Abstract
In Alzheimer’s disease (AD), accumulation of brain amyloid-β (Aβ) depends on imbalance between production and clearance of Aβ. Several pathways for Aβ clearance have been reported including transport across the blood-brain barrier (BBB) and hepatic clearance. The incidence of AD increases with age and failure of Aβ clearance correlates with AD. The cholinesterase inhibitors (ChEIs) donepezil and rivastigmine are used to ease the symptoms of dementia associated with AD. Besides, both drugs have been reported to provide neuroprotective and disease-modifying effects. Here, we investigated the effect of ChEIs on age-related reduced Aβ clearance. Findings from in vitro and in vivo studies demonstrated donepezil and rivastigmine to enhance 125I-Aβ40 clearance. Also, the increase in brain and hepatic clearance of 125I-Aβ40 was more pronounced in aged compared to young rats, and was associated with significant reduction in brain Aβ endogenous levels determined by ELISA. Furthermore, the enhanced clearance was concomitant with up-regulation in the expression of Aβ major transport proteins P-glycoprotein and LRP1. Collectively, our findings that donepezil and rivastigmine enhance Aβ clearance across the BBB and liver are novel and introduce an additional mechanism by which both drugs could affect AD pathology. Thus, optimizing their clinical use could help future drug development by providing new drug targets and possible mechanisms involved in AD pathology.
Keywords: Donepezil, rivastigmine, Alzheimer’s disease, amyloid-β clearance, BBB, liver
Graphical Abstract
Alzheimer’s disease (AD) is the most common cause of dementia and most prevalent in the older population.1,2 The etiopathology of AD is uncertain with about 90–95% of AD cases being sporadic, while less than 2% of cases are familial with genetic origin.3 AD is characterized by the presence of amyloid deposits in brain parenchyma (senile plaques) and blood vessels (cerebral amyloid angiopathy), and intraneuronal accumulations of abnormally phosphorelated tau protein (neurofibrilary tangles).4,5 The plaques consist mainly of amyloid-β peptides (Aβ40 and Aβ42) produced by sequential cleavage of amyloid precursor protein (APP) by β- and γ-secretases; the majority of these plaques’ content is the more hydrophobic Aβ42 that has more propensity to aggregate.6,7 In AD, failure in Aβ clearance and/or its overproduction in the brain are considered major factors that contribute to Aβ brain accumulation.8–10 Clearance of Aβ across the blood-brain barrier (BBB) is mediated mainly by low density lipoprotein receptor related protein-1 (LRP1) and P-glycoprotein (P-gp).11,12 Several studies have targeted clearance pathways of Aβ efflux across the BBB and/or enzymatic degradation as a possible therapeutic approach to reduce Aβ brain burden.13–15 For instance, the administration of oleocanthal extracted from extra-virgin olive oil to C57BL/6 wild-type mice enhanced the clearance of exogenously introduced Aβ40 from the brain across the BBB and increased its brain efflux by 29% compared to control mice, which was associated with up-regulation of P-gp and LRP1 expressions at the BBB.16 In addition, Aβ brain homeostasis is maintained by the peripheral sink condition that is created by the liver. Hepatic LRP1 and P-gp were also found to play role in Aβ clearance from the periphery.17,18
In addition to the major pathological features of AD, amyloid plaques and neurofibrilary tangles, disruption in the neurotransmitter levels of acetylcholine (ACh) and cholinergic dysfunction are observed in AD that are associated with early cognitive impairments, correlate with cognitive decline, and are the basis of the cholinergic hypothesis of AD.19 Based on the cholinergic dysfunction and associated reduction in brain levels of ACh, inhibitors of enzymes that degrade Ach, including the acetylcholinesterase and butyrylcholinesterase, have been developed in attempt to increase ACh levels and improve the cognitive function of AD patients. Some of these cholinesterase inhibitors (ChEIs) are United States Food and Drug Administration (FDA) approved drugs including tacrine, donepezil, rivastigmine, and galantamine, with a limited clinical use of tacrine due to its reported hepatotoxicity.20 In addition to ChEIs, N-methyl-d-aspartate (NMDA) receptor antagonists have been developed to inhibit neurotoxicity caused by NMDA receptors hypersensitivity and over activation mediated by various toxins including Aβ oligomers.21 Memantine is an AD marketed drug that inhibits NMDA receptors and protects neurons from the excitatory effect of excessive glutamate stimulation.22,23
In addition to their established mechanisms, these AD medications (ChEIs and NMDA antagonist) are reported to provide neuroprotective and disease-modifying effects,24–26 and to improve hippocampal-dependent memory in rodents;27 however, the mechanism by which these drugs produce this effect is not fully understood. Recently, researchers have started to explore possible mechanisms by which ChEIs could influence AD pathology other than of being merely inhibitors of ChEs. For example, available in vitro studies have shown that rivastigmine and donepezil were able to reduce Aβ production by shifting APP processing to α-secretase pathway precluding the formation of toxic Aβ.28,29 In addition, treatment of rat microglia with galantamine significantly enhanced microglial Aβ phagocytosis by inducing the influx of Ca2+ into microglia.30 Several mechanisms have been proposed for donepezil neuroprotective effect including down-regulation of NMDA receptors following stimulation of alpha7 nAChRs,24 increase production of insulin-like growth factor-I in the hippocampus,27 or inhibition of microglial activation through blocking MAPK and NF-kB signaling pathways.25
In this study, we investigated the AD drugs tacrine, donepezil, rivastigmine, galantamine, and the NMDA receptor antagonist memantine for their effect on Aβ brain and hepatic clearance in vitro and in vivo. For the in vitro studies, rat brain endothelial cells (RBE4) cultured on filters as a cell-based model of the BBB, and sandwich-cultured primary rat hepatocytes (SCHs) as a liver model were used to study the effect of the AD medications on 125I-Aβ40 transport across the BBB, and biliary clearance, respectively. Subsequently, we chose donepezil and rivastigmine to in vivo investigate their effect on Aβ clearance in rats as both drugs demonstrated the highest significant effect to enhance 125I-Aβ40 clearance across both in vitro models. For this, we examined the ability of the chosen drugs to rectify the reduced clearance of Aβ from the brain and the liver caused by aging17,31 in aged rats (24 months of age), and was compared to young rats (4 months of age). Understanding the influence of ChEIs on the major pathways that contribute to AD pathology could optimize their clinical potential and help future drug development endeavor by providing new drug targets and possible mechanisms involved in AD pathology.
RESULTS AND DISCUSSION
Currently, one NMDA receptor antagonist, memantine, and four ChEIs, tacrine, donepezil, rivastigmine, and galantamine, are approved for use in the treatment of AD. Although these AD medications do not stop the progression of the disease, they provide symptomatic relief and improve memory in patients with AD. Despite the fact that ChEIs are developed based on their inhibition of ChEs, thereby increasing synaptic ACh level and improving cognitive function, recent studies showed that ChEIs have noncholinergic mechanisms by which they influence Aβ brain level and show neuroprotective effects.25,26,28,30 In sporadic AD, failure in Aβ clearance from the brain has been suggested as a major mechanism contributing to brain accumulation of Aβ.8–10 Aβ clearance from the brain across the BBB is mediated by two major transport proteins, P-gp and LRP1.11,32 Zlokovic and co-workers12,33,34 proposed that reduced Aβ clearance across the BBB underlies Aβ brain accumulation, which suggest targeting mechanisms involved in Aβ brain clearance across the BBB as a therapeutic approach for AD treatment.13–15 Thus, in the present study, we assessed the effect of AD drugs on brain and hepatic clearance of Aβ as an additional mechanism by which they provide neuroprotective effect.
Effect of AD Medications on Aβ Clearance in Vitro
Although Aβ42 is more prone to aggregation compared to Aβ40, both Aβ peptides have been implicated in the pathogenesis of AD and participate in the formation of senile plaques with neurotoxicity potentials.35 Both Aβ40 and Aβ42 are substrates for P-gp and LRP1;32,33 however, due to the propensity of Aβ42 to rapidly aggregate and its slow clearance compared to Aβ40,36,37 in all our in vitro and in vivo studies we used 125I-Aβ40 to evaluate the effect of AD drugs on the clearance of Aβ.
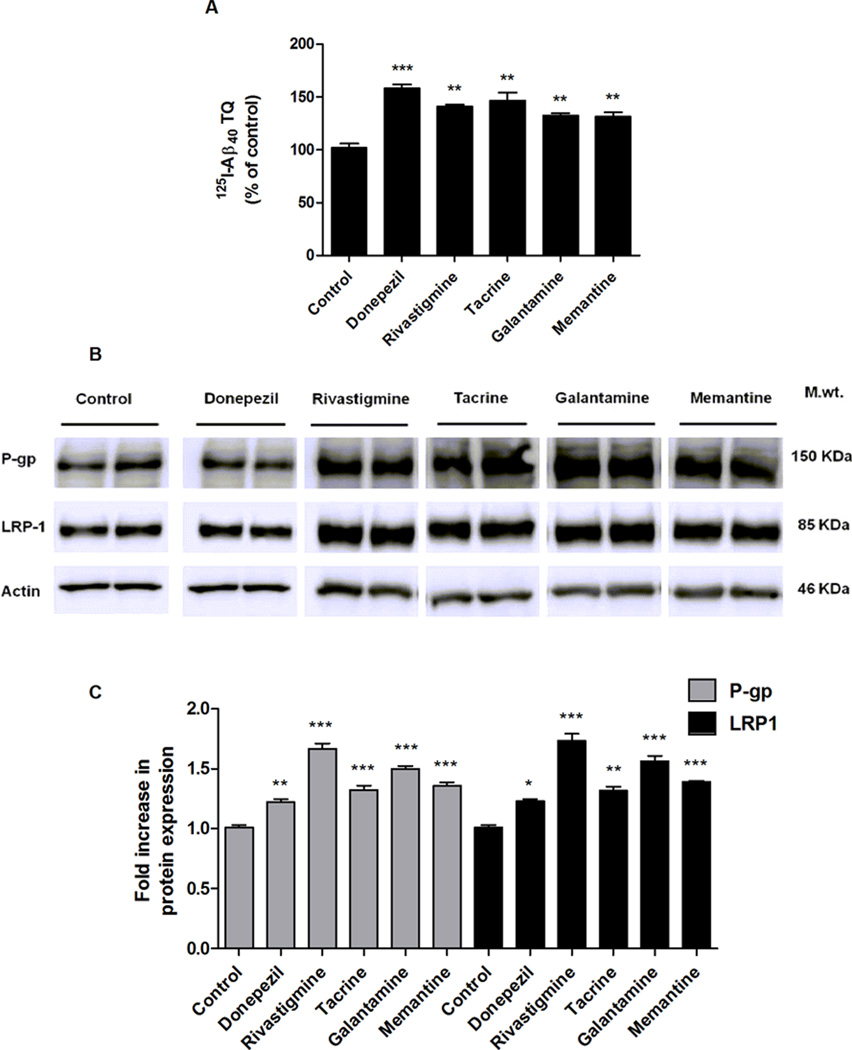
In vitro studies were first performed to screen for the effect of memantine, tacrine, donepezil, rivastigmine and galantamine on 125I-Aβ40 transport across RBE4 cells, as a cell-based model for the BBB, and 125I-Aβ40 biliary clearance in SCHs, a liver model. Transport of 125I-Aβ40 from the basolateral side (B; representative of brain) to the apical side (A; representative of blood) of RBE4 monolayer is mediated mainly by active transport processes. To examine the possible effect of AD medications on these processes, 48 h treatment with 5 µM of each AD medications were performed followed by 4 h washout period to avoid any possible interaction between Aβ and the drugs used. As shown in Figure 1A, 125I-Aβ40 transport quotient (125I-Aβ40 TQ) was significantly enhanced by all drugs with most pronounced effect observed following donepezil, which increased TQ by 57% (P < 0.001). The increase in 125I-Aβ40 TQ by the other drugs was also significant and ranged from 40 to 45% (P < 0.01). To examine the effect of the drugs on the expression of Aβ transport proteins, P-gp and LRP1, Western blotting was performed. As shown in Figure 1B and C, all the AD medications caused significant increase in the expressions of P-gp and LRP1 with the highest effect observed with rivastigmine by approximately 1.7-fold for both P-gp and LRP1 (P < 0.001). The effect of other drugs (donepezil, tacrine, galantamine, and memantine) on P-gp and LRP1 expressions was also significant and ranged from 1.2- to 1.5-fold (P < 0.05). These findings could explain, at least in part, the increase in 125I-Aβ40 TQ across RBE4 monolayer.
Figure 1.
Effect of AD medications on transport of 125I-Aβ40 across the BBB model, and on Aβ transport proteins expression in RBE4 cells. (A) Transport quotient (TQ) of 125I-Aβ40, as percent of control, in response to 48 h treatment of 5 µM of each drug. (B) Representative Western blots of P-gp and LRP1 following treatment with AD medications. (C) Graphical representation of normalized protein expression. Data presented as mean ± SEM (n = 4, *P < 0.05, **P < 0.01, ***P < 0.001, ns = not significant, compared to control untreated cells).
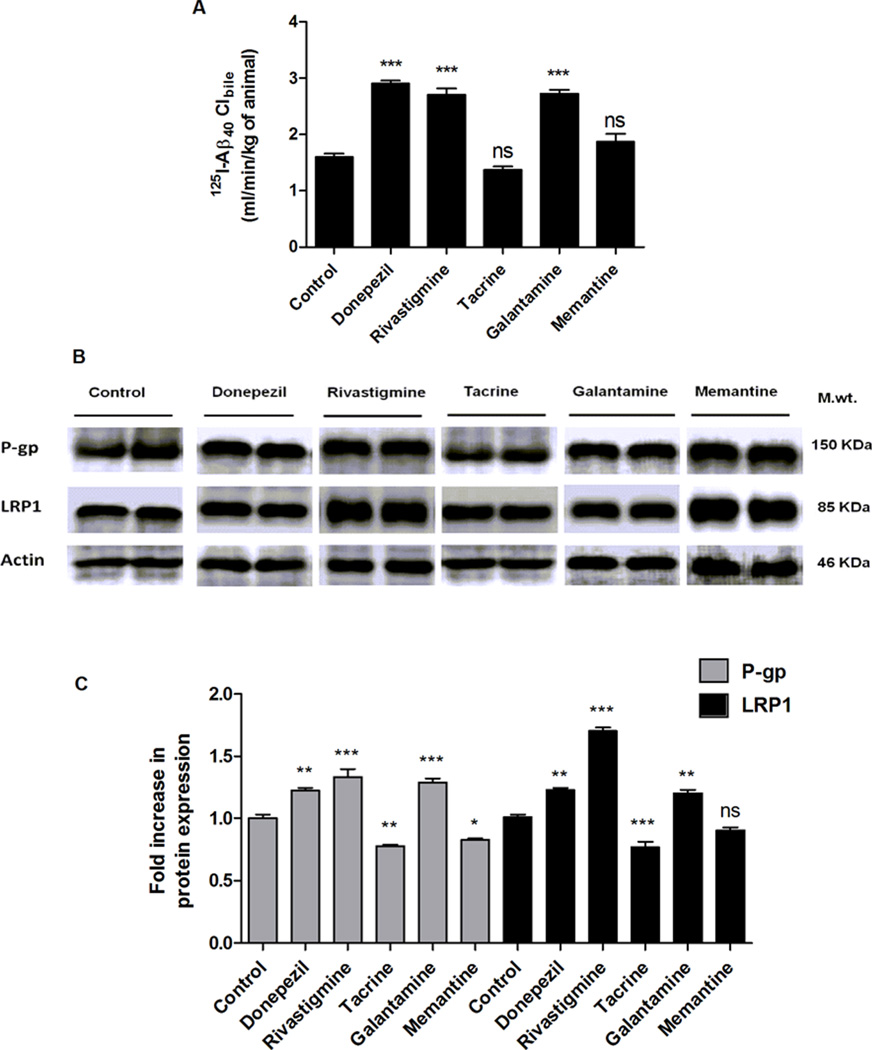
We have previously shown SCHs as a useful model to study Aβ hepatic disposition and its biliary clearance, which was also mediated by P-gp and LRP1.18 Hence, in this study, SCHs model was used to study the effect of AD medications on 125I-Aβ40 hepatic clearance as well as their effect on Aβ transport proteins expression. The 48 h treatment with AD medications, at 10 µM concentration, resulted in a differential effect between the drugs. Cells treated with donepezil, rivastigmine, and galantamine significantly increased the biliary clearance of 125I-Aβ40 by 64%, 55%, and 57%, respectively, compared to control untreated cells (1.6 ± 0.06 mL/min/kg; P < 0.001, Figure 2A). On the other hand, tacrine and memantine showed no significant effect on 125I-Aβ40 biliary clearance (P > 0.05; Figure 2A). Consistent with their effect on 125I-Aβ40 biliary clearance, SCHs treated with donepezil, rivastigmine, and galantamine upregulated the protein expression of Aβ transporters, P-gp and LRP1 (Figure 2B and C). The three drugs similarly increased P-gp expression by 1.3-fold compared to control cells while for LRP1, its expression was increased by 1.2-fold when SCHs was treated with donepezil and galantamine and 1.7 fold with rivastigmine. Conversely, tacrine down-regulated the expression of both LRP1 and P-gp by 20%, while memantine down-regulated P-gp expression and showed no effect on LRP1 expression, which was consistent with their lack of effect on 125I-Aβ40 biliary clearance. This correlation between the effect of the investigated drugs on 125I-Aβ40 biliary clearance and transport across the cell-based BBB model and their modulatory effect on the expression of Aβ transport proteins LRP1 and P-gp suggests the mechanism by which Aβ clearance is enhanced is due to, at least in part, up-regulation of P-gp and LRP1 expressions.
Figure 2.
Effect of AD medications on hepatic clearance of 125I-Aβ40, and on Aβ transport proteins expression in SCHs. (A) Biliary clearance (Clbile) of 125I-Aβ40 in response to 48 h treatment of 10 µM of each drug. (B) Representative Western blots of P-gp and LRP1 following treatment with AD medications. (C) Graphical representation of normalized protein expression. Data presented as mean ± SEM (n = 4, *P < 0.05, **P < 0.01, ***P < 0.001, ns = not significant, compared to control untreated cells).
Treatments of RBE4 cells and SCHs for 48 h with AD medications showed no significant effect on degradation of 125I-Aβ40 as determined by the TCA assay (data not shown). From this initial in vitro screening, donepezil, and rivastigmine were selected for in vivo evaluation due to their pronounced effect on 125I-Aβ40 clearance when compared to the other tested drugs.
Effect of Donepezil and Rivastigmine on Clearance of Aβ in Vivo
Several studies reported a progressive decline in the BBB expressions of P-gp and LRP1 during normal aging, and this decline was positively correlated with accumulation of Aβ in the brains of aged nondemented subjects and AD patients.38,39 Similarly, in rats the expression of P-gp and LRP1 are decreased with aging.17,31 Hence, we sought to study the effect of aging on brain and hepatic clearance of 125I-Aβ40 in rats, and whether donepezil and rivastigmine were able to rectify age-related reduction in Aβ clearance in vivo. First, we examined the effect of aging on the two pathways that regulate Aβ brain and plasma homeostasis where their dysfunction could contribute largely to Aβ brain accumulation and AD pathology. These pathways included the efflux of Aβ across the BBB (from brain to blood) and extraction of Aβ from the general circulation by the liver, which were determined by the brain efflux index (BEI%) and liver uptake index (LUI) methods, respectively. In addition, the effect of aging on the expression of Aβ transport proteins P-gp and LRP1 at the brain and liver was also evaluated. As shown in Figure 3A, the brain efflux index of 125I-Aβ40 in aged rats was significantly lower by 22% compared to young rats (P < 0.01). Age effect on extravascular extraction (E) of 125I-Aβ40 was more pronounced, and when compared to young animals, E was reduced by 44% in aged rats (Figure 3D; P < 0.01). These reductions in 125I-Aβ40 brain and hepatic clearances were consistent with downregulation of P-gp and LRP1 protein expressions in both brain and liver tissues of aged rats (Figure 3B, C, E, F). P-gp expression was reduced in aged as compared to young rats by 23% and 33% in brain and liver, respectively (P < 0.01). For LRP1, the reduction in its protein expression due to aging was also more pronounced in the liver compared to brain with 45% and 20%, respectively (Figure 3C and F; P < 0.05).
Figure 3.
Effect of age on brain clearance and hepatic extraction of 125I-Aβ40 and on Aβ transport proteins expression in young (4 months old) and aged (24 months old) rats. (A) Brain efflux index (BEI%) of 125I-Aβ40 in young and aged rats. (B) Representative Western blots of brain P-gp and LRP1 proteins expression. (C) Graphical representation of normalized brain proteins expression. (D) Hepatic extraction, E, of 125I-Aβ40 in young and aged rats. (E) Representative Western blots of hepatic P-gp and LRP1. (F) Graphical representation of normalized hepatic proteins expression. Data presented as mean ± SEM (n = 5, *P < 0.05, **P < 0.01).
Next step was to examine the effect of rivastigmine and donepezil on brain clearance and hepatic uptake of 125I-Aβ40 in young (4 months) and aged rats (24 months). To evaluate the ability of donepezil and rivastigmine to rectify the age-associated decline in 125I-Aβ40 clearance, young and aged rats were treated with clinically relevant doses of either donepezil (2 mg/kg/day) or rivastigmine (0.3 mg/kg/day) using Alzet pump to provide sustained release for 26 days.40 These doses were selected from previous studies that showed chronic treatment of rats with 2 mg/kg/day of donepezil or 0.3 mg/kg/ day of rivastigmine mitigated cholinergic neuronal degeneration, and improved cognitive performance.41,42 The results are shown in Figure 4. In young animals, BEI studies with both drugs demonstrated a significant increase in the efflux of stereotaxically microinjected 125I-Aβ40 across the BBB by 13 ± 2.9% for donepezil (P < 0.05) and 31 ± 3.7% for rivastigmine (P < 0.01; Figure 4A). In aged animals, donepezil treatment was able to enhance the BEI% of 125I-Aβ40 by 30 ± 3.3% compared to vehicle treated control (P < 0.05; Figure 4B). Similar to the effect on young rats, rivastigmine effect on aged rats was higher than donepezil and increased the BEI% of 125I-Aβ40 by 44 ± 4.3% compared to vehicle treated control (P < 0.001; Figure 4B). Interestingly, in aged rats, donepezil increased 125I-Aβ40 BEI% from 47 to 61%, a value that was comparable to that of vehicle-treated young rats (59%); and with rivastigmine the increase in 125I-Aβ40 BEI% was even higher reaching 67%. These results demonstrated that both drugs were able to rectify age-dependent reduction in Aβ brain clearance. In addition, these findings were consistent with those obtained from clinical trials that reported treating AD patients with rivastigmine for 2 weeks increased plasma level of Aβ42, which was associated with positive response to treatment after 6 months and 2 years of follow up.43,44 This effect could be related to enhanced brain to blood efflux of Aβ and reduced Aβ brain load due to rivastigmine treatment.
Figure 4.
Effect of 26 day treatment of donepezil (2.0 mg/kg/day) or rivastigmine (0.3 mg/kg/day) on brain clearance of exogenous 125I-Aβ40 across the BBB in young and aged rats. Brain efflux index (BEI%) of 125I-Aβ40 in young (A), and aged (B) rats treated with donepezil or rivastigmine. Data presented as mean ± SEM (n = 5, *P < 0.05, **P < 0.01, ***P < 0.001).
In addition to the BBB, targeting the hepatic clearance of Aβ may provide a unique mechanism for rapid and efficient elimination of Aβ from the general circulation, which could in turn reduce Aβ brain load.17,45 In our studies, the extravascular extraction or hepatic uptake of 125I-Aβ40 by a single pass into the portal vein was 51.9 ± 2.4% in vehicle-treated young rats (Figure 5A). The hepatic uptake of 125I-Aβ40 was significantly enhanced by 27% in donepezil-treated rats (from 51.9 ± 2.4% to 66 ± 3.2%; P < 0.05), and by 23% in rivastigmine-treated rats (from 51.9 ± 2.4% to 64 ± 3.7%; P < 0.05, Figure 5A) compared to vehicle-treated rats. In aged rats, the effect was more pronounced and reached values comparable to those of control young animals (Figure 5B), where donepezil treatment increased the hepatic uptake of 125I-Aβ40 by 67% (from 29.2 ± 4.6% to 48.7 ± 3.2%; P < 0.05) compared to vehicle-treated rats of the same age. For rivastigmine treated rats, the hepatic uptake of 125I-Aβ40 was greatly enhanced by 119% (from 29.2 ± 4.6% to 64.0 ± 4.9%; P < 0.001), reaching values greater than those of control young rats (Figure 5).
Figure 5.
Effect of 26 day treatment of donepezil (2.0 mg/kg/day) or rivastigmine (0.3 mg/kg/day) on hepatic extraction of exogenous 125I-Aβ40 in young and aged rats. Hepatic extraction (E) of 125I-Aβ40 in young (A) and aged (B) rats treated with donepezil or rivastigmine. Data presented as mean ± SEM (n = 5, *P < 0.05, ***P < 0.001).
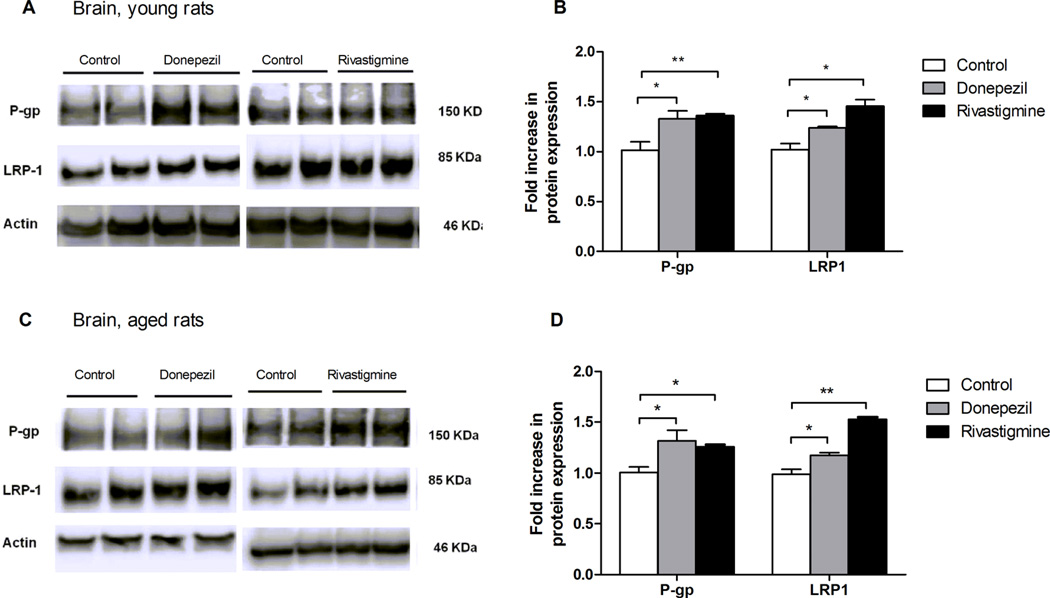
Western blot analysis of P-gp and LRP1 expressions in the brains and livers of treated and untreated animals of both ages showed consistent correlation with 125I-Aβ40 clearance. As shown in Figure 6A and B, in the brain, donepezil treatment of young rats enhanced the protein expression of both P-gp and LRP1 by 31% and 22%, respectively. Higher effect was observed with rivastigmine, which up-regulated the expression of both P-gp and LRP1 by 36% and 42%, respectively. For aged animals, donepezil was able to increase the expression of P-gp and LRP1 by 25% and 20%, respectively. Similarly, rivastigmine increased P-gp and LRP1 protein expression by 20% and 56%, respectively (Figure 6C and D). The inductive effect of both drugs on P-gp and LRP1 was also seen in the liver. In young animals, both drugs enhanced P-gp and LRP1 expressions by 47% and 41% for rivastigmine, and by 40% and 46% for donepezil, respectively (Figure 7A and B). For aged animals, donepezil was able to increase the protein expression level of Pgp and LRP1 by 49% and 31%, respectively. Rivastigmine treated animals up-regulated P-gp and LRP1 by 81% and 88%, respectively, compared to young animals, which was greater than the effect of donepezil by almost two times (Figure 7C and D). These findings suggested that the mechanism by which donepezil and rivastigmine ameliorated Aβ clearance was mediated, at least in part, by their ability to upregulate P-gp and LRP1, whereas both drugs had no effect on Aβ degradation as confirmed by the TCA assay (data not shown). Several studies reported that induction of LRP1, a major receptor involved in Aβ hepatic elimination, would enhance Aβ uptake by the liver. Ravindranath and colleagues reported that a 30 day course of oral administration of Withania somnifera root extract reversed behavioral deficits, plaque pathology, and accumulation of Aβ and oligomers in the brains of middle-aged and old APP/PS1 AD transgenic mice, and this effect was mainly attributed to the induction of peripheral “sink condition” via up-regulation of liver LRP1.46 In addition, we have shown previously, using SCHs, the induction of LRP1 and P-gp by rifampicin significantly increased the biliary clearance of 125I-Aβ.47 Interestingly, treatment of animals with donepezil and rivastigmine showed a significantly higher effect on the hepatic extraction of Aβ by the liver compared to their effect on Aβ efflux across the BBB. This observation could be related to differences in donepezil and rivastigmine accessibility to these tissues influencing their local concentrations thus their effect on transport proteins expression and consequently on Aβ clearance. In addition, this observed effect with donepezil and rivastigmine was more pronounced in aged compared to young rats, which could be attributed to pharmacokinetic changes with age that influenced plasma levels of the two drugs.48 It has been reported that the blood and brain concentrations of ChEIs are higher in aged than young rats.48
Figure 6.
Effect of 26 day treatment of donepezil (2.0 mg/kg/day) or rivastigmine (0.3 mg/kg/day) on Aβ transport proteins expression in brains of young and aged rats. (A) Representative Western blots of brain P-gp and LRP1 of young rats. (B) Graphical representation of normalized proteins expression of young rats. (C) Representative Western blots of brain P-gp and LRP1 of aged rats. (D) Graphical representation of normalized proteins expression of aged rats. Data presented as mean ± SEM (n = 5, *P < 0.05, **P < 0.01, ***P < 0.001).
Figure 7.
Effect of 26 day treatment of donepezil (2.0 mg/kg/day) or rivastigmine (0.3 mg/kg/day) on Aβ transport proteins expression in livers of young and aged rats. (A) Representative Western blots of hepatic P-gp and LRP1 in young rats. (B) Graphical representation of normalized proteins expression of young rats. (C) Representative Western blots of hepatic P-gp and LRP1 of aged rats. (D) Graphical representation of normalized proteins expression of aged rats. Data presented as mean ± SEM (n = 5, *P < 0.05, **P < 0.01, ***P < 0.001).
As both donepezil and rivastigmine were able to enhance the clearance of exogenously administered 125I-Aβ40 from the brain and liver, we hypothesized that chronic treatment with both drugs could reduce endogenous Aβ brain load. To determine the overall effect of donepezil and rivastigmine on endogenous levels of both forms of Aβ, Aβ40 and Aβ42, were extracted from the brain and their levels were determined in young and aged rats after 26 days of treatment with either drug. As expected, endogenous levels of both Aβ isoforms, Aβ40 and Aβ42, were significantly reduced by both drugs in the brains of aged rats, though this reduction was not observed in the brains of young rats (Figure 8). In aged rats, compared to vehicle-treated controls, donepezil reduced total Aβ40 levels by 29% from 3520 ± 383 to 2497 ± 142 pmol/g brain tissue, and reduced Aβ42 level by 41% from 991 ± 182 to 586 ± 31 pmol/g brain tissue (Figure 8). Rivastigmine, generated a much greater decline compared to donepezil with comparable reduction in the levels of both Aβ40 and Aβ42 by approximately 52%, from 3520 ± 383 to 1713 ± 260, and from 991 ± 182 to 466 ± 67 pmol/g brain tissue, for Aβ40 and Aβ42, respectively (Figure 8). These reduced levels of Aβ40 and Aβ42 in the brains of aged rats could be attributed to their enhanced brain and hepatic clearances mediated by increased expression of P-gp and LRP1. Although donepezil and rivastigmine showed a reduction trend in Aβ42 levels in young rats, it was not statistically significant (P = 0.07). In young rats, brain Aβ levels were already low and close to ELISA quantification limits of both peptides, making further decline difficult to be accurately captured. This, in addition to Aβ efficient clearance machinery in young compared to aged rats, could contribute to the limited effect.
Figure 8.
Effect of 26 day treatment of donepezil (2.0 mg/kg/day) or rivastigmine (0.3 mg/kg/day) on endogenous levels of total Aβ40 and Aβ42 in brains of young and aged rats determined using specific ELISA. (A) Levels of Aβ40 in young and aged rats. (B) Levels of Aβ42 in young and aged rats. Data presented as mean ± SEM (n = 5, *P < 0.05).
CONCLUSION
Aβ plays a central role in the degenerative process of neurons that lead to neuronal dysfunction in AD.49 Our findings here demonstrated that the ChEIs donepezil and rivastigmine are able to enhance both brain and hepatic Aβ clearance in young and aged rats, presenting an additional mechanism by which these drugs could implicate AD. Upregulation of P-gp and LRP1 by donepezil and rivastigmine could explain, at least in part, the improved clearance of Aβ across the BBB and the liver. The age-dependent attenuation of Aβ elimination from the brain and the liver confirms aging as a risk factor for AD, and according to our results ChEIs could reduce Aβ brain levels and thus may ameliorate neurodegeneration and memory impairment observed in AD. We thus support the notion that the clinical efficacy of donepezil and rivastigmine in AD are due not only to activation of cholinergic pathway, but also attenuation of Aβ-mediated neurotoxicity. Despite this effect of ChEIs on Aβ and their activation of cholinergic transmission, these agents are not capable of decreasing or altering the progression of AD. Donepezil was investigated in patients with mild to moderate AD in a placebo-controlled double-blind trial for its effect on the cognitive function; the results showed no difference between the treatment and placebo groups, which might indicate that ChEIs do not alter the course of AD. However, in most of the cases, ChEIs are prescribed to patients who already developed AD or at late stages of the disease, where irreversible neuronal damage and potential Aβ deposition and plaques are evident, and therapeutic benefits are expected to be very limited. Thus, we suggest giving donepezil and rivastigmine therapy to patients at very early stages of AD, or patients who are at high risk of developing the disease, which necessitate early diagnostic tools for early therapeutic intervention and better curative effect. In addition, since ChEIs therapeutic doses are determined based on their cholinergic activity, the doses for their noncholinergic effect may need to be adjusted for better therapeutic efficacy.
METHODS
Reagents and Antibodies
Synthetic monoiodinated and non-oxidized Aβ40 (125I-Aβ40; human, 2200 Ci/mmol) was purchased from PerkinElmer (Boston, MA). Fetal bovine serum (FBS) was purchased from ATLANTA biological (Flowery Branch, GA). Ham’s F12, MEM alpha medium (α-MEM), HEPES, and fetal calf serum (FCS) were obtained from Gibco (Grand Island, NY). Geneticin (G418) was purchased from Calbiochem (Gibbstown, NJ). Tacrine hydrochloride was obtained from Cayman Chemical Company (Ann Arbor, MI). Donepezil hydrochloride and rivastigmine l-tartrate were obtained from Tokyo Chemical Industry Co., LTD (TCI; Portland, OR). RBE4 cells were kindly provided by Dr. P. O. Couraud (Institut Cochin, Paris, France). Collagenase (type I, class I), rat tail collagen (type I), Dulbecco’s modified Eagle’s medium (DMEM), and insulin were purchased from Invitrogen (Carlsbad, CA). Galantamine hydrochloride, dexamethasone, bovine serum albumin (BSA), basic fibroblast growth factor (bFGF), and soybean trypsin inhibitor were purchased from Sigma-Aldrich (St. Louis, MO). ITS culture supplement (10 µg/mL insulin, 10 µg/mL transferrin, 10 ng/mL selenous acid) was obtained from BD Biosciences (San Jose, CA). Total protein measurement reagents with the bicinchoninic acid (BCA) method were obtained from Pierce (Rockford, IL). For Western blot, the mouse monoclonal antibody C-219 against P-gp was obtained from Covance Research Products (Dedham, MA); mouse monoclonal antibody against light chain LRP1 was obtained from Calbiochem; goat polyclonal antibodies against actin (C-11) and HRP-labeled secondary antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). All other chemicals and reagents were of analytical grade and were readily available from commercial sources.
RBE4 Cell Culture
The immortalized rat brain endothelial RBE4 cells were cultured in dishes or 24-well plates precoated with 150 µg/ mL rat-tail collagen solution. RBE4 cell media consisted of Ham’s F10 and α-MEM (1:1, v/v) supplemented with 10% FCS, 1 ng/mL bFGF, 300 mg/mL G418, and 20 mM HEPES. The RBE4 cells were used at a passage number between 26 and 36, and were incubated at 37 °C, air/ CO2 (95%/5%).
Treatment of RBE4 Cells with AD Medications
Cells were seeded in 10 mm cell culture dishes (Corning, NY) at a density of 1 × 106 cells per dish and allowed to grow in a humidified incubator (5% CO2/95% air) at 37 °C. At 50% confluency, cells were treated with 5 µM of tacrine, donepezil, rivastigmine, galantamine, memantine or vehicle (media contain 0.05% DMSO) for 48 h and replaced with fresh treatment after 24 h. At the end of treatment period, cells were washed with ice-cold phosphate buffer saline (PBS; Gibco) twice, scraped, collected, and lysed in RIPA buffer containing 1% protease inhibitors cocktail. Total protein was determined using the BCA protein assay. Samples were stored at −80 °C until used for Western blot analysis.
Effect of AD Drugs on 125I-Aβ40 Transport across the BBB Model
First, RBE4 cells cultured on inserts were treated for 48 h with 5 µM of tacrine, donepezil, rivastigmine, galantamine, memantine, or vehicle. Treatments were then removed and replaced with fresh media for 4 h, as a washout period. The basolateral to apical transport (B → A) was initiated by addition of media containing 0.1 nM 125I-Aβ40 and 14C-inulin (0.1 µCi/ml) to B side (lower chamber) and media alone to A side (upper chamber) for 30 min. Aliquots from both A and B sides were taken for radioactivity analysis using Wallac 1470 Wizard Gamma and Wallac 1414 WinSpectral liquid scintillation counters (PerkinElmer Inc. Waltham, MA). Degradation of 125I-Aβ40 was determined by the trichloroacetic acid (TCA) precipitation assay.32 Transport quotient (TQ) of 125I-Aβ40 was calculated using the following equation.51
| (1) |
where Aβ40(total) is the sum of counts per minute (cpm) in the apical and basolateral compartments and inulin(total) is the sum of dpm in the apical and basolateral compartments.
Animals
Sprague–Dawley male rats were purchased from Harlan Laboratories (Houston, TX). All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Louisiana at Monroe, and all surgical procedures were consistent with the IACUC policies and procedures. One set of male rats with 2–3 months of age were used for the isolation of hepatocytes. Another set of male rats were used for the in vivo studies, and were divided into two groups (n = 15 rat/group). Group 1 consists of young rats at 4 months of age, and group 2 consists of aged rats at 24 months of age. Both groups were used for intracerebral brain efflux and liver uptake index studies following treatment. Animals were maintained at 22 °C, 35% relative humidity and 12 h dark/light cycle and supplied with water and standard food.
Isolation of Primary Hepatocytes and Preparation of Sandwich Culture (SCHs)
Hepatocytes were isolated from rats’ livers by the two steps collagenase perfusion method,52 with slight modification as previously described by us.18 Hepatocytes were purified from other liver cell populations by a series of centrifugations and resuspended in serum-free medium. Using trypan blue assay, only cells with ≥85% viability were used in the preparation of SCHs. Collagen, 50 µg/mL, coated plates were seeded with 0.7 × 106 cells/ mL hepatocytes in serum-free medium supplemented with 1% ITS, 100 U/mL penicillin, 100 g/mL streptomycin, and 0.1 µM dexamethasone. On the second day after seeding, a second layer of 0.25 mg/mL matrigel in ice-cold medium was added over the cells and incubated at 37 °C for 24 h to form a sandwich configuration. The media was replaced with fresh media every day up to day 4 where hepatocytes form extensive bile canaliculi.
Treatment of SCHs with AD Medications
SCHs prepared in 6-well plates were treated with 10 or 25 µM of tacrine, donepezil, rivastigmine, galantamine, memantine, or vehicle for 48 h starting from day 2 of sandwich culture. Treatments were removed and replaced with fresh media for 4 h, as a washout period, followed by biliary clearance experiments. For protein expression studies, after 48 h of treatment cells were collected and processed as explained under Western blot analysis.
Determination of 125I-Aβ40 Biliary Clearance (Clbile) in SCHs Model
The biliary clearance of Aβ in SCHs was determined by the cumulative uptake method as previously described.47 Treated SCHs were first gently washed with standard HBSS containing calcium or HBSS without calcium (to maintain or break tight junctions that reserve bile canaliculi in culture, respectively) and then incubated with 0.1 nM 125I-Aβ40 in respective HBSS buffers containing 2% FBS. After 30 min of cumulative uptake, treatment was removed and cells were washed twice with ice-cold HBSS and once with HBSS containing 2% BSA to reduce Aβ nonspecific binding, and once again with HBSS alone to remove BSA residues. Cells were lysed by adding 300 µL of 1% triton buffer containing 1% protease inhibitors cocktail. Aliquots were taken to determine 125I-Aβ40 intracellular accumulation using the gamma counter. Degradation of 125I-Aβ40 was determined by TCA precipitation assay.32 Cell lysate aliquots were mixed with TCA (final concentration 10%) in 1:1 volume and centrifuged at 14 000g at 4 °C for 10 min. Data were normalized to total protein in each well using the BCA protein assay kit. Biliary clearance (Clbile) of Aβ was calculated using the B-CLEAR technology (Qualyst, Inc., Research Triangle Park, NC) according to eq 2.53
| (2) |
AUCmedium = incubation time × substrate initial concentration in the incubation medium. Biliary clearance values have a unit of mL/min/kg of body weight, based on 200 mg of protein/g of liver and 40 g of liver/kg of rat body weight.52
Treatment of Animals
Young, 4 months, and aged, 24 months, Sprague Dawely rats were treated chronically with donepezil (2.0 mg/ kg/day), rivastigmine (0.3 mg/kg/day) or vehicle (sterile distilled water) for 26 days using Alzet (DURECT Corporation, Cupertino, CA) osmotic minipumps (model 2ML4); n = 5 rats/treatment. Pumps were implanted subcutaneously by making a small cut in the midscapulary region, inserting the pump, and closing the wound with clips. Pumps delivered 2 mL of solution at a rate of 2.5 µL/h for 26 days. During surgery, rats were anesthetized via isofluorane inhalation. The implanted pumps were removed 1 day before the BEI/ LUI surgeries as a washout period to avoid any possible interaction between the drugs used and the injected Aβ inside the brain or the liver as described below.
Brain Efflux Index (BEI) Method
The brain clearance of Aβ was performed using the intracerebral microinjection technique as reported previously.54 In brief, rats were anaesthetized by an intramuscular injection with xylazine hydrochloride (1.25 mg/kg) and ketamine hydrochloride (125 mg/kg). Then, the coordinates that coincide with the parietal cortex area 2 were determined using stereotaxic apparatus (Stoelting Co., Wood Dale, IL). A small hole was made 5.5 mm lateral and 0.2 mm anterior to the bregma in which a 1 µL microinjection needle was inserted to a 4.5 mm depth. After that, a 0.5 µL of 0.02 µCi/mL 125I-Aβ40 and 0.01 µCi/mL inulin mixture prepared in extracellular fluid (ECF) buffer (122 mM NaCl, 25 mM NaHCO3, 3 mM KCl, 1.4 mM CaCl2, 1.2 mM MgSO4, 0.4 mM K2HPO4, 10 mM d-glucose, and 10 mM HEPES, pH 7.4) was administered into Par 2 region over 30 s and the microinjection was left in place for 4 min to avoid any backflow of the administered solution. At 60 min after microinjection, the left and right cerebrum and cerebellum were excised and homogenized in two volume of brain tissue in DPBS buffer (PBS, 5 mM glucose, 1 mM Na-pyruvate). Aliquots were taken for gamma radioactivity measurement using the Wallac gamma counter. Another set of samples was mixed with liquid scintillation mixture (1:10 ratio) for beta radioactivity measurement using the Wallac beta counter. Degradation of 125I-Aβ40 was determined by the TCA precipitation assay.32 The BEI and percentage of substrate remaining in the ipsilateral cerebrum (100-BEI) were determined using eqs 3 and 4, respectively.54
| (3) |
| (4) |
Liver Uptake Index (LUI) Method
The hepatic extraction of 125I-Aβ40 was determined by the unidirectional uptake of a single injection into the portal vein.55 Before performing the liver uptake index (LUI) studies, we tested the livers of a separate group of animals (n = 3 rats) that only underwent BEI experiments with 125I-Aβ40, and no radioactivity was detected. Thus, LUI studies were performed on the same rats that underwent BEI experiments. In brief, before the end of the BEI study, a midline incision into the rat abdomen was made to expose the portal vein. The hepatic artery was ligated, followed by a bolus injection of 200 µL of Ringer’s HEPES buffer, pH 7.4, containing 0.2 µCi of 125I-Aβ40 and 4.0 µCi of 3H water, internal reference, into the portal vein. After 18 s, the major lobe of the liver was excised and homogenized and dissolved for radioactivity analysis. The hepatic extraction (E) of 125I-Aβ40 was determined by measuring LUI that was defined by eq 5 and calculated using eq 6.17
| (5) |
| (6) |
ET and ER are the fractions of 125I-Aβ40 and 3H water, respectively, extracted by the liver on a single pass. By applying the ER value of 3H water, 65 ± 4 in rats as reported previously,55 in eq 5, and determining the value of LUI experimentally by eq 6, the ET value can be estimated. The extravascular extraction (E), represents intracellular uptake of 125I-Aβ40, can be determined using eq 7:
| (7) |
Ens is the fractional extraction for distribution in the vascular and extracellular space. Since uptake of 125I-Aβ40 upon a single injection into the portal vein represents the apparent extraction by the liver (intracellular uptake, distribution to the interstitial space, and retention in the vascular space), to calculate E, Ens was applied to eq 7. Here, we used an Ens value of 13 ± 3 as reported previously.55
Western Blot Analysis
SCHs and RBE4 cells were collected in 1.5 mL eppendorf tubes and centrifuged at 2000g for 10 min at 4 °C. Cells were then lysed in RIPA buffer containing 1% protease inhibitors cocktail and centrifuged at 15 000g for 15 min at 4 °C to remove cell debris. Supernatants were stored at −80 °C for subsequent Western blot analyses. For animals, samples from brain and liver tissues were homogenized and lysed in RIPA buffer containing protease inhibitor, followed by centrifugation at 15 000g for 15 min at 4 °C to collect supernatants for Western blot analysis. Next, 25 µg of protein samples was loaded and resolved using 7.5% SDS-polyacrylamide gel at 140 V for 1 h and transferred electrophoretically onto nitrocellulose membranes at 300 mA for 3 h. After that, membranes were incubated in 2% BSA blocking solution with rocking for 1 h at room temperature followed by overnight incubation at 4 °C with the primary antibodies for P-gp (C-219), LRP1 (light chain), or β-actin (C-11). Secondary antibodies used were anti-mouse IgG antibody for P-gp, anti-rabbit IgG antibody for LRP1, and anti-goat IgG antibody for β-actin, all labeled with horseradish peroxidase (HRP). Protein blots were developed using a chemiluminescence detection kit (SuperSignal West Femto substrate; Thermo Scientific, Waltham, MA). Bands were visualized using a C-DiGit Blot scanner (LI-COR Biosciences; Lincoln, NE), and band intensities were measured by densitometric analysis.
Aβ Enzyme-Linked Immunosorbent Assay (ELISA)
The levels of endogenous Aβ40 and Aβ42 in the homogenates of brains from young and aged rats treated with donepezil, rivastigmine, or vehicle (n = 5/treatment) were determined using sandwich ELISA. Total Aβ (soluble and insoluble) was extracted from brain tissues by ploytron homogenization in 70% formic acid (FA) and centrifuged for 2 h at 15 000g at 4 °C. Supernatant was collected and diluted 1:20 with TB buffer (1 M Tris base, 0.5 M NaHPO4) for neutralization, followed by further dilution 1:1 with antigen capture buffer provided by the ELISA kit. For Aβ40 detection, we used the Sensolyte anti-mouse/rat Aβ40 quantitative ELISA kit (AnaSpec, Inc., Fremont, CA), and for Aβ42 we used the high sensitivity ELISA kit from Wako Chemicals (Richmond, VA), both according to the manufacturers’ instructions. All antibodies used were provided by the kits. For specific detection of Aβ40, a mouse monoclonal antibody-coated plates and a rabbit HRP-conjugated anti-Aβ40 were used as capture and detection antibodies, respectively. For specific detection of Aβ42 antibody, (BNT77)-coated plates and HRP-conjugated antibody (BC05) were used as capture and detection antibodies, respectively. Total Aβ concentrations were expressed as pg/g brain tissue weight.
Statistical Analysis
All data were expressed as mean ± SEM. The experimental results were statistically analyzed for significant difference using two-tailed Student’s t test. A P-value less than 0.05 was considered statistically significant. All statistical analyses were done using GraphPad Prism, version 5.03.
Acknowledgments
Funding
This project was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under Grant Number P20GM103424, and by Louisiana Board of Reagent’s Research Competitive Program under grant number LEQSF-(2013-16)-RD-A-16..
ABBREVIATIONS
- APP
amyloid precursor protein
- ACh
acetylcholine
- AD
Alzheimer’s disease
- Aβ
amyloid-β
- BEI%
biliary excretion index
- BCA
bicinchoninic acid
- BBB
blood-brain barrier
- BSA
bovine serum albumin
- ChEIs
cholinesterase inhibitors
- Clbile
biliary clearance
- DMEM
Dulbecco’s modified Eagle’s medium
- ELISA
enzyme-linked immunosorbent assay
- E
hepatic extraction
- ECE
endothelin-converting enzyme-1
- EGTA
ethylene glycol tetraacetic acid
- FBS
fetal bovine serum
- FCS
fetal calf serum
- HBSS
Hank’s balanced salt solution
- LUI
liver uptake index
- LRP1
low density lipoprotein receptor related protein 1
- NMDA
N-methyl-d-aspartate
- P-gp
P-glycoprotein
- RBE4
rat brain endothelial cells
- SCHs
sandwich cultured primary rat hepatocytes
- TQ
transport quotient
- TCA
trichloroacetic acid
Footnotes
Author Contributions
All experiments were executed by L.A.M. and H.Q. L.A.M and A.K. designed the experiments and wrote the manuscript.
The authors declare no competing financial interest.
REFERENCES
- 1.Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, Hebert LE, Hennekens CH, Taylor JO. Prevalence of Alzheimer’s Disease in a Community Population of Older Persons. Higher than Previously Reported. JAMA, J. Am. Med. Assoc. 1989;262:2551–2556. [PubMed] [Google Scholar]
- 2.Sagare AP, Bell RD, Zlokovic BV. Neurovascular Defects and Faulty Amyloid-β Vascular Clearance in Alzheimer’s Disease. J. Alzheimer’s Dis. 2013:S87–S100. doi: 10.3233/JAD-2012-129037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardy J. The Alzheimer Family of Diseases: Many Etiologies, One Pathogenesis? Proc. Natl. Acad. Sci. U. S. A. 1997;94:2095–2097. doi: 10.1073/pnas.94.6.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selkoe DJ. Toward a Comprehensive Theory for Alzheimer’s Disease. Hypothesis: Alzheimer’s Disease Is Caused by the Cerebral Accumulation and Cytotoxicity of Amyloid Beta-Protein. Ann. N.Y. Acad. Sci. 2000;924:17–25. doi: 10.1111/j.1749-6632.2000.tb05554.x. [DOI] [PubMed] [Google Scholar]
- 5.Selkoe DJ. The Molecular Pathology of Alzheimer’s Disease. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- 6.Selkoe DJ. Alzheimer’s Disease: Genes, Proteins, and Therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 7.Cam JA, Bu G. Modulation of Beta-Amyloid Precursor Protein Trafficking and Processing by the Low Density Lipoprotein Receptor Family. Mol. Neurodegener. 2006;1:8. doi: 10.1186/1750-1326-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sommer B. Alzheimer’s disease and the amyloid cascade hypothesis: ten years on. Curr. Opin Pharmacol. 2002;2:87–92. doi: 10.1016/s1471-4892(01)00126-6. [DOI] [PubMed] [Google Scholar]
- 9.Sagare AP, Bell RD, Zlokovic BV. Neurovascular Dysfunction and Faulty Amyloid Beta-Peptide Clearance in Alzheimer Disease. Cold Spring Harbor Perspect. Med. 2012 doi: 10.1101/cshperspect.a011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ. Decreased Clearance of CNS Beta-Amyloid in Alzheimer’s Disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhnke D, Jedlitschky G, Grube M, Krohn M, Jucker M, Mosyagin I, Cascorbi I, Walker LC, Kroemer HK, Warzok RW, Vogelgesang S. MDR1-P-Glycoprotein (ABCB1) Mediates Transport of Alzheimer’s Amyloid-Beta Peptides–Implications for the Mechanisms of Abeta Clearance at the Blood-Brain Barrier. Brain Pathol. 2007;17:347–353. doi: 10.1111/j.1750-3639.2007.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, Spijkers P, Guo H, Song X, Lenting PJ, Van Nostrand WE, Zlokovic BV. LRP/ Amyloid Beta-Peptide Interaction Mediates Differential Brain Efflux of Abeta Isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Abuznait AH, Qosa H, O’Connell ND, Akbarian-Tefaghi J, Sylvester PW, El Sayed KA, Kaddoumi A. Induction of Expression and Functional Activity of P-Glycoprotein Efflux Transporter by Bioactive Plant Natural Products. Food Chem. Toxicol. 2011;49:2765–2772. doi: 10.1016/j.fct.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qosa H, Abuznait AH, Hill RA, Kaddoumi A. Enhanced Brain Amyloid-Beta Clearance by Rifampicin and Caffeine As a Possible Protective Mechanism against Alzheimer’s Disease. J. Alzheimer’s Dis. 2012;31:151–165. doi: 10.3233/JAD-2012-120319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Citron M. Alzheimer’s Disease: Strategies for Disease Modification. Nat. Rev. Drug Discovery. 2010;9:387–398. doi: 10.1038/nrd2896. [DOI] [PubMed] [Google Scholar]
- 16.Abuznait AH, Qosa H, Busnena BA, El Sayed KA, Kaddoumi A. Olive-Oil-Derived Oleocanthal Enhances Beta-Amyloid Clearance As a Potential Neuroprotective Mechanism against Alzheimer’s Disease: In Vitro and in Vivo Studies. ACS Chem. Neurosci. 2013;4:973–982. doi: 10.1021/cn400024q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamaki C, Ohtsuki S, Iwatsubo T, Hashimoto T, Yamada K, Yabuki C, Terasaki T. Major Involvement of Low-Density Lipoprotein Receptor-Related Protein 1 in the Clearance of Plasma Free Amyloid Beta-Peptide by the Liver. Pharm. Res. 2006;23:1407–1416. doi: 10.1007/s11095-006-0208-7. [DOI] [PubMed] [Google Scholar]
- 18.Mohamed LA, Kaddoumi A. In Vitro Investigation of Amyloid-beta Hepatobiliary Disposition in Sandwich Cultured Primary Rat Hepatocytes. Drug Metab. Dispos. 2013;41:1787–1796. doi: 10.1124/dmd.113.052514. [DOI] [PubMed] [Google Scholar]
- 19.Perry E, Walker M, Grace J, Perry R. Acetylcholine in Mnd: A Neurotransmitter Correlate of Consciousness? Trends Neurosci. 1999;22:273–280. doi: 10.1016/s0166-2236(98)01361-7. [DOI] [PubMed] [Google Scholar]
- 20.Watkins PB, Zimmerman HJ, Knapp MJ, Gracon SI, Lewis KW. Hepatotoxic Effects of Tacrine Admin-istration in Patients with Alzheimer’s Disease. JAMA, J. Am. Med. Assoc. 1994;271:992–998. [PubMed] [Google Scholar]
- 21.Cacabelos R, Takeda M, Winblad B. The Glutamatergic System and Neurodegeneration in Dementia: Preventive Strategies in Alzheimer’s Disease. Int. J. Geriatr Psychiatry. 1999;14:3–47. doi: 10.1002/(sici)1099-1166(199901)14:1<3::aid-gps897>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Keilhoff G, Wolf G. Memantine Prevents Quinolinic Acid-Induced Hippocampal Damage. Eur. J. Pharmacol. 1992;219:451–454. doi: 10.1016/0014-2999(92)90487-o. [DOI] [PubMed] [Google Scholar]
- 23.Willard LB, Hauss-Wegrzyniak B, Danysz W, Wenk GL. The Cytotoxicity of Chronic Neuroinflammation upon Basal Forebrain Cholinergic Neurons of Rats Can Be Attenuated by Glutamatergic Antagonism or Cyclooxygenase-2 Inhibition. Exp. Brain Res. 2000;134:58–65. doi: 10.1007/s002210000446. [DOI] [PubMed] [Google Scholar]
- 24.Shen H, Kihara T, Hongo H, Wu X, Kem WR, Shimohama S, Akaike A, Niidome T, Sugimoto H. Neuroprotection by Donepezil against Glutamate Excitotoxicity Involves Stimulation of α7 Nicotinic Receptors and Internalization of NMDA Receptors. Br. J. Pharmacol. 2010;161:127–139. doi: 10.1111/j.1476-5381.2010.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim HG, Moon M, Choi JG, Park G, Kim AJ, Hur J, Lee KT, Oh MS. Donepezil Inhibits the Amyloid-Beta Oligomer-Induced Microglial Activation in Vitro and in Vivo. Neurotoxicology. 2014;40:23–32. doi: 10.1016/j.neuro.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Ballard CG, Chalmers KA, Todd C, McKeith IG, O’Brien JT, Wilcock G, Love S, Perry EK. Cholinesterase Inhibitors Reduce Cortical Abeta in Dementia with Lewy Bodies. Neurology. 2007;68:1726–1729. doi: 10.1212/01.wnl.0000261920.03297.64. [DOI] [PubMed] [Google Scholar]
- 27.Narimatsu N, Harada N, Kurihara H, Nakagata N, Sobue K, Okajima K. Donepezil Improves Cognitive Function in Mice by Increasing the Production of Insulin-Like Growth Factor-I in the Hippocampus. J. Pharmacol. Exp. Ther. 2009;330:2–12. doi: 10.1124/jpet.108.147280. [DOI] [PubMed] [Google Scholar]
- 28.Bailey JA, Ray B, Greig NH, Lahiri DK. Rivastigmine Lowers Abeta and Increases sAPPalpha levels, Which Parallel Elevated Synaptic Markers and Metabolic Activity in Degenerating Primary Rat Neurons. PLoS One. 2011;6:e21954. doi: 10.1371/journal.pone.0021954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugimoto H. The NeW Approach in Development of Anti-Alzheimer’s Disease Drugs via the Cholinergic Hypothesis. Chem. Biol. Interact. 2008;175:204–208. doi: 10.1016/j.cbi.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 30.Takata K, Kitamura Y, Saeki M, Terada M, Kagitani S, Kitamura R, Fujikawa Y, Maelicke A, Tomimoto H, Taniguchi T, Shimohama S. Galantamine-Induced Amyloid-{beta} Clearance Mediated via Stimulation of Microglial Nicotinic Acetylcho-line Receptors. J. Biol. Chem. 2010;285:40180–40191. doi: 10.1074/jbc.M110.142356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silverberg GD, Messier AA, Miller MC, Machan JT, Majmudar SS, Stopa EG, Donahue JE, Johanson CE. Amyloid Efflux Transporter Expression at the Blood-Brain Barrier Declines in Normal Aging. J. Neuropathol. Exp. Neurol. 2010;69:1034–1043. doi: 10.1097/NEN.0b013e3181f46e25. [DOI] [PubMed] [Google Scholar]
- 32.Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer’s Amyloid-ss(1–40) Peptide from Brain by LDL Receptor-Related Protein-1 at the Blood-Brain Barrier. J. Clin. Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, Paul SM, Zlokovic BV, Piwnica-Worms D, Holtzman DM. P-Glycoprotein Deficiency at the Blood-Brain Barrier Increases Amyloid-Beta Deposition in an Alzheimer Disease Mouse Model. J. Clin. Invest. 2005;115:3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zlokovic BV. Neurovascular Mechanisms of Alzheimer’s Neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Hardy J. A Hundred Years of Alzheimer’s Disease Research. Neuron. 2006;52:3–13. doi: 10.1016/j.neuron.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 36.Zlokovic BV, Yamada S, Holtzman D, Ghiso J, Frangione B. Clearance of Amyloid Beta-Peptide from Brain: Transport or Metabolism? Nat. Med. 2000;6:718–719. doi: 10.1038/77397. [DOI] [PubMed] [Google Scholar]
- 37.Ito S, Ohtsuki S, Terasaki T. Functional Characterization of the Brain-to-Blood Efflux Clearance of Human Amyloid-Beta Peptide (1–40) across the Rat Blood-Brain Barrier. Neurosci. Res. 2006;56:246–252. doi: 10.1016/j.neures.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Vogelgesang S, Warzok RW, Cascorbi I, Kunert-Keil C, Schroeder E, Kroemer HK, Siegmund W, Walker LC, Pahnke J. The Role of P-Glycoprotein in Cerebral Amyloid Angiopathy; Implications for the Early Pathogenesis of Alzheimer’s Disease. Curr. Alzheimer Res. 2004;1:121–125. doi: 10.2174/1567205043332225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vogelgesang S, Cascorbi I, Schroeder E, Pahnke J, Kroemer HK, Siegmund W, Kunert-Keil C, Walker LC, Warzok RW. Deposition of Alzheimer’s Beta-Amyloid Is Inversely Correlated with P-Glycoprotein Expression in the Brains of Elderly Non-Demented Humans. Pharmacogenetics. 2002;12:535–541. doi: 10.1097/00008571-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Barnes CA, Meltzer J, Houston F, Orr G, McGann K, Wenk GL. Chronic Treatment of Old Rats with Donepezil or Galantamine: Effects on Memory, Hippocampal Plasticity and Nicotinic Receptors. NeuroScience. 2000;99:17–23. doi: 10.1016/s0306-4522(00)00180-9. [DOI] [PubMed] [Google Scholar]
- 41.Ginestet L, Ferrario JE, Raisman-Vozari R, Hirsch EC, Debeir T. Donepezil Induces a Cholinergic Sprouting in Basocortical Degeneration. J. Neurochem. 2007;102:434–440. doi: 10.1111/j.1471-4159.2007.04497.x. [DOI] [PubMed] [Google Scholar]
- 42.Carageorgiou H, Sideris AC, Messari I, Liakou CI, Tsakiris S. The Effects of Rivastigmine Plus Selegiline on Brain Acetylcholinesterase, (Na, K)-, Mg-ATPase Activities, Antioxidant Status, and Learning Performance of Aged Rats. Neuropsychiatr. Dis. Treat. 2008;4:687–699. doi: 10.2147/ndt.s3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sobow T, Flirski M, Liberski P, Kloszewska I. Plasma Abeta Levels as Predictors of Response to Rivastigmine Treatment in Alzheimer’s Disease. Acta Neurobiol. Exp. 2007;67:131–139. doi: 10.55782/ane-2007-1640. [DOI] [PubMed] [Google Scholar]
- 44.Sobow T, Kloszewska I, Flirski M, Liberski P. Predictors of Long-Term Treatment Effect of Rivastigmine in Alzheimer’s Disease: A Role for Beta-Amyloid Plasma Levels? Neurol. Neurochir. Pol. 2009;43:507–516. [PubMed] [Google Scholar]
- 45.Sagare AP, Deane R, Zetterberg H, Wallin A, Blennow K, Zlokovic BV. Impaired Lipoprotein Receptor-Mediated Peripheral Binding of Plasma Amyloid-Beta Is an Early Biomarker for Mild Cognitive Impairment Preceding Alzheimer’s Disease. J. Alzheimer’s Dis. 2011;24:25–34. doi: 10.3233/JAD-2010-101248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sehgal N, Gupta A, Valli RK, Joshi SD, Mills JT, Hamel E, Khanna P, Jain SC, Thakur SS, Ravindranath V. Withania Somnifera Reverses Alzheimer’s Disease Pathology by Enhancing Low-Density Lipoprotein Receptor-Related Protein in Liver. Proc. Natl. Acad. Sci. U. S. A. 2012;109:3510–3515. doi: 10.1073/pnas.1112209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohamed LA, Kaddoumi A. In Vitro Investigation of Amyloid-beta Hepatobiliary Disposition in Sandwich-Cultured Primary Rat Hepatocytes. Drug Metab. Dispos. 2013;41:1787–1796. doi: 10.1124/dmd.113.052514. [DOI] [PubMed] [Google Scholar]
- 48.Goh CW, Aw CC, Lee JH, Chen CP, Browne ER. Pharmacokinetic and Pharmacodynamic Properties of Cholinesterase Inhibitors Donepezil, Tacrine, and Galantamine in Aged and Young Lister Hooded Rats. Drug Metab. Dispos. 2011;39:402–411. doi: 10.1124/dmd.110.035964. [DOI] [PubMed] [Google Scholar]
- 49.Mucke L, Selkoe DJ. Neurotoxicity of Amyloid beta-Protein: Synaptic and Network Dysfunction. Cold Spring Harb Perspect. Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, Double-Blind, Placebo-Controlled Trial of Donepezil in Patients with Alzheimer’s Disease. Donepezil Study Group. Neurology. 1998;50:136–145. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 51.Qosa H, Abuasal BS, Romero IA, Weksler B, Couraud PO, Keller JN, Kaddoumi A. Differences in Amyloid-Beta Clearance Across Mouse and Human Blood-Brain Barrier Models: Kinetic Analysis and Mechanistic Modeling. Neuropharmacology. 2014;79:668–678. doi: 10.1016/j.neuropharm.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seglen PO. Preparation of Isolated Rat Liver Cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 53.Liu X, LeCluyse EL, Brouwer KR, Gan LS, Lemasters JJ, Stieger B, Meier PJ, Brouwer KL. Biliary Excretion in Primary Rat Hepatocytes Cultured in a Collagen-Sandwich Configuration. Am. J. Physiol. 1999;277:G12–G21. doi: 10.1152/ajpgi.1999.277.1.G12. [DOI] [PubMed] [Google Scholar]
- 54.Kakee A, Terasaki T, Sugiyama Y. Brain Efflux Index As a Novel Method of Analyzing Efflux Transport at the Blood-Brain Barrier. J. Pharmacol. Exp. Ther. 1996;277:1550–1559. [PubMed] [Google Scholar]
- 55.Pardridge WM, Mietus LJ. Transport of Protein-Bound Steroid Hormones into Liver in Vivo. Am. J. Physiol. 1979;237:E367–E372. doi: 10.1152/ajpendo.1979.237.4.E367. [DOI] [PubMed] [Google Scholar]