Abstract
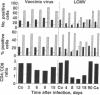
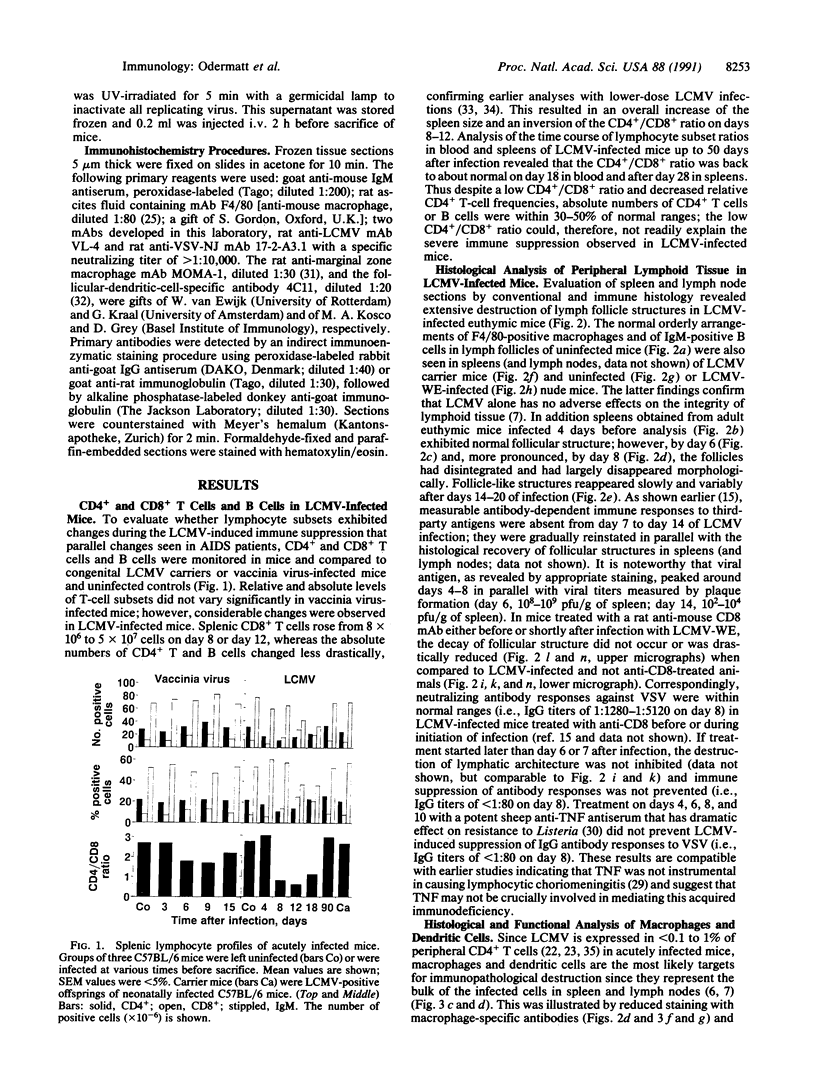
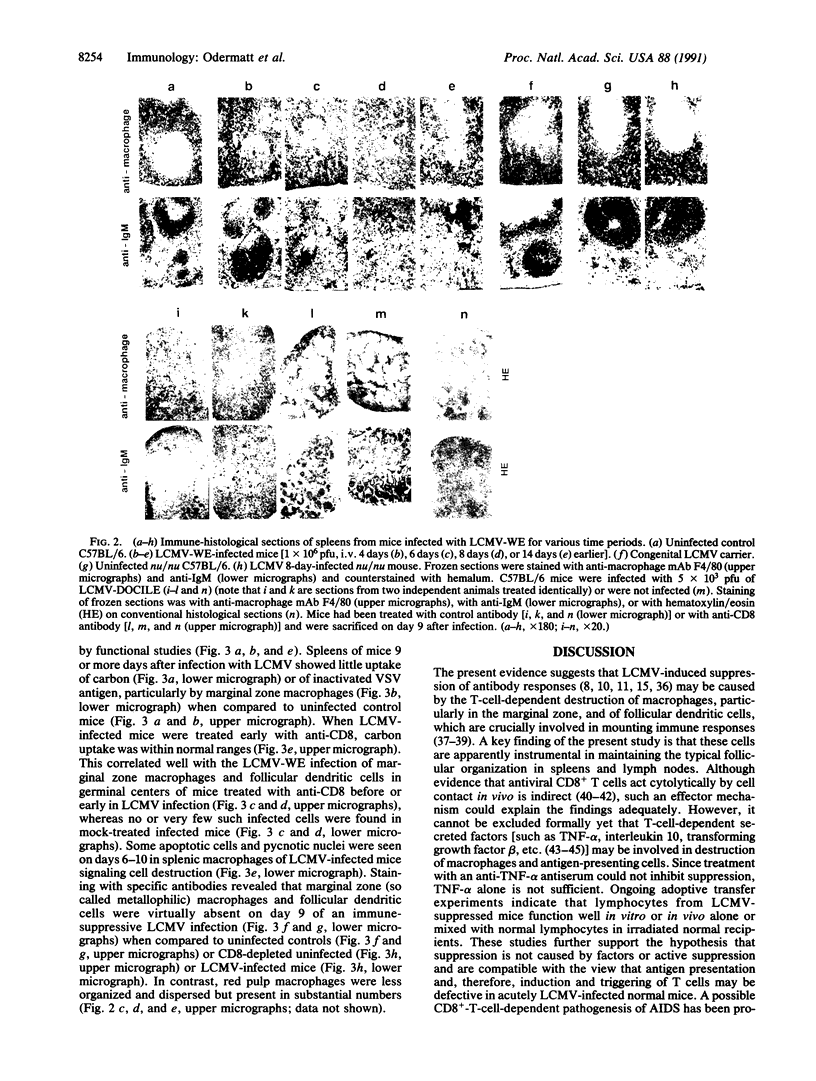
Virus-induced acquired immune suppression in mice infected with lymphocytic choriomeningitis virus is shown here to be caused by the CD8+-T-cell-dependent elimination of macrophages/antigen-presenting cells. Surprisingly, this is associated with severe destruction of the follicular organization of lymphoid organs, indicating a crucial role for dendritic cells and marginal zone macrophages in maintaining follicular structure. Once established, this immunopathology cannot be readily reversed by the elimination of CD8+ effector cells. Such a T-cell-mediated pathogenesis may play a pivotal role in acquired virus-induced immunosuppression and may represent one strategy by which virus escapes immune surveillance and establishes persistent infections in initially immunocompetent hosts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., King C. C., Oldstone M. B. Virus-lymphocyte interaction: T cells of the helper subset are infected with lymphocytic choriomeningitis virus during persistent infection in vivo. J Virol. 1987 May;61(5):1571–1576. doi: 10.1128/jvi.61.5.1571-1576.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., Salmi A., Butler L. D., Chiller J. M., Oldstone M. B. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984 Aug 1;160(2):521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. A., Horne R. Follicular dendritic cells and virus-like particles in AIDS-related lymphadenopathy. Lancet. 1984 Aug 18;2(8399):370–372. doi: 10.1016/s0140-6736(84)90540-3. [DOI] [PubMed] [Google Scholar]
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Biberfeld P., Ost A., Porwit A., Sandstedt B., Pallesen G., Böttiger B., Morfelt-Månsson L., Biberfeld G. Histopathology and immunohistology of HTLV-III/LAV related lymphadenopathy and AIDS. Acta Pathol Microbiol Immunol Scand A. 1987 Jan;95(1):47–65. doi: 10.1111/j.1699-0463.1987.tb00009_95a.x. [DOI] [PubMed] [Google Scholar]
- Brenan M., Zinkernagel R. M. Influence of one virus infection on a second concurrent primary in vivo antiviral cytotoxic T-cell response. Infect Immun. 1983 Aug;41(2):470–475. doi: 10.1128/iai.41.2.470-475.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bro-Jorgensen K., Güttler F., Jorgensen P. N., Volkert M. T lymphocyte function as the principal target of lymphocytic choriomeningitis virus-induced immunosuppression. Infect Immun. 1975 Apr;11(4):622–629. doi: 10.1128/iai.11.4.622-629.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bro-Jorgensen K., Volkert M. Defects in the immune system of mice infected with lymphocytic choriomeningitis virus. Infect Immun. 1974 Apr;9(4):605–614. doi: 10.1128/iai.9.4.605-614.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier M. J., Welsh R. M., Dutko F. J., Oldstone M. B. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- Ceredig R., Allan J. E., Tabi Z., Lynch F., Doherty P. C. Phenotypic analysis of the inflammatory exudate in murine lymphocytic choriomeningitis. J Exp Med. 1987 Jun 1;165(6):1539–1551. doi: 10.1084/jem.165.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cihak J., Lehmann-Grube F. Persistent infection of mice with the virus of lymphocytic choriomeningitis: virus-specific immunological tolerance. Infect Immun. 1974 Nov;10(5):1072–1076. doi: 10.1128/iai.10.5.1072-1076.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Allan J. E., Lynch F., Ceredig R. Dissection of an inflammatory process induced by CD8+ T cells. Immunol Today. 1990 Feb;11(2):55–59. doi: 10.1016/0167-5699(90)90019-6. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M., Ramshaw I. A. Specificity and development of cytotoxic thymus-derived lymphocytes in lymphocytic choriomeningitis. J Immunol. 1974 Apr;112(4):1548–1552. [PubMed] [Google Scholar]
- Doyle M. V., Oldstone M. B. Interactions between viruses and lymphocytes. I. In vivo replication of lymphocytic choriomeningitis virus in mononuclear cells during both chronic and acute viral infections. J Immunol. 1978 Oct;121(4):1262–1269. [PubMed] [Google Scholar]
- Dunlop M. B., Blanden R. V. Mechanisms of suppression of cytotoxic T-cell responses in murine lymphocytic choriomeningitis virus infection. J Exp Med. 1977 May 1;145(5):1131–1143. doi: 10.1084/jem.145.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Fenyö E. M., Morfeldt-Månson L., Chiodi F., Lind B., von Gegerfelt A., Albert J., Olausson E., Asjö B. Distinct replicative and cytopathic characteristics of human immunodeficiency virus isolates. J Virol. 1988 Nov;62(11):4414–4419. doi: 10.1128/jvi.62.11.4414-4419.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A., Frei K., Bodmer S., Hofer E., Schreier M. H., Palladino M. A., Jr, Zinkernagel R. M. Transforming growth factor-beta inhibits the generation of cytotoxic T cells in virus-infected mice. J Immunol. 1989 Nov 15;143(10):3230–3234. [PubMed] [Google Scholar]
- HOTCHIN J. The biology of lymphocytic choriomeningitis infection: virus-induced immune disease. Cold Spring Harb Symp Quant Biol. 1962;27:479–499. doi: 10.1101/sqb.1962.027.001.046. [DOI] [PubMed] [Google Scholar]
- Hauser T., Frei K., Zinkernagel R. M., Leist T. P. Role of tumor necrosis factor in Listeria resistance of nude mice. Med Microbiol Immunol. 1990;179(2):95–104. doi: 10.1007/BF00198530. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Rota T. R., Hirsch M. S. Infection of monocyte/macrophages by human T lymphotropic virus type III. J Clin Invest. 1986 May;77(5):1712–1715. doi: 10.1172/JCI112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoot G. P., Kettman J. R. Extra-thymic immature T cells: development in polyoma virus-induced murine salivary gland tumors. Eur J Immunol. 1989 Nov;19(11):1991–1998. doi: 10.1002/eji.1830191104. [DOI] [PubMed] [Google Scholar]
- Jacobs R. P., Cole G. A. Lymphocytic choriomeningitis virus-induced immunosuppression: a virus-induced macrophage defect. J Immunol. 1976 Sep;117(3):1004–1009. [PubMed] [Google Scholar]
- Janossy G., Pinching A. J., Bofill M., Weber J., McLaughlin J. E., Ornstein M., Ivory K., Harris J. R., Favrot M., Macdonald-Burns D. C. An immunohistological approach to persistent lymphadenopathy and its relevance to AIDS. Clin Exp Immunol. 1985 Feb;59(2):257–266. [PMC free article] [PubMed] [Google Scholar]
- Klaus G. G., Humphrey J. H., Kunkl A., Dongworth D. W. The follicular dendritic cell: its role in antigen presentation in the generation of immunological memory. Immunol Rev. 1980;53:3–28. doi: 10.1111/j.1600-065x.1980.tb01038.x. [DOI] [PubMed] [Google Scholar]
- Koenig S., Gendelman H. E., Orenstein J. M., Dal Canto M. C., Pezeshkpour G. H., Yungbluth M., Janotta F., Aksamit A., Martin M. A., Fauci A. S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986 Sep 5;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Kraal G., Janse M. Marginal metallophilic cells of the mouse spleen identified by a monoclonal antibody. Immunology. 1986 Aug;58(4):665–669. [PMC free article] [PubMed] [Google Scholar]
- Lehmann-Grube F., Cihak J., Varho M., Tijerina R. The immune response of the mouse to lymphocytic choriomeningitis virus. II. Active suppression of cell-mediated immunity by infection with high virus doses. J Gen Virol. 1982 Feb;58(Pt 2):223–235. doi: 10.1099/0022-1317-58-2-223. [DOI] [PubMed] [Google Scholar]
- Lehmann-Grube F., Niemeyer I., Löhler J. Lymphocytic choriomeningitis of the mouse. IV. Depression of the allograft reaction. Med Microbiol Immunol. 1972;158(1):16–25. doi: 10.1007/BF02122004. [DOI] [PubMed] [Google Scholar]
- Leist T. P., Cobbold S. P., Waldmann H., Aguet M., Zinkernagel R. M. Functional analysis of T lymphocyte subsets in antiviral host defense. J Immunol. 1987 Apr 1;138(7):2278–2281. [PubMed] [Google Scholar]
- Leist T. P., Rüedi E., Zinkernagel R. M. Virus-triggered immune suppression in mice caused by virus-specific cytotoxic T cells. J Exp Med. 1988 May 1;167(5):1749–1754. doi: 10.1084/jem.167.5.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist T. P., Zinkernagel R. M. Treatment with anti-tumor necrosis factor alpha does not influence the immune pathological response against lymphocytic choriomeningitis virus. Cytokine. 1990 Jan;2(1):29–34. doi: 10.1016/1043-4666(90)90040-z. [DOI] [PubMed] [Google Scholar]
- Levy J. A. Mysteries of HIV: challenges for therapy and prevention. Nature. 1988 Jun 9;333(6173):519–522. doi: 10.1038/333519a0. [DOI] [PubMed] [Google Scholar]
- Löhler J., Lehmann-Grube F. Immunopathologic alterations of lymphatic tissues of mice infected with lymphocytic choriomeningitis virus. I. Histopathologic findings. Lab Invest. 1981 Mar;44(3):193–204. [PubMed] [Google Scholar]
- MacNeil I. A., Suda T., Moore K. W., Mosmann T. R., Zlotnik A. IL-10, a novel growth cofactor for mature and immature T cells. J Immunol. 1990 Dec 15;145(12):4167–4173. [PubMed] [Google Scholar]
- Macatonia S. E., Taylor P. M., Knight S. C., Askonas B. A. Primary stimulation by dendritic cells induces antiviral proliferative and cytotoxic T cell responses in vitro. J Exp Med. 1989 Apr 1;169(4):1255–1264. doi: 10.1084/jem.169.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre K. W., Bukowski J. F., Welsh R. M. Exquisite specificity of adoptive immunization in arenavirus-infected mice. Antiviral Res. 1985 Oct;5(5):299–305. doi: 10.1016/0166-3542(85)90044-0. [DOI] [PubMed] [Google Scholar]
- Mims C. A. Immunofluorescence study of the carrier state and mechanism of vertical transmission in lymphocytic choriomeningitis virus infection in mice. J Pathol Bacteriol. 1966 Apr;91(2):395–402. doi: 10.1002/path.1700910214. [DOI] [PubMed] [Google Scholar]
- Mims C. A., Wainwright S. The immunodepressive action of lymphocytic choriomeningitis virus in mice. J Immunol. 1968 Oct;101(4):717–724. [PubMed] [Google Scholar]
- Moskophidis D., Cobbold S. P., Waldmann H., Lehmann-Grube F. Mechanism of recovery from acute virus infection: treatment of lymphocytic choriomeningitis virus-infected mice with monoclonal antibodies reveals that Lyt-2+ T lymphocytes mediate clearance of virus and regulate the antiviral antibody response. J Virol. 1987 Jun;61(6):1867–1874. doi: 10.1128/jvi.61.6.1867-1874.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Tishon A., Chiller J. M., Weigle W. O., Dixon F. J. Effect of chronic viral infection on the immune system. I. Comparison of the immune responsiveness of mice chronically infected with LCM virus with that of noninfected mice. J Immunol. 1973 May;110(5):1268–1278. [PubMed] [Google Scholar]
- Pfau C. J., Valenti J. K., Pevear D. C., Hunt K. D. Lymphocytic choriomeningitis virus killer T cells are lethal only in weakly disseminated murine infections. J Exp Med. 1982 Jul 1;156(1):79–89. doi: 10.1084/jem.156.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher H., Moskophidis D., Rohrer U., Bürki K., Hengartner H., Zinkernagel R. M. Viral escape by selection of cytotoxic T cell-resistant virus variants in vivo. Nature. 1990 Aug 16;346(6285):629–633. doi: 10.1038/346629a0. [DOI] [PubMed] [Google Scholar]
- Popescu M., Löhler J., Lehmann-Grube F. Infectious lymphocytes in lymphocytic choriomeningitis virus carrier mice. J Gen Virol. 1979 Mar;42(3):481–492. doi: 10.1099/0022-1317-42-3-481. [DOI] [PubMed] [Google Scholar]
- Rácz P., Tenner-Rácz K., Kahl C., Feller A. C., Kern P., Dietrich M. Spectrum of morphologic changes of lymph nodes from patients with AIDS or AIDS-related complexes. Prog Allergy. 1986;37:81–181. doi: 10.1159/000318442. [DOI] [PubMed] [Google Scholar]
- Sakai K., Dewhurst S., Ma X. Y., Volsky D. J. Differences in cytopathogenicity and host cell range among infectious molecular clones of human immunodeficiency virus type 1 simultaneously isolated from an individual. J Virol. 1988 Nov;62(11):4078–4085. doi: 10.1128/jvi.62.11.4078-4085.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman S. L., Jacobs R. P., Cole G. A. Mechanisms of hemopoietic and immunological dysfunction induced by lymphocytic choriomeningitis virus. Infect Immun. 1978 Feb;19(2):533–539. doi: 10.1128/iai.19.2.533-539.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenner-Rácz K., Rácz P., Dietrich M., Kern P. Altered follicular dendritic cells and virus-like particles in AIDS and AIDS-related lymphadenopathy. Lancet. 1985 Jan 12;1(8420):105–106. doi: 10.1016/s0140-6736(85)91994-4. [DOI] [PubMed] [Google Scholar]
- Tishon A., Southern P. J., Oldstone M. B. Virus-lymphocyte interactions. II. Expression of viral sequences during the course of persistent lymphocytic choriomeningitis virus infection and their localization to the L3T4 lymphocyte subset. J Immunol. 1988 Feb 15;140(4):1280–1284. [PubMed] [Google Scholar]
- Walker C. M., Moody D. J., Stites D. P., Levy J. A. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986 Dec 19;234(4783):1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- Wu-Hsieh B., Howard D. H., Ahmed R. Virus-induced immunosuppression: a murine model of susceptibility to opportunistic infection. J Infect Dis. 1988 Jul;158(1):232–235. doi: 10.1093/infdis/158.1.232. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Haenseler E., Leist T., Cerny A., Hengartner H., Althage A. T cell-mediated hepatitis in mice infected with lymphocytic choriomeningitis virus. Liver cell destruction by H-2 class I-restricted virus-specific cytotoxic T cells as a physiological correlate of the 51Cr-release assay? J Exp Med. 1986 Oct 1;164(4):1075–1092. doi: 10.1084/jem.164.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]