Abstract
Background: The success of surgery for thyroid cancer hinges on thorough and accurate preoperative imaging, which enables complete clearance of the primary tumor and affected lymph node compartments. This working group was charged by the Surgical Affairs Committee of the American Thyroid Association to examine the available literature and to review the most appropriate imaging studies for the planning of initial and revision surgery for thyroid cancer.
Summary: Ultrasound remains the most important imaging modality in the evaluation of thyroid cancer, and should be used routinely to assess both the primary tumor and all associated cervical lymph node basins preoperatively. Positive lymph nodes may be distinguished from normal nodes based upon size, shape, echogenicity, hypervascularity, loss of hilar architecture, and the presence of calcifications. Ultrasound-guided fine-needle aspiration of suspicious lymph nodes may be useful in guiding the extent of surgery. Cross-sectional imaging (computed tomography with contrast or magnetic resonance imaging) may be considered in select circumstances to better characterize tumor invasion and bulky, inferiorly located, or posteriorly located lymph nodes, or when ultrasound expertise is not available. The above recommendations are applicable to both initial and revision surgery. Functional imaging with positron emission tomography (PET) or PET-CT may be helpful in cases of recurrent cancer with positive tumor markers and negative anatomic imaging.
Introduction
Though thyroid cancer is associated with high overall survival rates exceeding 90% for most subtypes, the risk of recurrence has been reported to be as high as 35% (1). Most of these recurrences are detected within the first five years after diagnosis, and thus may actually represent persistent rather than truly recurrent disease. It is known that the majority of reoperations for thyroid cancer are preventable, and that inadequate preoperative imaging frequently is the root cause of incomplete initial surgery (2).
Thorough and accurate preoperative imaging enables complete clearance of the primary tumor as well as affected lymph node compartments, and is thus an essential component in the planning of thyroid cancer surgery. As stated in the Revised American Thyroid Association Management Guidelines for Patients with Thyroid Nodules and Differentiated Thyroid Cancer, ultrasound is the first-line imaging modality for patients with known or suspected thyroid cancer (Recommendation 21) (3). In addition, cross-sectional imaging, including computed tomography (CT) scan with contrast, has a role in primary and nodal preoperative assessment in some patients with well-differentiated thyroid cancer. Herein, we summarize the available literature to describe the role of ultrasound, cross-sectional imaging, and functional imaging in the planning of initial and subsequent surgery for thyroid cancer.
Ultrasound Hardware, Technology, and Technique
Equipment
Currently, a variety of high-quality ultrasound machines produced by different manufacturers are available. Several key components are required. First, a high frequency linear transducer (12 mHz) is preferable. This permits higher resolution imaging of the more superficial tissues of the neck (up to about 4 cm below the skin surface). The transducer should allow for variable frequency selection, so that the frequency can be decreased to 10 mHz or even 8 mHz, for deeper fields of view. Lower frequency imaging is useful in patients with prominent soft tissue and larger circumference necks, especially when examining the deeper paratracheal areas, the tracheoesophageal groove, and level 4 lymph nodes that tend to be located posterior to the carotid/jugular sheath. Second, because vascular assessment of lymph nodes is critical for assessing malignancy risk, color flow Doppler and/or power Doppler are required with variable settings. Third, sonographic imaging is improved if at least two focal zones are available and the focal zones can be shifted to the depth of interest. Fourth, ultrasound machines should possess technology for total gain correction or time-gain compensation, allowing for increasing the gain in the far field, deeper in the neck to maximize imaging, while decreasing the gain in the near field, since the skin is generally not a site for metastatic disease. Finally, an optional small sector or convex transducer with variable frequency of up to 8.5 mHz can be useful for evaluation of the upper mediastinum because the curved footprint can be placed in the sternal notch and angled inferiorly to view the mediastinum.
Aims of Preoperative Ultrasound for Thyroid Cancer Surgery
The objectives of preoperative sonographic imaging in patients with known or suspected thyroid cancer are (a) to assess the primary tumor and (b) to identify abnormal lymph nodes in the central (4) and lateral (5) neck that should be targeted for compartment-oriented surgical removal. The procedure described below should be performed in all patients undergoing initial surgery, as well as after thyroidectomy in thyroid cancer patients as part of a surveillance strategy. Prior to removal of the thyroid, examination of the central neck compartment is more challenging because of the thyroid's presence.
Scanning Technique
Essential points of scanning technique are summarized in Table 1. Proper positioning of the patient, the examiner, and the ultrasound machine are essential for optimizing the sonographic examination. Typically, for a right-handed operator, both the ultrasound console and the operator are positioned on the patient's right-hand side. This configuration may be modified to maximize the ergonomic advantage of the individual operator and for patient comfort. To optimize imaging of the lower cervical lymph node neck levels (level 4, inferior level 6, and upper mediastinum), the patient's neck should be hyperextended and supported with a pillow placed under the shoulders or a dedicated shoulder bolster. The room should have window shades and adjustable lighting, so that it is dark while the examination is being performed.
Table 1.
Scanning Technique: Preoperative Ultrasound for Thyroid Cancer Surgery
| • Equipment: High-frequency linear array probe. |
| • Positioning: Hyperextension of neck. |
| • Primary lesion: Assess size, multiplicity, margin, invasion of deep structures. |
| • Central compartment lymph nodes (level 6): Scan from submental area to sternal notch. Scan three distinct areas: pretracheal, right paratracheal, and left paratracheal. Turn head away from side of interest to image tracheoesophageal groove. Angle transducer inferiorly to examine mediastinum. |
| • Lateral compartment lymph nodes (levels 2, 3, and 4): Scan from mandible to clavicle. Angle transducer inferiorly at clavicle to image infraclavicular nodes at base of level 4. |
| • Posterior compartment lymph nodes (level 5): Sweep laterally along clavicle to posterior border of sternocleidomastoid muscle, then trace posterior border superiorly to mastoid process. |
Scanning of the neck should begin in the transverse plane. It is preferable initially to have the patient looking straight ahead with the chin up rather than rotating the head. This position permits imaging lymph nodes in the anatomically neutral position. Turning of the head to the left or the right, away from the side of interest, may facilitate imaging of tracheoesophageal or deep level 6 lymph nodes because this position causes these structures to rotate more anteriorly. However, labeling of lymph node location should be done with the head in the anatomically neutral position. Given that the anatomic borders of the compartments are not always straightforward to identify sonographically, additional descriptors for the locations of abnormal lymph nodes, such as their positions relative to major vessels and bony landmarks, may be useful.
To scan the lateral compartments, levels 2–4, the transducer should be positioned inferior to the mandible, at the level of the submandibular gland. In the transverse plane, the transducer should be moved inferiorly to the clavicle with the field of vision centered on the carotid/jugular sheath. Imaging should not just involve one sweep from superior to inferior, but the examiner should move the transducer several times up and down the vascular sheath to best identify lymph nodes. On both sides, the transducer should be angled inferiorly at the clavicle to image infraclavicular nodes at the base of level 4. Imaging of the posterior triangle, level 5, requires scanning laterally along the clavicle and posterior to the sternocleidomastoid muscle (SCM). The transducer should be moved laterally from level 4 along the edge of the clavicle to the posterior border of the SCM, which evaluates the supraclavicular lymph nodes (level 5b). Then, scanning continues by tracing the posterior SCM border superiorly to the angle of the mandible (level 5a).
Comprehensive scanning of the central neck cannot be accomplished with one sweep of the transducer. Each of the three areas of the central neck should be scanned separately—right paratracheal, left paratracheal, and pretracheal/prelaryngeal. For imaging the pretracheal area, scanning begins under the chin, in the submental area. Although this area is seldom affected by metastatic thyroid cancer, thyroglossal duct cysts, which may be a source of persistent thyroglobulin production in radioiodine-ablated post-thyroidectomy patients, can occasionally be found. From the submental area, the transducer sweep continues inferiorly to the sternal notch. The region of interest is the field just anterior to the airway. This includes imaging of the prelaryngeal lymph nodes. At the sternal notch, the transducer should be angled inferiorly into the mediastinum, and here, if available, a small convex transducer is ideal. The paratracheal areas bilaterally are evaluated by focusing on the region lateral to the trachea and medial to the carotid artery. Superiorly, imaging commences at the level of the hyoid and continues inferiorly until the sternal notch. All three sweeps in the central neck should be repeated several times.
The described technique for evaluating the central and lateral cervical lymph node compartments is one suggested protocol. However, the operator may adapt this sequence as needed provided that all compartments are comprehensively evaluated. In addition, consistency in technique should be maintained. Any identified abnormal lymph nodes or abnormal soft tissue should be imaged in both transverse and longitudinal views, measured, and labeled with the specific location (laterality, level, and pre- or paratracheal). Color flow Doppler imaging should be performed with the appropriate settings of a low pulse repetition frequency (<800 Hz) and a low wall filter, and findings documented.
Ultrasound Assessment of the Primary Tumor
The primary tumor should be assessed for its size, location, the clarity of its margin, multifocality, and local invasion. The finding of a posteromedially located lesion near the recurrent laryngeal nerve may influence patient counseling, preoperative evaluation, and surgical strategy. Minor invasion of the strap muscles is somewhat common and generally does not influence surgical strategy significantly. However, invasion of the visceral or vascular structures of the neck, which can appear sonographically as a blurry or indistinct deep margin, may prompt further imaging and alterations of the surgical approach. The thyroid should also be assessed for additional tumor foci, as contralateral foci may raise clinical suspicion for positive contralateral lymph nodes (6,7).
Ultrasound Features of Benign and Malignant Lymph Nodes
Central and lateral neck lymph nodes are affected by metastatic papillary thyroid carcinoma (PTC) in up to 70% of cases (8). Metastatic involvement of lymph nodes may be noted upon initial evaluation or later in cases of recurrence. Ultrasound is acknowledged to be the first-line imaging modality for lymph node assessment in both circumstances (9,10). The sensitivity of ultrasound in detecting abnormal lymph nodes varies widely in the literature, ranging from 25% to 60% for the central neck and from 70% to 95% for the lateral neck (11,12). Factors influencing sensitivity include practitioner expertise and, in many studies, the prophylactic dissection of microscopically positive central neck nodes, which are not reliably detected by any imaging modality. The specificity of ultrasound in detecting lymph nodes affected by metastatic PTC is high, ranging from 80% to 95% in both the central and lateral neck (13).
In contrast to some authors, the Committee members have generally found it straightforward to detect abnormal central neck lymph nodes in the presence of the thyroid within their own practices. We note that identification of all abnormal central neck nodes in a given patient may not be a priority, as the detection of a single abnormal central neck lymph node is sufficient to prompt the appropriate clinical decision, that is, performance of a compartmental lymph node dissection. As the neck region contains numerous lymph nodes (∼100–200), any of which could be enlarged related to underlying benign or malignant processes, it is important to understand the ultrasound findings that distinguish benign lymph nodes from those affected by malignancy. Sonographic features of benign and malignant lymph nodes are described below.
Characteristics of Benign Lymph Nodes
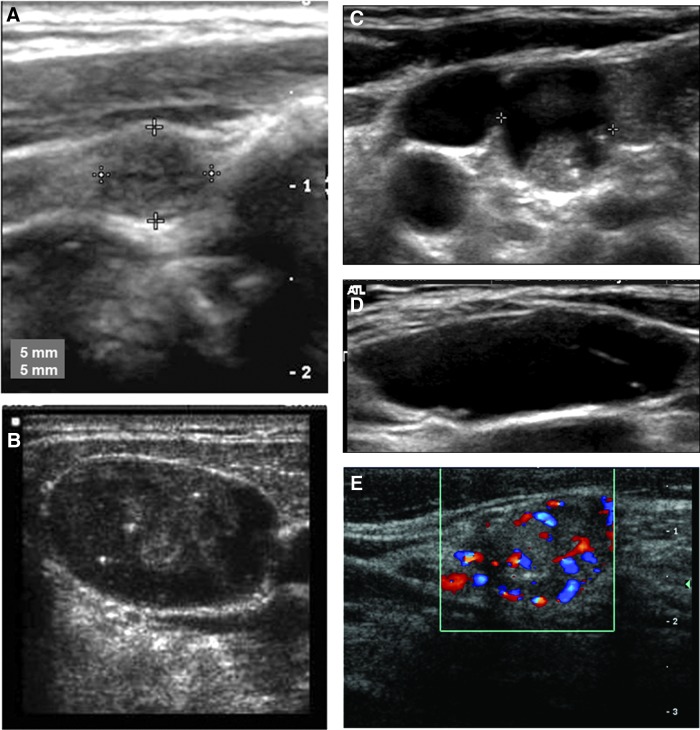
When assessing lymph nodes for the presence of malignancy, one must be able to recognize the appearance of a benign lymph node. Many normal lymph nodes are not visualized by routine ultrasound. Enlarged lymph nodes may represent a benign reactive process or malignancy. Benign lymph nodes typically are oval, with a hyperechoic central stripe and vascular flow being present in the center of the node (Fig. 1) (14). Loss of the hilum is felt to represent interruption of lymphatic flow by tumor invasion. However, the hilum may not always be easily defined by ultrasound in a benign lymph node, and the specificity for lack of a hilum has been reported to be only 29% for predicting the presence of cancer (10).
FIG. 1.
Ultrasound features of benign lymph nodes. (A) Benign fusiform lymph node. (B) Benign lymph node with hyperechoic central fatty hilum. (C) Hilar blood flow pattern in a normal lymph node.
Location
Lymph node location is another important factor when assessing for potential metastatic involvement. Cervical lymph nodes are subdivided by location into six levels, as previously described (4). When initial lymph node dissections are performed routinely, cervical metastases related to thyroid cancer are most commonly found in ipsilateral level 6 (prevalence 50–70%), followed by ipsilateral levels 2, 3, and 4 (prevalence 30–45%) (8,15,16). In the absence of bilateral primary tumors >1 cm, contralateral level 6 is less commonly affected (prevalence 5–25%), and contralateral levels 2, 3, and 4 are rarely affected (prevalence 5–14%) (17,18). Ipsilateral levels 2b (above the spinal accessory nerve) and 5 (posterior triangle) are involved in approximately 5% of patients with PTC (19). Some evidence exists for a stepwise progression of PTC metastases, starting generally in ipsilateral level 6 and then proceeding laterally to levels 3 and 4, and less commonly 2. A different pattern of lymph node metastases, affecting the lateral neck without the central neck, may occur in primary tumors of the superior pole (20).
Size
Though lymph nodes >1 cm in maximum diameter have conventionally been considered more likely to harbor malignancy, many benign or reactive nodes will exceed this size while having the strong tendency to remain fusiform in shape (21). In a study that carefully matched the ultrasound and histologic findings of lymph nodes in thyroid cancer patients, a lymph node size of >1 cm was associated with only a 68% sensitivity and 75% specificity for malignancy. Ultrasound features predictive of malignant lymph node involvement are summarized in Table 2.
Table 2.
Ultrasound Features Predictive of Malignant Lymph Node Involvement
| Criterion | Sensitivity | Specificity |
|---|---|---|
| Size >1 cm | 68% | 75% |
| Shape (ratio of long axis to short axis <2.0) | 46% | 64% |
| Punctate calcifications | 46% | 100% |
| Peripheral hypervascularity | 86% | 82% |
Adapted from Leboulleux et al. (10).
Shape
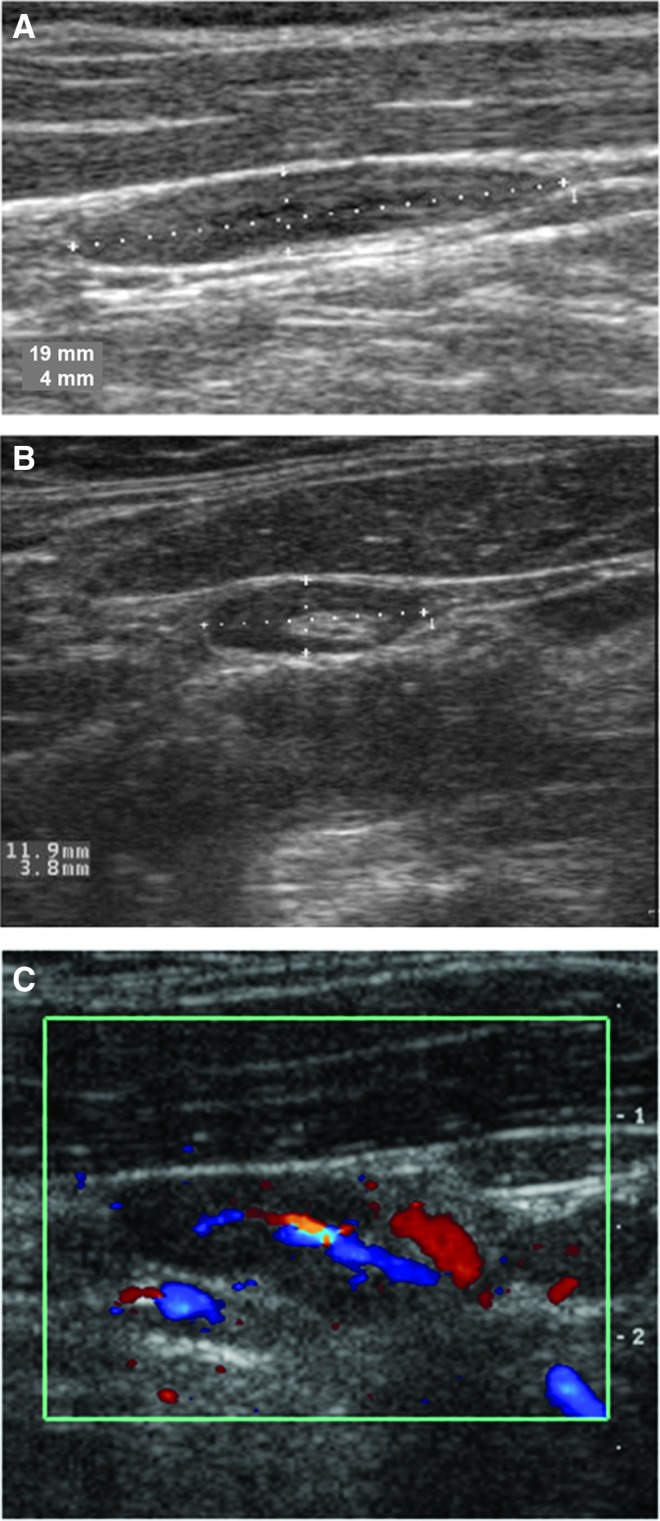
Malignant lymph nodes tend to become rounded in shape, distinguishing them from fusiform benign lymph nodes. The shortest diameter of the lymph node can be measured to predict the likelihood of metastatic involvement. Lymph nodes containing malignancy tend to have a shortest diameter >0.5 cm. However, the specificity (96%) for this criterion is superior to its sensitivity (61%) (10). The Solbiati index is another available tool to evaluate lymph node shape objectively for the presence of malignancy. The Solbiati index is calculated by using a ratio comparing the long and short axes of the lymph node. A ratio of >2.0 is consistent with a benign lymph node, while a value of <2.0 is considered suspicious for the presence of malignancy (Fig. 2) (14).
FIG. 2.
Ultrasound features of malignant lymph nodes. (A) Solid, rounded lymph node with metastatic papillary thyroid carcinoma (PTC). (B) Punctate internal calcifications in a lymph node with metastatic PTC. (C) Complex solid/cystic lymph node with metastatic PTC. (D) Completely cystic lymph node with metastatic PTC. (E) Peripheral hypervascular pattern in a lymph node with metastatic PTC.
Calcifications
Just as with thyroid nodules, microcalcifications within lymph nodes can be seen with metastases from both PTC and occasionally medullary thyroid carcinoma (MTC) (22). Microcalcifications are a highly specific finding: a lymph node containing microcalcifications in a patient with known or suspected PTC should be presumed to be positive for malignancy until proven otherwise (Fig. 2) (10).
Echogenicity
The echogenicity of lymph nodes can also be useful for detecting metastatic involvement. Benign lymph nodes usually are uniformly hypoechoic except for the central hilar stripe. Lymph nodes affected by malignancy may appear hyperechoic, have a mixed echogenicity, or even exhibit cystic changes to the point of total cystic replacement. Cystic nodes may be related to increased tumor aggressiveness, and appear to be more frequent in children and younger adults (23). Most lymph nodes with cystic changes appear complex and can have septations, a thickened outer wall, and solid internal components (Fig. 2). Typically, cystic metastases occur ispilateral to the primary foci and involve level 3 and 4 nodes.
Peripheral Hypervascularity
As mentioned previously, the vascular flow of lymph nodes, by either color Doppler or power Doppler, can be assessed. Benign lymph nodes exhibit flow in the hilum (center of the node), while malignancy interrupts this flow (24). As tumor involvement increases within the node, vascular flow is noted in the periphery. A disordered flow throughout the node can be seen with extensive tumor involvement (Fig. 3) (25). The presence of peripheral hypervascularity is reported to have the highest sensitivity and specificity for the detection of cervical lymph nodes affected by metastatic thyroid cancer (Table 2).
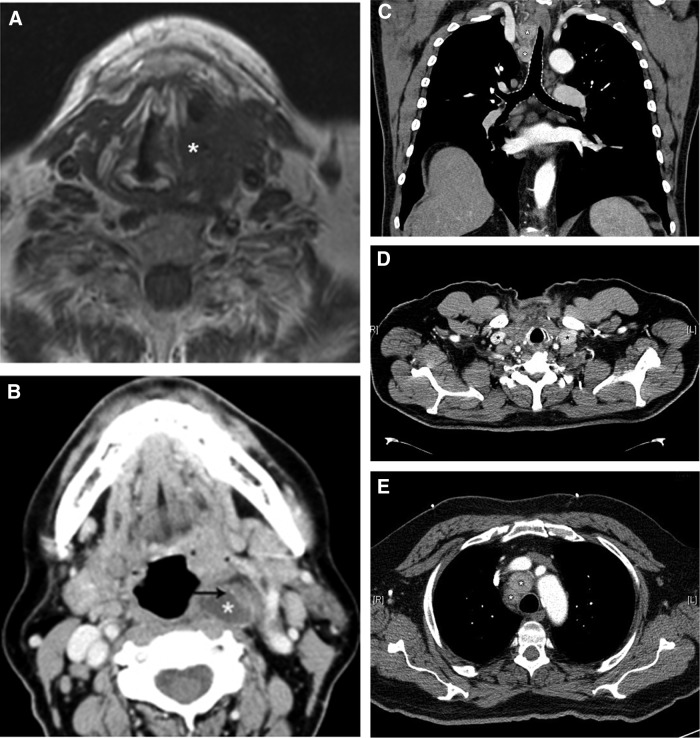
FIG. 3.
Cross-sectional images of invasive primary tumors and malignant lymph nodes. (A) T1-weighted magnetic resonance imaging (MRI) of tall cell variant papillary thyroid carcinoma (PTC) with cartilaginous invasion. (B) Contrast computed tomography (CT) of complex cystic and solid lymph node containing metastatic PTC in retropharynx (*) with calcification (arrow). (C) Contrast CT of hypervascular lymph nodes in inferior right level VI/level VII (metastatic PTC). (D) Contrast CT of hypervascular lymph nodes in bilateral infraclavicular areas (metastatic PTC). (E) Contrast CT demonstrating periaortic adenopathy (metastatic medullary thyroid carcinoma).
Ultrasound-Guided Fine-Needle Aspiration of Cervical Lymph Nodes
Once the decision has been made to perform ultrasound-guided fine-needle aspiration (FNA) of a sonographically suspicious lymph node, several techniques for this procedure can be used. However, for all, it is mandatory that the needle tip is visualized within the target lymph node during the FNA to assure specimen accuracy. If the entire lymph node is sonographically abnormal, then the FNA specimen can be obtained by moving the needle tip throughout the lymph node. However, if the lymph node maintains some normal architecture and only the periphery is sonographically suspicious, the needle tip should be directed to the suspicious component of the lymph node. Lastly, if the lymph node is partially cystic and partially solid, to obtain cells, the needle tip should be targeted to the solid vascular portion for cytology assessment (unless fluid is obtained for thyroglobulin analysis, see below).
Either a parallel or a perpendicular approach can be employed for ultrasound-guided FNA. In the parallel approach, the needle is visualized along its length as it enters the lymph node. In the perpendicular approach, only the tip of the needle can be seen. To obtain the cytology specimen, either a 25-gauge or a 27-gauge needle is appropriate. Once the needle is confirmed to be within the lymph node by ultrasound imaging, as with a thyroid nodule FNA, the cytology specimen is obtained with either the capillary or the aspiration technique. In the capillary technique, the needle tip is moved back and forth through the diameter of the target without suction. In the aspiration technique, suction is applied as the needle tip is moved. For thyroid nodule FNA, recent publications have reported low nondiagnostic rates using smaller gauge needles and the capillary action technique (26), but no such data exist for lymph node FNA.
The cytology specimen can be prepared by making a slide smear or using a liquid preparation. The decision about which method to use should be based upon the experience of the cytologist and the success in obtaining specimen adequacy. Therefore, a continued dialogue between the physician who performs the procedure and the cytologist is required for quality assessment and improvement. Also, the number of separate passes used should be based on whether on-site assessment of cytology is available, and whether additional studies are planned. In the absence of on-site adequacy assessment, the preferred number is three to four passes (27).
In addition to cytology analysis, thyroglobulin measurement from the FNA needle washout (FNA-Tg) has been shown to improve the sensitivity of ultrasound FNA cytology alone (28). Published reports have suggested a cutoff FNA-Tg level in the 1 ng/mL range for washouts prepared with up to 1 mL of diluent, though the serum thyroglobulin level, which may be higher in patients with a large volume of residual cancer or an intact thyroid gland, must be considered (29,30). The use of FNA-Tg is especially important for cystic lymph nodes, where cytology may reveal only macrophages without epithelial cells, and the diagnosis may hinge on a positive FNA-Tg test of the aspirated lymph node fluid or the rinse of the needle used to aspirate a solid lesion. Even in patients with circulating serum antithyroglobulin antibodies, FNA-Tg is accurate as long as there is no significant contamination of the specimen with peripheral blood (28). In MTC, FNA washout for calcitonin measurement can also be performed for diagnosis of metastatic lymph nodes (31).
Ultrasound in the Planning of Initial Surgery
Preoperative Ultrasound in the Setting of Malignant Cytology
Ultrasound evaluation (“mapping”) of bilateral lymph node compartments 1–6 should be performed routinely in the preoperative evaluation of patients with definitive cytologic evidence of carcinoma (positive FNA). This should ideally be performed by an experienced practitioner in the outpatient clinic setting, with the purpose of guiding a complete resection of the primary tumor as well as a compartment-oriented dissection of affected lymph node basins as necessary. Preoperative ultrasound should be performed either by the operating surgeon or in close collaboration with the operating surgeon in order to facilitate patient counseling regarding surgical risks, since the addition of central or lateral neck dissection to total thyroidectomy is accompanied by different types and/or increased rates of operative complications. As patient positioning is optimal under general anesthesia, surgeons may elect to repeat the ultrasound examination immediately prior to incision in order to have an anatomic “roadmap” fresh in their minds. This may be paired with a formal accounting system to ensure that all sonographic abnormalities are retrieved and represented among the surgical specimens at the end of the case.
After the primary lesion has been confirmed as malignant on FNA, FNA of sonographically suspicious lymph nodes can be performed to provide firm justification for adding lymph node dissection to initial surgery. Some centers perform this routinely and would not perform lateral neck dissection in the absence of a positive FNA in a lateral neck lymph node (32). There will be cases in which lymph nodes are unequivocally abnormal based on imaging findings alone. In such cases, it is acceptable to bypass FNA of these nodes and proceed directly with lymph node dissection in keeping with sound clinical judgment.
Preoperative ultrasound may provide insight into the need for further imaging prior to surgery. The finding of an irregular or indistinct margin between the primary tumor and the airway, esophagus, or major vessels of the neck may prompt cross-sectional imaging. Furthermore, patients with significant adenopathy in levels 6 and 4 may benefit from cross-sectional imaging of inferiorly located (substernal or infraclavicular) lymph nodes that may be difficult to examine by ultrasound (discussed further below, under Assessment of Lymph Nodes with Cross-Sectional Imaging).
Preoperative Ultrasound in the Setting of Indeterminate or Suspicious Cytology
Approximately 10–40% of thyroid aspirates yield indeterminate cytology (33) with the risk of malignancy stratified by the Bethesda System for Reporting Thyroid Cytopathology. Subcategories within the umbrella of indeterminate cytology include “atypia of undetermined significance” or “follicular lesion of undetermined significance” (malignancy risk 5–15%), “follicular neoplasm” or “suspicious for follicular neoplasm” (malignancy risk 15–30%), and “suspicious for malignancy” (malignancy risk 60–75%) (34). While a great number of patients with indeterminate cytology ultimately undergo surgical resection, the majority of nodules within the indeterminate classification are ultimately diagnosed as benign (35,36).
There are many published studies examining the utility of ultrasound to predict the risk of malignancy in patients with indeterminate FNA cytology. Though it is beyond the scope of the present statement to completely review this literature, several findings are commonly used to help guide preoperative planning. Hypoechogenicity, increased intranodular vascularity, irregular infiltrative margins, the presence of microcalcifications, and taller-than-wide shape all correlate with an increased risk of malignancy (3). These sonographic findings may be used in a supplementary fashion to predict increased malignant potential in cytologically indeterminate nodules (37,38).
Ultrasound interrogation of the central and lateral neck lymph nodes may inform the clinician in the planning surgery for cytologically indeterminate nodules. The discovery of lymph node metastases in this setting affords the opportunity to move forward with complete initial resection of all tumor tissue, that is, oncologic surgery. In contrast, if abnormal lymph nodes are not appreciated preoperatively in the setting of an underlying malignancy, initial diagnostic surgery (lobectomy) will obligate at least one, and often two or more, preventable reoperations (2). Patients found to have sonographically abnormal central (level 6) or lateral neck lymph nodes (levels 2, 3, and 4) should undergo FNA of at least one of these nodes. The finding of cells of thyroid origin within lymph nodes clinches the diagnosis of metastatic thyroid cancer and may significantly alter surgical management in the patient with indeterminate thyroid cytology as described above. Alternatively, the surgeon may elect to forego FNA in favor of intraoperative frozen section analysis of suspicious lymph nodes.
Ultrasound in the Planning of Revision Surgery
Ultrasound is an important tool for thyroid cancer surveillance and aids in the detection, localization, and planning of revision surgery for recurrent/persistent disease. A known limitation of ultrasound and other imaging modalities in the reoperative setting arises from postoperative inflammation, scarring, and reactive adenopathy. For this reason, it is generally advisable to allow approximately six months to elapse after surgery in order for postoperative changes to resolve prior to re-evaluating recently manipulated compartments of the neck. Any treatment decisions based on ultrasound imaging prior to this time point should be made with caution, as false-positive findings are common, particularly in the central neck. There are certain exceptions to this rule, that is, circumstances where persistent disease may be readily detected soon after initial surgery. These most often arise from incomplete surgery, which may arise from inadequate imaging prior to initial surgery and/or inadequate compartment-oriented nodal clearance during initial surgery.
After total thyroidectomy, visualization of the central compartment (levels 6 and 7) is often improved with regard to node detection. There should be little intervening tissue between the trachea and carotid arteries bilaterally, so abnormal pre- and paratracheal lymph nodes can often be readily apparent. Annual surveillance ultrasound is generally performed in the follow-up of thyroid cancer patients (3). FNA of suspicious lymph nodes should only be performed if further surgery or other interventions are planned and generally should be considered only for nodes ≥0.8 cm or with evidence of disease progression (3).
In the setting of suspected persistent or recurrent thyroid cancer, it is strongly advised that masses in the central neck/thyroid bed and any abnormal lymph nodes be evaluated with FNA. Establishing a tissue diagnosis provides justification for embarking on high-risk revision surgery. Rare exceptions can occur in cases where (a) abnormal lymph nodes are inaccessible or anatomically risky to biopsy, usually due to their location with respect to major vessels, and (b) unequivocally abnormal lymph nodes are found on imaging and surgery would be recommended regardless of FNA results. In either of these cases, the presence of an elevated serum thyroglobulin level may greatly assist clinical decision making by influencing the pretest probability of persistent/recurrent disease. The cellular diagnosis provided by a positive FNA is often helpful for patient counseling in preparation for revision surgery.
In assessing abnormal lymph nodes for potential revision surgery, it is important to consider any sonographic findings in the context of the original pathology report, operative report, and TNM staging. Up to 25% of patients who present initially with positive lateral neck lymph nodes will experience ipsilateral lymph node recurrences. As such, any ipsilateral abnormality should be scrutinized carefully and likely undergo FNA, especially if the serum thyroglobulin is elevated. Patients who present with contralateral lymph node abnormalities during surveillance should also undergo FNA, albeit with a different clinical perspective. In those cases, the negative predictive value of FNA would be leveraged to potentially avoid revision surgery. In patients with locally invasive primary tumors, the risk of local recurrence is the principal concern, and cross-sectional imaging may be helpful in such cases.
Ultrasound Expertise
Clinic-based ultrasound has become commonplace in many endocrinology and surgical practices treating thyroid disease. For the majority of providers, the skills of performing and interpreting ultrasound are obtained during postgraduate subspecialty training. Additional training is often pursued through one- to two-day basic or advanced ultrasound courses, most commonly associated with annual society meetings.
The Committee does not support defining expertise based on successful completion of a specific training or certification course or by a specific accreditation body. Certification and accreditation courses afford an organized approach to initial and continuing education in ultrasonography but do not ensure expertise. Expertise should not be defined by specialty and can only be achieved through regular clinical practice, participation in continuing medical education didactics, and mentored bedside practice of thyroid and neck sonography. Although ultrasound is widely available and essential in the preoperative assessment of central and lateral neck disease, improved sensitivity and specificity arises within high volume centers staffed by dedicated clinicians (radiologists, surgeons, and endocrinologists) performing the procedure. We recommend attempts to focus preoperative evaluation at high volume centers for radiological evaluation, as the diagnostic performance of staging ultrasound for thyroid malignancy varies according to experience (27,39).
Cross-Sectional Imaging
Ultrasound is the first-line imaging modality for assessing thyroid nodules and cervical lymph nodes because it is widely available, inexpensive, provides detailed high-resolution anatomic information, avoids ionizing radiation, and facilitates ultrasound-guided FNA of suspicious lesions (4). Current ATA guidelines do not recommend the routine use of other imaging beyond neck ultrasound prior to initial surgery for differentiated thyroid cancers.
Ultrasound does have certain limitations related to the underlying technology, particularly in the imaging of deep structures and those acoustically shielded by air or bone. For this reason, cross-sectional imaging with CT or magnetic resonance imaging (MRI) may play a supplemental role in preoperative imaging for thyroid cancer in the minority of cases. These circumstances may include (a) clinical or sonographic evidence of an invasive primary tumor, (b) presence of a large primary tumor or bulky nodal disease incompletely imaged with ultrasound, (c) presence or extension of nodal disease into the mediastinum or deep structures of the neck (parapharyngeal/retropharyngeal regions) incompletely imaged with ultrasound, and (d) absence of sonographic expertise in evaluation of cervical lymph nodes (Table 3). Advanced thyroid malignancy with extrathyroidal spread occurs in 10–15% of patients presenting with differentiated thyroid cancer (DTC) (40,41). Screening for distant metastasis is generally not performed prior to initial surgery for differentiated thyroid cancers (3).
Table 3.
Findings That May Prompt Axial Imaging
| • Hoarseness with vocal cord paresis/paralysis |
| • Progressive dysphagia or odynophagia |
| • Mass fixation to surrounding structures |
| • Respiratory symptoms, hemoptysis, stridor, or positional dyspnea |
| • Large size of tumor or mediastinal extension, incompletely imaged on ultrasound |
| • Rapid progression/enlargement |
| • Sonographic suspicion for significant extrathyroidal invasion (cT4) |
| • Bulky, posteriorly located, or inferiorly located lymph nodes incompletely imaged by ultrasound |
| • Ultrasound expertise not available |
Technical Aspects of Cross-Sectional Imaging
CT is performed on a helical scanner acquiring contiguous 3 mm thick sections. Intravenous contrast enhancement aids greatly in the optical differentiation of tissue types and should be used in all cases unless contraindicated by allergy or concern for contrast nephropathy. Modern CT contrast agents are nonionic and either low-osmolal or iso-osmolal (42); all contain iodine (see Use of Iodinated Contrast and Impact on Subsequent Treatment, below). Intravenous contrast aids in distinguishing blood vessels from lymph nodes, and more importantly in allowing detection of architectural abnormalities (necrosis, cystic changes) within pathologic nodes. Sagittal and coronal reconstructed images may be generated and can be helpful when correlated with the axial images in surgical localization. Images should be reviewed in soft tissue and bone detail.
MRI is performed with sagittal and axial T1W, axial fat-saturated T2W, and enhanced axial and coronal fat-saturated T1W images. Section thickness is usually 5 mm. MRI should be performed both with and without intravenous contrast, since many pathologic nodes contain thyroglobulin, which appears hyperintense on noncontrast T1W images. Paramagnetic contrast agents contain gadolinium and thus present an alternative to iodinated contrast agents used in CT. Gadolinium should be used with caution in patients with advanced renal failure, who are at risk for nephrogenic systemic fibrosis (43).
Assessment of the Primary Tumor with Cross-Sectional Imaging
Both CT and MRI provide axial imaging from skull base to mediastinum in a standardized, reproducible fashion that is relatively user independent. The following symptoms and signs are suggestive of a locally invasive primary tumor and may prompt preoperative axial scanning: voice change, difficulty swallowing, respiratory compromise, rapid tumor enlargement, vocal cord paralysis, and mass fixation to the airway. Certain sonographic features of the primary tumor, including extrathyroidal extension and extension into the mediastinum, may also prompt axial imaging. Chest CT is useful in defining the inferior limit of disease and in determining the extent to which the great vessels and tracheobronchial tree are involved in cases with significant caudal spread. CT findings may influence management by indicating the need for sternotomy and/or tracheal resection/reconstruction, both of which require the mobilization of additional resources and personnel in advance of surgery. Neck CT with contrast is useful in delineating the extent of laryngeal, tracheal, and/or esophageal involvement in tumors displaying aggressive local invasion (Fig. 3). Preoperative knowledge of these features of the primary tumor would significantly influence the surgical plan. Hence, the Committee supports selective use of preoperative cross-sectional imaging based on clinical suspicion.
Assessment of Lymph Nodes with Cross-Sectional Imaging
CT or MRI imaging may be obtained in order to evaluate lymphadenopathy fully when ultrasound expertise in nodal assessment is lacking or when physical exam and/or ultrasound suggest bulky or extensive nodal metastasis incompletely evaluable with ultrasound. Indeed, the presence of abnormal lymph nodes at the periphery or limits of the sonographically accessible field were felt by the Committee to be one of the strongest indications for cross-sectional imaging. A recent ATA publication summarized evidence demonstrating that the prognostic significance of lymph node metastases in PTC is a function of the size and number of positive nodes, and that extranodal tumor extension is an independent predictor of poor outcome (44). Large nodes (>3 cm) often demonstrate extracapsular extension into surrounding vessels and/or musculature, which may be best characterized by axial scanning. There are limited data suggesting that the combination of CT with ultrasound in preoperative lymph node evaluation is superior to either single modality (13,45). These studies have reported that combined imaging yields a 15% gain in sensitivity with a concomitant 5–10% loss in specificity.
Patients displaying extensive lymph node metastases may present with involvement of nodal regions beyond the typical levels, such as the mediastinum and infraclavicular, retropharyngeal, and parapharyngeal regions, which are difficult to evaluate fully with ultrasound (Fig. 3) (46). Patients found to have significant adenopathy in the inferior aspects of level 4 and 6 on ultrasound may benefit from cross-sectional imaging of the lower neck/upper chest to assess the deep tracheoesophageal groove and infraclavicular space, respectively, to delineate the inferior extent of disease (Fig. 3). This information may alter surgical planning in cases where the disease cannot be completely accessed via a transcervical approach.
Axial Scanning in the Setting of Revision Surgery
In the previously treated patient, CT or MR imaging should be considered in the following scenarios: (a) in patients with new dysphagia, respiratory symptoms, hoarseness, or vocal cord paralysis, for assessment of invasive central neck disease; and (b) in patients with rising thyroglobulin levels and negative ultrasound exams (functional imaging may also be considered).
Use of Iodinated Contrast and Impact on Subsequent Treatment
CT imaging of the neck is optimized by iodinated intravenous contrast. This advantage must be balanced against the impact the iodine load will have in causing what is usually a minor delay in subsequent postoperative radioactive iodine ablation. Thus, preoperative communication between the surgeon and endocrinologist is important. Noncontrast CT is generally lacking in definition, and its utility is thus limited to gross evaluation of the extent of mediastinal disease. MRI with gadolinium contrast is an alternative axial scanning modality that avoids the use of iodine, but may be less informative to surgeons as compared to CT in the central compartment due to motion artifact arising from swallowing and respiration. Despite these issues, it is generally preferable to noncontrast CT in preoperative imaging for thyroid cancer. After the administration of iodinated contrast, a waiting period of at least one month is recommended to allow urinary iodine levels to return to baseline levels before moving forward with radioactive iodine ablation (47). At present, there is no evidence to suggest that delays of this scale adversely affect thyroid cancer outcomes.
Functional Imaging
Radioiodine whole body scanning has traditionally been the primary functional imaging modality for patients suspected of persistent/recurrent well-differentiated thyroid carcinoma (3). In patients who have undergone prior remnant ablation, these scans have a high specificity but low sensitivity, and the low resolution has traditionally been insufficient for surgical planning. Newer technology utilizing radioiodine with SPECT/CT fusion significantly improves anatomic localization of radioiodine avid disease and may be used to guide surgical intervention with or without radioguidance (48–50).
Given the slow growth rate of most thyroid cancers, the Committee does not recommend the use of positron emission tomography (PET) or PET-CT prior to initial surgery for thyroid cancer. PET using F-18 fluoro-2-deoxyglucose (18F-FDG) as a radiopharmaceutical has been widely accepted as a method for detecting recurrence of differentiated thyroid cancer, particularly in noniodine avid disease (50–52). PET avidity is inversely correlated with iodine avidity, and the former has been shown to be a strong predictor of poor outcome in patients with metastatic thyroid cancer (53). However, PET alone does not provide specific anatomic localization of recurrent lesions. Co-registered PET-CT has allowed for enhanced anatomic localization of metabolically active sites of recurrent thyroid carcinoma and distant metastases, facilitating surgical excision for diagnostic or therapeutic purposes. In a recent report, PET-CT demonstrated a sensitivity of 81%, specificity of 89%, positive predictive value of 95%, and negative predictive value of 65% in the detection of recurrent differentiated thyroid cancer. Information gleaned from PET-CT altered treatment in 28.2% of patients and guided additional surgery in 21% of patients (54).
Furthermore, PET-CT can potentially explain false positive FDG uptake in brown fat, muscles, and sites of inflammation. In addition, when PET-CT includes a “diagnostic” quality CT scan, nonmetabolically active metastases can be identified due to the identification of concerning morphologic features. A number of studies have shown that PET is effective in localizing resectable recurrence(s) in patients with differentiated thyroid cancer, thus facilitating revision surgery (55–58). The sensitivity of PET-CT appears to be enhanced with thyrotropin stimulation (59).
An additional benefit of PET-CT is its ability to detect unrecognized distant metastatic disease. PET can also identify lesions in which continued growth near critical structures can lead to serious morbidity. These include lesions in the brain, weight-bearing bones, and structures near the great vessels (60). Thus, the ability of PET-CT to detect distant metastatic disease can guide management and aid in palliative measures in patients with incurable disease.
Conclusions
Thorough preoperative imaging facilitates complete initial surgical clearance of thyroid cancer and associated cervical lymph node metastases. Ultrasound is the primary imaging modality for thyroid cancer and should ideally be performed by an expert clinician using a standardized methodology that the Committee has described herein. The Committee supports the selective use of cross-sectional imaging with CT or MRI in the minority of patients based on clinical and sonographic indicators of extensive disease. The suggested use of functional imaging is currently limited to the detection of recurrent disease.
Contributor Information
Collaborators: for the American Thyroid Association Surgical Affairs Committee Writing Task Force
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Mazzaferri EL, Kloos RT. 2001. Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab 86:1447–1463 [DOI] [PubMed] [Google Scholar]
- 2.Kouvaraki MA, Shapiro SE, Fornage BD, Edeiken-Monro BS, Sherman SI, Vassilopoulou-Sellin R, Lee JE, Evans DB. 2003. Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer. Surgery 134:946–954; discussion 954–945. [DOI] [PubMed] [Google Scholar]
- 3.Cooper DS, Doherty GM, Haugen BR, Hauger BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM, American Thyroid Association Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer 2009. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214 [DOI] [PubMed] [Google Scholar]
- 4.Carty SE, Cooper DS, Doherty GM, Duh QY, Kloos RT, Mandel SJ, Randolph GW, Stack BC, Steward DL, Terris DJ, Thompson GB, Tufano RP, Tuttle RM, Udelsman R, American Thyroid Association Surgery Working Group 2009. Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid 19:1153–1158 [DOI] [PubMed] [Google Scholar]
- 5.Stack BC, Jr., Ferris RL, Goldenberg D, Haymart M, Shaha A, Sheth S, Sosa JA, Tufano RP. 2012. American Thyroid Association consensus review and statement regarding the anatomy, terminology, and rationale for lateral neck dissection in differentiated thyroid cancer. Thyroid 22:501–508 [DOI] [PubMed] [Google Scholar]
- 6.Koo BS, Choi EC, Park YH, Kim EH, Lim YC. 2010. Occult contralateral central lymph node metastases in papillary thyroid carcinoma with unilateral lymph node metastasis in the lateral neck. J Am Coll Surg 210:895–900 [DOI] [PubMed] [Google Scholar]
- 7.Zhao Q, Ming J, Liu C, Shi L, Xu X, Nie X, Huang T. 2013. Multifocality and total tumor diameter predict central neck lymph node metastases in papillary thyroid microcarcinoma. Ann Surg Oncol 20:746–752 [DOI] [PubMed] [Google Scholar]
- 8.Ito Y, Higashiyama T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Kuma K, Miyauchi A. 2007. Risk factors for recurrence to the lymph node in papillary thyroid carcinoma patients without preoperatively detectable lateral node metastasis: validity of prophylactic modified radical neck dissection. World J Surg 31:2085–2091 [DOI] [PubMed] [Google Scholar]
- 9.Langer JE, Mandel SJ. 2008. Sonographic imaging of cervical lymph nodes in patients with thyroid cancer. Neuroimaging Clin N Am 18:479–489, vii–viii. [DOI] [PubMed] [Google Scholar]
- 10.Leboulleux S, Girard E, Rose M, Travagli JP, Sabbah N, Caillou B, Hartl DM, Lassau N, Baudin E, Schlumberger M. 2007. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J Clin Endocrinol Metab 92:3590–3594 [DOI] [PubMed] [Google Scholar]
- 11.Choi JS, Kim J, Kwak JY, Kim MJ, Chang HS, Kim EK. 2009. Preoperative staging of papillary thyroid carcinoma: comparison of ultrasound imaging and CT. AJR Am J Roentgenol 193:871–878 [DOI] [PubMed] [Google Scholar]
- 12.Hwang HS, Orloff LA. 2011. Efficacy of preoperative neck ultrasound in the detection of cervical lymph node metastasis from thyroid cancer. Laryngoscope 121:487–491 [DOI] [PubMed] [Google Scholar]
- 13.Kim E, Park JS, Son KR, Kim JH, Jeon SJ, Na DG. 2008. Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography. Thyroid 18:411–418 [DOI] [PubMed] [Google Scholar]
- 14.Solbiati L, Osti V, Cova L, Tonolini M. 2001. Ultrasound of thyroid, parathyroid glands and neck lymph nodes. Eur Radiol 11:2411–2424 [DOI] [PubMed] [Google Scholar]
- 15.Popadich A, Levin O, Lee JC, Smooke-Praw S, Ro K, Fazel M, Arora A, Tolley NS, Palazzo F, Learoyd DL, Sidhu S, Delbridge L, Sywak M, Yeh MW. 2011. A multicenter cohort study of total thyroidectomy and routine central lymph node dissection for cN0 papillary thyroid cancer. Surgery 150:1048–1057 [DOI] [PubMed] [Google Scholar]
- 16.Gimm O, Rath FW, Dralle H. 1998. Pattern of lymph node metastases in papillary thyroid carcinoma. Br J Surg 85:252–254 [DOI] [PubMed] [Google Scholar]
- 17.Sadowski BM, Snyder SK, Lairmore TC. 2009. Routine bilateral central lymph node clearance for papillary thyroid cancer. Surgery 146:696–703; discussion 703–705. [DOI] [PubMed] [Google Scholar]
- 18.Machens A, Hinze R, Thomusch O, Dralle H. 2002. Pattern of nodal metastasis for primary and reoperative thyroid cancer. World J Surg 26:22–28 [DOI] [PubMed] [Google Scholar]
- 19.Khafif A, Medina JE, Robbins KT, Silver CE, Weber RS, Rinaldo A, Owen RP, Shaha AR, Ferlito A. 2013. Level V in therapeutic neck dissections for papillary thyroid carcinoma. Head Neck 35:605–607 [DOI] [PubMed] [Google Scholar]
- 20.Lee YS, Shin SC, Lim YS, Lee JC, Wang SG, Son SM, Kim IJ, Lee BJ. 2014. Tumor location-dependent skip lateral cervical lymph node metastasis in papillary thyroid cancer. Head Neck 36:887–891 [DOI] [PubMed] [Google Scholar]
- 21.Kuna SK, Bracic I, Tesic V, Kuna K, Herceg GH, Dodig D. 2006. Ultrasonographic differentiation of benign from malignant neck lymphadenopathy in thyroid cancer. J Ultrasound Med 25:1531–1537; quiz 1538–1540. [DOI] [PubMed] [Google Scholar]
- 22.Sipos JA. 2009. Advances in ultrasound for the diagnosis and management of thyroid cancer. Thyroid 19:1363–1372 [DOI] [PubMed] [Google Scholar]
- 23.Wunderbaldinger P, Harisinghani MG, Hahn PF, Daniels GH, Turetschek K, Simeone J, O'Neill MJ, Mueller PR. 2002. Cystic lymph node metastases in papillary thyroid carcinoma. AJR Am J Roentgenol 178:693–697 [DOI] [PubMed] [Google Scholar]
- 24.Ahuja AT, Ying M, Ho SS, Metreweli C. 2001. Distribution of intranodal vessels in differentiating benign from metastatic neck nodes. Clin Radiol 56:197–201 [DOI] [PubMed] [Google Scholar]
- 25.Görges R, Eising EG, Fotescu D, Renzing-Köhler K, Frilling A, Schmid KW, Bockisch A, Dirsch O. 2003. Diagnostic value of high-resolution B-mode and power-mode sonography in the follow-up of thyroid cancer. Eur J Ultrasound 16:191–206 [DOI] [PubMed] [Google Scholar]
- 26.Kim MJ, Kim EK, Park SI, Kim BM, Kwak JY, Kim SJ, Youk JH, Park SH. 2008. US-guided fine-needle aspiration of thyroid nodules: indications, techniques, results. Radiographics 28:1869–1886; discussion 1887. [DOI] [PubMed] [Google Scholar]
- 27.Bhatki AM, Brewer B, Robinson-Smith T, Nikiforov Y, Steward DL. 2008. Adequacy of surgeon-performed ultrasound-guided thyroid fine-needle aspiration biopsy. Otolaryngol Head Neck Surg 139:27–31 [DOI] [PubMed] [Google Scholar]
- 28.Boi F, Baghino G, Atzeni F, Lai ML, Faa G, Mariotti S. 2006. The diagnostic value for differentiated thyroid carcinoma metastases of thyroglobulin (Tg) measurement in washout fluid from fine-needle aspiration biopsy of neck lymph nodes is maintained in the presence of circulating anti-Tg antibodies. J Clin Endocrinol Metab 91:1364–1369 [DOI] [PubMed] [Google Scholar]
- 29.Snozek CL, Chambers EP, Reading CC, Sebo TJ, Sistrunk JW, Singh RJ, Grebe SK. 2007. Serum thyroglobulin, high-resolution ultrasound, and lymph node thyroglobulin in diagnosis of differentiated thyroid carcinoma nodal metastases. J Clin Endocrinol Metab 92:4278–4281 [DOI] [PubMed] [Google Scholar]
- 30.Cunha N, Rodrigues F, Curado F, Ilheu O, Cruz C, Naidenov P, Rascao MJ, Ganho J, Gomes I, Pereira H, Real O, Figueiredo P, Campos B, Valido F. 2007. Thyroglobulin detection in fine-needle aspirates of cervical lymph nodes: a technique for the diagnosis of metastatic differentiated thyroid cancer. Eur J Endocrinol 157:101–107 [DOI] [PubMed] [Google Scholar]
- 31.Boi F, Maurelli I, Pinna G, Atzeni F, Piga M, Lai ML, Mariotti S. 2007. Calcitonin measurement in wash-out fluid from fine needle aspiration of neck masses in patients with primary and metastatic medullary thyroid carcinoma. J Clin Endocrinol Metab 92:2115–2118 [DOI] [PubMed] [Google Scholar]
- 32.Grant CS, Stulak JM, Thompson GB, Richards ML, Reading CC, Hay ID. 2010. Risks and adequacy of an optimized surgical approach to the primary surgical management of papillary thyroid carcinoma treated during 1999–2006. World J Surg 34:1239–1246 [DOI] [PubMed] [Google Scholar]
- 33.Nikiforov YE, Steward DL, Robinson-Smith TM, Haugen BR, Klopper JP, Zhu Z, Fagin JA, Falciglia M, Weber K, Nikiforova MN. 2009. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab 94:2092–2098 [DOI] [PubMed] [Google Scholar]
- 34.Cibas ES, Ali SZ. 2009. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid 19:1159–1165 [DOI] [PubMed] [Google Scholar]
- 35.Yang J, Schnadig V, Logrono R, Wasserman PG. 2007. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer 111:306–315 [DOI] [PubMed] [Google Scholar]
- 36.Banks ND, Kowalski J, Tsai HL, Somervell H, Tufano R, Dackiw AP, Marohn MR, Clark DP, Umbricht CB, Zeiger MA. 2008. A diagnostic predictor model for indeterminate or suspicious thyroid FNA samples. Thyroid 18:933–941 [DOI] [PubMed] [Google Scholar]
- 37.Yoon JH, Kwak JY, Kim EK, Moon HJ, Kim MJ, Kim JY, Koo HR, Kim MH. 2010. How to approach thyroid nodules with indeterminate cytology. Ann Surg Oncol 17:2147–2155 [DOI] [PubMed] [Google Scholar]
- 38.Roy R, Kouniavsky G, Venkat R, Felger EA, Shiue Z, Schneider E, Zeiger MA. 2012. The role of preoperative neck ultrasounds to assess lymph nodes in patients with suspicious or indeterminate thyroid nodules. J Surg Oncol 105:601–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moon HJ, Kim EK, Yoon JH, Kwak JY. 2012. Differences in the diagnostic performances of staging US for thyroid malignancy according to experience. Ultrasound Med Biol 38:568–573 [DOI] [PubMed] [Google Scholar]
- 40.Andersen PE, Kinsella J, Loree TR, Shaha AR, Shah JP. 1995. Differentiated carcinoma of the thyroid with extrathyroidal extension. Am J Surg 170:467–470 [DOI] [PubMed] [Google Scholar]
- 41.McCaffrey TV, Bergstralh EJ, Hay ID. 1994. Locally invasive papillary thyroid carcinoma: 1940–1990. Head Neck 16:165–172 [DOI] [PubMed] [Google Scholar]
- 42.From AM, Al Badarin FJ, McDonald FS, Bartholmai BJ, Cha SS, Rihal CS. 2010. Iodixanol versus low-osmolar contrast media for prevention of contrast induced nephropathy: meta-analysis of randomized, controlled trials. Circ Cardiovasc Interv 3:351–358 [DOI] [PubMed] [Google Scholar]
- 43.Yang L, Krefting I, Gorovets A, Marzella L, Kaiser J, Boucher R, Rieves D. 2012. Nephrogenic systemic fibrosis and class labeling of gadolinium-based contrast agents by the Food and Drug Administration. Radiology 265:248–253 [DOI] [PubMed] [Google Scholar]
- 44.Randolph GW, Duh QY, Heller KS, LiVolsi VA, Mandel SJ, Steward DL, Tufano RP, Tuttle RM, The American Thyroid Association Surgical Affairs Committee's Taskforce on Thyroid Cancer Nodal Surgery 2012. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid 22:1144–1152 [DOI] [PubMed] [Google Scholar]
- 45.Lesnik D, Cunnane ME, Zurakowski D, Acar GO, Ecevit C, Mace A, Kamani D, Randolph GW. 2013. Papillary thyroid carcinoma nodal surgery directed by a preoperative radiographic map utilizing CT scan and ultrasound in all primary and reoperative patients. Head Neck 36:191–202 [DOI] [PubMed] [Google Scholar]
- 46.Ahn JE, Lee JH, Yi JS, Shong YK, Hong SJ, Lee DH, Choi CG, Kim SJ. 2008. Diagnostic accuracy of CT and ultrasonography for evaluating metastatic cervical lymph nodes in patients with thyroid cancer. World J Surg 32:1552–1558 [DOI] [PubMed] [Google Scholar]
- 47.Padovani RP, Kasamatsu TS, Nakabashi CC, Camacho CP, Andreoni DM, Malouf EZ, Marone MM, Maciel RM, Biscolla RP. 2012. One month is sufficient for urinary iodine to return to its baseline value after the use of water-soluble iodinated contrast agents in post-thyroidectomy patients requiring radioiodine therapy. Thyroid 22:926–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Avram AM. 2012. Radioiodine scintigraphy with SPECT/CT: an important diagnostic tool for thyroid cancer staging and risk stratification. J Nucl Med 53:754–764 [DOI] [PubMed] [Google Scholar]
- 49.Francis CL, Nalley C, Fan C, Bodenner D, Stack BC., Jr. 2012. 18F-fluorodeoxyglucose and 131I radioguided surgical management of thyroid cancer. Otolaryngol Head Neck Surg 146:26–32 [DOI] [PubMed] [Google Scholar]
- 50.Grünwald F, Kälicke T, Feine U, Lietzenmayer R, Scheidhauer K, Dietlein M, Schober O, Lerch H, Brandt-Mainz K, Burchert W, Hiltermann G, Cremerius U, Biersack HJ. 1999. Fluorine-18 fluorodeoxyglucose positron emission tomography in thyroid cancer: results of a multicentre study. Eur J Nucl Med 26:1547–1552 [DOI] [PubMed] [Google Scholar]
- 51.Schlüter B, Bohuslavizki KH, Beyer W, Plotkin M, Buchert R, Clausen M. 2001. Impact of FDG PET on patients with differentiated thyroid cancer who present with elevated thyroglobulin and negative 131I scan. J Nucl Med 42:71–76 [PubMed] [Google Scholar]
- 52.Wang W, Macapinlac H, Larson SM, Yeh SD, Akhurst T, Finn RD, Rosai J, Robbins RJ. 1999. [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography localizes residual thyroid cancer in patients with negative diagnostic (131)I whole body scans and elevated serum thyroglobulin levels. J Clin Endocrinol Metab 84:2291–2302 [DOI] [PubMed] [Google Scholar]
- 53.Robbins RJ, Wan Q, Grewal RK, Reibke R, Gonen M, Strauss HW, Tuttle RM, Drucker W, Larson SM. 2006. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metab 91:498–505 [DOI] [PubMed] [Google Scholar]
- 54.Razfar A, Branstetter BF, Christopoulos A, Lebeau SO, Hodak SP, Heron DE, Escott EJ, Ferris RL. 2010. Clinical usefulness of positron emission tomography-computed tomography in recurrent thyroid carcinoma. Arch Otolaryngol Head Neck Surg 136:120–125 [DOI] [PubMed] [Google Scholar]
- 55.Finkelstein SE, Grigsby PW, Siegel BA, Dehdashti F, Moley JF, Hall BL. 2008. Combined [18F]Fluorodeoxyglucose positron emission tomography and computed tomography (FDG-PET/CT) for detection of recurrent, 131I-negative thyroid cancer. Ann Surg Oncol 15:286–292 [DOI] [PubMed] [Google Scholar]
- 56.Kim SJ, Lee TH, Kim IJ, Kim YK. 2009. Clinical implication of F-18 FDG PET/CT for differentiated thyroid cancer in patients with negative diagnostic iodine-123 scan and elevated thyroglobulin. Eur J Radiol 70:17–24 [DOI] [PubMed] [Google Scholar]
- 57.Mirallié E, Guillan T, Bridji B, Resche I, Rousseau C, Ansquer C, Bodet-Milin C, Curtet C, Carnaille B, Murat A, Charbonnel B, Kraeber-Bodéré F. 2007. Therapeutic impact of 18FDG-PET/CT in the management of iodine-negative recurrence of differentiated thyroid carcinoma. Surgery 142:952–958; discussion 952–958. [DOI] [PubMed] [Google Scholar]
- 58.Shammas A, Degirmenci B, Mountz JM, McCook BM, Branstetter B, Bencherif B, Bencherif BB, Joyce JM, Carty SE, Kuffner HA, Avril N. 2007. 18F-FDG PET/CT in patients with suspected recurrent or metastatic well-differentiated thyroid cancer. J Nucl Med 48:221–226 [PubMed] [Google Scholar]
- 59.Leboulleux S, Schroeder PR, Busaidy NL, Auperin A, Corone C, Jacene HA, Ewertz ME, Bournaud C, Wahl RL, Sherman SI, Ladenson PW, Schlumberger M. 2009. Assessment of the incremental value of recombinant thyrotropin stimulation before 2-[18F]-Fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography imaging to localize residual differentiated thyroid cancer. J Clin Endocrinol Metab 94:1310–1316 [DOI] [PubMed] [Google Scholar]
- 60.Tuttle RM, Leboeuf R, Martorella AJ. 2007. Papillary thyroid cancer: monitoring and therapy. Endocrinol Metab Clin North Am 36:753–778, vii. [DOI] [PubMed] [Google Scholar]