Abstract
Bud growth in yeast is guided by myosin-driven delivery of secretory vesicles from the mother cell to the bud. We find transport occurs along two sets of actin cables assembled by two formin isoforms. The Bnr1p formin assembles cables that radiate from the bud neck into the mother, providing a stable mother-bud axis. These cables also depend on septins at the neck and are required for efficient transport from the mother to the bud. The Bni1p formin assembles cables that line the bud cortex and target vesicles to varying locations in the bud. Loss of these cables results in morphological defects as vesicles accumulate at the neck. Assembly of these cables depends on continued polarized secretion, suggesting vesicular transport provides a positive feedback signal for Bni1p activation, possibly by rho-proteins. By coupling different formin isoforms to unique cortical landmarks, yeast uses common cytoskeletal elements to maintain stable and dynamic axes in the same cell.
INTRODUCTION
Proper cell polarization requires a balance between dynamism and stability. Persistent systems, such as the apical and basolateral domains of epithelial cells, show a higher stability than flexible systems, such as cells undergoing chemotaxis. However, both types can use similar cytoskeletal components, so an important task in understanding the control of polarity is to identify features that influence persistence, and how those features integrate with the core cytoskeletal machinery providing the physical expression of polarity.
The process of bud growth of the yeast Saccharomyces cerevisiae provides a model for examining the control of polarity (for reviews, see Bretscher, 2003; Chang and Peter, 2003). Secretory organelles, such as the Golgi and endoplasmic reticulum, are distributed throughout the bud and mother cell, whereas post-Golgi secretory vesicles concentrate at discrete growth sites at the cell cortex. These vesicles are guided to these sites along what are essentially two axes of polarity: a stable axis directing traffic from the mother into the bud, and a dynamic axis that fine-tunes delivery from the bud neck to specific regions in the bud, sending vesicles first to the small bud tip, then to the entire bud surface, and finally back toward the neck during mother/daughter separation. On delivery to these locations, post-Golgi vesicles fuse with the cell surface to promote localized cell expansion.
Two class-V myosins, with heavy chains encoded by MYO2 (Johnston et al., 1991) and MYO4 (Haarer et al., 1994), propel cellular components, including vesicles, organelles, mRNAs, and microtubules, from the mother into the bud during growth and organelle segregation. The myosins move along cables of actin filaments that radiate throughout the cell in arrays oriented toward growth sites (Adams and Pringle, 1984; Kilmartin and Adams, 1984).
Assembly of these cables depends on two formin homologues, Bni1p and Bnr1p (Evangelista et al., 2002; Sagot et al., 2002a), members of a family of cytoskeletal regulatory proteins defined by conserved formin homology (FH)1 and FH2 domains (for reviews, see Evangelista et al., 2003; Wallar and Alberts, 2003). In vitro, the FH2 domain nucleates actin filaments from monomers (Pruyne et al., 2002; Sagot et al., 2002b; Kovar et al., 2003; Li and Higgs, 2003; Harris et al., 2004; Kobielak et al., 2004), but distinct from other nucleators, it remains associated with the growing barbed end to promote elongation, even in the presence of barbed-end capping proteins (Pruyne et al., 2002; Kovar et al., 2003; Li and Higgs, 2003; Pring et al., 2003; Harris et al., 2004; Kobielak et al., 2004; Moseley et al., 2004). The FH1 region is a proline-rich stretch that recruits profilin, an actin-monomer binding protein essential for formin function in vivo (Evangelista et al., 2002), to participate in FH2-mediated nucleation in vitro (Sagot et al., 2002b; Kovar et al., 2003; Pring et al., 2003). Other regions present in Bni1p and Bnr1p are a regulatory NH2-terminal rho-GTPase-binding domain that subjects many formins to rho-dependent activation (Kohno et al., 1996; Evangelista et al., 1997; Imamura et al., 1997; Dong et al., 2003), and an FH3 motif that helps localize formins within the cell (Petersen et al., 1998).
With loss of formin function, actin cables disassemble in 2 min, and a cytokinetic ring is unable to assemble, but actin patches remain intact (Evangelista et al., 2002; Sagot et al., 2002a; Tolliday et al., 2002). Other actin nucleators, particularly the Arp2/3 complex, play no apparent role in cable or cytokinetic ring assembly in budding yeast (Winter et al., 1999; Evangelista et al., 2002; Tolliday et al., 2002), suggesting the formins might be in vivo nucleators for these actin filaments. However, the loss of Bni1p or Bnr1p individually results in different phenotypes. To determine whether these distinct phenotypes relate to actin assembly, we examined the relationship between the two formins and patterns of filament assembly in the yeast cell.
MATERIALS AND METHODS
Plasmid Construction
To allow for overexpression of CLN2, a 694-base pair BamHI/EcoRI fragment from pRS316-GAL1 (Liu et al., 1992) containing the promoter of the GAL1 and GAL10 genes, was cloned into pRS306 (Sikorski and Hieter, 1989) to generate pDP132. The CLN2 ORF with 201 base pairs downstream sequence and introduced flanking BamHI and NotI sites was amplified by polymerase chain reaction (PCR) from genomic DNA by using primers GGCGGATCCATGGCTAGTGCTGAACCAAGACCCC and GGGCGGCCGCTCTACACAGAATTTAGTTGGCTGT (restriction sites underlined), cloned into pCR2.1 by the TOPO-TA Cloning reaction (Invitrogen, Carlsbad, CA), and sequenced to verify the absence of mutations. The BamHI/NotI fragment of this was then cloned into pDP132 to generate pDP134.
To allow for visualization of Bud6p, a 4.0-kb EcoRI/SalI fragment from pRB2190 (Amberg et al., 1997), containing a functional NH2-terminal green fluorescent protein (GFP)S65T fusion of the BUD6 open reading frame (ORF) flanked by the ACT1 promoter and terminator, was subcloned into pRS306 to generate pDP128. For visualization of Cdc12p, a 2.8-kb EcoRI/SstI fragment from pRS315::GFP:CDC12 (Richman et al., 1999), containing an NH2-terminal GFP-fusion of CDC12 behind the CDC12 promoter, was cloned into pRS306 to generate pDP135. For visualization of Bni1p in vivo, the BNI1 ORF plus promoter from plasmid p39C2 (CEN LEU2; kindly provided by H. Fares and J.R. Pringle, University of North Carolina, Chapel Hill, NC) was COOH-terminally tagged in-frame with GFPF64L/S65T by a PCR-based method (Longtine et al., 1998). A 11.6-kb SphI/SpeI fragment from this plasmid, carrying BNI1 promoter, BNI1:GFP ORF, and KanR, was subcloned into YEplac181 (2 μm LEU2; Gietz and Sugino, 1988) to generate YEp181-BNI1:GFP::KanR.
To allow for deletion of TPM2 by using a TRP1 marker, three individual PCRs were done to amplify the TRP1 sequence by using primers CGCGGCGGGTGTGGTGGTTA and AGCGGGTGTTGGCGGGTGTA, bases–716 to –209 upstream of TPM2 by using primers GACCAATGGGCACGGAAGG (with endogenous KpnI site) and GTAACCACCACACCCGCCGCGTGTTGAAATACTTGTAAAAA, and bases +488 to +780 downstream of TPM2 by using primers AAAGCTGACGCAACGATTACTAT (with endogenous NdeI site) and GACACCCGCCAACACCCGCTCCCATTGATAAGACTAAAA (TRP1-complementing sequences underlined). Resultant products were combined by double fusion PCR (Amberg et al., 1995) to generate TRP1 flanked by TPM2 upstream and downstream sequences, and then was cloned into pCR2.1 by the TOPO-TA Cloning method (Invitrogen) to generate pDP116.
For overproduction of NH2-terminally truncated Bnr1p in yeast, plasmid p015 containing Bnr1p residues 386-1375 behind the GAL1-10 promoter was isolated as a suppressor of the temperature-sensitive growth of ABY2000 (bni1-11 bnr1Δ) by using a GAL1-regulated cDNA expression library (Liu et al., 1992). For production of recombinant Bnr1p sequences including the FH1, FH2, and COOH-terminal residues fused to glutathione S-transferase (GST) [called GSTBnr1p(FH1FH2)], BNR1 sequence from +2269 to +4284 was amplified from genomic DNA by using primers CCGGAATTCCCCAAGTAGTACCTGAAGTTGTTAAACTACC and GCAGCATGGCGGCCGCGAATCTGTCCATCTCCAAATC (EcoRI and NotI restriction sites, respectively, underlined) and cloned into the EcoRI/NotI sites of pGEX-6P-3 (Amersham Biosciences, Piscataway, NJ) to generate p080. The BNR1 sequence was analyzed to verify the absence of mutations.
Yeast Strains and Growth Conditions
The genotypes of yeast strains used are listed in Table 1. Growth conditions and temperature shifts were done as described previously (Pruyne et al., 1998; Evangelista et al., 2002).
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| ABY501 | MATa/α ade2-101/ade2-101 his3-Δ200/his3-Δ200 leu2-3,112/leu2-3,112 lys2-801/lys2-801 ura3-52/ura3-52 | Derived from ABY531 (Schott et al., 1999) |
| ABY506 | MATa/α ade2-101/ade2-101 his3-Δ200/his3-Δ200 leu2-3,112/leu2-3,112 lys2-801/lys2-801 ura3-52/ura3-52 myo2-16::LEU2/myo2-16::HIS3 | Derived from ABY536 (Schott et al., 1999) |
| ABY944 | MATa his3-Δ200 leu2-3,112 lys2-801 trp1-1(am) ura3-52 tpm1-2::LEU2 tpm2::HIS3 | Pruyne et al. (1998) |
| ABY945 | MATa his3-Δ200 leu2-3,112 lys2-801 trp1-1(am) ura3-52 tpm2::HIS3 | Pruyne et al. (1998) |
| ABY971 | MATa/α his3-Δ200/his3-Δ200 leu2-3,112/leu2-3,112 lys2-801/lys2-801 trp1-1(am)/trp1-1(am) ura3-52/ura3-52 tpm1-2::LEU2/tpm1-2::LEU2 tpm2::HIS3/tpm2::HIS3 | Pruyne et al. (1998) |
| ABY973 | MATa/α his3-Δ200/his3-Δ200 leu2-3,112/leu2-3,112 lys2-801/lys2-801 trp1-1(am)/trp1-1(am) ura3-52/ura3-52 tpm2::HIS3/tpm2::HIS3 | Pruyne et al. (1998) |
| ABY993 | MATa/α his3-Δ200/his3-Δ200 leu2-3,112/leu2-3,112 lys2-801/lys2-801 trp1-1(am)/trp1-1(am) ura3-52/ura3-52 sec6-4/sec6-4 | Derived from EHY52 (Harsay and Schekman, 2002) |
| ABY994 | MATa/α ura3-52/ura3-52 leu2-3,112::SEC8:3×-c-myc::LEU2/leu2-3,112::SEC8:3×-c-myc::LEU2 sec4-8/sec4-8 | Derived from NY1373 (Finger et al., 1998) |
| ABY995 | MATa/α ura3-52/ura3-52 leu2-3,112::SEC8:3×-c-myc::LEU2/leu2-3,112::SEC8:3×-c-myc::LEU2 sec10-2/sec10-2 | Derived from NY1389 (Finger et al., 1998) |
| ABY996 | MATa/α ura3-52/ura3-52 leu2-3,112::SEC8:3×-c-myc::LEU2/leu2-3,112::SEC8:3×-c-myc::LEU2 sec15-1/sec15-1 | Derived from NY1390 (Finger et al., 1998) |
| ABY1166 | MATα his3-Δ200 leu2-3,112 lys2-801 trp1-1(am) ura3-52 tpm2::HIS3 3×-HA:RHO1::URA3 | This study |
| ABY1171 | MATa/α his3-Δ200/his3-Δ200 leu2-3,112/leu2-3,112 lys2-801/lys2-801 trp1-1(am)/trp1-1(am) ura3-52::ACT1promoter:GFPS65T:BUD6::URA3/ura3-52::ACT1promoter:GFP S65T:BUD6::URA3 tpm1-2::LEU2/tpm1-2::LEU2 tpm2::HIS3/tpm2::HIS3 SEC8:3×-HA::TRP1/SEC8:3×-HA::TRP1 | This study |
| ABY1197 | MATa/α his3-Δ200/his3-Δ200 leu2-3,112/leu2-3,112 lys2–801/lys2-801 trp1-1(am)/trp1-1(am) ura3-52::SPA2:GFPS65T::URA3/ura3-52::SPA2:GFPS65T::URA3 | This study |
| ABY1198 | MATa/α his3-Δ200/his3-Δ200 leu2-3,112/leu2-3,112 lys2-801/lys2-801 trp1-1(am)/trp1-1(am) ura3-52::SPA2S65T:GFP::URA3/ura3-52::SPA2:GFPS65T::URA3 tpm2::HIS3/tpm2::HIS3 | This study |
| ABY1199 | MATa/α his3-Δ200/his3-Δ200 leu2-3,112/leu2-3,112 lys2-801/lys2-801 trp1-1(am)/trp1-1(am) ura3-52::ACT1promoter:GFPS65T:BUD6::URA3/ura3-52::ACT1promoter:GFPS 65T:BUD6::URA3 tpm2::HIS3/tpm2::HIS3 | This study |
| ABY1601 | MATa/α his3-Δ200/his3-Δ200 leu2-3,112/leu2-3,112 lys2-801/lys2-801 trp1-1(am)/trp1-1(am) ura3-52/ura3-52 tpm2::HIS3/tpm2::HIS3 3×-HA:RHO1::URA3/3×-HA:RHO1::URA3 | This study |
| ABY1602 | MATa/α his3-Δ200/his3-Δ200 leu2-3,112/leu2-3,112 lys2-801/lys2-801 trp1-1(am)/trp1-1(am ura3-52/ura3-52 tpm1-2::LEU2/tpm1-2::LEU2 tpm2::HIS3/tpm2::HIS3 3×-HA:RHO1::URA3/3×-HA:RHO1::URA3 | This study |
| ABY1637 | MATa his3-Δ200 leu2-3,112 lys2-801 trp1-1(am) ura3-52 tpm2::HIS3 cdc10-1 | This study |
| ABY1638 | MATα his3-Δ200 leu2-3,112 lys2-801 trp1-1(am) ura3-52 tpm2::HIS3 cdc10-1 | This study |
| ABY1639 | MATa his3-Δ200 leu2-3,112 trp1-1(am) ura3-52 tpm1-2::LEU2 tpm2::HIS3 cdc10-1 | This study |
| ABY1641 | MATa his3-Δ200 leu2-3,112 lys2-801 trp1-1(am) ura3-52 tpm2::HIS3 cdc12-6 | This study |
| ABY1643 | MATa his3-Δ200 leu2-3,112 lys2-801 trp1-1(am) ura3-52 tpm1-2::LEU2 tpm2::HIS3 cdc12-6 | This study |
| ABY1662 | MATa/α his3-Δ200/his3-Δ200 leu2-3,112/leu2-3,112 lys2-801/lys2-801 trp1-1(am)/trp1-1(am) ura3-52/ura3-52 tpm2::HIS3/tpm2::HIS3 cdc10-1/cdc10-1 | This study |
| ABY1665 | MATa/α his3? his4? leu2-3,112/leu2-3,112 lys2-801/lys2-801 trp1-1(am)/trp1-1(am) tpm2::HIS3/tpm2::HIS3 sec2-41/sec2-41 | This study |
| ABY1801 | MATa/α his3Δ1/his3Δ1 met15? leu2Δ0/leu2Δ0 lys2? ura3Δ0/ura3Δ0 bnr1Δ::KanR/bnr1Δ::KanR | Evangelista et al. (2002) |
| ABY1802 | MATα his3Δ0 leu2Δ0 lys2Δ0 ura3Δ0 bni1Δ::KanR | Evangelista et al. (2002) |
| ABY1803 | MATa/α his3Δ1/his3Δ1 met15? leu2Δ0/leu2Δ0 lys2? ura3Δ0/ura3Δ0 bni1-11::URA3/bni1-11::URA3 bnr1Δ::KanR/bnr1Δ::KanR | Evangelista et al. (2002) |
| ABY1804 | MATα his3Δ leu2 lys2 trp1? ura3 tpm1-2:LEU2 tpm2::HIS3 bnr1Δ::KanR | Evangelista et al. (2002) |
| ABY1805 | MATα his3Δ leu2 lys2 trp1-1(am) ura3 tpm1-2::LEU2 tpm2::HIS3 bni1-12::URA3 | Evangelista et al., 2002 |
| ABY1806 | MATα his3Δ leu2 lys2 trp1? ura3 tpm1-2::LEU2 tpm2::HIS3 bni1-12::URA3 bnr1Δ::KanR | Evangelista et al. (2002) |
Strains ABY1637 (tpm2Δ cdc10-1), ABY1638 (tpm2Δ cdc10-1), ABY1639 (tpm1-2 tpm2Δ cdc10-1), ABY1641 (tpm2Δ cdc12-6), ABY1643 (tpm1-2 tpm2Δ cdc12-6) were generated through crosses between M-195 (cdc10-1) or M-239 (cdc12-6) (both kindly provided by M. Longtine, Oklahoma State University, Stillwater, OK) and ABY944 (tpm1-2 tpm2Δ) or ABY945 (tpm2Δ), followed by backcross of the appropriate isolates to ABY944 or ABY945 and sporulation. ABY1662 (cdc10-1/cdc10-1 tpm2Δ/tpm2Δ) was generated from ABY1637 mated to ABY1638.
For comparison of bni1 and septin mutant phenotypes, ABY1637 was crossed with Y4133 (bni1-11 bnr1Δ) and sporulated to generate ABY2201 (cdc10-1 tpm2Δ), ABY2209 (bni1-11), ABY2218 (bni1-11 bnr1Δ), and ABY2226 (bni1-11 cdc10-1), or progeny were mated appropriately to generate ABY2247 (bni1-11/bni1-11 tpm2Δ/tpm2Δ) and ABY2248 (bni1-11/bni1-11 cdc10-1/cdc10-1 tpm2Δ/tpm2Δ). The retention of tpm2Δ in many of the BNR1+ strains reflects genetic linkage between the TPM2 and BNR1 loci. However, in the presence of wild-type TPM1, we find no effects from tpm2Δ and believe this deletion has not influenced results obtained.
To generate yeast with inducible CLN2, SpeI-linearized pDP134 was transformed into Y1239 (wild-type) and ABY1807 (tpm1-2 tpm2Δ), resulting in integration at the CLN2 locus to yield ABY2251 (GAL-CLN2) and ABY2252 (tpm1-2 tpm2Δ GAL-CLN2). To generate yeast bearing a control plasmid or an inducible NH2-terminally truncated Bni1p or Bnr1p, Y1239 was transformed with pRS316 (Sikorski and Hieter, 1989), p356 (Bni1p residues 452-1953) (Evangelista et al., 2002), or p015, respectively.
For visualization of Sec4p in vivo, ABY1848 (wild type), ABY1867 (bni1Δ/bni1Δ), and ABY1801 (bnr1Δ/bnr1Δ) were transformed with pRC651, a construct identical to reported pRC556 (pRS315::GFP:SEC4; Schott et al., 2002). For visualization of Bni1p in vivo, ABY1848 was transformed with YEp181-BNI1:GFP::KanR.
For visualization of Bud6p, pDP128 was StuI-linearized and transformed into ABY987 (tpm2Δ/tpm2Δ) and ABY988 (tpm1-2/tpm1-2 tpm2Δ/tpm2Δ) for integration at the ura3-52 locus. Sporulation and mating of Ura+ haploids yielded ABY1199 (tpm2Δ/tpm2Δ GFP:BUD6/GFP:BUD6) and ABY1171 (tpm1-2/tpm1-2 tpm2Δ/tpm2Δ GFP:BUD6/GFP:BUD6).
For visualization of Spa2p, pRS406S2G bearing a COOH-terminal GFPS65T-fusion of SPA2 (Arkowitz and Lowe, 1997) was StuI linearized and integrated at the ura3-52 locus in ABY971 (tpm1-2/tpm1-2 tpm2Δ/tpm2Δ) and ABY973 (tpm2Δ/tpm2Δ) to generate ABY1197 (tpm1-2/tpm1-2 tpm2Δ/tpm2Δ SPA2:GFP/SPA2:GFP) and ABY1198 (tpm2Δ/tpm2Δ SPA2:GFP/SPA2:GFP).
For visualization of Cdc12p, pDP135 was MluI linearized and integrated at one CDC12 locus of ABY1848 to generate ABY1896 (CDC12/CDC12::URA3::GFP:CDC12), into ABY1662 to generate ABY2257 (CDC12/CDC12::URA3::GFP:CDC12 cdc10-1/cdc10-1 tpm2Δ/tpm2Δ), into ABY1867 to generate ABY1898 (bni1Δ/bni1Δ CDC12/CDC12::URA3::GFP:CDC12), and into ABY1807 to generate ABY1897 (tpm1-2 tpm2Δ CDC12::URA3::GFP:CDC12). ABY1897 was mated with ABY1894 (MATα tpm1-2 tpm2Δ) to generate ABY1899 (tpm1-2/tpm1-2 tpm2Δ/tpm2Δ CDC12/CDC12::URA3:: GFP:CDC12).
For visualization of Bnr1p, one chromosomal copy of BNR1 in YEF473 (Bi and Pringle, 1996) was COOH-terminally tagged in-frame with GFPF64L/S65T by a PCR-based method (Longtine et al., 1998). This strain was sporulated and appropriate progeny mated to generate YEF2255 (BNR1:GFP/BNR1:GFP). To test for functionality of BNR1:GFP, YEF2255 was sporulated to yield haploid ABY1882. This strain was mated to ABY1802 (bni1Δ), and the resultant diploid sporulated. No lethality of the progeny was observed, and the bni1Δ phenotype of wide necks and rounded buds and the Bnr1pGFP-associated fluorescence segregated 2:2 independently, showing no synthetic growth deficits. However, isolation of the homozygous ABY2256 (bni1Δ/bni1Δ BNR1:GFP/BNR1:GFP) was extremely difficult, even through ABY2256 displayed no growth defect beyond that expected for bni1Δ/bniΔ cells, and the haploid bni1Δ BNR1:GFP were able to mate with wild-type cells. Because no similar difficulty had been observed in previously isolating ABY1867, Bnr1pGFP may specifically be defective in supporting mating compared with Bnr1p.
To visualize Bnr1p in conditional tropomyosin mutants, ABY1882 was transformed with NdeI/KpnI-linearized pDP116 to generate ABY1885 (tpm2Δ::TRP1 BNR1:GFP), and then transformed with BsmAI/BcgI-linearized pDP115 (Pruyne et al., 1998), to generate ABY1886 (tpm1-2::LEU2 tpm2Δ::TRP1 BNR1:GFP). TPM2 deletion was confirmed by Western analysis, and tpm1-2 replacement confirmed by observation of rapid temperature-sensitive cable disassembly and Myo2p delocalization. Diploid ABY1891 (tpm1-2/tpm1-2 tpm2Δ/tpm2Δ BNR1:GFP/BNR1:GFP) was generated by transformation of ABY1886 with YCp50-HO12 mating-type switching plasmid (Russell et al., 1986), followed by isolation of HO12-free yeast on 5-fluorootic acid–containing medium.
To visualize Bnr1p in a conditional septin mutant, ABY1637 was mated to ABY1881 (a haploid segregant of YEF2255), and the resultant diploid sporulated. Temperature-sensitive, KanR segregants were remated to isolate the homozygous diploid ABY2262 (cdc10-1/cdc10-1 BNR1:GFP/BNR1:GFP).
For visualization of RHO1, a fusion of RHO1 upstream DNA sequences (–161 to –1), followed by ATG and a triple hemagglutinin (HA)-tag, followed by the RHO1 ORF and downstream sequence to +766, followed by URA3, followed by further RHO1 downstream sequence (+767 to +876) was generated by five PCR reactions by using the primer pairs GATCATTCCTCTGAGTATTG and CTTTCTAGTATAATTTTTAAAGTTCTATG (with genomic DNA template), AAAAATTATACTAGAAAGATGGCCTACCCATACGATGTT and GTTACCAACTTGTTGTGACATGCACTGAGCAGCGTAATC (with pCS124 template; kindly provided by C. Shamu, Harvard Medical School, Cambridge, MA), ATGTCACAACAAGTTGGTAAC and CTGGGAGAAAAACAAGT (genomic DNA template), ACTTGTTTTTCTCCCAGGCAGATTGTACTGAGAGTGC and GAATAAGATTATACACATAGATGCGGTATTTTCTCCT (pRS306 template), and TATGTGTATAATCTTATTC and GGATACGCACTTGTAAAT (genomic DNA template), followed by stepwise combination of products by fusion PCR. The final product was transformed into ABY946 (tpm2Δ) to replace RHO1 at the genomic locus, generating ABY1166 (tpm2Δ HA3RHO1::URA3). Replacement and functionality of HA3:RHO1 were confirmed by PCR amplification of the RHO1 locus, sequencing of the RHO1 ORF, demonstration of normal morphology at 18 and 37°C, normal polarization of Myo2p at 18 and 34.5°C, and normal Myo2p depolarization at 38°C. Through mating ABY1166 with ABY944, followed by sporulation and mating of progeny, ABY1601 (tpm2Δ/tpm2Δ HA3RHO1/HA3RHO1) and ABY1602 (tpm1-2/tpm1-2 tpm2Δ/tpm2Δ HA3RHO1/HA3RHO1) were isolated.
For ease of immunofluorescence imaging, previously described haploid myo2 and sec strains were converted to diploids (Table 1). ABY1665 (sec2-41/sec2-41 tpm2Δ/tpm2Δ) was obtained through crossing of ABY945 and NY132 (sec2-41) and subsequent sporulation and mating of progeny.
Production of GSTBnr1p(FH1FH2) Recombinant Protein
Protease-deficient bacterial strain ER2508 (New England Biolabs, Beverly, MA) was transformed with either pGEX-6P-3 or p080 and grown in 2-liter culture at 30°C to OD600 = 1.0 before induction with 1 mM isopropyl β-d-thiogalactoside for 3 h at 30°C. Cultures were resuspended in GST-buffer A (20 mM Tris-HCl, pH 7.4, 0.2 mM EDTA, 1 M NaCl) with 1 mM dithiothreitol (DTT) and protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO), and then sonicated. High-speed supernatants were incubated against glutathione-agarose (Sigma-Aldrich), washed, and eluted in GST-buffer C (20 mM Tris-HCl, pH 7.4, 0.02 mM EDTA, 0.1 M NaCl, 5 mM reduced glutathione).
Pyrene-Actin Polymerization Assays
Equal amounts of purified rabbit muscle actin (purified as described by MacLean-Fletcher and Pollard, 1980) and pyrene rabbit muscle actin (Cytoskeleton, Denver, CO) were combined in G-buffer (10 mM Tris-HCl, pH 7.5, 0.2 mM MgCl2, 1 mM DTT, 0.5 mM ATP) and precleared at 180,000 × g for 100 min at 4°C before use. Varying concentrations of GST or GSTBnr1p(FH1FH2) were combined with 3 μM actin and polymerization was induced by addition of F-buffer (giving final concentrations of 0.5 mM ATP, 5 mM MgCl2, 12.5 mM KCl). Pyrene fluorescence at 407 nm was monitored under excitation at 365 nm with a model 814 photomultiplier detection system (Photon Technology International, Monmouth Junction, NJ).
Generation of Bni1p-specific Antibodies
A GST-fusion protein containing the Bni1p residues 1227–1953 was expressed from p4565 in BL21 Escherichia coli and purified as described previously (Pruyne et al., 2002). Protein was resolved by SDS-PAGE, excised, eluted, and dialyzed against phosphate-buffered saline (PBS), yielding 3 μg of protein for injection into two rabbits. Collected antiserum from one rabbit diluted 1:2000 recognized a 9E10 (anti-myc) reactive band on a blot of yeast extract producing Bni1p-myc (Evangelista et al., 2002), which was absent from noninduced yeast extract and which was not recognized by preimmune serum. Affinitypurified serum was produced by incubation of crude serum with GST-Bni1p (1227–1953) CNBr-coupled to Sepharose beads (Sigma-Aldrich), followed by elution with 0.1 M glycine-HCl, pH 2.5, and buffering to pH 7.4 with unbuffered 1 M Tris. Immunofluorescence of cells overexpressing Bni1p-myc showed complete costaining of anti-Bni1p and 9E10. Wild-type and bni1Δ yeast showed diffuse cytoplasmic staining and a faint nuclear stain, but only wild-type cells showed a polarized stain characteristic of Bni1p.
Light Microscopy and Imaging
Immunofluorescence microscopy for Tpm1p, Myo2p, HA epitope, and Cdc42p was performed as described previously (Pruyne et al., 1998; Kozminski et al., 2000). Immunofluorescence staining for GFP-fusions of Bud6p, Cdc12p, and Spa2p by using GFP-specific antibodies (kindly provided by P. Silver, Dana-Farber Cancer Institute, Boston, MA) was performed in the same manner, with the primary antibodies diluted 1:250 in PBS/1% bovine serum albumin. Anti-Bni1p staining or anti-GFP staining for Bnr1pGFP required 30-min fixation, and 3-h incubation with primary antibodies. Anti-Bni1p was diluted 1:30. Cells were observed on an Axiovert 100 TV microscope (Carl Zeiss, Thornwood, NY), and images were captured through using an RTE (round thermoelectric passive)/chargecoupled device (CCD) digital camera (Princeton Scientific Instruments, Monmouth Junction, NJ), and processed using the MetaMorph Imaging System (Universal Imaging, Downingtown, PA).
For fluorescence microscopy of Bni1pGFP, Bnr1pGFP, or GFPCdc12p in fixed cells, yeast were fixed for 30 s by direct addition of 37% HCHO to a final concentration of 5%, and then washed twice in equal volumes of PBS and resuspended in 1/10 volume of PBS. Images were acquired and processed as for immunofluorescence.
For live cell fluorescence microscopy of cells expressing GFPSec4p, Bni1pGFP, or Spa2pGFP, cells were mounted on 2% agarose containing standard synthetic complete medium and observed at 22°C under on a Nikon eclipse TE-2000U microscope (Nikon, Tokyo, Japan) by using the UltraVIEW LCI confocal imaging system (PerkinElmer Life and Analytical Sciences, Boston, MA). Movies of single focal planes were acquired through consecutive 80-ms exposures by using a C4742-95-12ERG 12-bit digital output CCD camera (Hamamatsu Photonics, Bridgewater, NJ). All still images presented were processed nonlinearly through Adobe Photoshop (Adobe Systems, Mountain View, CA) to reduce background haze but preserve faint structures.
Assays for Scoring Cytoskeletal Organization and Membrane Trafficking
For indicated assays, yeast cells were visually assigned as unbudded, small-budded (bud length <1/3 mother cell length), medium-budded (1/3 mother cell length ≤ bud length <2/3 mother cell length), or large-budded (bud length ≥2/3 mother cell length). For scoring the distribution of actin cables in unbudded cells, cells treated for Tpm1p immunofluorescence were visually scored for the presence or absence of cables associated with a single point on the cell cortex. For assessing actin cable distribution for cells of other budding categories were visually scored based on whether Tpm1p displayed a strong stain in the bud, a strong association with cables that intersected the bud neck, a combination of the two, or neither.
For assessing the location of actin cable assembly or the location of Myo2p, Bni1p, HARho1p, GFPBud6p, Spa2pGFP, Cdc42p, Bnr1pGFP, or GFPCdc12p in budded cells, cells of the appropriate budding categories stained for the appropriate epitope were visually scored based on whether the stain was concentrated over background levels at the bud tip, in puncta along the bud cortex, at the bud neck, at a combination of the bud tip and neck, or at none of these. For assessing the distribution of any of these markers except GFPCdc12p in unbudded cells, cells were scored based on whether the stain was localized to a single point on the cell cortex or was delocalized. GFPCdc12p localization in unbudded cells was categorized as either associated with a large ring indicative of a cytokinetic remnant, with a small patch or ring presumed to reflect the nascent bud site, with both structures, or with neither.
For scoring GFPSec4p-associated particle density, movies of 19 small- or medium-budded cells of each strain acquired at a focal plane containing the bud neck were examined frame-by-frame by using QuickTime Player (Apple Computer, Cupertino, CA). The mother cells were visually divided into halves near or away from the bud. The number of discrete GFPSec4p-associated particles in each half was counted every 2 s over the 40-s movies. True fluorescent particles were discriminated from noise by observation of three successive movie frames (encompassing ∼0.24 s), with fluorescent bodies in-focus in at least one frame and present in at least two consecutive frames being considered true particles. Cell areas were determined by importing a representative frame from each movie into MetaMorph Imaging System (Universal Imaging) and measuring the area in pixels, with cell areas calculated based on the TIF pixels corresponding to a calculated 0.00416 μm2 based on a 6.45 × 6.45-μm camera pixel size and use of a 100× objective.
For scoring the likelihood of GFPSec4p-associated particles to undergo directed movements, movies of 23 wild-type, 25 bnr1Δ/bnr1Δ, and 19 bni1Δ/bni1Δ were observed for 5-s intervals, and fluorescent particles were scored as positive if seen to commence movement along a single straight or curved trajectory for ≥1/3 the mother cell length over four consecutive frames, and as negative if they disappeared or exhibited only random motions.
RESULTS
Bni1p and Bnr1p Maintain Distinct Sets of Actin Cables
Although Bni1p and Bnr1p show partial redundancy with each other for actin cable assembly, only Bni1p has been shown to stimulate actin assembly in vitro or in vivo. In the absence of regulatory NH2-terminal sequences, overexpression of the Bni1p FH1 and FH2 domains in yeast stimulates assembly of cable-like filaments (Evangelista et al., 1997, 2002). To determine whether Bnr1p exhibits the same properties, we overexpressed NH2-terminally truncated Bnr1p (residues 386-1375) in wild-type cells and stained for actin (Act1p) distribution by immunofluorescence microscopy. Cells expressing this Bnr1p construct showed an overabundance of actin within the bud to the degree that discrete cortical actin patches were no longer visible, just as occurs with overexpression of NH2-terminally truncated Bni1p (Figure 1A), indicating the Bnr1p COOH-terminal region, including the FH1 and FH2 domains can stimulate the assembly of cable-like filaments in vivo.
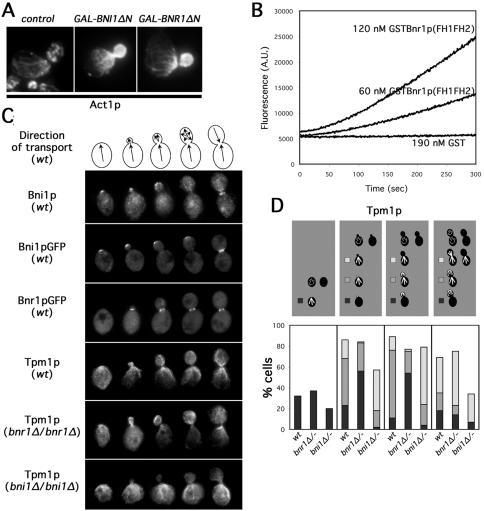
Figure 1.
Bni1p and Bnr1p are important for distinct sets of actin cables. (A) Wild-type cells (Y1239) were grown 2 h in galactose to induce a control vector or NH2-terminally truncated Bni1p (BNI1ΔN) or Bnr1p (BNR1ΔN) and stained for Act1p distribution by immunofluorescence microscopy. (B) Recombinant GST (190 nM) or GSTBnr1p(FH1FH2) (60 or 120 nM) were combined with 3 μM G-actin (∼5% pyrene labeled), and actin assembly was monitored through increases in pyrene fluorescence (measured in arbitrary units). (C) Models depict the direction of polarized transport in yeast at various stages of bud growth that correspond with cell images below. Bni1p is shown in selected wild-type cells (ABY1848) treated for anti-Bni1p immunofluorescence or expressing Bni1pGFP from a high-copy plasmid. Bnr1pGFP fluorescence is shown in selected wild-type cells (YEF2255). The distribution of actin cables in selected wild-type (ABY1848), bnr1Δ/bnr1Δ (ABY1801), and bni1Δ/bni1Δ (ABY1867) cells is shown by anti-Tpm1p immunofluorescence microscopy. (D) One hundred cells of each indicated budding categories of wild-type (ABY1848), bnr1Δ/bnr1Δ (ABY1801), and bni1Δ/bni1Δ (ABY1867) strains were processed for localization of Tpm1p by immunofluorescence microscopy as a reporter for actin cable organization. Unbudded cells scored as either having organized cables (black) or disorganized cables (blank). Budded cells were categorized as having unorganized cables (blank), cables predominantly associated with the bud cortex (black), cables associated with bud cortex and neck (gray), or cables predominantly associated with the bud neck (white).
In vitro, the Bni1p FH2 domain can nucleate actin filaments from monomers, with the FH1 enhancing the efficiency of this reaction (Pruyne et al., 2002; Sagot et al., 2002b). To determine whether the Bnr1p FH1 and FH2 domains also nucleate actin filaments in vitro, a recombinant GST-fusion construct encompassing the Bnr1p FH1 and FH2 domains plus COOH-terminal sequences (residues 757-1375) was examined for the ability to accelerate the assembly of pyrene-conjugated actin (Figure 1B). As has been seen for similar Bni1p-derived constructs, this construct stimulated the polymerization of actin from monomers, whereas GST did not. Thus, the conserved COOH-terminal regions of the two yeast formins stimulate actin assembly.
Based on studies of overexpressed Bni1p and Bnr1p, the NH2 termini of these formins help confer distinct localizations on the two (Jansen et al., 1996; Evangelista et al., 1997; Fujiwara et al., 1998; Kamei et al., 1998; Ozaki-Kuroda et al., 2001). We wished to examine whether these localizations impart differences in the distribution of the actin filaments assembled by the two isoforms. However, overexpression of either formin alters actin organization and cell morphology (Kamei et al., 1998; Evangelista et al., 2002; Sagot et al., 2002a). To avoid this complication, we observed the distribution of the formins and formin-dependent actin filaments in cells that expressed Bni1p and Bnr1p at endogenous levels.
Immunofluorescence staining of endogenous Bni1p agreed with the reported distribution for overexpressed epitope-tagged Bni1p, concentrating at the nascent bud sites of unbudded cells, at the tips of small buds, in a crescent over the surfaces of medium buds, and at the septal planes between dividing cells (Figures 1C and S1A). A COOH-terminal GFP-fusion of full-length Bnr1p expressed from the BNR1 locus also displayed a localization in agreement with previous overexpression studies (Kamei et al., 1998), showing a polarized distribution in very few unbudded cells (5% of unbudded cells), but a ring of fluorescence around the neck of cells with buds of all sizes (Figures 1C and S1A).
In yeast, formin-dependent actin filaments can be distinguished from the Arp2/3 complex-dependent filaments by their association with the F-actin binding protein tropomyosin, encoded by the TPM1 and TPM2 genes (Liu and Bretscher, 1989; Pruyne et al., 1998; Evangelista et al., 2002; Sagot et al., 2002a; Tolliday et al., 2002). To determine whether Bni1p or Bnr1p make distinct contributions to assembling these filaments, we examined Tpm1p distribution in wild-type yeast and strains lacking Bni1p (bni1Δ/bni1Δ) or Bnr1p (bnr1Δ/bnr1Δ) (Figure 1, C and D). Importantly, for both deletion strains, the localization of the remaining formin isoform was not perturbed compared with wild-type cells (Figure S1A and B).
In asynchronous wild-type cultures, unbudded cells are either undergoing depolarized growth or bud initiation, and thus, Tpm1p was present in either disorganized cables or cables radiating from the nascent bud site. Unbudded bnr1Δ/bnr1Δ cells showed a similar distribution as wild-type yeast, whereas unbudded bni1Δ/bni1Δ cells displayed a higher incidence of unorganized cables, and when filaments were organized, they formed a patch at one pole rather than an array of cables (Figure 1, C and D). Thus, Bni1p, which is polarized in unbudded cells, is important for organizing actin cables in these cells, whereas Bnr1p, which is largely depolarized in these cells, is not important for this.
Cells with small- and medium-sized buds are undergoing bud growth. In wild-type cells, Tpm1p-stained cables formed a meshwork in the bud that was concentrated at the tip, and an array that radiated from the bud neck into the mother cell (Figure 1, C and D). In bnr1Δ/bnr1Δ cells, the cable meshwork in the bud was present, but the proportion of cells with prominent cables in the mother (Figure 1D, gray + white bars) was reduced from 76% of wild-type cells to 25% of bnr1Δ/bnr1Δ cells. In contrast, cables were prominent in bni1Δ/bni1Δ mother cells, but the percentage with Tpm1p stain in the bud (Figure 1D, gray + black bars) was reduced from 72% of wild-type cells to 21% of bni1Δ/bni1Δ cells. Thus, there are correlations between bud-associated cables and Bni1p activity and between neck-associated cables and Bnr1p activity.
Large-budded cells are either continuing bud growth, undergoing cytokinesis, or depositing new cell wall between divided cells. Thus, Tpm1p-staining in wild-type cells either resembled small- and medium-budded cells, labeled a contractile ring around the neck plus disorganized cables, or labeled cables radiating from the septal plane into the mother and daughter cells (Figure 1, C and D). Both formins support the assembly of the contractile ring (Vallen et al., 2000), so we did not examine that structure. Cables in bnr1Δ/bnr1Δ cells were similar to wild-type yeast, but they were more likely to be disorganized in bni1Δ/bni1Δ cells (Figure 1, C and D).
These results show that Bni1p and Bnr1p have distinct functions in organizing actin cables. Although the two formins show some ability to compensate for the loss of the other isoform (Figure 1D), Bni1p shows a strong preference for maintaining cables at the nascent bud site and along the growing bud cortex, whereas Bnr1p shows a strong preference for maintaining neck-associated cables in the mother during bud growth.
Yeast Lacking Bni1p or Bnr1p Exhibit Myosin-V Transport Defects
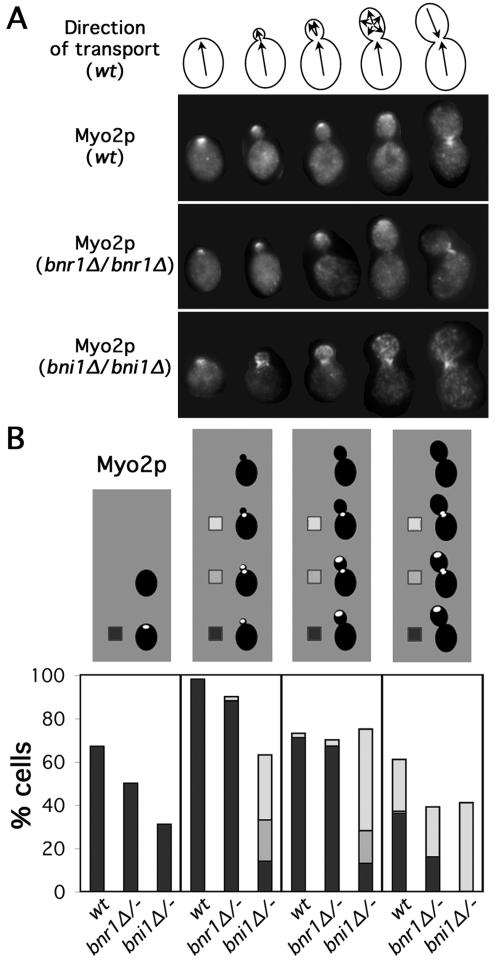
The myosin-V Myo2p rapidly translocates along actin cables to concentrate at growth sites (Johnston et al., 1991; Lillie and Brown, 1994; Pruyne et al., 1998; Schott et al., 2002). We examined the distribution of Myo2p in wild-type, bni1Δ/bni1Δ, and bnr1Δ/bnr1Δ cells by immunofluorescence staining (Figure 2, A and B). For unbudded cells of all three types, Myo2p localized to a patch that presumably marked the nascent bud site. For all three, the proportion of cells with Myo2p was greater than the proportion that showed polarized cables (Figure 1D), which we believe reflects the greater ease at detecting a single spot of Myo2p than resolving the overall organization of cables that extend throughout the cell. Consistent with the poor organization of actin cables in unbudded bni1Δ/bni1Δ yeast compared with wild-type or bnr1Δ/bnr1Δ yeast, unbudded bni1Δ/bni1Δ cells showed a reduced proportion of cells with properly organized Myo2p compared with the other two strains (Figure 2B). In wild-type and bnr1Δ/bnr1Δ cells, Myo2p also associated with the growing bud tip and the septal plane of dividing cells, but in bni1Δ/bni1Δ cells with buds, Myo2p localized to the bud neck and diffusely over the bud cortex, but almost never concentrated at the bud tip (Figure 2, A and B).
Figure 2.
Myo2p localization is perturbed in bni1Δ cells. (A) Models as in Figure 1A are depicted with selected wild-type (ABY1848), bnr1Δ/bnr1Δ (ABY1801), and bni1Δ/bni1Δ (ABY1867) cells stained to show Myo2p distribution. (B) One hundred cells of each budding category treated as described in A were scored for Myo2p localization. Unbudded cells were categorized as having Myo2p delocalized (blank) or localized to one site on the cell surface (black). Budded cells were scored as having Myo2p localized to the bud cortex (though not necessarily the bud tip) (black), the bud cortex and neck (gray), localized to the bud neck (white), or delocalized (blank).
Myo2p polarizes growth by delivering secretory vesicles along actin cables (Johnston et al., 1991; Walch-Solimena et al., 1997; Pruyne et al., 1998; Schott et al., 1999). The in vivo movement and distribution of vesicles can be observed using a GFP-tagged secretory vesicle-associated rab-GTPase Sec4p (Goud et al., 1988; Mulholland et al., 1997; Walch-Solimena et al., 1997; Schott et al., 2002). GFPSec4p occurs in large fluorescent masses within yeast cells, as well as discrete fluorescent particles in the mother cell. The large masses are interpreted to correspond to the cluster of post-Golgi secretory vesicles accumulated at growth sites as seen by electron microscopy, whereas the discrete particles are interpreted to be individual or small clusters of vesicles (Schott et al., 2002).
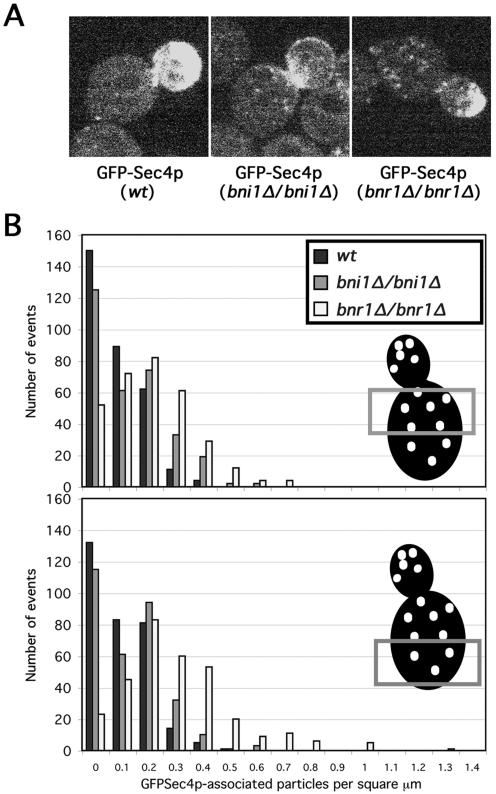
We expressed GFPSec4p in wild-type, bni1Δ/bni1Δ, and bnr1Δ/bnr1Δ yeast, and observed living cells (for sample 12-s movies, see Mov. S1–3). GFPSec4p accumulated at the bud tips of small- and medium-budded wild-type and bnr1Δ/bnr1Δ cells, whereas in bni1Δ/bni1Δ cells, GFPSec4p concentrated at the bud neck, with a more diffuse accumulation over the bud cortex, consistent with the Myo2p distribution in these cells (Figure 3A). When the discrete particles in the mother cell were examined for each strain, we found that in the half of the mother cell closer to the bud neck, wild-type yeast displayed an average density of 0.06 vesicles/μm2 (with a SD of 0.08 vesicles/μm2), bni1Δ/bni1Δ yeast showed 0.10 ± 0.11 vesicles/μm2, and bnr1Δ/bnr1Δ cells showed 0.17 ± 0.13 vesicles/μm2 (315 observations for each strain). When the density of discrete GFPSec4p-associated particles in the half of the mother cell further from the bud neck was examined, wild-type cells showed 0.07 ± 0.08 vesicles/μm2, bni1Δ/bni1Δ showed 0.10 ± 0.10 vesicles/μm2, and bnr1Δ/bnrΔ showed 0.25 ± 0.20 vesicles/μm2 (315 observations for each strain). Thus, bnr1Δ/bnr1Δ cells tended to have an increased number of visible vesicles dispersed in the mother cell compared with BNR1+ strains, particularly in the region further from the bud neck (Figure 3, A and B).
Figure 3.
Bni1Δ and bnr1Δ yeast have unique perturbations in vesicle dynamics. (A) Still GFPSec4p fluorescence images from movies of living wild-type (ABY1848; Mov. S1), bni1Δ/bni1Δ (ABY1867; Mov. S2), and bnr1Δ/bnr1Δ (ABY1801; Mov. S3) cells are shown. (B) Results of density counts for individual fluorescent particles from individual frames from 19 movies of GFPSec4p-expressing small- or medium-budded cells of each strain in A are summarized. The density of discrete particles counted in the indicated portions of each mother cell was plotted against the number of times that density was counted (with 315 total counting events for mother cell-half for each strain).
The discrete GFPSec4p-associated particles alternatively undergo Brownian motion or Myo2p-dependent directed movements (Schott et al., 2002). We examined whether the three yeast strains exhibited different tendencies in the behavior of the fluorescent particles. When small- and medium-budded cells were examined for 5-s intervals, GFPSec4p-associated particles had a 24% chance to engage in directed movements in wild-type cells (71 of 270 observations), 29% chance in bni1Δ/bni1Δ cells (147 of 500 observations), but only 10% chance in bnr1Δ/bnr1Δ cells (96 of 920 observations). For wild-type and bnr1Δ/bnr1Δ cells, these directed movements were from the mother cell into the bud, whereas for bni1Δ/bni1Δ cells, 17% of directed movements were away from the bud, suggesting some cables were misoriented. These results together indicate Bnr1p promotes efficient engagement of Myo2p-associated cargoes in directed transport in the mother cell, whereas Bni1p promotes Myo2p-dependent traffic from the bud neck to the bud tip.
Formin-stimulated Filament Assembly Occurs at the Bud Cortex and Neck
The ability of formins to stimulate actin assembly in vitro suggests that formin-rich regions of the cell might correspond with cable filament assembly sites. Consistent with this, actin cables decorated in vivo with a GFPAbp140p fusion protein show that new material incorporates into cables somewhere near their bud-directed ends (Yang and Pon, 2002), but the high density of cables in the bud and neck in a growing yeast cell prevents resolution of the exact filament assembly sites.
To identify the assembly sites of actin cable filaments, we examined yeast with temperature-sensitive actin cables. When yeast with conditional tropomyosin mutations (tpm1-2 tpm2Δ) are shifted from 18 to 34.5°C, actin cables disassemble in 1 min, but when restored to 18°C, staining for Tpm1p reveals cable reassembly after 1 min (Pruyne et al., 1998). With cable disassembly followed by 1 min of filament reassembly at 18°C, the background of preformed cables is eliminated to reveal only newly assembled filaments at assembly sites. When tpm1-2/tpm1-2 tpm2Δ/tpm2Δ cells were shifted to 34.5°C for 2 min to disassemble actin cables, and then returned to 18°C for 1 min and stained for Tpm1p distribution, filament assembly was seen at the nascent bud site of unbudded cells, at the bud cortex and neck of small- and medium-budded cells, and at the bud neck/septal plane of large-budded cells (small- and medium-budded cells shown in Figure 4A).
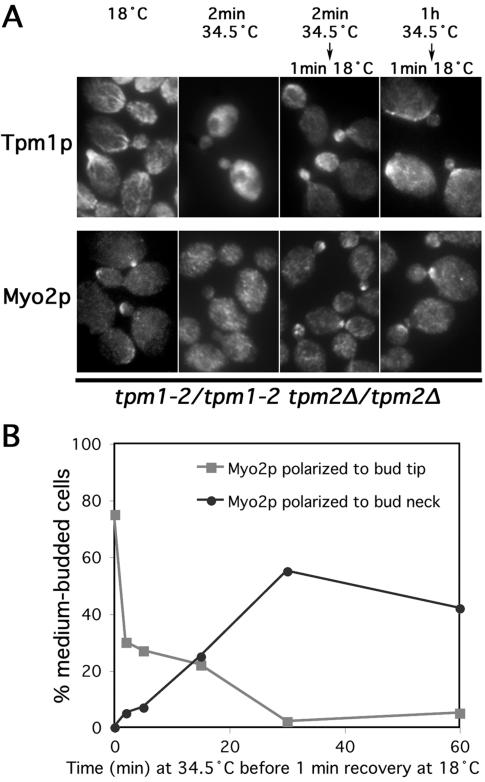
Figure 4.
Actin cable filaments assemble at the bud cortex and neck. (A) Tpm1-2/tpm1-2 tpm2Δ/tpm2Δ mutants (ABY971) were subjected to the indicated conditions and stained to show Tpm1p and Myo2p distributions. (B) Tpm1-2/tpm1-2 tpm2Δ/tpm2Δ cells (ABY971) were shifted to 34.5°C for varying lengths of time before restoration to 18°C for 1 min and treatment for anti-Myo2p immunofluorescence microscopy. One hundred medium-budded cells from each time point were scored for the presence of Myo2p at the bud tip (gray squares) or neck (black circles).
Myo2p provides another probe for actin cable assembly sites. In this assay, Myo2p delocalizes after 2 min of cable disassembly, but it begins to repolarize at cable assembly sites after 1 min at 18°C (Pruyne et al., 1998). With filament disassembly and reassembly in these cells, Myo2p began to reappear at the nascent bud site, at small- and medium-sized bud tips, and at the septal plane (small-, medium-, and large-budded cells shown in Figure 4A), indicating the newly formed filaments are functional cables capable of recruiting the myosin. Both sets of results show a correlation between cable assembly sites and formin-rich regions of the cell cortex (the bud cortex and neck).
To examine the persistence of these filament assembly sites, cables were disassembled in tpm1-2/tpm1-2 tpm2Δ/tpm2Δ cells for varying periods of time at 34.5°C before returning to 18°C for 1 min. After a prolonged absence of actin cables (1 h), cable reassembly and Myo2p recruitment at 18°C occurred at the bud neck rather than the bud cortex (Figure 4A). Looking specifically at Myo2p distribution, we found that the proportion of tpm1-2/tpm1-2 tpm2Δ/tpm2Δ cells that were able to recruit the myosin to the bud cortex decreased to near zero after 30 min at 34.5°C, whereas the proportion that recruited Myo2p to the neck increased over the same period (Figure 4B), indicating that the ability to assemble cables at the neck persisted through this time, but the ability to assemble cables at the bud cortex (which are required to transport Myo2p to the bud tip) was lost under these conditions.
However, this was not a permanent reorientation of the actin cytoskeleton to the neck for many cells. When tpm1-2/tpm1-2 tpm2Δ/tpm2Δ cells were shifted to 34.5°C for 1 h, and then restored to 18°C for 20 min, Myo2p returned to the bud tip in the majority of small-budded cells (78%, compared with 95% with tip-associated Myo2p before loss of cables), whereas tip-directed polarity was regained in a smaller proportion of medium-budded cells (23%, compared with 62% before with tip-associated Myo2p before loss of cables). Thus, the neck-associated cable assembly site is persistent, whereas the cortex-associated cable assembly site is not under these conditions.
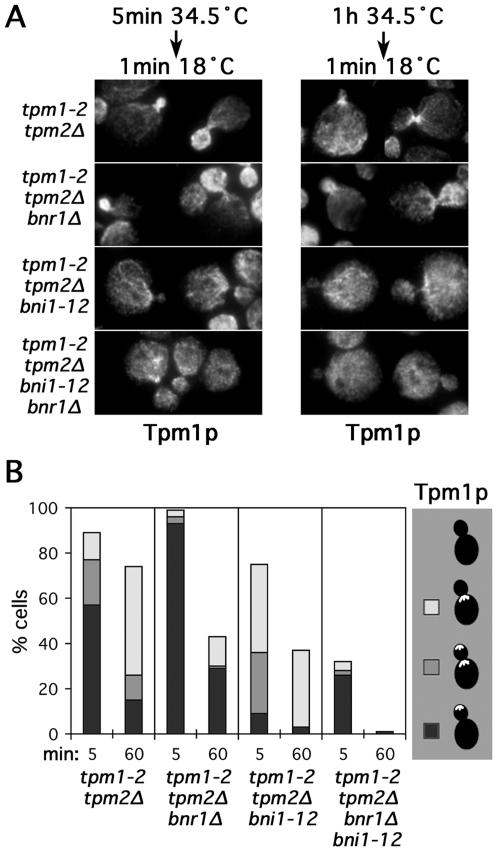
To determine whether bud cortex- and neck-associated filament assembly correlated with the activity of the appropriate formin, we examined cable assembly in tpm1-2 tpm2Δ yeast bearing bnr1Δ and/or an irreversible temperature-sensitive mutation of BNI1 (bni1-12) (Evangelista et al., 2002). These strains were subjected to short (5-min) or long (60-min) shifts to 34.5°C before returning to 18°C for 1 min (Figure 5, A and B). In tpm1-2 tpm2Δ bnr1Δ cells after the short shift, filament reassembly generally occurred at the bud cortex and not at the bud neck, and after the long shift, assembly was either disorganized or occurred at the bud cortex and not the neck (Figure 5B, black bars), in contrast to tpm1-2 tpm2Δ controls, where assembly was primarily at the neck (Figure 5B, white bars), showing the absence of Bnr1p diminishes neck-associated filament assembly. The remaining neck-associated assembly suggests that in some cells, Bni1p is present at this location, despite our inability to detect it. For the tpm1-2 tpm2Δ bni1-12 cells, filament assembly at the bud cortex was reduced compared with control tpm1-2 tpm2Δ cells after short and long shifts (Figure 5B, black + gray bars), indicating the importance of Bni1p for bud cortex-associated filament assembly, although again a small amount of residual activity at the bud cortex suggests an undetectable amount of Bnr1p is present at that location. Finally, tpm1-2 tpm2Δ bni1-12 bnr1Δ cells were nearly unable to assemble cables from the neck under any condition (Figure 5B, absence of gray + white bars), and after the long shift (when bni1-12 was completely inactivated), were unable to assemble cables at all, consistent with the formin dependence of cable assembly. This correlation between the respective formins and distinct sites of filament assembly suggests the presence of formins at these sites stimulates actin cable assembly there and provides an explanation for the dependence of subsets of actin cables on the two formin isoforms.
Figure 5.
Bni1p and Bnr1p differentially direct bud cortex- and bud neck-associated filament assembly. (A) Strains bearing tpm1-2 tpm2Δ (ABY944), tpm1-2 tpm2Δ bnr1Δ (ABY1804), tpm1-2 tpm2Δ bni1-12 (ABY1805), and tpm1-2 tpm2Δ bni1-12 bnr1Δ (ABY1806) mutations were subjected to the indicated conditions and stained to show Tpm1p distribution. (B) One hundred medium-budded cells of each strain in A were shifted to 34.5°C for the indicated times before restoration to 18°C for 1 min and staining to show Tpm1p distribution. Cells were scored for the presence of Tpm1p in the bud (black), in the bud and at the neck (gray), at the neck (white), or delocalized (blank). Results for small-budded cells were similar.
Actin Assembly at the Bud Neck Requires the Septins
The persistence of cable assembly activity at the bud neck suggests it associates with a stable landmark. A ring of proteins called septins, encoded by the genes CDC3, CDC10, CDC11, CDC12, and SHS1, is a prominent landmark of the neck. Septins are recruited to the nascent bud site in a Cdc42p-dependent manner, and then remain at that location as a ring around the neck of the emerging and growing bud, and finally divide into two rings at cell separation (Figure S2A and B; for review, see Field and Kellogg, 1999; Gladfelter et al., 2001; Faty et al., 2002; Longtine and Bi, 2003). However, the septins play a variety of roles, promoting the proper activation of the B-cyclins for cell cycle progression (Barral et al., 1999; McMillan et al., 1999; Shulewitz et al., 1999; Longtine et al., 2000; Hanrahan and Snyder, 2003), maintaining a diffusion barrier between the bud and mother cortices (Barral et al., 2000), and recruiting cytokinetic (Bi et al., 1998; Lippincott and Li, 1998) and cell wall-synthesizing machinery (DeMarini et al., 1997) to the bud neck. To determine whether septins influence actin cable organization, we examined Tpm1p distribution in yeast with a temperature-sensitive CDC10 septin (cdc10-1) (Hartwell, 1971).
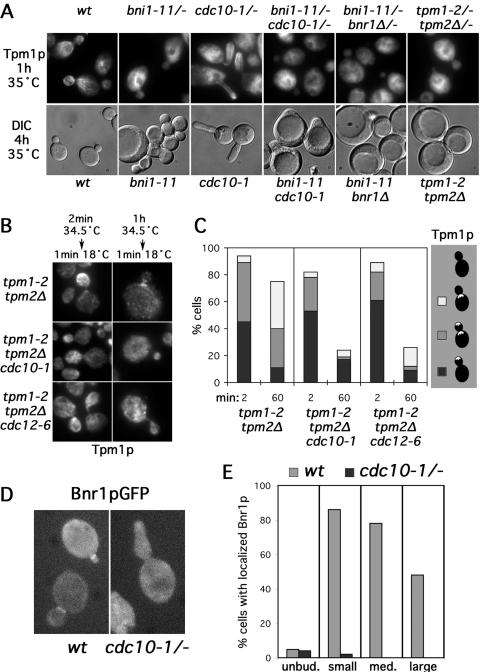
When cdc10-1/cdc10-1 cells were shifted to 35°C for 1 h, the septin scaffold at the bud neck disassembled completely as judged by the absence of a NH2-terminally GFP-tagged Cdc12p septin from the neck, with GFPCdc12p being largely delocalized or showing a very faint enrichment at cell tips (Figure S3, white arrows). Under these conditions, the cdc10-1/cdc10-1 cells displayed prominent cables at the tip and along the sides of the bud, but rarely at the bud neck or in the mother (Figure 6A), similar to bnr1Δ cells. With 4-h incubation at 35°C, cdc10-1 cells grew in a highly polarized manner, consistent with the dispensability of neck-associated cables for polarized growth as long as bud-associated actin cables are present.
Figure 6.
Septins localize Bnr1p-dependent cable assembly to the bud neck. (A) Diploid wild-type (ABY1848), bni1-11/bni1-11 (ABY2247), cdc10-1/cdc10-1 (ABY1662), bni1-11/bni1-11 cdc10-1/cdc10-1 (ABY2248), bni1-11/bni1-11 bnr1Δ/bnr1Δ (ABY1803), and tpm1-2/tpm1-2 tpm2Δ/tpm2Δ (ABY2250) were shifted to 35°C for 1 h and stained to show Tpm1p distribution. Corresponding haploid strains (Y1239, ABY2209, ABY2201, ABY2226, ABY2218, and ABY1807) were shifted to 35°C for 4 h and observed by differential interference contrast microscopy. (B) Tpm1-2 tpm2Δ (ABY944), tpm1-2 tpm2Δ cdc10-1 (ABY1639), and tpm1-2 tpm2Δ cdc12-6 (ABY1643) cells were subjected to indicated conditions and stained for Tpm1p distribution. (C) Cells of each strain in B were shifted to 34.5°C for the indicated times before 1 min restoration to 18°C and staining to show Tpm1p distribution. One hundred medium-budded cells were scored for the presence of Tpm1p in the bud (black), in the bud and at the neck (gray), at the neck (white), or delocalized (blank). Results for small-budded cells were similar. (D) Bnr1pGFP-fluorescence is shown for wild-type (YEF2255) and cdc10-1/cdc10-1 mutant cells (ABY2262) after 1 h at 34.5°C. (E) Fifty cells each of unbudded (unbud.) and small- (small), medium- (med.), and large-budded (large) cells treated as in D were assayed for the localization of Bnr1pGFP to a single point on the cell cortex (for unbudded cells) or the bud neck (for budded cells).
To determine whether filament assembly at the neck, rather than simply cable anchorage, is dependent on septins, we examined where cable assembly occurred in tropomyosin mutant yeast bearing either of two temperature-sensitive septin mutations (tpm1-2 tpm2Δ cdc10-1 and tpm1-2 tpm2Δ cdc12-6). Cables were disassembled in these cells and tpm1-2 tpm2Δ control cells for short (2-min) or long (60-min) periods at 34.5°C before 1 min recovery at 18°C. After short periods of disassembly, cable reassembly was detected at the bud cortex of all strains, but after long periods of disassembly, the tpm1-2 tpm2Δ controls but not the tpm1-2 tpm2Δ cdc10-1 and tpm1-2 tpm2Δ cdc12-6 strains assembled cables at the neck (Figure 6, B and C). Rather, the septin mutations resulted in the sparse assembly of filaments in a disorganized manner, showing that the septins are important for localizing actin cable assembly activity to the bud neck.
A conditional cdc12-6 mutation has been reported to have only a partial effect on the localization of an overexpressed Bnr1p-derived construct (Kikyo et al., 1999), with only ∼50% of cdc12-6 cells losing polarization of the protein after 6 h under restrictive conditions. However, the Bnr1p-derived construct was expressed from the strong GAL1-10 promoter. To determine whether Bnr1p expressed at the endogenous level depends on the septins for localization, we examined Bnr1pGFP in cdc10-1/cdc10-1 cells. Compared with wild-type controls, Bnr1pGFP in cdc10-1/cdc10-1 cells lost nearly all localization after 1 h at 35°C (Figure 6, D and E), demonstrating a strong dependence of Bnr1p on septins for its proper localization.
This loss of neck-associated Bnr1p and cables may explain the synthetic lethality observed between the cdc12-6 septin mutation and deletion of BNI1 (which led to the gene name Bud Neck Interacting 1) (Longtine et al., 1996) and between the cdc10-1 septin mutation and deletion of SPA2 (Flescher et al., 1993), which encodes a Bni1p-binding protein. Specifically, combinations in defects in septin and Bni1p function would be expected to eliminate all cable organization. To address this, we examined yeast harboring the cdc10-1 mutation and a conditional bni1-11 mutation (Evangelista et al., 2002). Alone, bni1-11 caused loss of Tpm1p-staining cables from the bud at 35°C, and after prolonged growth, resulted in cells with a variety of morphological defects, varying from highly depolarized cells to those with round buds and a wide neck typical of bni1Δ yeast (Figure 6A). In bni1-11/bni1-11 cdc10-1/cdc10-1 cells, cables were no longer present in the bud or mother after 1 h at 35°C, just as with bni1-11/bni1-11 bnr1Δ/bnr1Δ or tpm1-2/tpm1-2 tpm2Δ/tpm2Δ strains (Figure 6A). Prolonged growth of bni1-11 cdc10-1 cells at 35°C showed uniformly severe polarized growth defects greater than those caused by bni1-11 or cdc10-1 alone. The bni1-11 cdc10-1 cells enlarged to rounded cells that were unable to form buds, similar to bni1-11 bnr1Δ and tpm1-2 tpm2Δ yeast that completely lack actin cables (Figure 6A). However, the bni1-11 cdc10-1 cells retained some polarity in that they were each able to form a blunted projection, indicating the cells retained some Bnr1p-dependent polarity, possibly reflecting a partial septin independence of Bnr1p localization in unbudded cells (Figure 6E), or the small amount of cell tip-associated GFP-Cdc12p septin in cdc10-1/cdc10-1 yeast at 35°C (Figure S3).
Polarized Secretion Maintains Cable Assembly Activity at the Bud Cortex
The loss of cable assembly activity from the buds of tpm1-2 tpm2Δ cells after 1 h at 34.5°C suggests some instability in that activity under those conditions. Two possibilities presented themselves. The first possibility was that the retention of actin cable assembly activity within the bud specifically depends on the presence of actin cables. Alternatively, the loss might reflect a nonspecific instability in the association of cable assembly activity with the bud cortex at 34.5°C, whether or not cables are present. Thus, loss of the activity from the bud cortex might be due to trivial factors such as progression through the cell cycle or a limited half-life of components directing cable assembly at the bud cortex.
To resolve whether cable assembly at the bud cortex is inherently unstable at 34.5°C even in the presence of actin cables, we examined yeast that normally undergo prolonged periods of polarized growth to determine whether they maintain bud cortex-associated actin cables for prolonged periods. Yeast polarity is regulated by the cell cycle. The appearance of G1-cyclins trigger initiation of bud growth, whereas B-cyclin activity in the cytoplasm specifically promotes the switch from tip-directed (apical) bud growth to isotropic bud growth (reviewed in Lew and Reed, 1995; Rua et al., 2001). Overproduction of G1-cyclins allows for bud initiation, but then it prolongs tip-directed growth, the latter likely by indirect inhibition of B-cyclin activity (Lew and Reed, 1993; Ahn et al., 2001).
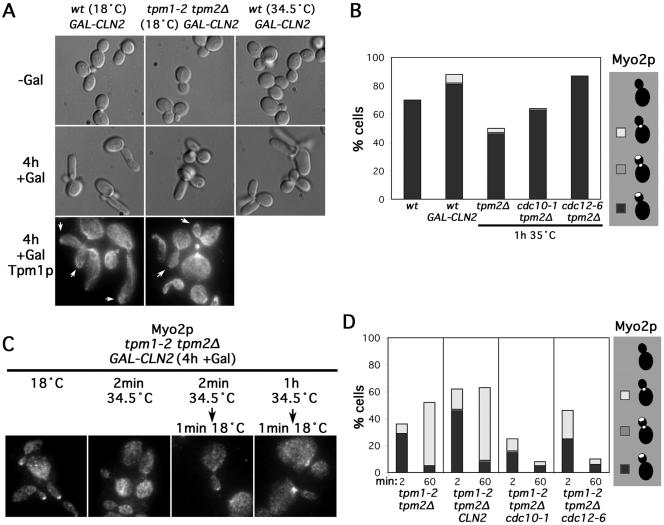
We introduced into wild-type yeast an inducible G1-cyclin–encoding CLN2 (GAL-CLN2) and induced Cln2p production for 4 h at either 18 or 34.5°C. At both temperatures, we found cells exhibited elongated buds, indicative of prolonged apical growth (Figure 7A). Tpm1p staining showed cables were associated with the bud tip and the cortex of these elongated buds as well as with the bud necks and in the mother cells, and Myo2p staining was concentrated at their bud tips (Figure 7, A and B). Thus, Cln2p-overproduction was able to prolong the time during which cable assembly occurred at the bud tip. Similar results were obtained with yeast bearing either conditional septin mutation cdc10-1 or cdc12-6, both of which also result in inhibition of B-cyclin activity (for a mechanistic explanation, see Moffat and Andrews, 2003), and which induced elongated buds after 1 h at 35°C with bud cortex-associated actin cables and Myo2p (Figures 6A and 7B). Thus, appropriate cell cycle manipulations suggest that the association of cable assembly activity with the bud cortex is not inherently unstable at elevated temperatures.
Figure 7.
Inhibition of the switch from apical to isotropic bud growth does not preserve bud cortex-associated cable assembly in the absence of actin cables. (A) GAL-CLN2–bearing wild-type (ABY2251) and tpm1-2 tpm2Δ (ABY 2252) cells either were (+Gal) or were not (-Gal) induced to overproduce Cln2p for 4 h at the indicated temperatures and observed by differential interference contrast microscopy or stained to show Tpm1p distribution. Arrows indicate bud tips with associated Tpm1p. (B) Wild-type (Y1239) or GAL-CLN2-bearing (ABY2251) cells induced for 4 h were grown at 18°C and stained for Myo2p. Tpm2Δ (ABY945), cdc10-1 tpm2Δ (ABY1637), and cdc12-6 tpm2Δ (AY1639) cells were grown at 35°C for 1 h before staining to show Myo2p distribution. One hundred cells of each strain with medium-sized and/or elongated buds were scored for the presence of Myo2p at the bud tip (black), at the bud tip and the neck (gray), at the bud neck (white), or not localized (blank). (C) Tpm1-2 tpm2Δ GAL-CLN2 cells (ABY2252) were subjected to the indicated conditions and stained to show Myo2p distribution. (D) Tpm1-2 tpm2Δ (ABY944) cells, tpm1-2 tpm2Δ GAL-CLN2 (ABY2252) cells grown 4 h in galactose, tpm1-2 tpm2Δ cdc10-1 (ABY1639) cells, and tpm1-2 tpm2Δ cdc12-6 (ABY1643) cells were shifted to 34.5°C for 2 or 60 min before restoration to 18°C for 1 min and Myo2p localized by immunofluorescence microscopy. One hundred medium-budded cells of each were scored for the presence of Myo2p at the bud tip (black), at the bud tip and neck (gray), at the bud neck (white), or not localized (blank).
To determine whether these cell cycle perturbations would be sufficient to preserve cable assembly activity at the bud cortex in the absence of actin cables, we examined the effects of Cln2p-overproduction in tpm1-2 tpm2Δ cells. At 18°C, Gal-induction of tpm1-2 tpm2Δ GAL-CLN2 cells promoted tip-directed growth and bud cortex-associated cables and Myo2p just as for wild-type cells (Figure 7, A and C). When shifted to 34.5°C, cables disassembled and Myo2p delocalized in the tpm1-2 tpm2Δ GAL-CLN2 cells, just as for tpm1-2 tpm2Δ cells (Figure 7C). When the tpm1-2 tpm2Δ GAL-CLN2 were then restored to 18°C for 1 min and stained for Myo2p, we found the results were nearly identical to those of the tpm1-2 tpm2Δ controls: after brief cable disassembly and reassembly, Myo2p was recruited along cables to the bud tip, but after prolonged actin cable disassembly followed by reassembly, Myo2p was recruited along cables to the bud neck with the same efficiency as for tpm1-2 tpm2Δ cells not overproducing Cln2p (Figure 7, C and D). Thus, Cln2p-overproduction had no impact on the ability of yeast to maintain actin cable assembly activity at the bud cortex in the absence of actin cables.
Similar results were seen when the septin mutations cdc10-1 and cdc12-6 were introduced into tpm1-2 tpm2Δ yeast. With prolonged incubation at 35°C, the ability to assemble actin cables on the bud cortex or recruit Myo2p to the bud tip upon restoration to 18°C was lost in tpm1-2 tpm2Δ cdc10-1 and tpm1-2 tpm2Δ cdc12-6, despite the expectation that passage through the cell cycle is inhibited (Figures 6, B and C, and 7D).
In tpm1-2 tpm2Δ cells without cell cycle perturbations, we also found evidence that loss of tip-directed polarity in the absence of cables was not dependent on passage through the cell cycle. Specifically, as mentioned previously, the majority of small-budded tpm1-2/tpm1-2 tpm2Δ/tpm2Δ were able to regain tip-associated Myo2p when allowed to recover for 20 min at 18°C after lacking actin cables for 1 h. Similarly, when haploid tpm1-2 tpm2Δ yeast were shifted to 34.5°C for 1 h, and then restored to 18°C for 15 min, Myo2p became associated with the bud cortex of 68% small-budded cells (compared with 96% before the loss of actin cables) and 35% medium-budded cells (compared with 60% before the loss of actin cables). The short period of recovery and the high proportion of small-budded cells with tip-associated Myo2p argue against these solely representing new buds that have formed, and their appearance is inconsistent with passage of these cells through the cell cycle to the point where the cytoskeleton reorients toward the neck for cell division. Rather, these results indicate that the ability to assemble actin cables at the bud cortex was lost specifically due to the absence of actin cables, not cell cycle progression.
One explanation for the dependence of bud cortex-associated cable assembly activity on the presence of actin cables is that the cables might directly anchor such a component. However, the loss of bud cortex-associated assembly activity was gradual (Figure 4B), suggesting an indirect dependence on actin cables. An alternative explanation for this cable dependence is that some factor important to cable assembly is gradually lost from the bud cortex but continually replenished by transport along the cables.
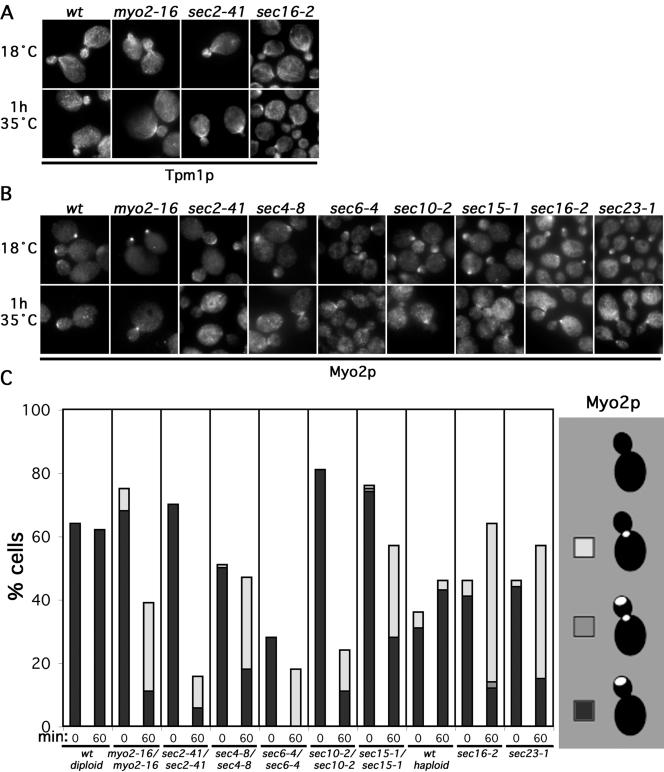
If Myo2p were important in transporting such a factor, we would expect defects in Myo2p to result in the loss of bud cortex-associated actin cables and Myo2p. We examined yeast bearing a temperature-sensitive mutation of the Myo2p COOH-terminal domain (myo2-16). At 35°C, this mutant Myo2p still polarizes in response to actin cables, but it is unable to deliver secretory vesicles (Schott et al., 1999). At 18°C, actin cables and Myo2p distribution in myo2-16/myo2-16 seemed normal, but after 1 h at 35°C, cables were largely absent from the bud, and the mutant Myo2p, when polarized, was found at the bud neck (Figure 8, A–C). Such a shift was not seen in wild-type yeast, suggesting maintenance of actin cable assembly activity at the bud cortex depends on Myo2p. The loss of tip-directed polarity in these cells occurred over a very similar time course as in tpm1-2 tpm2Δ yeast (compare Figures 4B and S4), consistent with a common mechanism.
Figure 8.
Disruption of polarized secretion depletes bud-associated cables. (A) Wild-type (ABY501), myo2-16/myo2-16 (ABY506), sec2-41/sec2-41 (ABY1665), and sec16-2 (RSY267) cells were subjected to the indicated conditions and stained to show Tpm1p distribution. (B) Wild-type (ABY501), myo2-16/myo2-16 (ABY506), sec2-41/sec2-41 (ABY1665), sec4-8/sec4-8 (ABY994), sec6-4/sec6-4 (ABY993), sec10-2/sec10-2 (ABY995), sec15-1/sec15-1 (ABY996), sec16-2 (RSY267), and sec23-1 (RSY281) cells were subjected to the indicated conditions and stained to show Myo2p distribution. (C) One hundred medium-budded cells of each sample in B plus the wild-type haploid RSY255 were scored for the presence of Myo2p at the bud tip (black), the bud tip and neck (gray), the neck (white), or delocalized (blank). Results were similar for small-budded cells.
To determine whether secretory vesicles might carry a putative cable-assembly factor, we investigated whether blocks in secretion cause a loss of cables or Myo2p from the bud. Among proteins critical for the fusion of secretory vesicles with the plasma membrane are the rab-GTPase Sec4p (Salminen and Novick, 1987), its guanine-nucleotide-exchange-factor Sec2p (Walch-Solimena et al., 1997), and a protein complex termed the exocyst (composed of Sec3p, 5p, 6p, 8p, 10p, 15p, Exo70p, and Exo84p) (TerBush and Novick, 1995; TerBush et al., 1996; Guo et al., 1999). Temperature-sensitive mutations of these result in accumulation of post-Golgi secretory vesicles and an exocytic block at restrictive temperatures (Novick et al., 1980, 1981; Guo et al., 1999). When a collection of such mutants (sec2-41/sec2-41, sec4-8/sec4-8, sec6-4/sec6-4, sec10-2/sec10-2, sec15-1/sec15-1) was grown at 18°C, Myo2p and Tpm1p distribution seemed normal, but after 1 h at 35°C, all showed a decreased proportion of cells with cables and Myo2p associated with the bud cortex and an increased proportion with Myo2p concentrated at the bud neck (Figure 8, A–C).
As with defects in actin cables or Myo2p function, the shift in actin-based polarity to the neck in these cells occurred gradually over the course of 1 h (Figure S4). This is consistent with a requirement for secretion to maintain cable assembly activity at the bud cortex. However, this interpretation is complicated in that for many of these sec mutants, the overall localization of Myo2p was very poor, yet Myo2p itself is required to maintain cable assembly activity at the bud cortex. Thus, it was possible that in these mutants, the loss of bud cortex-associated cables was an indirect effect of delocalizing Myo2p.
We hypothesized that the association of Myo2p with the accumulated post-Golgi vesicles in some late sec mutants (Wagner et al., 2002) might interfere with the localization of the myosin in these cells. To avoid this, we also examined cable and Myo2p distribution in yeast bearing conditional blocks in earlier steps of the secretory pathway. Temperature-sensitive mutations affecting a COPII component (sec23-1) (Barlowe et al., 1994) or a protein involved in packaging COPII-coated vesicles (sec16-2) (Campbell and Schekman, 1997) result in the accumulation of secretory products in the endoplasmic reticulum at the restrictive temperature (Novick et al., 1980, 1981). When such cells were shifted to 35°C for 1 h, both showed a depletion of actin cables from the bud and increased association of Myo2p with the bud neck (Figure 8, A–C). Unlike in some of the late sec mutants, Myo2p in these cells was polarized in a similar proportion of cells as a wild-type control, suggesting the altered cable distribution was not caused by direct interference of Myo2p function. Thus, depolarization or blockage of secretion reduces the presence of bud cortex-associated cables, consistent with the dependence of some cable assembly factor on the secretory pathway for delivery to the bud cortex.
Rho-GTPases Depend on Polarized Secretion for Bud Tip-Association
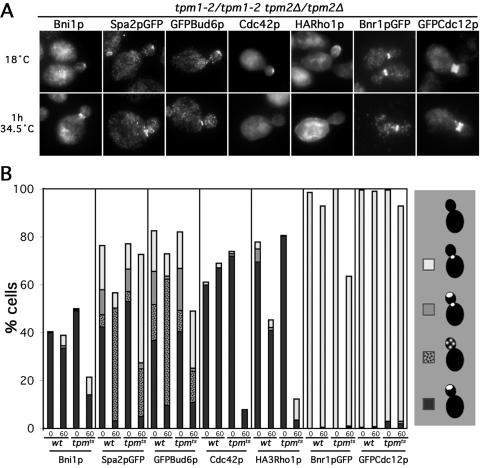
To determine whether any factors known to be important for cable assembly depend on actin cables for their localization, we examined the distribution of a variety of such proteins in wild-type and tpm1-2/tpm1-2 tpm2Δ/tpm2Δ yeast at 34.5°C. Serving as controls, Bnr1pGFP and GFPCdc12p largely remained associated with the bud neck of small- and medium-budded tpm1-2/tpm1-2 tpm2Δ/tpm2Δ cells, as judged by anti-GFP immunofluorescence (Figure 9, A and B), consistent with the persistence of the neck-associated cable assembly site. The association of Bni1p with the bud cortex was reduced in tpm1-2/tpm1-2 tpm2Δ/tpm2Δ cells after 1 h at 34.5°C (Figure 9, A and B). However, a reduction of cortical Bni1p also was seen in the TPM1/TPM1 tpm2Δ/tpm2Δ controls, and in small-budded cells in particular, the proportion of tpm1-2/tpm1-2 tpm2Δ/tpm2Δ cells retaining detectable Bni1p at the bud tip (36%) was nearly identical to that of the TPM1/TPM1 tpm2Δ/tpm2Δ control cells (39%). This suggests that loss of Bni1p localization in the absence of cables is not likely to account for the loss of cable assembly activity from the bud cortex of tropomyosin mutant yeast at 34.5°C. Consistent with this, wild-type yeast expressing Bni1pGFP from a high-copy plasmid did not display any fluorescent particles undergoing directed movements indicative of Myo2p-dependent transport of post-Golgi vesicles.
Figure 9.
Rho-proteins, but not other cable-assembly factors, strongly depend on cables for retention at the bud tip. (A) Tpm1-2/tpm1-2 tpm2Δ/tpm2Δ strains expressing Spa2pGFP (ABY1197), GFPBud6p (ABY1171), HARho1p (ABY1602), Bnr1pGFP (ABY1891), GFPCdc12p (ABY1899), or no epitope-tagged protein (ABY971) were subjected to the indicated growth conditions and stained to show Bni1p, GFP, Cdc42p, and HA-epitope distributions. Examples of aberrant Bni1p, Spa2pGFP, GFPBud6p, Cdc42p, and HARho1p localization at 34.5°C are shown. (B) Small- and medium-budded cells of each sample in A plus identically treated TPM1/TPM1 tpm2Δ/tpm2Δ control strains expressing Spa2pGFP (ABY1198), GFPBud6p (ABY1199), HARho1p (ABY1601), GFPCdc12p (ABY1896), or no epitope-tagged protein (ABY973), or TPM1/TPM1 TPM2/TPM2 Bnr1pGFP control strain (YEF2255), were scored for the presence of stain at the bud tip (black), as puncta along the bud cortex (speckled), at the bud tip and neck (gray), at the neck (white), or delocalized (blank). For strains ABY1171, 1197, 1198, 1199, 1601, and 1602, 200 small- and 200 medium-budded cells of each were scored and averaged. For the remaining strains, 100 small- and 100 medium-budded cells of each strain were scored and averaged.
Bud6p and Spa2p are two components of a Bni1p-binding protein complex called the polarisome (Sheu et al., 1998) that localizes to growth sites with Bni1p (Snyder, 1989; Amberg et al., 1997). Bud6p cooperates with Bni1p in promoting actin assembly in vivo (Evangelista et al., 2002; Sagot et al., 2002a) and in vitro (Moseley et al., 2004) and associates with secretory vesicles (Jin and Amberg, 2000), and in the absence of Bud6p, cables are very sparse (Amberg et al., 1997). Spa2p is required for the proper localization of Bni1p to the bud tip (Fujiwara et al., 1998), and in its absence, cables are specifically absent from the bud, similar to bni1Δ cells (our unpublished data). Shift of either control or tpm1-2/tpm1-2 tpm2Δ/tpm2Δ cells resulted in GFPBud6p and Spa2pGFP redistributing from the bud tip to puncta along the bud cortex and to the bud neck (Figure 9, A and B). Roughly half the proportion of tpm1-2/tpm1-2 tpm2Δ/tpm2Δ cells retained both fusion proteins within the bud compared with control cells, suggesting neither protein represents the critical cable-assembly factor lost under these conditions. As further support, Spa2pGFP in live yeast did not display directed movements of fluorescent particles, suggesting this protein also is not recruited to the bud cortex by targeted secretion.
The rho-GTPases Cdc42p and Rho1p also localize to growth sites (McCaffrey et al., 1991; Ziman et al., 1993; Yamochi et al., 1994) and interact with Bni1p in two-hybrid assays (Kohno et al., 1996; Evangelista et al., 1997). Cdc42p is required for recruitment of Bni1p to the nascent bud site (Jaquenoud and Peter, 2000; Ozaki-Kuroda et al., 2001) and when overexpressed, Cdc42p promotes Bni1p-dependent cable assembly (Dong et al., 2003). Rho1p is required for Bni1p- and Bnr1p-dependent cable assembly, at least at 37°C (Tolliday et al., 2002; Dong et al., 2003). Also, both rho-GTPases associate with secretory vesicles (Abe et al., 2003; Wedlich-Soldner et al., 2003). For endogenous Cdc42p and NH2-terminally HA-tagged Rho1p expressed from the RHO1 locus, actin cable disassembly in tpm1-2/tpm1-2 tpm2Δ/tpm2Δ cells resulted in delocalization (Figure 9, A and B). After 1 h at 34.5°C, the fraction of tropomyosin mutants retaining Cdc42p at the bud cortex was only 12% that of wild-type controls, and the fraction of mutants retaining HARho1p at the bud cortex was only 8% of that of controls. Thus, these two proteins are strong candidates for cable-dependent factors important for promoting Bni1p-stimulated filament assembly on the bud cortex.
DISCUSSION
Our results help reconcile the similar biochemical functions of the two formins, Bni1p and Bnr1p, with the distinct phenotypes of their loss of function. Both formins promote actin cable assembly (Evangelista et al., 2002; Sagot et al., 2002a), but the loss of Bnr1p causes only a mild delay in cell separation (Vallen et al., 2000), whereas the absence of Bni1p causes a widened bud neck (Jansen et al., 1996; Zahner et al., 1996; Mosch and Fink, 1997; Sheu et al., 2000), an inability to engage in tip-directed growth (Evangelista et al., 1997; Mosch and Fink, 1997; Sheu et al., 2000; Ozaki-Kuroda et al., 2001), a defect in bipolar bud site selection (Zahner et al., 1996), a partial defect in cytokinetic ring contraction (Vallen et al., 2000), a defect in early spindle alignment (Fujiwara et al., 1999; Lee et al., 1999), and a variety of synthetic lethal genetic interactions (Kohno et al., 1996; Longtine et al., 1996; Fujiwara et al., 1998; Fujiwara et al., 1999; Lee et al., 1999; Tong et al., 2001, 2004). We suggest that many, if not all, of these can be attributed to the actin assembly activity of the two formins in conjunction with their specific localizations.
Bnr1p becomes well localized only after bud emergence, associating with the bud neck throughout bud growth. It is required for the strong assembly of tropomyosin-enriched actin filaments at the neck, which gives rise to bundles of actin cables anchored at the neck that permeate the mother cell. These cables behave as a stable mother/bud axis of polarity, because their organization remains unchanged throughout growth of the bud, and this Bnr1p-dependent assembly site remains associated with the bud neck in the face of perturbations such as actin cable disassembly. The localization of Bnr1p depends upon the septins, a group of related proteins that form a scaffold of filaments girding the bud neck. The septin dependence of Bnr1p provides a likely explanation for the synthetic lethality between defects in Bni1p function and septins (Longtine et al., 1996), which recapitulate the lethality of the simultaneous loss of Bni1p- and Bnr1p-dependent cable assembly (Figure 5A). Septin-dependent recruitment of Bnr1p also might explain the delay in Bnr1p recruitment to the bud neck until after bud emergence, because during this transition the septins undergo significant changes in their organization (reviewed in Gladfelter et al., 2001; recent results in Caviston et al., 2003; Dobbelaere et al., 2003).
With the absence of Bnr1p-dependent cables, myosin-V–associated membranes are less efficient in initiating directed movements toward the bud, consistent with the reduced presence of actin cables in the mother cell. However, strains lacking Bnr1p still transport vesicles from the mother to concentrate at the bud tip (Figure 3A) and are able to grow with a normal morphology and rate (Imamura et al., 1997; Vallen et al., 2000), suggesting that the few Bni1p-dependent cables in the mother cell meet the transport requirements of the yeast cell under the conditions examined so far.
In unbudded cells, Bni1p localizes to the nascent bud site before bud emergence to promote assembly of an array of actin cables. This initial recruitment depends on Cdc42p, possibly through bridged interactions between the Cdc42p-effector Gic2p and the Bni1p-binding proteins Spa2p and Bud6p (Jaquenoud and Peter, 2000). The absence of Bni1p disrupts normal cable organization in these cells, resulting instead in formation of a disorganized mesh of Bnr1p-dependent filaments. Presumably, the septins, which are polarized in both wild-type and bni1Δ unbudded cells (Figure S2A and B), provide the cue by which Bnr1p is weakly polarized in unbudded cells, because yeast lacking both Bni1p function and septin function are unable to initiate a normal bud (Figure 6A). The guidance of bud emergence by this disorganized mesh of Bnr1p-dependent cables may provide an explanation for the widened neck of bni1Δ cells (Jansen et al., 1996; Zahner et al., 1996; Mosch and Fink, 1997; Sheu et al., 2000), a feature that also may indirectly explain the cytokinetic ring contraction defects (Vallen et al., 2000), and minor defects seen in the septin ring of bni1Δ yeast (Figure S2A, middle bni1Δ/bni1Δ cell).
In cells with growing buds, Bni1p shows a variable localization on the bud cortex that correlates with an axis of transport toward the changing location of growth sites through the cell cycle (Ozaki-Kuroda et al., 2001). Bni1p is required to efficiently assemble cable filaments within the bud, and the absence of Bni1p (by bni1Δ) or Bni1p-dependent cables (by disruption of polarized secretion) prevents the directed movements of myosin-associated cargoes from the bud neck to the growing bud tip. As a result, Myo2p and secretory vesicles accumulate at the bud neck or associate with the bud cortex diffusely, rather than concentrating at the bud tip. This provides an explanation for the inability of bni1Δ yeast to undergo tip-directed growth (Evangelista et al., 1997; Mosch and Fink, 1997; Sheu et al., 2000; Ozaki-Kuroda et al., 2001) or to bud in a bipolar manner (Zahner et al., 1996), which in turn requires tip-directed growth (Sheu et al., 2000). Other myosin-V–dependent cargoes also lose their tip-association in bni1Δ cells, including the microtubule anchoring protein Kar9p (Miller et al., 1999), cytoplasmic microtubules (Segal et al., 2000), and the ASH1 mRNA (Takizawa et al., 1997; Beach et al., 1999), explaining the inability of bni1Δ yeast to orient the mitotic spindle early in the cell cycle (Fujiwara et al., 1999; Lee et al., 1999) or to properly restrict Ash1p-mediated cell differentiation to daughter cells (Jansen et al., 1996).
Based on assays of filament reassembly after short periods of filament disassembly, Bni1p-dependent assembly seems to be more robust than Bnr1p-dependent assembly, with the majority of newly assembled filaments in budded cells occurring along the bud cortex rather than at the bud neck (Figure 4A). But unlike the Bnr1p-dependent axis of polarity, Bni1p-dependent polarity is only meta-stable in that its persistence depends on continued polarized secretion, which in turn depends on transport along actin cables. Thus, when filaments are reassembled after a prolonged period in the absence of actin cables, Bni1p-dependent assembly is reduced compared with Bnr1p-stimulated filament assembly. This loss of Bni1p-dependent cable assembly from the bud cortex occurs in a variety of defects in polarized secretion and does not depend on progression of the cell cycle (Figure 6). However, the loss of Bni1p-dependent cables from the bud occurred only gradually after disruption of polarized secretion, suggesting that some factor is gradually depleted from the bud cortex and continually replenished by association with secretory vesicles transported to the bud cortex along actin cables.
This reduction of Bni1p-dependent assembly does not correlate well with a loss of Bni1p from the bud cortex, nor the Bni1p-binding polarisome proteins Spa2p or Bud6p, despite the fact that Bud6p, at least, is transported on secretory vesicles (Jin and Amberg, 2000). However, two rho-GTPases, Cdc42p and Rho1p, strongly depend on actin cables for their maintenance at the bud cortex, and both have been shown to associate with secretory vesicles (Abe et al., 2003; Wedlich-Soldner et al., 2003). Because both of these have been implicated in promoting activation of Bni1p (although by unclear mechanisms; Dong et al., 2003), they represent two potential critical factors that are transported along the cables, although other factors are certainly possible. Interestingly, Bni1p, but not Bnr1p, also depends on the rho-GTPase Rho3p for activation (Dong et al., 2003). The requirement of Rho3p for efficient fusion of secretory vesicles with the plasma membrane (Adamo et al., 1999) may contribute to an explanation for this dependence.
The coupling of formin-activating molecules to secretion provides a potential explanation for why the Bni1p-dependent assembly system depends on the presence of polarized cables, whereas the Bnr1p-dependent system does not. Under normal growth conditions, rho-GTPases or other activators continually cycle through rounds of endocytosis and delivery back to the bud tip by secretion, resulting in enrichment of those factors near Bni1p and the preeminence of Bni1p-dependent filament assembly over Bnr1p-dependent assembly. With depolarization of secretion, the rho-GTPases or other critical factors are endocytosed from the bud and reappear at the mother cortex (Figure 9A). Bnr1p would then gain preeminence either by its continued contact with putative formin-activators now present on the mother cortex, or perhaps by being regulated by factors not subject to membrane traffic at all.
With the restoration of polarized secretion, Bnr1p-dependent cables would initially guide vesicles, resulting in vesicle accumulation at the bud neck and diffusely along the bud cortex (as seen with bni1Δ cells). As putative formin-activating factors become enriched in the bud, Bni1p would gradually resume function, restoring transport to the bud tip and reestablishing the tip-directed positive-feedback loop. Future studies may determine whether the dependence of the dynamic Bni1p-driven axis on this positive feedback signal serves as a point of regulation during polarized growth in response to pheromones, cell cycle progression, or stress responses, where realignment or temporary disruption of polarized growth reflects a reduced cable-dependent recruitment of Myo2p and vesicles to the growing cell tip.
Our results indicate budding yeast maintain two axes of polarity by coupling the common core cytoskeletal elements of formin-stimulated filaments to distinct cortical landmarks. One axis provides an entraining signal to direct polarity into the bud, whereas the other allows dynamic targeting of class-V myosins and their cargoes within the bud for proper morphogenesis, with the availability of activating factors providing a key difference between the two. Future studies to elucidate links between the formins, cortical landmarks, and factors important to their activation in yeast may serve as a blueprint for mechanisms used to control the persistence of cytoskeletal polarity in other systems.
Supplementary Material
Acknowledgments
We thank D. Amberg, R. Arokowitz, R. Collins, D. Drubin, H. Fares, D. Johnson, K. Kozminski, M. Longtine, P. Novick, J. Pringle, T. Richman, R. Schekman, C. Shamu, and P. Silver for kindly supplying reagents, and J. Ingraffea and B. Judson for providing technical assistance. This work was supported by National Institutes of Health grants GM-39066 (A.B.) and GM-59216 (E.B.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04–04–0296. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–04–0296.
Abbreviations used: FH, formin homology.
The online version of this article contains supplementary material accessible through http://www.molbiolcell.org.
References
- Abe, M., Qadota, H., Hirata, A., and Ohya, Y. (2003). Lack of GTP-bound Rho1p in secretory vesicles of Saccharomyces cerevisiae. J. Cell Biol. 162, 85-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo, J.E., Rossi, G., and Brennwald, P. (1999). The Rho GTPase Rho3 has a direct role in exocytosis that is distinct from its role in actin polarity. Mol. Biol. Cell 10, 4121-4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, A.E., and Pringle, J.R. (1984). Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J. Cell Biol. 98, 934-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, S.H., Tobe, B.T., Fitz Gerald, J.N., Anderson, S.L., Acurio, A., and Kron, S.J. (2001). Enhanced cell polarity in mutants of the budding yeast cyclin-dependent kinase Cdc28p. Mol. Biol. Cell 12, 3589-3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberg, D.C., Botstein, D., and Beasley, E.M. (1995). Precise gene disruption in Saccharomyces cerevisiae by double fusion polymerase chain reaction. Yeast 11, 1275-1280. [DOI] [PubMed] [Google Scholar]
- Amberg, D.C., Zahner, J.E., Mulholland, J.W., Pringle, J.R., and Botstein, D. (1997). Aip3p/Bud6p, a yeast actin-interacting protein that is involved in morphogenesis and the selection of bipolar budding sites. Mol. Biol. Cell 8, 729-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkowitz, R.A., and Lowe, N. (1997). A small conserved domain in the yeast Spa2p is necessary and sufficient for its polarized localization. J. Cell Biol. 138, 17-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe, C., Orci, L., Yeung, T., Hosobuchi, M., Hamamoto, S., Salama, N., Rexach, M.F., Ravazzola, M., Amherdt, M., and Schekman, R. (1994). COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77, 895-907. [DOI] [PubMed] [Google Scholar]
- Barral, Y., Mermall, V., Mooseker, M.S., and Snyder, M. (2000). Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol. Cell 5, 841-851. [DOI] [PubMed] [Google Scholar]
- Barral, Y., Parra, M., Bidlingmaier, S., and Snyder, M. (1999). Nim1-related kinases coordinate cell cycle progression with the organization of the peripheral cytoskeleton in yeast. Genes Dev. 13, 176-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach, D.L., Salmon, E.D., and Bloom, K. (1999). Localization and anchoring of mRNA in budding yeast. Curr. Biol. 9, 569-578. [DOI] [PubMed] [Google Scholar]
- Bi, E., Maddox, P., Lew, D.J., Salmon, E.D., McMillan, J.N., Yeh, E., and Pringle, J.R. (1998). Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J. Cell Biol. 142, 1301-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi, E., and Pringle, J.R. (1996). ZDS1 and ZDS2, genes whose products may regulate Cdc42p in Saccharomyces cerevisiae. Mol. Cell. Biol. 16, 5264-5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher, A. (2003). Polarized growth and organelle segregation in yeast: the tracks, motors, and receptors. J. Cell Biol. 160, 811-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviston, J.P., Longtine, M., Pringle, J.R., and Bi, E. (2003). The role of Cdc42p GTPase-activating proteins in assembly of the septin ring in yeast. Mol. Biol. Cell 14, 4051-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, J.L., and Schekman, R. (1997). Selective packaging of cargo molecules into endoplasmic reticulum-derived COPII vesicles. Proc. Natl. Acad. Sci. USA 94, 837-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, F., and Peter, M. (2003). Yeasts make their mark. Nat. Cell Biol. 5, 294-299. [DOI] [PubMed] [Google Scholar]
- DeMarini, D.J., Adams, A.E., Fares, H., De Virgilio, C., Valle, G., Chuang, J.S., and Pringle, J.R. (1997). A septin-based hierarchy of proteins required for localized deposition of chitin in the Saccharomyces cerevisiae cell wall. J. Cell Biol. 139, 75-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelaere, J., Gentry, M.S., Hallberg, R.L., and Barral, Y. (2003). Phosphorylation-dependent regulation of septin dynamics during the cell cycle. Dev. Cell 4, 345-357. [DOI] [PubMed] [Google Scholar]
- Dong, Y., Pruyne, D., and Bretscher, A. (2003). Formin-dependent actin assembly is regulated by distinct modes of Rho signaling in yeast. J. Cell Biol. 161, 1081-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista, M., Blundell, K., Longtine, M.S., Chow, C.J., Adames, N., Pringle, J.R., Peter, M., and Boone, C. (1997). Bni1p, a yeast formin linking cdc42p and the actin cytoskeleton during polarized morphogenesis. Science 276, 118-122. [DOI] [PubMed] [Google Scholar]
- Evangelista, M., Pruyne, D., Amberg, D.C., Boone, C., and Bretscher, A. (2002). Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nat. Cell Biol. 4, 260-269. [DOI] [PubMed] [Google Scholar]
- Evangelista, M., Zigmond, S., and Boone, C. (2003). Formins: signaling effectors for assembly and polarization of actin filaments. J. Cell Sci. 116, 2603-2611. [DOI] [PubMed] [Google Scholar]
- Faty, M., Fink, M., and Barral, Y. (2002). Septins: a ring to part mother and daughter. Curr. Genet 41, 123-131. [DOI] [PubMed] [Google Scholar]
- Field, C.M., and Kellogg, D. (1999). Septins: cytoskeletal polymers or signalling GTPases? Trends Cell Biol. 9, 387-394. [DOI] [PubMed] [Google Scholar]
- Flescher, E.G., Madden, K., and Snyder, M. (1993). Components required for cytokinesis are important for bud site selection in yeast. J. Cell Biol. 122, 373-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara, T., Tanaka, K., Inoue, E., Kikyo, M., and Takai, Y. (1999). Bni1p regulates microtubule-dependent nuclear migration through the actin cytoskeleton in Saccharomyces cerevisiae. Mol. Cell. Biol. 19, 8016-8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara, T., Tanaka, K., Mino, A., Kikyo, M., Takahashi, K., Shimizu, K., and Takai, Y. (1998). Rho1p–Bni1p–Spa2p interactions: implication in localization of Bni1p at the bud site and regulation of the actin cytoskeleton in Saccharomyces cerevisiae. Mol. Biol. Cell 9, 1221-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz, R.D., and Sugino, A. (1988). New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74, 527-534. [DOI] [PubMed] [Google Scholar]
- Gladfelter, A.S., Pringle, J.R., and Lew, D.J. (2001). The septin cortex at the yeast mother-bud neck. Curr. Opin. Microbiol. 4, 681-689. [DOI] [PubMed] [Google Scholar]
- Goud, B., Salminen, A., Walworth, N.C., and Novick, P.J. (1988). A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell 53, 753-768. [DOI] [PubMed] [Google Scholar]
- Guo, W., Grant, A., and Novick, P. (1999). Exo84p is an exocyst protein essential for secretion. J. Biol. Chem. 274, 23558-23564. [DOI] [PubMed] [Google Scholar]
- Haarer, B.K., Petzold, A., Lillie, S.H., and Brown, S.S. (1994). Identification of MYO4, a second class V myosin gene in yeast. J. Cell Sci. 107, 1055-1064. [DOI] [PubMed] [Google Scholar]
- Hanrahan, J., and Snyder, M. (2003). Cytoskeletal activation of a checkpoint kinase. Mol. Cell 12, 663-673. [DOI] [PubMed] [Google Scholar]
- Harris, E.S., Li, F., and Higgs, H.N. (2004). The mouse formin, FRLalpha, slows actin filament barbed end elongation, competes with capping protein, accelerates polymerization from monomers, and severs filaments. J. Biol. Chem. 297, 20076-20087. [DOI] [PubMed] [Google Scholar]
- Hartwell, L.H. (1971). Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp. Cell Res. 69, 265-276. [DOI] [PubMed] [Google Scholar]
- Imamura, H., Tanaka, K., Hihara, T., Umikawa, M., Kamei, T., Takahashi, K., Sasaki, T., and Takai, Y. (1997). Bni1p and Bnr1p: downstream targets of the Rho family small G-proteins which interact with profilin and regulate actin cytoskeleton in Saccharomyces cerevisiae. EMBO J. 16, 2745-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen, R.P., Dowzer, C., Michaelis, C., Galova, M., and Nasmyth, K. (1996). Mother cell-specific HO expression in budding yeast depends on the unconventional myosin myo4p and other cytoplasmic proteins. Cell 84, 687-697. [DOI] [PubMed] [Google Scholar]
- Jaquenoud, M., and Peter, M. (2000). Gic2p may link activated Cdc42p to components involved in actin polarization, including Bni1p and Bud6p (Aip3p). Mol. Cell. Biol. 20, 6244-6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, H., and Amberg, D.C. (2000). The secretory pathway mediates localization of the cell polarity regulator Aip3p/Bud6p. Mol. Biol. Cell 11, 647-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, G.C., Prendergast, J.A., and Singer, R.A. (1991). The Saccharomyces cerevisiae MYO2 gene encodes an essential myosin for vectorial transport of vesicles. J. Cell Biol. 113, 539-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei, T., Tanaka, K., Hihara, T., Umikawa, M., Imamura, H., Kikyo, M., Ozaki, K., and Takai, Y. (1998). Interaction of Bnr1p with a novel Src homology 3 domain-containing Hof1p. Implication in cytokinesis in Saccharomyces cerevisiae. J. Biol. Chem. 273, 28341-28345. [DOI] [PubMed] [Google Scholar]
- Kikyo, M., Tanaka, K., Kamei, T., Ozaki, K., Fujiwara, T., Inoue, E., Takita, Y., Ohya, Y., and Takai, Y. (1999). An FH domain-containing Bnr1p is a multi-functional protein interacting with a variety of cytoskeletal proteins in Saccharomyces cerevisiae. Oncogene 18, 7046-7054. [DOI] [PubMed] [Google Scholar]
- Kilmartin, J.V., and Adams, A.E. (1984). Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J. Cell Biol. 98, 922-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak, A., Pasolli, H.A., and Fuchs, E. (2004). Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat. Cell Biol. 6, 21-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno, H., et al. (1996). Bni1p implicated in cytoskeletal control is a putative target of Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 15, 6060-6068. [PMC free article] [PubMed] [Google Scholar]
- Kovar, D.R., Kuhn, J.R., Tichy, A.L., and Pollard, T.D. (2003). The fission yeast cytokinesis formin Cdc12p is a barbed end actin filament capping protein gated by profilin. J. Cell Biol. 161, 875-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski, K.G., Chen, A.J., Rodal, A.A., and Drubin, D.G. (2000). Functions and functional domains of the GTPase Cdc42p. Mol. Biol. Cell 11, 339-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, L., Klee, S.K., Evangelista, M., Boone, C., and Pellman, D. (1999). Control of mitotic spindle position by the Saccharomyces cerevisiae formin Bni1p. J. Cell Biol. 144, 947-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew, D.J., and Reed, S.I. (1993). Morphogenesis in the yeast cell cycle: regulation by Cdc28 and cyclins. J. Cell Biol. 120, 1305-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew, D.J., and Reed, S.I. (1995). Cell cycle control of morphogenesis in budding yeast. Curr. Opin. Genet Dev. 5, 17-23. [DOI] [PubMed] [Google Scholar]
- Li, F., and Higgs, H.N. (2003). The mouse Formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr. Biol. 13, 1335-1340. [DOI] [PubMed] [Google Scholar]
- Lillie, S.H., and Brown, S.S. (1994). Immunofluorescence localization of the unconventional myosin, Myo2p, and the putative kinesin-related protein, Smy1p, to the same regions of polarized growth in Saccharomyces cerevisiae. J. Cell Biol. 125, 825-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott, J., and Li, R. (1998). Sequential assembly of myosin II, an IQGAP-like protein, and filamentous actin to a ring structure involved in budding yeast cytokinesis. J. Cell Biol. 140, 355-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H., Krizek, J., and Bretscher, A. (1992). Construction of a GAL1-regulated yeast cDNA expression library and its application to the identification of genes whose overexpression causes lethality in yeast. Genetics 132, 665-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H.P., and Bretscher, A. (1989). Disruption of the single tropomyosin gene in yeast results in the disappearance of actin cables from the cytoskeleton. Cell 57, 233-242. [DOI] [PubMed] [Google Scholar]
- Longtine, M.S., and Bi, E. (2003). Regulation of septin organization and function in yeast. Trends Cell Biol. 13, 403-409. [DOI] [PubMed] [Google Scholar]
- Longtine, M.S., DeMarini, D.J., Valencik, M.L., Al-Awar, O.S., Fares, H., De Virgilio, C., and Pringle, J.R. (1996). The septins: roles in cytokinesis and other processes. Curr. Opin. Cell Biol. 8, 106-119. [DOI] [PubMed] [Google Scholar]
- Longtine, M.S., McKenzie, A., 3rd, Demarini, D.J., Shah, N.G., Wach, A., Brachat, A., Philippsen, P., and Pringle, J.R. (1998). Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953-961. [DOI] [PubMed] [Google Scholar]
- Longtine, M.S., Theesfeld, C.L., McMillan, J.N., Weaver, E., Pringle, J.R., and Lew, D.J. (2000). Septin-dependent assembly of a cell cycle-regulatory module in Saccharomyces cerevisiae. Mol. Cell. Biol. 20, 4049-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean-Fletcher, S., and Pollard, T.D. (1980). Mechanism of action of cytochalasin B on actin. Cell 20, 329-341. [DOI] [PubMed] [Google Scholar]
- McCaffrey, M., Johnson, J.S., Goud, B., Myers, A.M., Rossier, J., Popoff, M.R., Madaule, P., and Boquet, P. (1991). The small GTP-binding protein Rho1p is localized on the Golgi apparatus and post-Golgi vesicles in Saccharomyces cerevisiae. J. Cell Biol. 115, 309-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan, J.N., Longtine, M.S., Sia, R.A., Theesfeld, C.L., Bardes, E.S., Pringle, J.R., and Lew, D.J. (1999). The morphogenesis checkpoint in Saccharomyces cerevisiae: cell cycle control of Swe1p degradation by Hsl1p and Hsl7p. Mol. Cell. Biol. 19, 6929-6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, R.K., Matheos, D., and Rose, M.D. (1999). The cortical localization of the microtubule orientation protein, Kar9p, is dependent upon actin and proteins required for polarization. J. Cell Biol. 144, 963-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat, J., and Andrews, B. (2003). Ac`septin'a signal: kinase regulation by septins. Dev. Cell 5, 528-530. [DOI] [PubMed] [Google Scholar]
- Mosch, H.U., and Fink, G.R. (1997). Dissection of filamentous growth by transposon mutagenesis in Saccharomyces cerevisiae. Genetics 145, 671-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley, J.B., Sagot, I., Manning, A.L., Xu, Y., Eck, M.J., Pellman, D., and Goode, B.L. (2004). A conserved mechanism for Bni1- and mDia1-induced actin assembly and dual regulation of Bni1 by Bud6 and profilin. Mol. Biol. Cell 15, 896-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland, J., Wesp, A., Riezman, H., and Botstein, D. (1997). Yeast actin cytoskeleton mutants accumulate a new class of Golgi-derived secretary vesicle. Mol. Biol. Cell 8, 1481-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick, P., Ferro, S., and Schekman, R. (1981). Order of events in the yeast secretory pathway. Cell 25, 461-469. [DOI] [PubMed] [Google Scholar]
- Novick, P., Field, C., and Schekman, R. (1980). Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 21, 205-215. [DOI] [PubMed] [Google Scholar]
- Ozaki-Kuroda, K., Yamamoto, Y., Nohara, H., Kinoshita, M., Fujiwara, T., Irie, K., and Takai, Y. (2001). Dynamic localization and function of Bni1p at the sites of directed growth in Saccharomyces cerevisiae. Mol. Cell. Biol. 21, 827-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, J., Nielsen, O., Egel, R., and Hagan, I.M. (1998). FH3, a domain found in formins, targets the fission yeast formin Fus1 to the projection tip during conjugation. J. Cell Biol. 141, 1217-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pring, M., Evangelista, M., Boone, C., Yang, C., and Zigmond, S.H. (2003). Mechanism of formin-induced nucleation of actin filaments. Biochemistry 42, 486-496. [DOI] [PubMed] [Google Scholar]
- Pruyne, D., Evangelista, M., Yang, C., Bi, E., Zigmond, S., Bretscher, A., and Boone, C. (2002). Role of formins in actin assembly: nucleation and barbed-end association. Science 297, 612-615. [DOI] [PubMed] [Google Scholar]
- Pruyne, D.W., Schott, D.H., and Bretscher, A. (1998). Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J. Cell Biol. 143, 1931-1945. [DOI] [PubMed] [Google Scholar]
- Richman, T.J., Sawyer, M.M., and Johnson, D.I. (1999). The Cdc42p GTPase is involved in a G2/M morphogenetic checkpoint regulating the apical-isotropic switch and nuclear division in yeast. J. Biol. Chem. 274, 16861-16870. [DOI] [PubMed] [Google Scholar]
- Rua, D., Tobe, B.T., and Kron, S.J. (2001). Cell cycle control of yeast filamentous growth. Curr. Opin. Microbiol. 4, 720-727. [DOI] [PubMed] [Google Scholar]
- Russell, D.W., Jensen, R., Zoller, M.J., Burke, J., Errede, B., Smith, M., and Herskowitz, I. (1986). Structure of the Saccharomyces cerevisiae HO gene and analysis of its upstream regulatory region. Mol. Cell. Biol. 6, 4281-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagot, I., Klee, S.K., and Pellman, D. (2002a). Yeast formins regulate cell polarity by controlling the assembly of actin cables. Nat. Cell Biol. 4, 42-50. [DOI] [PubMed] [Google Scholar]
- Sagot, I., Rodal, A.A., Moseley, J., Goode, B.L., and Pellman, D. (2002b). An actin nucleation mechanism mediated by Bni1 and profilin. Nat. Cell Biol. 4, 626-631. [DOI] [PubMed] [Google Scholar]
- Salminen, A., and Novick, P.J. (1987). A ras-like protein is required for a post-Golgi event in yeast secretion. Cell 49, 527-538. [DOI] [PubMed] [Google Scholar]
- Schott, D., Ho, J., Pruyne, D., and Bretscher, A. (1999). The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. J. Cell Biol. 147, 791-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott, D.H., Collins, R.N., and Bretscher, A. (2002). Secretory vesicle transport velocity in living cells depends on the myosin-V lever arm length. J. Cell Biol. 156, 35-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal, M., Bloom, K., and Reed, S.I. (2000). Bud6 directs sequential microtubule interactions with the bud tip and bud neck during spindle morphogenesis in Saccharomyces cerevisiae. Mol. Biol. Cell 11, 3689-3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu, Y.J., Barral, Y., and Snyder, M. (2000). Polarized growth controls cell shape and bipolar bud site selection in Saccharomyces cerevisiae. Mol. Cell. Biol. 20, 5235-5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu, Y.J., Santos, B., Fortin, N., Costigan, C., and Snyder, M. (1998). Spa2p interacts with cell polarity proteins and signaling components involved in yeast cell morphogenesis. Mol. Cell. Biol. 18, 4053-4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulewitz, M.J., Inouye, C.J., and Thorner, J. (1999). Hsl7 localizes to a septin ring and serves as an adapter in a regulatory pathway that relieves tyrosine phosphorylation of Cdc28 protein kinase in Saccharomyces cerevisiae. Mol. Cell. Biol. 19, 7123-7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski, R.S., and Hieter, P. (1989). A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder, M. (1989). The SPA2 protein of yeast localizes to sites of cell growth. J. Cell Biol. 108, 1419-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa, P.A., Sil, A., Swedlow, J.R., Herskowitz, I., and Vale, R.D. (1997). Actin-dependent localization of an RNA encoding a cell-fate determinant in yeast. Nature 389, 90-93. [DOI] [PubMed] [Google Scholar]
- TerBush, D.R., Maurice, T., Roth, D., and Novick, P. (1996). The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 15, 6483-6494. [PMC free article] [PubMed] [Google Scholar]
- TerBush, D.R., and Novick, P. (1995). Sec6, Sec8, and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. J. Cell Biol. 130, 299-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolliday, N., VerPlank, L., and Li, R. (2002). Rho1 directs formin-mediated actin ring assembly during budding yeast cytokinesis. Curr. Biol. 12, 1864-1870. [DOI] [PubMed] [Google Scholar]
- Tong, A.H., et al. (2001). Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science 294, 2364-2368. [DOI] [PubMed] [Google Scholar]
- Tong, A.H., et al. (2004). Global mapping of the yeast genetic interaction network. Science 303, 808-813. [DOI] [PubMed] [Google Scholar]
- Vallen, E.A., Caviston, J., and Bi, E. (2000). Roles of Hof1p, Bni1p, Bnr1p, and myo1p in cytokinesis in Saccharomyces cerevisiae. Mol. Biol. Cell 11, 593-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, W., Bielli, P., Wacha, S., and Ragnini-Wilson, A. (2002). Mlc1p promotes septum closure during cytokinesis via the IQ motifs of the vesicle motor Myo2p. EMBO J. 21, 6397-6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena, C., Collins, R.N., and Novick, P.J. (1997). Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J. Cell Biol. 137, 1495-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallar, B.J., and Alberts, A.S. (2003). The formins: active scaffolds that remodel the cytoskeleton. Trends Cell Biol. 13, 435-446. [DOI] [PubMed] [Google Scholar]
- Wedlich-Soldner, R., Altschuler, S., Wu, L., and Li, R. (2003). Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science 299, 1231-1235. [DOI] [PubMed] [Google Scholar]
- Winter, D.C., Choe, E.Y., and Li, R. (1999). Genetic dissection of the budding yeast Arp2/3 complex: a comparison of the in vivo and structural roles of individual subunits. Proc. Natl. Acad. Sci. USA 96, 7288-7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamochi, W., Tanaka, K., Nonaka, H., Maeda, A., Musha, T., and Takai, Y. (1994). Growth site localization of Rho1 small GTP-binding protein and its involvement in bud formation in Saccharomyces cerevisiae. J. Cell Biol. 125, 1077-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H.C., and Pon, L.A. (2002). Actin cable dynamics in budding yeast. Proc. Natl. Acad. Sci. USA 99, 751-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahner, J.E., Harkins, H.A., and Pringle, J.R. (1996). Genetic analysis of the bipolar pattern of bud site selection in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 16, 1857-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziman, M., Preuss, D., Mulholland, J., O'Brien, J.M., Botstein, D., and Johnson, D.I. (1993). Subcellular localization of Cdc42p, a Saccharomyces cerevisiae GTP-binding protein involved in the control of cell polarity. Mol. Biol. Cell 4, 1307-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.