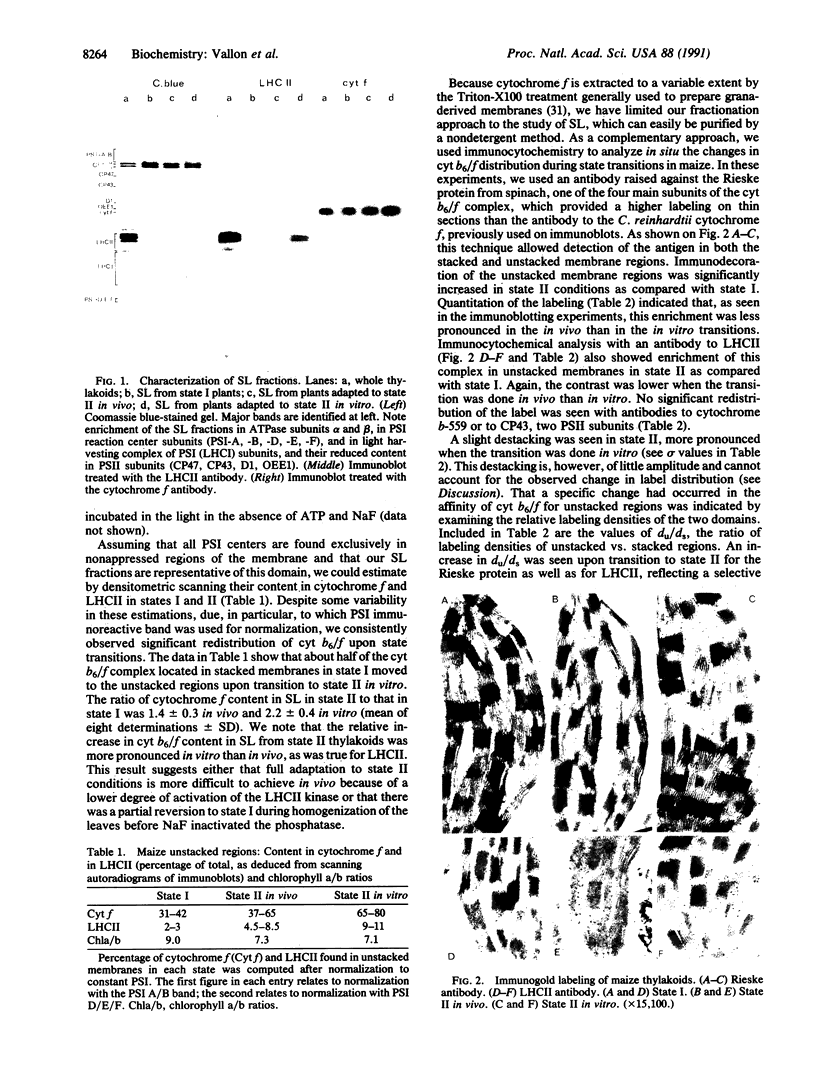
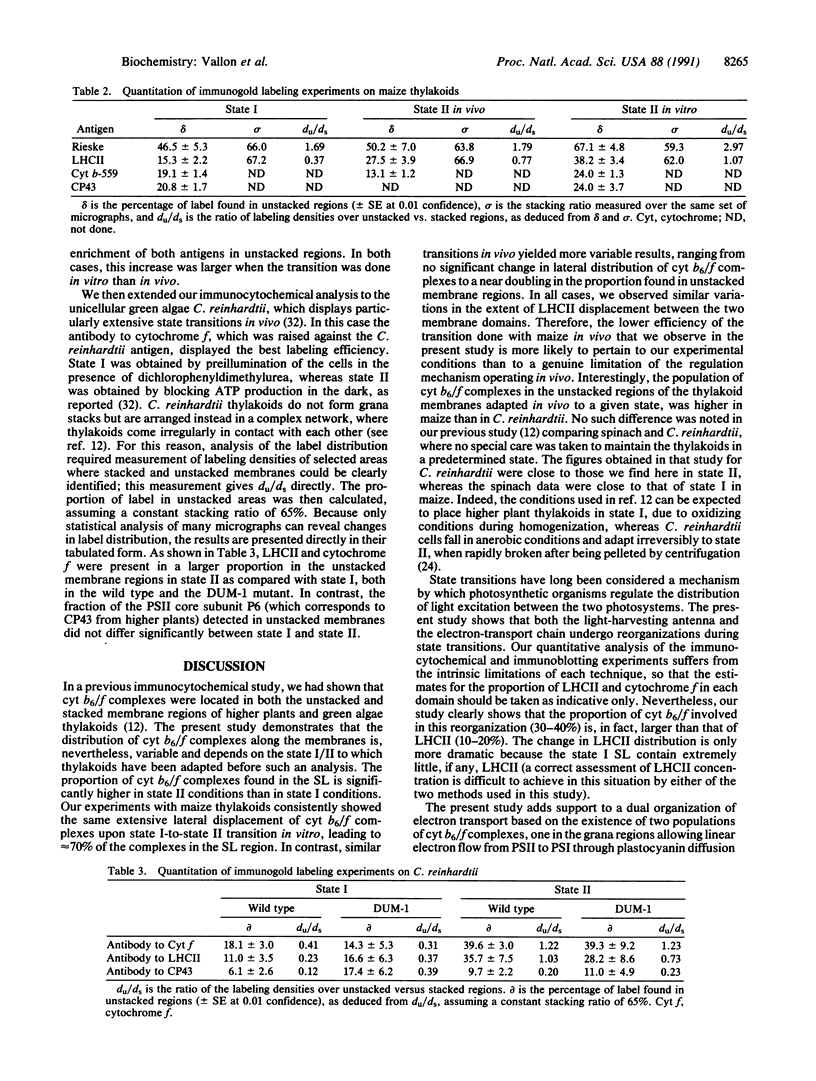
Abstract
The cytochrome b6/f complex operates in photosynthetic electron transfer either in linear electron flow from photosystem II to photosystem I or in cyclic flow around photosystem I. Using membrane fractionation and immunocytochemistry, we show a change in lateral distribution of cytochrome b6/f complexes along the thylakoid membranes during state transitions. This change is seen in maize as well as in the green algae Chlamydomonas reinhardtii. When either of the two organisms were adapted to state II in vivo, the proportion of cytochrome b6/f complexes found in the photosystem I-enriched stroma lamellae regions was significantly larger than after adaptation to state I. A similar observation was made upon state I to state II transitions done in vitro by illuminating, in the presence of ATP, broken maize chloroplasts prepared from dark-adapted leaves. This reorganization of the electron-transfer chain is concurrent with the change in light-energy distribution between the two photosystems, which requires lateral displacement of light-harvesting complex II. That the changes in lateral distribution of both cytochrome b6/f and light-harvesting II complexes seen upon state transition in vitro similarly required addition of exogenous ATP, suggests that the change in cytochrome b6/f organization also depends on kinase activity. The increased concentration of cytochrome b6/f complexes in the vicinity of photosystem I in state II is discussed in terms of an increase in cyclic electron flow, thus favoring ATP production. Because transition to state II can be triggered in vivo by ATP depletion, we conclude that state transitions should be regarded not only as a light-adaptation mechanism but also as a rerouting of photosynthetic electron flow, enabling photosynthetic organisms to adapt to changes in the cell demand for ATP.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allred D. R., Staehelin L. A. Lateral Distribution of the Cytochrome b(6)/f and Coupling Factor ATP Synthetase Complexes of Chloroplast Thylakoid Membranes. Plant Physiol. 1985 May;78(1):199–202. doi: 10.1104/pp.78.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi R., Høyer-Hansen G., Barbato R., Giacometti G. M., Simpson D. J. Chlorophyll-proteins of the photosystem II antenna system. J Biol Chem. 1987 Sep 25;262(27):13333–13341. [PubMed] [Google Scholar]
- Bennett J., Shaw E. K., Michel H. Cytochrome b6f complex is required for phosphorylation of light-harvesting chlorophyll a/b complex II in chloroplast photosynthetic membranes. Eur J Biochem. 1988 Jan 15;171(1-2):95–100. doi: 10.1111/j.1432-1033.1988.tb13763.x. [DOI] [PubMed] [Google Scholar]
- Berry E. A., Trumpower B. L. Isolation of ubiquinol oxidase from Paracoccus denitrificans and resolution into cytochrome bc1 and cytochrome c-aa3 complexes. J Biol Chem. 1985 Feb 25;260(4):2458–2467. [PubMed] [Google Scholar]
- Bonaventura C., Myers J. Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim Biophys Acta. 1969;189(3):366–383. doi: 10.1016/0005-2728(69)90168-6. [DOI] [PubMed] [Google Scholar]
- Cox R. P., Andersson B. Lateral and transverse organisation of cytochromes in the chloroplast thylakoid membrane. Biochem Biophys Res Commun. 1981 Dec 31;103(4):1336–1342. doi: 10.1016/0006-291x(81)90269-2. [DOI] [PubMed] [Google Scholar]
- Gal A., Hauska G., Herrmann R., Ohad I. Interaction between light harvesting chlorophyll-a/b protein (LHCII) kinase and cytochrome b6/f complex. In vitro control of kinase activity. J Biol Chem. 1990 Nov 15;265(32):19742–19749. [PubMed] [Google Scholar]
- Goodchild D. J., Anderson J. M., Andersson B. Immunocytochemical localization of the cytochrome b/f complex of chloroplast thylakoid membranes. Cell Biol Int Rep. 1985 Aug;9(8):715–721. doi: 10.1016/0309-1651(85)90079-7. [DOI] [PubMed] [Google Scholar]
- Lavergne J., Joliot P. Restricted diffusion in photosynthetic membranes. Trends Biochem Sci. 1991 Apr;16(4):129–134. doi: 10.1016/0968-0004(91)90054-y. [DOI] [PubMed] [Google Scholar]
- Matagne R. F., Michel-Wolwertz M. R., Munaut C., Duyckaerts C., Sluse F. Induction and characterization of mitochondrial DNA mutants in Chlamydomonas reinhardtii. J Cell Biol. 1989 Apr;108(4):1221–1226. doi: 10.1083/jcb.108.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N. Control of excitation transfer in photosynthesis. I. Light-induced change of chlorophyll a fluorescence in Porphyridium cruentum. Biochim Biophys Acta. 1969 Feb 25;172(2):242–251. doi: 10.1016/0005-2728(69)90067-x. [DOI] [PubMed] [Google Scholar]
- Rebeille F., Gans P. Interaction between Chloroplasts and Mitochondria in Microalgae: Role of Glycolysis. Plant Physiol. 1988 Dec;88(4):973–975. doi: 10.1104/pp.88.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone N., Sekimachi M., Kutoh E. Identification and properties of a quinol oxidase super-complex composed of a bc1 complex and cytochrome oxidase in the thermophilic bacterium PS3. J Biol Chem. 1987 Nov 15;262(32):15386–15391. [PubMed] [Google Scholar]
- Vallon O., Tae G. S., Cramer W. A., Simpson D., Hoyer-Hansen G., Bogorad L. Visualization of antibody binding to the photosynthetic membrane: the transmembrane orientation of cytochrome b-559. Biochim Biophys Acta. 1989 Jun 23;975(1):132–141. doi: 10.1016/s0005-2728(89)80211-7. [DOI] [PubMed] [Google Scholar]
- Wollman F. A., Delepelaire P. Correlation between changes in light energy distribution and changes in thylakoid membrane polypeptide phosphorylation in Chlamydomonas reinhardtii. J Cell Biol. 1984 Jan;98(1):1–7. doi: 10.1083/jcb.98.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]