Abstract
Epstein–Barr virus (EBV)-associated malignancies display distinct patterns of virus latent gene expression that reflect the complex interplay between the virus and its host cell. In the EBV-associated epithelial tumor nasopharyngeal carcinoma (NPC), the virus-encoded latent membrane protein LMP2A is consistently expressed whereas the oncogenic LMP1 protein appears to be restricted to only a proportion of tumors. In an attempt to understand the contribution of LMP2A to the pathogenesis of NPC, we established carcinoma cell lines stably infected in vitro with either a wild-type recombinant EBV (rEBV) or a mutant rEBV in which LMP2A is deleted (rEBV-2A). An NPC-like pattern of EBV gene expression including LMP2A but not LMP1 was consistently observed in carcinoma cells infected with rEBV. However, carcinoma cells infected with rEBV-2A expressed high levels of LMP1 from the signal transducer and activator of transcription (STAT)-regulated L1-TR promoter. Consistent with this effect, basal STAT activity was reduced in rEBV-infected carcinoma cells, and this repression was relieved in the absence of LMP2A. This modulation of STAT activity correlated with the ability of LMP2A to inhibit the autocrine secretion of IL-6 from carcinoma cell lines. Exogenous IL-6 was able to induce expression of LMP1 by means of STAT3 activation both in rEBV-infected carcinoma cell lines and in the EBV-positive C666-1 NPC cell line. The LMP2A-mediated suppression of IL-6 was a consequence of NF-κB inhibition. These data reveal that LMP2A modulates two key transcription factor pathways in carcinoma cells and suggest that this finding may be important in the pathogenesis of EBV-associated tumors.
Epstein–Barr virus (EBV) is a ubiquitous human herpesvirus associated with the development of both lymphoid and epithelial tumors (1). The pattern of EBV latent protein expression in these tumors is different from that observed in EBV-transformed lymphoblastoid cell lines (LCLs) (2–4). Thus, only EBV-encoded nuclear antigen 1 (EBNA1) is expressed in Burkitt's lymphoma whereas EBNA1 and two membrane proteins (LMP1 and LMP2A/B) are expressed in Hodgkin's lymphoma (HL) and nasopharyngeal carcinoma (NPC) (2, 4). The Qp promoter is responsible for specifically regulating EBNA1 expression in these tumors rather than the Cp promoter, which drives expression of all of the EBNAs in LCLs (2, 5). Likewise, in NPC and HL the expression of LMP1 is predominantly regulated by the L1-TR promoter rather than the EBNA2-responsive ED-L1 promoter used to drive LMP1 expression in B cells (6). Interestingly, the Qp, L1-TR, and ED-L1 promoters are positively regulated by the Janus kinase/signal transducer and activator of transcription (STAT) pathway (6, 7). Although the expression of the oncogenic LMP1 protein is a consistent feature of virus-associated HL, this is not the case in NPC, where expression is variable with only ≈20% of biopsies being unequivocally positive for LMP1 at the protein level (8). The mechanisms underlying differential LMP1 expression in NPC and the consequent effects on the NPC phenotype remain unknown.
Unlike LMP1, LMP2A and LMP2B are consistently expressed in NPC at the RNA level, and recent work confirms that LMP2A protein is frequently expressed in NPC (9, 10). This finding supports earlier work in which antibody responses to LMP2A and LMP2B were detected in sera from NPC patients but not in sera from patients with other EBV-related disorders (11). The immunoreceptor tyrosine-based activation motif (ITAM) of LMP2A, like that present in the B cell receptor (BCR), is tyrosine-phosphorylated and associates with the src family of protein tyrosine kinases and the syk protein tyrosine kinase (12). This association accounts for the ability of the LMP2A ITAM to block BCR signal transduction, thereby preventing the induction of lytic EBV replication in B cells (13). The consistent expression of LMP2A in NPC suggests an important function for this protein in the carcinogenic process, and this contention is supported by the demonstration that LMP2A can transform an epithelial cell line, an effect that is associated with activation of the phosphatidylinositol 3-kinase (PI3-K)/Akt survival pathway (14). LMP2A can also engage the extracellular signal-regulated kinase/JNK mitogen-activated protein (MAP) kinase and β-catenin signaling pathways in epithelial cells, but the consequences of this activation for NPC cells is unknown (15, 16). As the major EBV-encoded oncogene, LMP1 has diverse phenotypic effects, including up-regulation of antiapoptotic proteins and stimulation of cytokine production (2, 3). The ability of LMP1 to function as a constitutively activated member of the tumor necrosis factor receptor superfamily results in the activation of a diverse number of signaling pathways, including the NF-κB transcription factor pathway, the MAP kinase cascade, and the PI3K/Akt pathway (2, 3, 17).
An important consequence of epithelial infection with EBV is malignant transformation resulting in the development of NPC, a subset of gastric adenocarcinomas and of certain salivary gland carcinomas (1). Studies using recombinant EBV carrying the neomycin resistance gene (rEBV) to directly infect epithelial cell lines have demonstrated that stable infection can be established producing a pattern of EBV latent gene expression (EBNA1 and LMP2A) identical to that observed in the majority of NPC and virus-positive gastric carcinomas (18–20). To examine the possible role of LMP2A in carcinogenesis, we established carcinoma-derived cell lines infected with either wild-type rEBV or an rEBV in which the first exon of the LMP2A gene is disrupted (rEBV-2A) (21). These experiments reveal, for the first time, a relationship between LMP2A and LMP1 expression and highlight a significant role for LMP2A in modulating the Janus kinase/STAT and NF-κB transcription pathways.
Materials and Methods
Cell Lines and Tissue Culture. AGS (a human gastric-derived carcinoma cell line) and C666-1 (derived from undifferentiated EBV-positive NPC) (22) cells were cultured in Ham's F12 media. HONE-1 (an EBV-negative NPC cell line) and Ad/AH (a human adenocarcinoma of the nasopharynx) cells were cultured in RPMI medium 1640. All media were supplemented with 10% FCS, 2 mM l-glutamine, and 1% penicillin–streptomycin solution (Sigma-Aldrich). Cells were grown in six-well plates for recombinant IL-6 stimulations, transfections, RNA extraction, and immunoblot analysis or in 10-cm dishes for electrophoretic mobility-shift assays (EMSAs). Cells were grown on Tefloncoated microdot slides (Hendley, Loughton, U.K.) for immunofluorescence analysis. IL-6 secretion was analyzed by using the Pelikine human IL-6 ELISA kit (CLB, Amsterdam) according to the manufacturer's instructions.
Generation of EBV-Infected Carcinoma Cell Lines. Akata Burkitt's lymphoma cells carrying either rEBV or rEBV-2A were generated as previously described (18, 21, 23). Carcinoma cell lines infected with either rEBV or rEBV-2A were established by coincubation with anti-IgG-treated rEBV-carrying Akata Burkitt's lymphoma cells as previously described. The cells were cocultivated for 2–3 days before the removal of the nonadherent Akata cells by vigorous washing with PBS. Cells were then re-fed in their normal growth media supplemented with 50 μg/ml G418 (GIBCO) and left at 37°C for 1 week. Drug selection was increased to 400 μg/ml G418 over a period of 3 weeks and maintained at this level.
Immunofluorescence Staining and Immunoblotting. For LMP1 immunostaining, cells grown on slides and fixed in 4% paraformaldehyde were stained as previously described (17) by using the LMP1 antibody CS1-3 (diluted 1:1). Standard immunoblotting procedures (17) were used to detect EBNA1 by using a reference serum (AM, 1:200), LMP1 by using anti-LMP1 CS1-3 monoclonal antibodies (1:20), hemagglutinin (HA)-tagged LMP2A using a rabbit anti-HA antibody (Y11 diluted 1:200, Santa Cruz Biotechnology), STAT3 using either a phospho-STAT3-specific antibody or a total STAT3 antibody (1:1,000, Cell Signaling Technology). All immunoblots were repeated on at least three independent experiments.
EMSA. Nuclear extracts were prepared for EMSA as previously described (24) by using probes for NF-κB (5′-AGTTGAGGGGACT T TCCCAGGC-3′), STAT (5′-GATCCTTCTGGGAATTCCTAGATC-3′), and STAT3 (5′-GATCCTTCTGGGAATTCCTAGATC-3′). A total of 4 μg of protein was resolved by electrophoresis through a 5% polyacrylamide gel in a 0.55× TBE buffer (90 mM Tris/64.6 mM boric acid/2.5 mM EDTA, pH 8.3). Gels were then dried and exposed to x-ray film for autoradiography. All EMSAs were repeated on at least three independent experiments.
RNA Extraction, RT-PCR, and Southern Blotting. Preparation of total RNA and amplification by RT-PCR were performed as previously described (24) by using primers specific for L1-TR transcripts (5′-GCGTTACTCTGACGTAGCCG-3′ and 5′-ATACCGAAGACAAGTAAGCA-3′) or Qp promoter transcripts (5′-GTGCGCTACCGGATGGCG-3′ and 5′-CATTTCCAGGTCCTGTACCT-3′). PCR products for EBNA1 (273 bp) and LMP1 (490 bp) were visualized on 2% agarose gels and confirmed by Southern blotting by using either an EBNA1-specific probe (5′-AGACCTGGGAGCAGATTCAC-3′) or a LMP1-specific probe (5′-GAACAGCACAATTCCAAGGAACAATGCCTG-3′).
Small Interfering RNAs (siRNAs) Directed to LMP2A. siRNA sequence directed to LMP2A (5′-CUCCCAAUAUCCAUCUGCU-3′) and an irrelevant/control siRNA (5′-GGGUAGAUAGACUCUCGCU-3′) were designed and manufactured by Eurogentec (Brussels). Sequences were labeled with a fluorescent dye (5′-fluorescein, 6-FAM) to monitor transfection efficiency. HONE-1 rEBV-infected cells were transfected by using the transfection reagent Ribojuice (Novagen) as per the manufacturer's instructions and maintained at 37°C in 5% CO2 for 3 h before removal of siRNA. Solutions were replaced with complete growth medium, and RNA was collected at 24 and 48 h posttransfection.
Real-Time PCR (Quantitative PCR). RNA was extracted by using EZ-RNA total RNA isolation kit (Geneflow, Staffordshire, U.K.) and reverse-transcribed by using a cDNA reverse transcription kit (Promega) according to the manufacturer's instructions. Quantitative PCR for LMP2A (25) and LMP1 was performed by using LMP2 specific forward and reverse primers (5′-GCCTGGATTCTTACAGCAGGAT-3′ and 5′-GCAGCATCTAATGACCCCAAA-3′) and LMP1 specific forward and reverse primers (5′-CCTTGGTCTACTCCTACTGATGATCA-3′ and 5′-CAGCACAATTCCAAGGTACAATG-3′). All reactions were performed on a real-time PCR machine (PE7700 ABI Prism, PE Biosystems/Roche) with 18S ribosomal RNA as an internal standard. Cycle threshold (Ct) values were obtained graphically for LMP1, LMP2A, and the 18S internal standard. Gene expression was normalized to the 18S and represented as DCt values. For each sample the mean DCt values was calculated. Comparison of gene expression between control and treated samples was derived from subtraction of control DCt values from treatment DCt values to give a DDCt value, and relative gene expression was calculated as 2-DDCt. Relative gene expression was normalized to 1.0 (100%) of controls.
Transient Transfection and Adenovirus Infection. For luciferase assays the dual luciferase reporter assay (Promega) was performed according to manufacturer's instructions by using an IL-6 reporter plasmid and the Renilla plasmid to control for transfection efficiency. Transient transfection was performed by using increasing concentrations of pSG5 control and pSG5-LMP2A (expressing HA-tagged LMP2A) plasmids, and samples for EMSA were harvested after 24 h for incubation with NF-κB- and STAT-specific probes. HONE-1 cells infected with rEBV-2A were transfected with pSG5 control or pSG5-LMP2A. HONE-1 rEBV-2A cells were infected with a recombinant adenovirus expressing a dominantly active mutant of IκBα (RAd-IκBαSS/AA) kindly provided by R. T. Hay (University of St. Andrews, St. Andrews, U.K.). Cells were exposed to the recombinant virus at different multiplicities of infection for 2 h, and supernatants were harvested after 48 h for subsequent analysis of IL-6.
Results
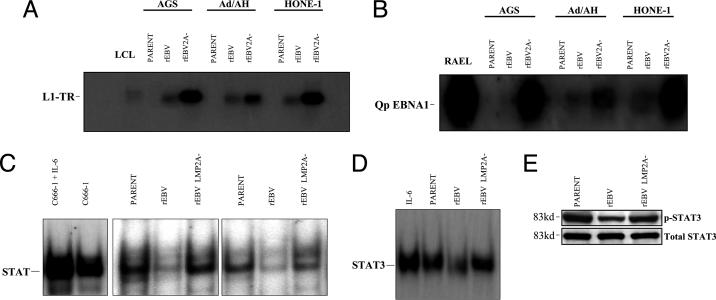
Infection with rEBV-2A Results in LMP1 Expression. The carcinoma cell line panel (AGS, Ad/AH, and HONE-1) was infected with either rEBV or rEBV-2A. Stable cell lines carrying EBV were selected in neomycin and maintained as polyclonal populations. No difference in the efficiency of selection between rEBV- and rEBV-2A-infected cells was observed. Immunoblot analysis of EBNA1 expression confirmed that the selected carcinoma cells were EBV-positive (Fig. 1A), and this was further supported by both immunostaining for EBNA1 and the detection of EBV-encoded small RNA expression (data not shown). The expression of LMP2A in the rEBV-infected carcinoma cells was confirmed by quantitative PCR and, interestingly, shown to be at a relatively low level compared with that expressed in the X50-7 LCL (Table 1). However, the LMP2A levels in rEBV-infected HONE-1 and Ad/AH cells were similar to that observed in the EBV-positive C666-1 NPC cell line (Table 1). Whereas carcinoma cells infected with rEBV did not express LMP1, those infected with rEBV-2A expressed significant levels of LMP1 that were detectable by both immunostaining (Fig. 1B) and immunoblotting (Fig. 1C). RT-PCR analysis demonstrated that the LMP1 expressed in rEBV-2A-infected carcinoma cells was predominantly driven from the STAT-responsive L1-TR promoter (Fig. 2A), with low-level expression also detected from the ED-L1 promoter (data not shown). Only extremely low levels of LMP1 expression were detected by RT-PCR in the rEBV-infected carcinoma cells, and these low levels did not result in LMP1 protein expression. Because a previous study had found that the Qp promoter responsible for EBNA1 expression in NPC is also STAT-responsive (7), we examined the activity of this promoter using RT-PCR and found a significant induction of Qp in carcinoma cells infected with rEBV-2A compared with those infected with rEBV (Fig. 2B).
Fig. 1.
Expression of EBNA1 and LMP1 in EBV-infected AGS, Ad/AH, and HONE-1 cell lines. Immunoblot analysis demonstrates EBNA1 (A) and LMP1 (C) expression in cells infected with rEBV- and rEBV-2A-infected cell lines. The LCL X50-7 was used as a positive control for both EBNA1 and LMP1. EBNA1 reference serum (AM) was used to detect EBNA1, and CS1-3 was used to detect LMP1. (B) Immunofluorescence staining using CS1-3 demonstrates LMP1 expression in cell lines infected with rEBV-2A (Bottom). LMP1 is absent from parental cells (Top) and cells infected with wild-type rEBV (Middle).
Table 1. Relative expression of LMP2A.
| Cell line | LMP2A expression, % |
|---|---|
| X50-7 | 100 |
| HONE-1 | 0.3 |
| AGS | 30.4 |
| Ad/AH | 0.8 |
| C666-1 | 0.8 |
Quantitative PCR analysis to determine the level of LMP2A expression in EBV-infected cell lines was performed as described in ref. 25. The X50-7 LCL was used as a reference for LMP2A levels in comparison with those in rEBV-infected HONE-1 AGS and Ad/AH cell lines as well as the EBV-positive C666-1 NPC cell line. Values assigned were calculated as a percentage of LMP2A expression in X50-7 LCLs.
Fig. 2.
Cells infected with rEBV-2A show increased transcriptional activity from the alternative LMP1 promoter L1-TR and EBNA1 Qp promoter. (A) Transcription of LMP1 from the alternative L1-TR (3.7 kb) is significantly elevated in cells infected with rEBV-2A as demonstrated by RT-PCR and Southern blotting. LMP1 expression in the X50-7 LCL was predominantly from the 2.8-kb promoter, and low-level L1-TR activity was detected in both X50-7 and Akata virus-transformed LCLs (data not shown). Uninfected parental cells served as a negative control. (B) Cells infected with rEBV-2A display increased transcription from the EBNA1 Qp promoter as detected using RT-PCR and Southern blotting. RAEL, a type 1 latently infected Burkitt's lymphoma cell line that expresses EBNA1 transcribed from the Qp promoter, served as a positive control. (C) EMSA demonstrates that LMP2A deletion results in derepression of STAT activity in nuclear extracts isolated from AGS and Ad/AH EBV-infected cells. The amount of activated STAT was determined by measuring its binding to a 32P-labeled generic STAT probe. Parental cells lines show constitutive levels of STAT activity comparable to that of cells infected with rEBV-2A. Basal STAT activity is reduced in cells infected with rEBV. IL-6 stimulated C666-1 NPC cells served as a positive control for STAT activation. (D) EMSA analysis of nuclear extracts isolated from HONE-1 parental and rEBV-infected cell lines confirms that LMP2A deletion results in derepression of STAT3 activity. IL-6-stimulated HONE-1 parental cells serve as a positive control for STAT3 activation. (E) Immunoblotting for phosphorylation of STAT3 confirms the ability of LMP2A to suppress STAT3 activity. Total STAT3 served as a loading control.
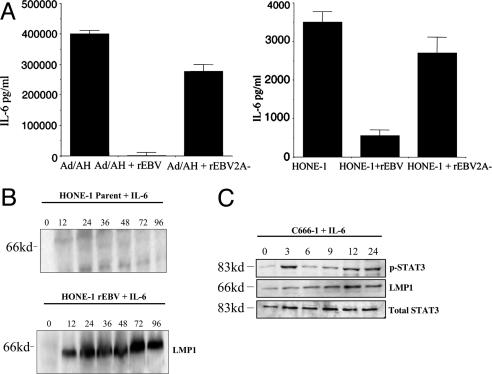
LMP2A Regulates STAT Activity and IL-6 Secretion. The up-regulation of both the L1-TR and Qp promoters in rEBV-2A-infected carcinoma cells suggested that LMP2A might be regulating STAT activity. To investigate this possibility, nuclear extracts from the infected carcinoma cell panel were examined by EMSA by using a generic STAT probe. The results of this assay confirmed that the constitutive levels of STAT activity observed in uninfected carcinoma cells were down-regulated upon rEBV infection and that this repression was relieved in cells infected with rEBV-2A (Fig. 2C). Given the proposed role of STAT3 in regulating LMP1 L1-TR and EBNA1 Qp and its activation in NPC cells (6, 7), we examined STAT3 activity in the HONE-1 NPC cell line. Consistent with our previous data in AGS and Ad/AH cells, STAT3 activity was reduced in rEBV-infected HONE-1 cells but up-regulated in cells infected with rEBV-2A as determined by both EMSA and phosphorylation of STAT3 (Fig. 2 D and E). The ability of IL-6 to activate STAT3, confirmed in HONE-1 cells in response to exogenous IL-6 (Fig. 2D), suggested that the effects of LMP2A could result from modulation of IL-6 expression. To address this possibility, supernatants from the infected carcinoma panel were examined for IL-6 secretion in ELISAs. Consistent with the constitutive activation of STAT3 in these cells, both HONE-1 and Ad/AH cells but not AGS cells expressed IL-6, but this was down-regulated upon infection with rEBV (Fig. 3A). Infection with rEBV-2A relieved this inhibition of IL-6, resulting in cytokine levels equivalent to those observed in uninfected control cells (Fig. 3A). Because IL-6 can stimulate the L1-TR promoter (6), we proposed that exogenous IL-6 might be able to overcome the LMP2A-induced inhibition of LMP1 expression. Thus, rEBV-infected HONE-1 cells were exposed to IL-6 for different times and subsequently harvested for examination of LMP1 expression. IL-6 was found to induce abundant expression of LMP1 in these cells, and this induction was maintained up to the latest time point at which cells were harvested (Fig. 3B). To confirm the ability of IL-6 to induce LMP1 expression in a more representative background, we examined the effects of exogenous IL-6 on the C666-1 EBV-positive NPC cell line, which has been reported as expressing LMP2A but very low levels of LMP1 (22). Exogenous IL-6 induced a biphasic response in STAT3 activation in C666-1 cells as determined by STAT3 phosphorylation. Thus, STAT3 activation was elevated after 3 h of IL-6 treatment but then declined and was subsequently induced again after 12 h (Fig. 3C). In contrast, a steady increase in LMP1 expression was observed, with maximum levels being achieved after 12 h of IL-6 treatment (Fig. 3C). These effects can be explained by the ability of LMP1 itself to induce IL-6 and STAT activation (24). Thus, IL-6 induced STAT3 activity results in up-regulation of LMP1 expression by means of the L1-TR promoter, and the induced LMP1 activates IL-6 secretion, resulting in a second wave of STAT3 activation.
Fig. 3.
IL-6 secretion is inhibited by LMP2A and controls LMP1 expression. (A) The ability of LMP2A to down-regulate IL-6 secretion in rEBV-infected HONE-1 and Ad/AH cell lines was determined by using an IL-6 ELISA. The histograms are representative of at least three separate experiments, and the mean ± SE of triplicate determinations is shown. (B) Addition of human recombinant IL-6 to the HONE-1 rEBV-infected cell line induces LMP1 expression as demonstrated by immunoblot analysis. (C) Addition of human recombinant IL-6 to the C666-1 NPC cell line induces LMP1 expression and phosphorylation of STAT3 as demonstrated by immunoblot analysis. Total STAT3 served as a loading control.
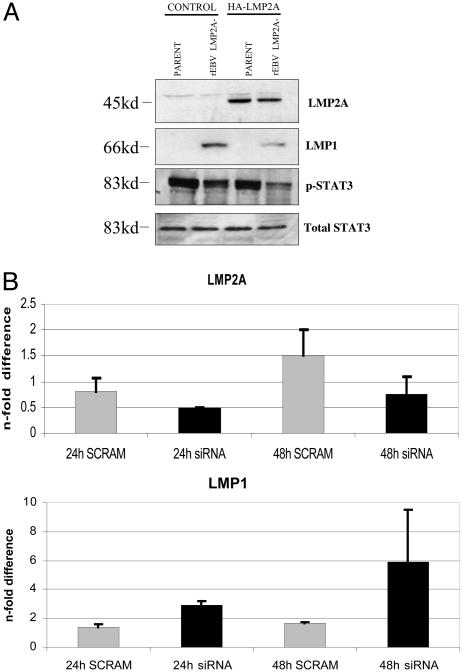
LMP2A Regulates LMP1 Expression. Experiments were performed to confirm the specificity of the inhibitory effect of LMP2A on LMP1 expression. The HONE-1 cell line infected with rEBV-2A was transiently transfected with a vector expressing HA-tagged LMP2A (pSG-LMP2A) in an attempt to transcomplement the LMP2A deletion. The expression of LMP2A in the transfected rEBV-2A-infected HONE-1 cells was confirmed by using HA immunoblotting to detect the HA–LMP2A fusion (Fig. 4A). Immunoblotting of the same extracts revealed down-regulation of LMP1 expression, which was accompanied by a decrease in the levels of phosphorylated STAT3 (Fig. 4A). To further examine the effect of LMP2A on LMP1 expression, HONE-1 cells infected with rEBV were treated with siRNA specific to LMP2A, and after 24 or 48 h RNA was harvested for quantitative PCR determination of both LMP2A and LMP1 expression. Although no effect of treatment with a control scrambled siRNA was observed, LMP2A-specific siRNA induced a decrease in LMP2A levels with a reduction of 50% at 48 h relative to the scrambled siRNA-treated control (Fig. 4C). Consistent with this decrease in LMP2A expression, the LMP2A-specific siRNA induced a significant increase in LMP1 RNA levels equating to a 6-fold induction at 48 h (Fig. 4C).
Fig. 4.
Confirmation that LMP2A regulates LMP1 expression. (A) Transient delivery of LMP2A represses LMP1 expression in rEBV-2A-infected HONE-1 cells. HONE-1 cells infected with rEBV-2A were transiently transfected with a vector (pSG-LMP2A) expressing HA-tagged LMP2A or with a control vector (pSG5), and, after 48 h, the protein expression was assessed by immunoblotting with antibodies to HA for detection of tagged LMP2A, LMP1, phospho-STAT3, and total STAT3. LMP2A expression resulted in decreased expression of both LMP1 and phospho-STAT3. pSG5 control transfected cells were used as a negative control. (B) HONE-1 rEBV-infected cells were transiently transfected with siRNA targeted to LMP2A or with a control scrambled siRNA. This treatment resulted in a 50% reduction in the levels of LMP2A relative to control (Upper) and a 6-fold increase in LMP1 relative to control (Lower) at 48 h posttransfection. Data shown are representative of two separate experiments with triplicate determination in each experiment, and data are expressed as the mean ± SE.
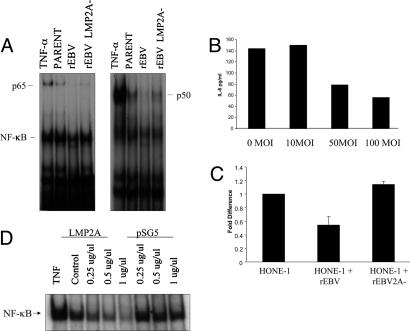
LMP2A Modulates NF-κB Activation. The ability of LMP2A to down-regulate IL-6 secretion and the observation that this effect can be overcome by exogenous IL-6 suggests that LMP2A directly influences IL-6 expression. Given that IL-6 transcription is directly regulated by the NF-κB pathway (24), we examined the effect of LMP2A on NF-κB activity. EMSAs using an NF-κB probe revealed that infection of HONE-1 cells with rEBV resulted in a reduction in NF-κB activity and that this was relieved in rEBV-2A-infected HONE-1 cells (Fig. 5A). To confirm the influence of NF-κB activity on IL-6 expression, HONE-1 cells infected with rEBV-2A were infected with a recombinant adenovirus expressing a dominantly active mutant of IκBα (RAd-IκBαSS/AA). Blockade of NF-κB activity with this recombinant virus induced a dose-dependent inhibition of IL-6 secretion (Fig. 5B). That these effects were caused by transcriptional regulation was confirmed by using reporter assays to monitor IL-6 promoter activity. Although the IL-6 promoter was active to a similar level in control HONE-1 cells and in HONE-1 cells infected with rEBV-2A, there was a marked reduction in promoter activity in rEBV-infected HONE-1 cells (Fig. 5C). These effects were consistent with the NF-κB activity levels detected in these cells (Fig. 5A). The effect of LMP2A on NF-κB activity was confirmed by transient transfection of LMP2A into HONE-1 cells. Thus, LMP2A induced a dose-dependent inhibition of the constitutive levels of NF-κB activity in HONE-1 cells (Fig. 5D).
Fig. 5.
LMP2A negatively regulates IL-6 secretion by means of inhibition of NF-κB. (A) EMSA demonstrates that infection of HONE-1 cells with rEBV inhibits NF-κB activity and that this effect is relieved in cells infected with rEBV-2A. Nuclear extracts isolated from HONE-1 cells or HONE-1 cells infected with rEBVs were analyzed for basal NF-κB activity. Tumor necrosis factor α-stimulated HONE-1 parental cells were used as a positive control. The presence of p65 and p50 subunits in the NF-κB complexes was confirmed by using antibodies to these subunits. (B) Blockade of NF-κB activity in rEBV-2A-infected HONE-1 cells with the RAd-IκBαSS/AA virus inhibited IL-6 secretion in a dose-dependent manner as determined by IL-6 ELISA. Data are representative of two separate experiments with triplicate determination, and the mean ± SE were all <5 pg/ml and thus unable to be accurately depicted on the histograms. (C) Transient transfection of the HONE-1 cell panel with an IL-6 reporter plasmid (p-IL-6-luc651) confirms that LMP2A modulates IL-6 expression. Thus, IL-6 promoter acting was similar in parental HONE-1 cells and rEBV-2A-infected HONE-1 cells but significantly reduced in rEBV-infected HONE-1 cells. Data are representative of three experiments and are presented as the mean ± SE of triplicate determinations. (D) NF-κB activity was inhibited in HONE-1 parent cells transiently transfected with a LMP2A-expressing plasmid as determined by EMSA. The pSG5 control vector was used as a negative control. Tumor necrosis factor α-stimulated HONE-1 parent cells served as a positive control for NF-κB activity.
Discussion
Previous studies examining the function of LMP2A have focused on either EBV-transformed LCLs carrying LMP2A mutations or overexpression of LMP2A in B cell and carcinoma cell lines (14, 26). In this study we have used the more physiologically relevant system of EBV infection of carcinoma cell lines to reveal a hitherto unknown function of LMP2A, namely the ability to down-regulate the STAT and NF-κB pathways in carcinoma cells. One consequence of this effect is repression of LMP1 expression, although it is likely that many cellular genes targeted by these transcription factors will also be down-regulated. Repressive effects of LMP2A expression have recently been reported in human and murine B cells and many of these target B cell-specific factors, resulting in a phenotype similar to that of the malignant HRS cells in HL and of germinal center B cells (27, 28). Alongside these effects, LMP2A was also found to induce expression of a range of genes involved in cell cycle induction, apoptosis inhibition, and suppression of cell-mediated immunity. Thus, the ability of LMP2A to both repress and induce cellular gene expression has been established in B cells, but differential effects due to variable levels of LMP2A expression or to cellular context have not been examined. Although our studies demonstrate a repressive effect of LMP2A on the NF-κB pathway in carcinoma cells, no such effect has been observed in B cells.
Quantitative examination of LMP2A expression at the RNA level showed that LMP2A is expressed at extremely low levels in EBV-infected carcinoma cell lines and in the EBV-positive C666-1 NPC cell line relative to the levels observed in an LCL. This finding may account for the difficulties in detecting LMP2A at the protein level in NPC biopsies and in EBV-infected carcinoma cell lines. These relatively low levels of LMP2A expression in EBV-infected carcinoma cells were nevertheless functional, because infection with rEBV-2A rather than wild-type EBV resulted in expression of LMP1, secretion of IL-6, and relief of STAT and NF-κB transcription factor repression. Although LMP2A was able to repress STAT activity in the AGS cell line, this effect was not caused by IL-6 suppression, because these cells fail to produce this cytokine. This finding suggests that other factors are likely to contribute along with IL-6 to the observed consequences of LMP2A expression. It was also interesting that EBV-infected AGS cells expressed relatively higher levels of LMP2A than either of the virus-infected NPC cell lines (Ad/AH and HONE-1). Whether the lack of IL-6 production and high levels of LMP2A expression in AGS cells are linked in any way requires further investigation. Transient expression of LMP2A in rEBV-2A-infected carcinoma cells suppressed LMP1 expression and STAT3 activity. Down-regulation of LMP2A using siRNA resulted in the induction of LMP1 in rEBV-infected carcinoma cell lines. These studies confirmed the specificity of LMP2A function and support a key role for LMP2A in modulating the STAT and NF-κB pathways in epithelial cells.
A previous study proposed a role for the STAT pathway in regulating LMP1 expression in carcinoma cells and suggested the establishment of an autoregulatory positive-feedback loop in which STAT-induced LMP1 expression results in up-regulation of IL-6 with consequent STAT activation inducing LMP1 by means of the L1-TR promoter and EBNA1 by means of the Qp promoter (29). Our study refines this model by demonstrating that LMP2A indirectly modulates LMP1 expression by repressing NF-κB activity. This effect results in suppression of IL-6 secretion with consequent down-regulation of STAT activity. Our observations explain the lack of detectable LMP1 expression at the protein level in most EBV-infected carcinoma cell lines as well as in NPC- and EBV-positive gastric carcinomas (8, 18, 19, 30, 31). In our studies we used carcinoma cell lines derived from either the nasopharynx or stomach, tissues that are naturally targeted by EBV in vivo, and confirmed that EBV infection of these cells results in the establishment of a latent virus infection with a pattern of EBV latent gene expression identical to that observed in the majority of NPC tumors and EBV-positive gastric adenocarcinomas. Importantly, we used the HONE-1 cell line that was originally EBV-positive but lost the EBV genome in culture and that carries the range of genetic changes evident in NPC (32, 33).
The observation that LMP2A can inhibit LMP1 expression and that this effect is relieved by exposure to IL-6 suggests an important contribution of the cellular environment to the modulation of LMP2A function. Thus, the consistent expression of LMP1 together with LMP2A in EBV-positive HL may be attributable to the autocrine production of IL-6 by malignant HRS cells (34). Furthermore, the critical role of IL-6 as an autocrine growth factor for EBV-transformed LCLs may be explained by its ability to induce LMP1 expression even in the presence of LMP2A (35). Our data suggest that the level of LMP2A expression may also determine the ability of NPC cells to express LMP1, thus raising questions about the factors responsible for regulating LMP2A transcription in these cells. The consistent expression of LMP1 in high-grade premalignant lesions (36) suggests that the effects of LMP2A on LMP1 expression may be modulated either by factors influencing LMP2A transcription or by local production of IL-6.
Constitutive activation of the STAT and NF-κB pathways commonly occurs in both hematopoietic malignancies and carcinomas as a consequence of either genetic or autocrine/paracrine alterations (37, 38). Our study links these two pathways by demonstrating that constitutive activation of NF-κB in carcinoma cell lines, a result of relA gene amplification in gastric and head and neck carcinomas (38, 39), induces IL-6 production, and this leads to the subsequent activation of the STAT pathway. Apart from its ability to promote cell growth and survival, the NF-κB pathway also mediates inflammatory responses by means of induction of cytokines and chemokines, which can result in the recruitment and activation of immune cells with consequent stimulation of anti-tumor immunity (38). Thus, a balance must exist between the ability of NF-κB to promote tumor development and its role in activating anti-tumor immune responses. This balance suggests that the developing cancer cell will need to alter its profile of NF-κB target genes at certain stages, e.g., to suppress apoptosis without activating the inflammatory response. LMP2A may contribute to this effect by modulating NF-κB activity while inducing the prosurvival phosphatidylinositol 3-kinase/Akt pathway (16, 26). Another important consideration is the context-dependent effects of NF-κB, particularly in epithelial cells, where this pathway has been shown to promote cell cycle withdrawal and differentiation in stratified squamous epithelium (40, 41). The demonstration that blockade of NF-κB function induces epidermal hyperplasia suggests a potential contribution of LMP2A-induced NF-κB repression to the pathogenesis of NPC, an undifferentiated tumor originating from squamous nasopharyngeal epithelium (40). Further studies in appropriate epithelial and carcinoma cell backgrounds are required to define the precise mechanisms underlying the effect of LMP2A on NF-κB and to determine the downstream consequences on gene transcription and cellular phenotype.
Acknowledgments
This work was supported by Cancer Research UK. We thank Ron Hay (University of St. Andrews, St. Andrews, U.K.) and Richard Longnecker (Northwestern University, Chicago) for providing reagents and Dolly Huang (Chinese University, Hong Kong) for providing the C666-1 cell line.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: EBV, Epstein–Barr virus; rEBV, wild-type recombinant EBV; rEBV-2A, mutant rEBV in which LMP2A is deleted; STAT, signal transducer and activator of transcription; NPC, nasopharyngeal carcinoma; LCL, lymphoblastoid cell line; HL, Hodgkin's lymphoma; EBNA, EBV-encoded nuclear antigen; siRNA, small interfering RNA; HA, hemagglutinin.
References
- 1.Rickinson, A. B. & Kieff, E. (2001) in Fields Virology, eds. Knipe, D. M. & Howley, P. M. (Lippincott, Philadelphia), pp. 2575-2627.
- 2.Kieff, E. & Rickinson, A. B. (2001) in Fields Virology, eds. Knipe, D. M. & Howley, P. M. (Lippincott, Philadelphia), pp. 2511-2574.
- 3.Young, L. S. & Murray, P. G. (2003) Oncogene 22, 5108-8121. [DOI] [PubMed] [Google Scholar]
- 4.Raab-Traub, N. (2002) Semin. Cancer Biol. 12, 431-441. [DOI] [PubMed] [Google Scholar]
- 5.Nonkwelo, C., Skinner, J., Bell, A., Rickinson, A. B. & Sample, J. (1996) J. Virol. 70, 623-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, H., Lee, J. M., Zong, Y. S., Borowitz, M., Ng, M. H., Ambinder, R. F. & Hayward, S. D. (2001) J. Virol. 75, 2929-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, H., Smith, P., Ambinder, R. F. & Hayward, S. D. (1999) Blood 93, 3026-3032. [PubMed] [Google Scholar]
- 8.Niedobitek, G., Young, L. S., Sam, C. K., Brooks, L., Prasad, U. & Rickinson, A. B. (1992) Am. J. Pathol. 140, 879-887. [PMC free article] [PubMed] [Google Scholar]
- 9.Brooks, L., Yao, Q. Y., Rickinson, A. B. & Young, L. S. (1992) J. Virol. 66, 2689-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heussinger, N., Buttner, M., Ott, G., Brachtel, E., Pilch, B. Z., Kremmer, E. & Niedobitek, G. (2004) J. Pathol., in press. [DOI] [PubMed]
- 11.Frech, B., Zimber-Strobl, U., Yip, T. T. C., Lau, W. H. & Mueller-Lantzsch, N. (1993) J. Gen. Virol. 74, 811-811. [DOI] [PubMed] [Google Scholar]
- 12.Fruehling, S. & Longnecker, R. (1997) J. Virol. 235, 241-251. [DOI] [PubMed] [Google Scholar]
- 13.Miller, C. L., Burkhardt, A. L., Lee, J. H., Stealey, B., Longnecker, R., Bolen, J. B. & Kieff, E. (1995) Immunity 2, 155-166. [DOI] [PubMed] [Google Scholar]
- 14.Scholle, F., Bendt, K. M. & Raab-Traub, N. (2000) J. Virol. 74, 10681-10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, S.-Y., Lu, J., Shih, Y.-C. & Tsai, C.-H. (2002) J. Virol. 76, 9556-9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrison, J. A., Klingelhutz, A. J. & Raab-Traub, N. (2003) J. Virol. 77, 12276-12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawson, C. W., Tramountanis, G., Eliopoulos, A. G. & Young, L. S. (2003) J. Biol. Chem. 278, 3694-3704. [DOI] [PubMed] [Google Scholar]
- 18.Imai, S., Nishikawa, J. & Takada, K. (1998) J. Virol. 72, 4371-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshiyama, H., Imai, S., Shimizu, N. & Takada, K. (1997) J. Virol. 71, 5688-5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fingeroth, J. D., Diamond, M. E., Sage, D. R., Hayman, J. & Yates, J. L. (1999) J. Virol. 73, 2115-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konishi, K., Maruo, S., Kato, K. & Takada, K. (2001) J. Gen. Virol. 82, 1451-1456. [DOI] [PubMed] [Google Scholar]
- 22.Cheung, S. T., Huang, D. P., Hui, A. B. Y., Lo, K.-W., Ko, C. W., Tsang, Y. S., Wong, N., Whitney, B. M. & Lee, J. C. K. (1999) Int. J. Cancer 83, 121-126. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu, N., Yoshiyama, H. & Takada, K. (1996) J. Virol. 70, 7260-7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eliopoulos, A. G., Stack, M., Dawson, C. W., Kaye, K. M., Hodgkin, L., Sihota, S., Rowe, M. & Young, L. S. (1997) Oncogene 14, 2899-2916. [DOI] [PubMed] [Google Scholar]
- 25.Moody, C. A., Scott, R. S., Su, T. & Sixbey, J. W. (2003) J. Virol. 77, 8555-8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swart, R., Ruf, I. K., Sample, J. & Longnecker, R. (2000) J. Virol. 74, 10838-10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Portis, T. & Longnecker, R. (2003) J. Virol. 77, 105-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Portis, T., Dyck, P. & Longnecker, R. (2003) Blood 102, 4166-4178. [DOI] [PubMed] [Google Scholar]
- 29.Chen, H., Hutt-Fletcher, L. M., Cao, L. & Hayward, S. D. (2003) J. Virol. 77, 4139-4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugiura, M., Imai, S., Tokunaga, M., Koizumi, S., Uchizawa, M., Okamoto, K. & Osato, T. (1996) Br. J. Cancer 74, 625-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishikawa, J., Imai, S., Oda, T., Kojima, T., Okita, K. & Takada, K. (1999) J. Virol. 73, 1286-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao, K. T., Zhang, H. Y., Zhu, H. C., Wang, F. X., Li, G. Y., Wen, D. S., Li, Y. P., Tsai, C. H. & Glaser, R. (1990) Int. J. Cancer 45, 83-89. [DOI] [PubMed] [Google Scholar]
- 33.Cheng, Y., Poulos, N. E., Lung, M. L., Hampton, G., Ou, B., Lerman, M. I. & Stanbridge, E. J. (1998) Proc. Natl. Acad. Sci. USA 95, 3042-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beck, A., Pazolt, D., Grabenbauer, G., Nicholls, J. M., Herbst, H., Young, L. S. & Niedobitek, G. (2001) J. Pathol. 194, 145-151. [DOI] [PubMed] [Google Scholar]
- 35.Tosato, G., Tanner, J., Jones, K. D., Ravel, M. & Pike, S. E. (1990) J. Virol. 64, 3033-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pathmanathan, R., Prasad, U., Sadler, R., Flynn, K. & Raab-Traub, N. (1995) N. Engl. J. Med. 333, 693-698. [DOI] [PubMed] [Google Scholar]
- 37.Yu, H. & Jove, R. (2004) Nat. Rev. Cancer 4, 97-105. [DOI] [PubMed] [Google Scholar]
- 38.Karin, M., Cao, Y., Greten, F. R. & Li, Z.-W. (2002) Nat. Rev. Cancer 2, 301-310. [DOI] [PubMed] [Google Scholar]
- 39.Rayet, B. & Gelinas, C. (1999) Oncogene 18, 6938-6947. [DOI] [PubMed] [Google Scholar]
- 40.Seitz, C. S., Lin, Q., Deng, H. & Khavari, P. A. (1998) Proc. Natl. Acad. Sci. USA 95, 2307-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaufman, C. K. & Fuchs, E. (2000) J. Cell Biol. 149, 999-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]