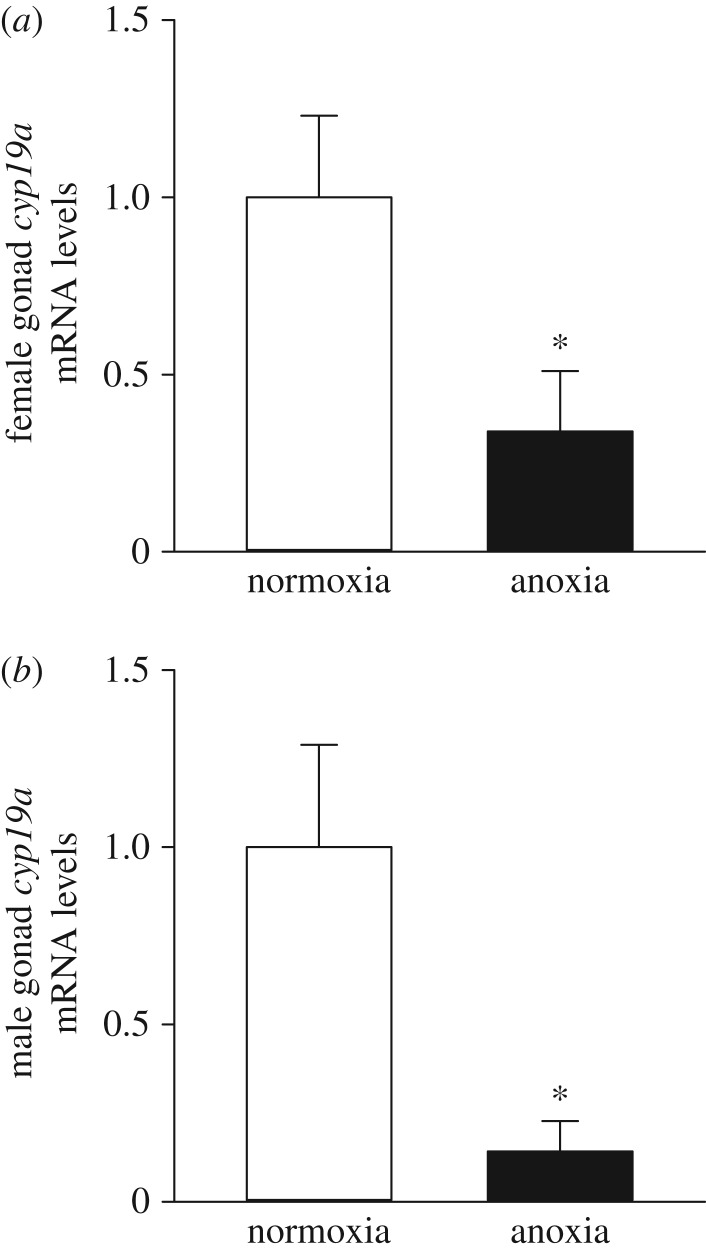
Gonadal aromatase gene expression, cyp19a, was significantly lower in anoxia- than normoxia-derived adults (figure 5). Overall, cyp19a mRNA levels were threefold (p = 0.009; figure 5a) and sevenfold lower, respectively (p = 0.002; figure 5b) in the ovaries and testes of anoxia- than normoxia-derived adults.
Figure 5.
Effects of exposing 36 hpf embryos to 4 h of either normoxia or anoxia on subsequent mRNA levels of gonadal aromatase (cyp19a) in (a) female and (b) male adult zebrafish. The mRNA levels are normalized with ef1a and presented relative to the normoxia treatment. Values are mean + s.e.m. (n = 12). A significant difference is indicated by an asterisk (Welch two-sample t-test, p < 0.05).
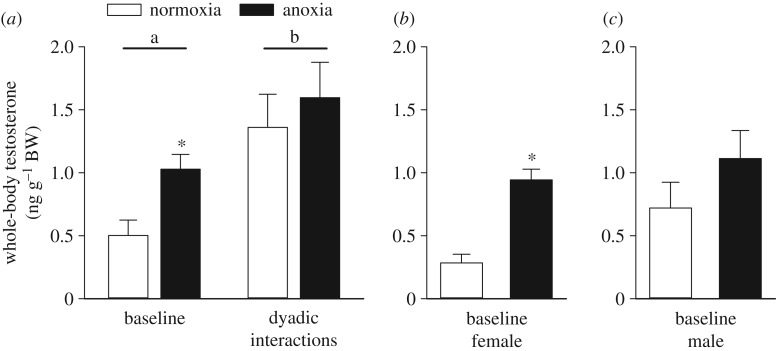
Dyadic interactions were observed to increase whole-body testosterone twofold when compared with baseline individuals, regardless of the embryonic treatment (p = 0.001; figure 6a). Independently, baseline anoxia-derived adults had higher testosterone levels compared with normoxia-derived adults (p = 0.001), although embryonic treatment had no effect on testosterone amount in fish from the dyadic social interactions (p = 0.558). In the baseline fish, comparison within sex between embryonic treatments found that testosterone levels were higher in anoxia- than normoxia-derived females (p < 0.001; figure 6b). In contrast, anoxia-derived males only exhibited a trend for increased whole-body testosterone, but were not significantly different from normoxia-derived males (p = 0.224; figure 6c). In the fish from the dyadic social interactions, whole-body testosterone levels did not differ between embryonic treatments within females or males (p = 0.369 and p = 0.150, respectively).
Figure 6.
Effects of exposing 36 hpf embryos to 4 h of either normoxia or anoxia on subsequent whole-body testosterone levels of (a) control adult zebrafish (baseline; n = 12) or adult zebrafish allowed to interact for 1 h in a dyadic social interaction contest (n = 10). Significant differences between the baseline and the dyadic interactions of adult fish are indicated by dissimilar letters, and a significant difference between embryonic treatments within a group of adult fish is indicated by an asterisk (two-way ANOVA and Tukey's post hoc test, p < 0.05). Baseline values are also shown for (b) female (n = 6) and (c) male adult fish (n = 6). A significant difference between embryonic treatments within a gender is indicated by an asterisk (Welch two-sample t-test, p < 0.05). Values are mean + s.e.m.
4. Discussion
Although acute anoxia exposure at 36 hpf does not affect embryo survival in zebrafish [13], here we demonstrate that this homeostatic challenge does have long-term endocrine and behavioural consequences. Specifically, we have shown that a 4 h exposure to anoxia during embryonic development enhances the competitive ability of adult zebrafish during dyadic social interactions and results in fish that are more aggressive, with lower gonadal aromatase gene expression and higher whole-body testosterone levels. In contrast, the same acute anoxia challenge did not affect basal cortisol levels or the cortisol response to hypoxia in adult fish, suggesting that the programming effects of embryonic anoxia exposure are context-dependent.
Acute embryonic anoxia exposure did not affect basal cortisol levels or the cortisol response to hypoxia in adult zebrafish. Because the fish derived from the anoxia- and normoxia-exposed embryos have the same hypoxia tolerance as adults [13], our results suggest that acute embryonic anoxia exposure does not have long-term effects on the hypoxia-responsive neurocircuitry that regulates HPI axis activity. Similarly, although chronic exposure to moderate hypoxia throughout development reduced basal cortisol levels in 120 hpf zebrafish larvae, it did not affect basal cortisol levels or the response to a net/handling stress protocol in adults [5]. In rats, while several studies have shown that hypoxia can programme the responsiveness of the HPA axis, whether the adult corticosterone stress response is reduced, enhanced or unaffected by fetal or perinatal hypoxia depends on when during development the hypoxic stressor is applied, its severity and duration [20,21]. To what extent HPI axis programming by hypoxia depends on similar exposure windows in zebrafish awaits further experimentation.
In contrast, in response to dyadic social interaction, adult zebrafish derived from anoxia-exposed embryos had significantly lower whole-body cortisol levels than adults derived from normoxia-exposed embryos. While the formation of a social hierarchy during dyadic social interactions can be associated with elevated plasma cortisol levels in the dominant and subordinate fish, previous studies have shown that the increase in circulating cortisol levels is transient in the former and chronic in the latter [17]. Therefore, our results suggest that in response to dyadic social interactions, adult zebrafish derived from anoxia- and normoxia-exposed embryos had whole-body cortisol levels that are characteristic of dominant and subordinate fish, respectively. In accord with this interpretation, our behavioural analysis of the dyadic interactions showed that adults derived from embryonic anoxia initiated more chases, bit more often and entered fewer freezes than adults derived from embryonic normoxia, a behavioural profile that is characteristic of dominant fish in dyadic interactions [14,17,22]. Overall, the behavioural analysis showed that 90% of the adults derived from anoxia-exposed embryos were dominant.
Consistent with their behavioural responses to dyadic interactions, the adults derived from embryonic anoxia performed more bites towards their mirror image than adults derived from embryonic normoxia. Because fish treat their mirror image as an intruding individual and respond with aggressive behaviours [23], our results suggest that the adults derived from embryonic anoxia are more aggressive than those derived from embryonic normoxia. Similarly, neonatal male rats exposed to anoxia are more aggressive as adults [24]. Although chronic hypoxia from fertilization to 75 dpf also influences aggression in adult zebrafish, the effects are acclimation history dependent [11]. Using a mirror image stimulation, Marks et al. [11] observed that zebrafish chronically reared in hypoxic conditions were most aggressive in hypoxia, while normoxia-reared fish were most aggressive in normoxia. In this study, we also observed that the amount of parallel inspections at the mirror was not significantly different between treatments, but this behaviour is meant to allow the fish to gain information about their rival and is not normally viewed as an act of aggression [25]. Lastly, the amount of time spent moving and stationary were similar between treatments, allowing us to suggest that there is no difference in activity levels between our primary treatment groups that could confound our results. Overall, findings from the mirror image tests suggest that adult aggression in zebrafish may be predisposed by a brief period of O2 deprivation during embryonic development.
Results from the light/dark box test suggest that the adult zebrafish derived from the anoxia- and normoxia-exposed embryos have similar anxiety-like behaviour. In general, the scototaxic response of adult zebrafish to the light/dark box test in this study was consistent with the behavioural response previously observed for this paradigm [18]. In mammals, highly anxious individuals often display a subordinate status [26]. Therefore, assuming that trait anxiety also has an important consequence for social status in zebrafish, we would predict that adults derived from embryonic anoxia would have lower anxiety levels. In general, however, the relationships between anxiety, behavioural coping styles, aggression and social status in fish are still poorly understood [27,28]. Moreover, because zebrafish have been shown to respond variably in different anxiety tests [29], our results from the light/dark box test may not generalize across anxiety tests and a more complete characterization of the anxiety-like phenotype of the adults derived from embryonic anoxia is needed before definitive conclusions can be reached.
While previous studies with zebrafish and other fish species have shown that chronic exposure to hypoxia leads to lower expression of gonadal aromatase, the enzyme that converts androgens to oestrogens and higher testosterone levels in adult fish [30–32], here we show that a 4 h exposure to anoxia in 36 hpf embryos produces the same outcome. In zebrafish and Atlantic croaker (Micropogonias undulatus), the decline in ovarian aromatase expression leads to ovarian masculinization and to male-biased populations [30,32]. Similarly, the acute embryonic anoxia exposure used in this study leads to a zebrafish population with 71% males compared with 41% males in the normoxic treatment [13]. Moreover, 48 hpf zebrafish embryos chronically exposed to 10% DO from fertilization are characterized with a reduction in brain aromatase [30], an increase in testosterone and a reduction in oestradiol levels [33]. Consistent with our results, Shang et al. [30] also observed that the disruption in the balance of sex steroids associated with chronic hypoxia in zebrafish is more pronounced in females than in males. Overall, these results suggest that in zebrafish exposure to low O2 levels during a critical window of embryogenesis leads to a persistent downregulation of aromatase expression, which affects sex determination and disrupts testosterone and oestradiol production. In contrast, the acute embryonic anoxia challenge does not appear to affect the androgen response to social challenge [34]. As previously observed in zebrafish and other species [22,35], both dominant and subordinate individuals in this study had similarly elevated testosterone levels immediately after the social dyadic interaction. Whether exposure to low O2 levels during embryogenesis affects the longer-term differences in androgen levels observed following social status establishment [34,36] remains to be established.
The reduction in aromatase expression and associated increase in whole-body testosterone probably contribute to the behavioural profile of the adults derived from embryonic anoxia. Gonadal and brain aromatase activity, testosterone and 11-ketotestosterone, the main bioactive androgen in male teleosts, have all been shown to affect social status and aggression in fish [36–38]. For example, as observed here, some studies have shown a positive relationship between testosterone levels and aggressive behaviour [15,39], whereas others have shown that manipulation of androgen levels with receptor agonists and antagonists or by castration can affect aggression [40]. These results argue for a causal role of testosterone for the aggressive and dominant behaviour of the adults derived from embryonic anoxia. However, the role of testosterone in aggression is equivocal and in general the balance of evidence suggests that androgens act as facilitators of aggressive behaviour rather than mediators [34,41].
Alternatively, studies of the proximate causes of aggression and dominance in zebrafish have implicated serotonin, dopamine, histamine, arginine vasotocin and oestradiol in the regulation of these behaviours [28,42]. For example, subordinate zebrafish in dyadic interactions have elevated hindbrain serotonergic activity [14] and pharmacological approaches that block serotonergic signalling increase aggression [43]. Interestingly, in addition to suppressing aromatase activity and plasma oestradiol levels, chronic hypoxia exposure in Atlantic croakers inhibited hypothalamic tryptophan hydroxylase, the rate-limiting enzyme in serotonin production [32,44], and treatment with oestradiol partially restored tryptophan hydroxylase activity and serotonin content [45]. In zebrafish, treatment with the synthetic oestradiol 17α-ethinylestradiol suppressed aggression in dominant male, inhibited their dominant status in social hierarchy, and affected the expression of serotonin, dopamine and arginine vasopressin pathway genes [46]. Therefore, a hypoxia-induced inhibition of aromatase activity and decreased oestradiol levels may provide an explanation for the increase in aggression and dominant status of the adult zebrafish derived from embryonic anoxia. This potential mechanism warrants further investigation.
Finally, altered serotonergic neurotransmission may also be a proximate cause for the cortisol response to the dyadic social interaction stressor in the adult zebrafish derived from embryonic anoxia. The sustained cortisol stress response of subordinates in dyadic social interactions is associated with a chronic activation of the serotonergic system, and it is generally believed that serotonin is responsible for the stimulation of the HPI axis in subordinate individuals [17,47]. Therefore, we hypothesize that a sustained anoxia-induced downregulation of aromatase and tryptophan hydroxylase expression may limit the capacity for serotonin synthesis during the formation of social hierarchies and in return affect the responsiveness of the HPI axis to social stress.
5. Conclusion
We have shown that a single 4 h exposure to anoxia during a critical window of hypoxia sensitivity during embryonic development can have long-lasting consequences for the hormonal and behavioural responses to social interactions in zebrafish. Specifically, our data suggest that acute embryonic anoxia exposure favours the development of a dominant and aggressive phenotype in adult zebrafish. Moreover, our results indicate that a reduction in aromatase expression and associated disruption in sex steroid production may contribute to the programming effects of environmental hypoxia. Overall, these findings will have implications for the interpretation of the impact of embryonic environmental hypoxia on adult performance in fish. In general, while social dominance and aggression can enhance access to limited resources and increase reproductive success, the literature on coping styles also suggests that non-aggressive individuals do better in a variable or unpredictable environment [48]. As such, it remains to be determined whether the developmental response to embryonic anoxia has adaptive value.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Dr Pat Wright for her valuable comments on experimental design and are grateful to the staff of the Hagen Aqualab for technical support.
Ethics
All animal care and experimentation was conducted according to the University of Guelph animal care protocol number 1256.
Data accessibility
Supporting data can be accessed at Dryad [49].
Authors' contributions
C.M.I. and N.J.B. designed the study. C.M.I., C.E.R. and N.J.B. ran experimental procedures. C.M.I and N.J.B. analysed the data. C.M.I., C.E.R. and N.J.B. wrote the paper. All authors discussed results and commented on the manuscript.
Competing interests
The authors declare no competing financial interests.
Funding
This work was financially supported by a Natural Sciences and Engineering Research Council (NSERC) of Canada Discovery Grants (N.J.B.).
References
- 1.Glover V, O'Connor TG, O'Donnell K. 2010. Prenatal stress and the programming of the HPA axis. Neurosci. Biobehav. Rev. 35, 17–22. ( 10.1016/j.neubiorev.2009.11.008) [DOI] [PubMed] [Google Scholar]
- 2.Denver RJ. 2009. Structural and functional evolution of vertebrate neuroendocrine stress systems. Ann. N.Y. Acad. Sci. 1163, 1–16. ( 10.1111/j.1749-6632.2009.04433.x) [DOI] [PubMed] [Google Scholar]
- 3.Auperin B, Geslin M. 2008. Plasma cortisol response to stress in juvenile rainbow trout is influenced by their life history during early development and by egg cortisol content. Gen. Comp. Endocrinol. 158, 234–239. ( 10.1016/j.ygcen.2008.07.002) [DOI] [PubMed] [Google Scholar]
- 4.Weinstock M. 2008. The long-term behavioural consequences of prenatal stress. Neurosci. Biobehav. Rev. 32, 1073–1086. ( 10.1016/j.neubiorev.2008.03.002) [DOI] [PubMed] [Google Scholar]
- 5.Wilson KS, Tucker CS, Al-Dujaili EAS, Holmes MC, Hadoke PWF, Kenyon CJ, Denvir MA. 2016. Early-life glucocorticoids programme behaviour and metabolism in adulthood in zebrafish. J. Endocrinol. 230, 125–142. ( 10.1530/JOE-15-0376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandi C, Haller J. 2015. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat. Rev. Neurosci. 16, 290–304. ( 10.1038/nrn3918) [DOI] [PubMed] [Google Scholar]
- 7.Diaz RJ, Breitburg DL. 2009. The hypoxic environment. In Hypoxia (eds Richards JG, Farrell AP, Brauner CJ), pp. 2–23. Amsterdam, The Netherlands: Academic Press. [Google Scholar]
- 8.Doney SC, et al. 2012. Climate change impacts on marine ecosystems. Annu. Rev. Mar. Sci. 4, 11–37. ( 10.1146/annurev-marine-041911-111611) [DOI] [PubMed] [Google Scholar]
- 9.Chapman LJ, Galis F, Shinn J. 2000. Phenotypic plasticity and the possible role of genetic assimilation: Hypoxia-induced trade-offs in the morphological traits of an African cichlid. Ecol. Lett. 3, 387–393. ( 10.1046/j.1461-0248.2000.00160.x) [DOI] [Google Scholar]
- 10.Marks C, Kaut KP, Moore FB, Bagatto B. 2012. Ontogenetic oxygen changes alter zebrafish size, behavior, and blood glucose. Physiol. Biochem. Zool. 85, 635–644. ( 10.1086/666508) [DOI] [PubMed] [Google Scholar]
- 11.Marks C, West TN, Bagatto B, Moore FBG. 2005. Developmental environment alters conditional aggression in zebrafish. Copeia 4, 901–908. ( 10.1643/0045-8511(2005)005%3C0901:DEACAI%3E2.0.CO;2) [DOI] [Google Scholar]
- 12.Alderman SL, Bernier NJ. 2009. Ontogeny of the corticotropin-releasing factor system in zebrafish. Gen. Comp. Endocrinol. 164, 61–69. ( 10.1016/j.ygcen.2009.04.007) [DOI] [PubMed] [Google Scholar]
- 13.Robertson CE, Wright PA, Koblitz L, Bernier NJ. 2014. Hypoxia-inducible factor-1 mediates adaptive developmental plasticity of hypoxia tolerance in zebrafish, Danio rerio. Proc. R. Soc. B 281, 20140637 ( 10.1098/rspb.2014.0637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahlborn SJ, Backström T, Lundstedt-Enkel K, Winberg S. 2012. Aggression and monoamines: effects of sex and social rank in zebrafish (Danio rerio). Behav. Brain Res. 228, 333–338. ( 10.1016/j.bbr.2011.12.011) [DOI] [PubMed] [Google Scholar]
- 15.Chang C, Li CY, Earley RL, Hsu Y. 2012. Aggression and related behavioural traits: the impact of winning and losing and the role of hormones. Integr. Comp. Biol. 52, 801–813. ( 10.1093/icb/ics057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber DN, Ghorai JK. 2013. Experimental design affects social behavior outcomes in adult zebrafish developmentally exposed to lead. Zebrafish 10, 294–302. ( 10.1089/zeb.2012.0780) [DOI] [PubMed] [Google Scholar]
- 17.Johnsson JI, Winberg S, Sloman KA. 2006. Social interactions. In Behaviour and physiology of fish (eds Balshine S, Sloman KA, Wilson RW), pp. 151–196. Amsterdam, The Netherlands: Academic Press. [Google Scholar]
- 18.Stewart A, et al. 2011. Neurophenotyping of adult zebrafish using the light/dark box paradigm. In Zebrafish neurobehavioral protocols (eds Kalueff AV, Cachat JM), pp. 157–167. Totowa, NJ: Humana Press Inc. [Google Scholar]
- 19.Fuzzen MLM, Van Der Kraak G, Bernier NJ. 2010. Stirring up new ideas about the regulation of the hypothalamic–pituitary–interrenal axis in zebrafish (Danio rerio). Zebrafish 7, 349–358. ( 10.1089/zeb.2010.0662) [DOI] [PubMed] [Google Scholar]
- 20.Venerosi A, Valanzano A, Cirulli F, Alleva E, Calamandrei G. 2004. Acute global anoxia during C-section birth affects dopamine-mediated behavioural responses and reactivity to stress. Behav. Brain Res. 154, 155–164. ( 10.1016/j.bbr.2004.02.008) [DOI] [PubMed] [Google Scholar]
- 21.Chintamaneni K, Bruder ED, Raff H. 2014. Programming of the hypothalamic–pituitary–adrenal axis by neonatal intermittent hypoxia: effects on adult male ACTH and corticosterone responses are stress specific. Endocrinology 155, 1763–1770. ( 10.1210/en.2013-1736) [DOI] [PubMed] [Google Scholar]
- 22.Filby AL, Paull GC, Bartlett EJ, Van Look KJW, Tyler CR. 2010. Physiological and health consequences of social status in zebrafish (Danio rerio). Physiol. Behav. 101, 576–587. ( 10.1016/j.physbeh.2010.09.004) [DOI] [PubMed] [Google Scholar]
- 23.Desjardins JK, Fernald RD. 2010. What do fish make of mirror images? Biol. Lett. 6, 744–747. ( 10.1098/rsbl.2010.0247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakazawa M, Tang A. 2006. Adult aggression during an initial social encounter: effects of neonatal anoxia and relation to juvenile open-field activity. Neurosci. Lett. 408, 119–123. ( 10.1016/j.neulet.2006.08.064) [DOI] [PubMed] [Google Scholar]
- 25.Archard GA, Braihwaite VA. 2011. Variation in aggressive behaviour in the poeciliid fish Brachyrhaphis episcopi: population and sex differences. Behav. Process. 86, 52–57. ( 10.1016/j.beproc.2010.09.002) [DOI] [PubMed] [Google Scholar]
- 26.Hollis F, Van Der Kooij MA, Zanoletti O, Lozano L, Cantó C, Sandi C. 2015. Mitochondrial function in the brain links anxiety with social subordination. Proc. Natl Acad. Sci. USA 112, 15 486–15 491. ( 10.1073/pnas.1512653112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Øverli Ø, Sørensen C, Pulman KGT, Pottinger TG, Korzan W, Summers CH, Nilsson GE. 2007. Evolutionary background for stress-coping styles: Relationships between physiological, behavioral, and cognitive traits in non-mammalian vertebrates. Neurosci. Biobehav. Rev. 31, 396–412. ( 10.1016/j.neubiorev.2006.10.006) [DOI] [PubMed] [Google Scholar]
- 28.Jones LJ, Norton WHJ. 2015. Using zebrafish to uncover the genetic and neural basis of aggression, a frequent comorbid symptom of psychiatric disorders. Behav. Brain Res. 276, 171–180. ( 10.1016/j.bbr.2014.05.055) [DOI] [PubMed] [Google Scholar]
- 29.Sørensen C, Johansen IB, Øverli Ø. 2013. Neural plasticity and stress coping in teleost fishes. Gen. Comp. Endocrinol. 181, 25–34. ( 10.1016/j.ygcen.2012.12.003) [DOI] [PubMed] [Google Scholar]
- 30.Shang EHH, Yu RMK, Wu RSS. 2006. Hypoxia affects sex differentiation and development, leading to a male-dominant population in zebrafish (Danio rerio). Environ. Sci. Technol. 40, 3118–3122. ( 10.1021/es0522579) [DOI] [PubMed] [Google Scholar]
- 31.Landry CA, Steele SL, Manning S, Cheek AO. 2007. Long term hypoxia suppresses reproductive capacity in the estuarine fish, Fundulus grandis. Comp. Biochem. Physiol. A 148, 317–323. ( 10.1016/j.cbpa.2007.04.023) [DOI] [PubMed] [Google Scholar]
- 32.Thomas P, Rahman MS. 2011. Extensive reproductive disruption, ovarian masculinization and aromatase suppression in Atlantic croaker in the northern Gulf of Mexico hypoxic zone. Proc. R. Soc. B 279, 28–38. ( 10.1098/rspb.2011.0529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shang EHH, Wu RSS. 2004. Aquatic hypoxia is a teratogen and affects fish embryonic development. Environ. Sci. Technol. 38, 4763–4767. ( 10.1021/es0496423) [DOI] [PubMed] [Google Scholar]
- 34.Oliveira RF. 2009. Social behavior in context: hormonal modulation of behavioral plasticity and social competence. Integr. Comp. Biol. 49, 423–440. ( 10.1093/icb/icp055) [DOI] [PubMed] [Google Scholar]
- 35.Desjardins JK, Hazelden MR, Van Der Kraak GJ, Balshine S. 2006. Male and female cooperatively breeding fish provide support for the ‘challenge hypothesis’. Behav. Ecol. 17, 149–154. ( 10.1093/beheco/arj018) [DOI] [Google Scholar]
- 36.Taves MD, Desjardins JK, Mishra S, Balshine S. 2009. Androgens and dominance: sex-specific patterns in a highly social fish (Neolamprologus pulcher). Gen. Comp. Endocrinol. 161, 202–207. ( 10.1016/j.ygcen.2008.12.018) [DOI] [PubMed] [Google Scholar]
- 37.Huffman LS, O'Connell LA, Hofmann HA. 2013. Aromatase regulates aggression in the African cichlid fish Astatotilapia burtoni. Physiol. Behav. 112–113, 77–83. ( 10.1016/j.physbeh.2013.02.004) [DOI] [PubMed] [Google Scholar]
- 38.Ramallo MR, Birba A, Honji RM, Morandini L, Moreira RG, Somoza GM, Pandolfi M. 2015. A multidisciplinary study on social status and the relationship between inter-individual variation in hormone levels and agonistic behavior in a Neotropical cichlid fish. Horm. Behav. 69, 139–151. ( 10.1016/j.yhbeh.2015.01.008) [DOI] [PubMed] [Google Scholar]
- 39.Parikh VN, Clement TS, Fernald RD. 2006. Androgen level and male social status in the African cichlid, Astatotilapia burtoni. Behav. Brain Res. 166, 291–295. ( 10.1016/j.bbr.2005.07.011) [DOI] [PubMed] [Google Scholar]
- 40.Maruska KP. 2014. Social regulation of reproduction in male cichlid fishes. Gen. Comp. Endocrinol. 207, 2–12. ( 10.1016/j.ygcen.2014.04.038) [DOI] [PubMed] [Google Scholar]
- 41.Almeida O, Canário AVM, Oliveira RF. 2014. Castration affects reproductive but not aggressive behavior in a cichlid fish. Gen. Comp. Endocrinol. 207, 34–40. ( 10.1016/j.ygcen.2014.03.018) [DOI] [PubMed] [Google Scholar]
- 42.Norton WHJ, Bally-Cuif L. 2012. Unravelling the proximate causes of the aggression-boldness behavioural syndrome in zebrafish. Behaviour 149, 1063–1079. ( 10.1163/1568539X-00003012) [DOI] [Google Scholar]
- 43.Filby AL, Paull GC, Hickmore TFA, Tyler CR. 2010. Unravelling the neurophysiological basis of aggression in a fish model. BMC Genomics 11, 498 ( 10.1186/1471-2164-11-498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas P, Rahman MS, Khan IA, Kummer JA. 2007. Widespread endocrine disruption and reproductive impairment in an estuarine fish population exposed to seasonal hypoxia. Proc. R. Soc. B 274, 2693–2701. ( 10.1098/rspb.2007.0921) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahman MS, Thomas P. 2013. Interactive effects of hypoxia with estradiol-17β on tryptophan hydroxylase activity and serotonin levels in the Atlantic croaker hypothalamus. Gen. Comp. Endocrinol. 192, 71–76. ( 10.1016/j.ygcen.2013.03.001) [DOI] [PubMed] [Google Scholar]
- 46.Filby AL, Paull GC, Searle F, Ortiz-Zarragoitia M, Tyler CR. 2012. Environmental estrogen-induced alterations of male aggression and dominance hierarchies in fish: a mechanistic analysis. Environ. Sci. Technol. 46, 3472–3479. ( 10.1021/es204023d) [DOI] [PubMed] [Google Scholar]
- 47.Summers CH, Winberg S. 2006. Interactions between the neural regulation of stress and aggression. J. Exp. Biol. 209, 4581–4589. ( 10.1242/jeb.02565) [DOI] [PubMed] [Google Scholar]
- 48.Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MAW, Blokhuis HJ. 1999. Coping styles in animals: current status in behaviour and stress-physiology. Neurosci. Biobehav. Rev. 23, 925–935. ( 10.1016/S0149-7634(99)00026-3) [DOI] [PubMed] [Google Scholar]
- 49.Ivy CM, Robertson CE, Bernier NJ. 2017. Data from: Acute embryonic anoxia exposure favours the development of a dominant and aggressive phenotype in adult zebrafish. Dryad Digital Repository. ( 10.5061/dryad.s0t8b) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supporting data can be accessed at Dryad [49].