Abstract
Recombination is a fundamental mechanism for the generation of genetic variation. Helicobacter pylori strains have different frequencies of intragenomic recombination, arising from deletions and duplications between DNA repeat sequences, as well as intergenomic recombination, facilitated by their natural competence. We identified a gene, hp1523, that influences recombination frequencies in this highly diverse bacterium and demonstrate its importance in maintaining genomic integrity by limiting recombination events. HP1523 shows homology to RecG, an ATP-dependent helicase that in Escherichia coli allows repair of damaged replication forks to proceed without recourse to potentially mutagenic recombination. Cross-species studies done show that hp1523 can complement E. coli recG mutants in trans to the same extent as E. coli recG can, indicating that hp1523 has recG function. The E. coli recG gene only partially complements the hp1523 mutation in H. pylori. Unlike other recG homologs, hp1523 is not involved in DNA repair in H. pylori, although it has the ability to repair DNA when expressed in E. coli. Therefore, host context appears critical in defining the function of recG. The fact that in E. coli recG phenotypes are not constant in other species indicates the diverse roles for conserved recombination genes in prokaryotic evolution.
Genetic recombination is an important driving force behind evolution of microbial pathogens, generating new genotypes more rapidly than is possible by mutation alone (11, 22). The acquisition of virulence factors, antigenic determinants, and antibiotic resistance is facilitated by recombination, which creates phenotypically diverse variants while alleviating the cost of high mutation rates by purging deleterious mutations (39, 47).
Helicobacter pylori is a gram-negative, microaerophilic gastric bacterium that persistently colonizes a large proportion of the world's human population (50). The panmictic population structure of H. pylori is believed to result from frequent recombination during mixed colonization by unrelated strains (2, 18, 59). Computational analysis of the fully sequenced H. pylori strains 26695 and J99 also has identified a large number of direct DNA repeats (1, 6, 54). Intragenomic recombination between such repeats allows deletion or duplication of intervening DNA segments, generating novel subtypes with changes in virulence effectors, such as CagA and CagY, and alterations in restriction modification systems (5, 7, 8).
Although the role of recombination in the generation of H. pylori variants with differential pathogenicity is well documented (6, 29, 51, 55, 58), knowledge of the molecular mechanisms that mediate genetic exchange remain rudimentary. Much research about bacterial recombination has focused on Escherichia coli; several enzymes that suppress recombination have been identified, including the RecG and RecQ helicases (23, 44), and the mismatch repair proteins MutS, MutH, and MutL (19, 53). Although H. pylori lacks homologs of MutS, MutH, and MutL, a putative RecG homolog, HP1523, has been identified by in silico analysis of sequenced H. pylori strain 26695 (JHP1412 in strain J99). Helicases, such as RecG, limit genome rearrangement by acting at damaged replication forks to repair lesions without recourse to recombination (44), a function possibly important in maintaining the genomic integrity of highly recombining species, such as H. pylori.
Therefore, we studied HP1523 to determine whether it has RecG functions and whether these functions influenced inter- and intragenomic recombination. Cross-species complementation studies performed between E. coli and H. pylori suggest that their RecG proteins are functionally interchangeable in E. coli but display phenotypic differences reflecting divergent intracellular environments. Unlike other RecG homologs (34, 41, 48), the H. pylori RecG (encoded by hp1523), is not required for recovery from DNA damage, although it plays a role in limiting recombination. These findings suggest that HP1523, influencing genomic plasticity in H. pylori without involvement in DNA repair pathways, functions in a new role for RecG helicase homologs.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The E. coli and H. pylori strains and plasmids used in this study are listed in Table 1. E. coli strains 1157 (9) and N4452, kindly provided by Robert Lloyd, were routinely grown in Luria-Bertani broth (LB) at 37°C. H. pylori strains were routinely grown on Trypticase soy agar (TSA) plates at 37°C in a 5% CO2 incubator.
TABLE 1.
Plasmids and bacterial strains used in this study
| Plasmid or strain | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Plasmids | ||
| p1523 | HP1523 fragment (bp 343-1520) in pGEMT-Easy | This work |
| pVacA | VacA fragment (bp 798-2003) in pGEMT-Easy | This work |
| pRecA | RecA fragment (bp 1-1040) in pGEMT-Easy | This work |
| pRecGKm | HP1523::aphA in pGEMT-Easy | This work |
| pVacAKm | vacA::aphA in pGEMT-Easy | This work |
| pRecAKm | recA::aphA in pGEMT-Easy | This work |
| pVacA-0 | vacA with 0-bp deletion cassette | 6 |
| pVacA-50 | vacA with 50-bp deletion cassette | 6 |
| pVacA-100 | vacA with 100-bp deletion cassette | 6 |
| pRecG-0 | HP1523 with 0-bp deletion cassette | This work |
| pRecG-50 | HP1523 with 50-bp deletion cassette | This work |
| pRecG-100 | HP1523 with 100-bp deletion cassette | This work |
| pAD1 | ureA promoter | 4 |
| pAD1-HPrG | pAD1 with HP1523 | This work |
| pAD1-ECrG | pAD1 with E. coli recG | This work |
| Strains | ||
| E. coli | ||
| AB1157 | recG+ (wild type) | 11 |
| N4452 | AB1157 recG::cat | 29 |
| N4452-AD1 | N4452 with pAD1 (control) | This work |
| N4452-HPrG | N4452 complemented with HP1523 | This work |
| N4452-ECrG | N4452 complemented with E. coli recG | This work |
| H. pylori | ||
| JP26 | Wild-type strain | |
| JP26/HP1523::aphA | ΔHP1523 | This work |
| JP26/vacA::aphA | ΔVacA | This work |
| JP26/recA::aphA | ΔRecA | This work |
| JP26 HPrG | HP1523::aphA complemented with HP1523 | This work |
| JP26 ECrG | HP1523::aphA complemented with E. coli recG | This work |
| JP26/HP1523:0 | HP1523 interrupted with 0-bp deletion cassette | This work |
| JP26/HP1523:50 | HP1523 interrupted with 50-bp deletion cassette | This work |
| JP26/HP1523:100 | HP1523 interrupted with 100-bp deletion cassette | This work |
| JP26/vacA::0 | vacA interrupted with 0-bp deletion cassette | 6 |
| JP26/vacA::50 | vacA interrupted with 50-bp deletion cassette | 6 |
| JP26/vacA::100 | vacA interrupted with 100-bp deletion cassette | 6 |
Construction of H. pylori mutants used to assess susceptibility to UV, intergenomic recombination frequencies, and spontaneous mutation frequencies.
Fragments of HP1523 (recG homolog), HP0887 (vacA), and HP0153 (recA) open reading frames (ORFs) were amplified by PCR using primers based on sequenced strain H. pylori 26695 or J99 (Table 2) and cloned into pGEMT-Easy (Promega, Madison, Wis.) to create p1523, pVacA, and pRecA, respectively. Next, p1523 was cut with EcoRI and ligated to an aphA cassette from pUC4K (GenBank accession no. X06404), which confers kanamycin resistance (Kmr), to create p1523Km. Inverse PCR was performed on pVacA and pRecA to introduce BamHI sites (see Table 2 for primers), and the PCR products were digested and subsequently ligated to aphA to create pVacAKm and pRecAKm, respectively. The vacA locus was chosen as a control for the presence of the aphA cassette, since it is not involved in recombination. The recA locus was interrupted as another control, since several phenotypes related to this study have been characterized (6, 62). By natural transformation as described previously (67), H. pylori strain JP26 was transformed to Kmr with p1523Km, pVacAKm, and pRecAKm to create JP26 1523::aphA, vacA::aphA, and recA::aphA. Chromosomal DNA was isolated from mutant strains, and the correct insertion of the aphA cassette into the expected ORF was confirmed by PCR in each case.
TABLE 2.
Primers used in this study
| Primer | Gene | Locationa | Sequence (5′ → 3′)b |
|---|---|---|---|
| F343 | HP1523 | 1553772-1553794 | ATCCTTACCGAGTTTGGCAAGA |
| R1520 | HP1523 | 1552617-1552637 | GAAATGCCCACCTCAATGAG |
| R483 | HP0153 (recA) | 163411-163430 | TTGGATCCGCATGTGGGCTTGCAAGCA |
| F477 | HP0153 (recA) | 163385-163405 | CTTGGATCCCCATCAATCTCCGCTTTAGG |
| F1 | HP0153 (recA) | 162928-163405 | ATGGCAATAGATGAAGACAAAC |
| R1040 | HP0153 (recA) | 163944-163968 | TCCATTTCTTCTAAAGGCTCATCC |
| R1060 | HP0887 (vacA) | 939456-939475 | TACGGGATCCTTTTCCTGCTTGAATGCGC |
| F1082 | HP0887 (vacA) | 939497-939517 | AAGAGGATCCAAGGAATACGACTTATACA |
| F798 | HP0887 (vacA) | 939213-939231 | CGTGAAAGCGAAAAACAAG |
| R2003 | HP0887 (vacA) | 940400-940418 | CATCGGCTTTAGTGTTGA |
HP1523 locations based on the sequence of strain J99. HP0153 (recA) and HP0887 (vacA) locations based on the sequence of strain 26695.
BamHI restriction sites underlined.
Construction of H. pylori mutants used to assess intragenomic (deletion) frequencies.
A unique BamHI site was created in p1523 and pVacA by inverse PCR using primers based on sequenced strain 26695 (Table 2), and each ORF was subsequently interrupted with a deletion cassette containing either 0-, 50-, or 100-bp repeats to create p1523-0, -50, and -100 and pVacA-0, -50, and -100. The construction and use of the deletion cassettes have been documented previously (6). H. pylori strain JP26 was subsequently transformed to chloramphenicol resistance (Catr) with these plasmids by natural transformation to create JP26 HP1523::0, -50, and -100 and JP26 vacA::0, -50, and -100. Chromosomal DNA was isolated from mutant strains, and the correct insertion of the deletion cassette into the expected ORF was confirmed by PCR in each case.
Deletion frequency assays in H. pylori to assess intragenomic recombination.
To assess recombination frequencies in the H. pylori strains containing the deletion cassette or control cassettes, the cells were grown on TSA plates for 48 h at 37°C (5% CO2), allowing deletions to occur. The cells were harvested and washed twice in phosphate-buffered saline (PBS), and 25-, 100-, and 200-μl aliquots were spread on brucella broth agar (BA) plates supplemented with newborn calf serum (NCS) and 25 μg of kanamycin per ml. For additional controls, 200 μl from each suspension was inoculated onto BA plates containing NCS, kanamycin (25 μg/ml), and chloramphenicol (20 μg/ml); as expected, in no experiments were strains with double resistance identified, confirming the specificity of the deletion process (6). Total CFU and numbers of Kmr deletion mutants were determined by plating serial dilutions onto TSA plates alone or TSA plates with kanamycin (25 μg/ml), respectively. Plates were incubated at 37°C in a 5% CO2 environment for 96 h, colonies were counted, and deletion frequencies were calculated.
Streptomycin resistance frequency assay to assess intergenomic recombination.
H. pylori strains were grown on TSA plates with 5% sheep blood agar. After 48 h, the recipient H. pylori cells were harvested and placed in 1 ml of PBS, and 25 μl of the resulting suspension was combined with 30 ng of donor DNA by spotting onto a TSA plate. The TSA plate was incubated for 18 h at 37°C in 5% CO2. Donor DNA was an 800-bp PCR product of H. pylori rpsL from streptomycin-resistant (Str) strain JP26 with A128G. The transformation mixture was then harvested and placed in 1 ml of PBS, and 100-μl portions of the appropriate serial dilutions were plated onto either TSA plates or BA plates containing 10% NCS and 25 μg of streptomycin per μl. The plates were incubated for 4 days at 37°C in 5% CO2, and the total recombination frequency was determined by the number of Str colonies divided by the total CFU. For a negative control, H. pylori strains with no DNA added were also tested in parallel with each experiment; no colonies were seen in any case.
Assays to examine recovery from DNA damage.
H. pylori cells to be tested were grown on TSA plates for 48 h and suspended in 1 ml of brucella broth. Equal amounts of suspension were inoculated on TSA plates to produce 100 to 500 CFU per plate. Cells were exposed to UV radiation at a wavelength of 312 nm (Stratagene Transilluminator) for 0 to 60 s and incubated at 37°C in 5% CO2 for 96 h. Colonies were counted, and the percentage of survival was calculated.
Ciprofloxacin E-test strips (AB Biodisk, Solna, Sweden) were used to determine the MICs for both wild-type and mutant strains of H. pylori and E. coli, according to the manufacturer's instructions. Plates were incubated for 48 and 24 h, respectively, and MIC determinations were repeated at least three times for each sample.
Morphology of H. pylori mutant cells.
Cells of wild-type and mutant H. pylori and E. coli strains, grown to stationary phase at 48 and 24 h, respectively, were spread onto glass slides and Gram stained. After examining the cells with a light microscope, the percentages of H. pylori and E. coli cells showing filamentation were determined.
Complementation of the H. pylori JP26 recG::aphA mutant.
Plasmid pAD1-HPrG, with ORF HP1523 placed downstream of the ureAB promoter, was constructed by using the same methods as those used to make pANDO2 (4), except HP1523 replaces HP0333. pAD1-HPrG was used to introduce HP1523 in trans into the genome of mutant JP26 recG::aphA via natural transformation as described previously (4, 67) to create JP26 HPrG comp. Transformants were selected on the basis of Catr, and the correct insertion of recG and flanking regions into ureA was confirmed by PCR of the chromosomal DNA. In parallel experiments, pAD1-ECrG (see below) was used to construct JP26 ECrG comp, exactly as described above.
Complementation of E. coli recG mutant.
pAD1-ECrG was constructed by inserting the E. coli recG ORF downstream of the ureA promoter, as described above, based on the construction of pANDO2 (4). E. coli strain N4452 (recG null mutant) was transformed with either pAD1-HPrG, pAD1-ECrG, or pAD1 (no insert) to create strains N4452-HPrG, N4452-ECrG, and N4452-AD1, respectively. For E. coli strains 1157 (wild type), N4452, N4452-HPrG, N4452-ECrG, and N4452-AD1, recovery after exposure to DNA damage was assessed using cultures that were grown overnight and serially diluted onto TSA plates and exposed to 312-nm-wavelength UV light (Stratagene Transilluminator) for 0 to 80 s. After incubation of plates overnight at 37°C, total numbers of colonies were counted, and survival was determined.
Statistical analyses.
Student's t test, unpaired with equal variance, was used to determine statistical significance in all cases. A P value of <0.05 was defined as statistically significant.
RESULTS
Comparison of HP1523 with RecG helicases.
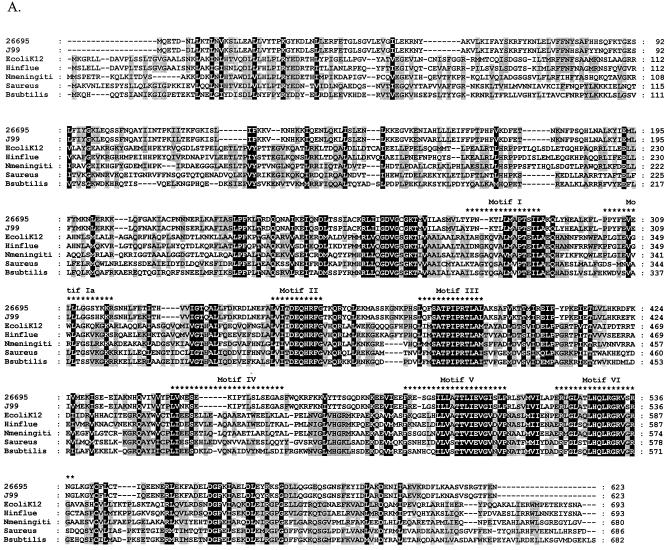
Previous analyses have shown that RecG is highly conserved in a wide range of bacteria and in plants (37, 56). A RecG homolog, HP1523, was identified in H. pylori sequenced strain 26695 using the clusters of orthologous groups of proteins (COG) database (http://www.ncbi.nlm.nih.gov/COG) (61), with 98.7% similarity to the product of JHP1412 in H. pylori strain J99 and 53.6% similarity to E. coli RecG. We first sought to determine the extent of similarity between HP1523 and members of the RecG family. The amino acid sequences of HP1523 and JHP1412 were aligned with RecG sequences from several representative bacterial species (Fig. 1A). The N terminus of RecG recognizes branched DNA structures and displays less similarity among the different bacterial species than do other parts of the protein (38, 56); the H. pylori products are no exception. The C-terminal region contains the helicase active site (38, 56), and the highly conserved RecG helicase motifs (35) are present in HP1523 and JHP1412. These studies support the annotation of HP1523 and JHP1412 as encoding RecG homologs (3, 64).
FIG. 1.
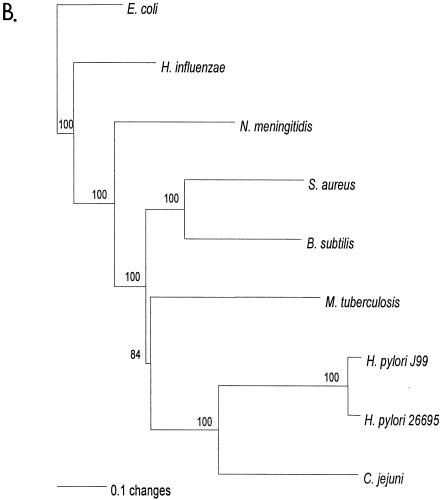
Relationship of deduced H. pylori products with RecG homologs. A. Alignment of amino acid sequences of H. pylori HP1523, JHP1412, and RecG homologs from five bacterial species (E. coli K-12, Haemophilus influenzae [Hinflue], Neisseria meningitidis [Nmeningiti], S. aureus, and Bacillus subtilis). Protein sequences were aligned with ClustalW, and the locations of conserved RecG helicase motifs (35) are indicated with asterisks. The program Genedoc (www.psc.edu/biomed/genedoc) was used to visualize the alignment in quantify mode, which highlights the one, two, or three most-frequent residues found in each column of the alignment. Conservative substitutions (e.g., I, L, V, M) were treated as if identical. Amino acids conserved in all species are indicated by white letters on black background. Gaps introduced to maximize alignment are indicated by dashes. Black letters on gray shading represent 60% or greater identity. White letters on black shading represent 100% identity. B. Phylogeny of RecG orthologs. Phylogenetic analysis of RecG orthologs was performed on 8 representative bacterial species, with bootstrap values listed next to the branches. M. tuberculosis, Mycobacterium tuberculosis.
Phylogenetic analysis of the deduced HP1523 and JHP1412 products with RecG from several representative bacterial species (Fig. 1B) shows that the closest relationship is with Campylobacter jejuni, as expected. However, unexpectedly, the H. pylori and C. jejuni orthologs are most closely related to those in gram-positive bacteria, rather than other gram-negative bacteria.
Effect of HP1523 on recombination frequency.
In E. coli, RecG influences recombination by converting halted replication forks into Holliday junctions and facilitating direct repair of such lesions (44). As a helicase that recognizes a variety of branched DNA structures, RecG also acts at D-loops, which are recombination intermediates formed during strand invasion of double-stranded DNA (43). By facilitating the direct repair of replication fork lesions without the need for recombination and through the dissolution of recombination intermediates, RecG helicase limits recombination (24, 43, 45).
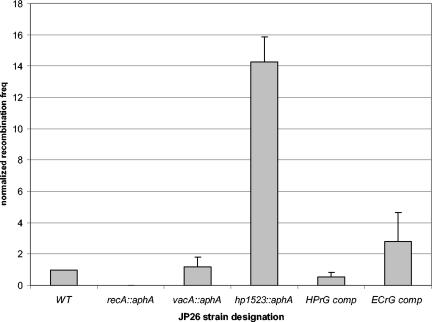
To determine whether HP1523 has a similar role in limiting recombination, we compared the frequency of natural transformation in wild-type and hp1523 mutant H. pylori strains. The hp1523 mutant displayed a >10-fold increase in intergenomic recombination frequencies over the wild type (Fig. 2) and a parallel aphA mutant of vacA, which has no known recombination activity. As expected, in a recA mutant, no transformants were detected. To confirm that the observed phenotype was not due to polar effects on genes downstream of HP1523, the hp1523 mutant was complemented in trans through integration of the hp1523 sequence downstream of a strong (ureA) promoter at a distant chromosomal locus. Complementation restored the wild-type phenotype (Fig. 2). In total, these results are consistent with a recG function for hp1523.
FIG. 2.
Intergenomic recombination frequencies (freq) of H. pylori wild-type and mutant strains. H. pylori strains were transformed to streptomycin resistance with a 800-bp rpsL A128G PCR product. The values are the means ± standard deviations (error bars) of transformation frequency normalized to that of the wild-type strain from four to eight replicate experiments. The H. pylori strains that were transformed were JP26 (wild type [WT]) and its recA, vacA (control), and hp1523 mutants, as well as the hp1523 mutant with a copy of either hp1523 or E. coli recG in trans downstream of the ureAB promoter to create JP26 HPrG comp and ECrG comp, respectively. As expected, there was no significant difference in transformation frequencies between the wild-type strain and the vacA (control) mutant, and in the recA mutant, no transformants were observed. The intergenomic recombination frequency in the recG mutant is consistently higher (14-fold) and significantly (P < 0.05) different from the transformation frequency of wild-type JP26. Complementation of the hp1523 mutant with hp1523 led to a recombination frequency that was not significantly different from that of the wild type. Complementation of the hp1523 mutant with E. coli recG led to a recombination frequency that was lower than the hp1523 mutant but still significantly (P value < 0.05) higher than that of the wild type.
Effect of HP1523 on intragenomic recombination between direct DNA repeats.
Several types of DNA damage can lead to DNA strand breakage (14, 32, 46, 65). Generation of loose DNA ends, which can participate in recombinogenic events, are hypothesized to promote deletion and duplication of intervening sequences between direct DNA repeats. Since the RecG helicase acts at damaged replication forks to repair DNA damage, it limits the generation of loose ends that can participate in both inter- and intragenomic recombination events. Analysis of the H. pylori genome reveals the existence of numerous direct DNA repeat sequences and the potential for genome rearrangements (1, 6, 54). Since the activity of helicases, such as RecG, that can limit genomic plasticity (44), may be especially important in organisms prone to genomic instability, such as H. pylori, we examined whether HP1523 could influence intragenomic recombination.
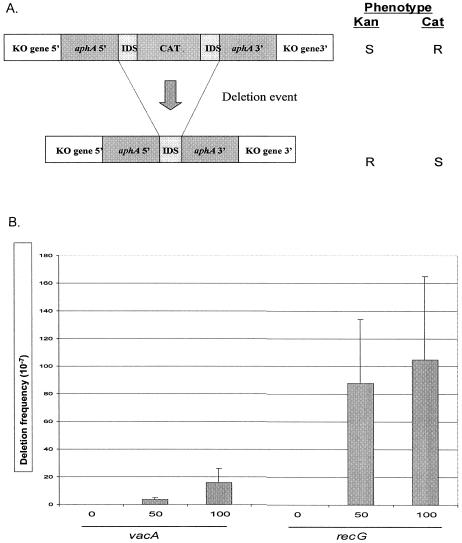
To determine whether HP1523 could influence intragenomic recombination, we used a previously validated deletion cassette (6), with identical DNA repeats (IDS) of 0, 50, or 100 bp (Fig. 3A). The deletion cassette was introduced into hp1523 or vacA (as a control) to assess the effect of the hp1523 product on intragenomic recombination between direct repeats. Consistent with the hypothesis that hp1523 is a recG homolog, the hp1523 mutant showed a significant (>10-fold) increase in recombination frequency compared to vacA mutant control strains for the IDS repeat sizes tested (Fig. 3B). These findings are consistent with hp1523 playing a role in limiting intragenomic recombination between direct DNA repeats.
FIG. 3.
Intragenomic recombination in H. pylori wild-type and mutant strains. A. Schematic of constructs used to assess deletion frequency in H. pylori. H. pylori mutants were created by inserting a deletion cassette construct with either 0-bp (control), 50-bp, or 100-bp flanking identical repeat segments (IDS) in recA, vacA, and recG (indicated as knockout [KO] gene 5′ and KO gene 3′). One copy of the IDS is part of the 5′ region of aphA, and the other has been ligated to the chloramphenicol (CAT) cassette. Insertion of the complete cassette into a host H. pylori strain confers resistance to chloramphenicol. The chloramphenicol cassette can subsequently be deleted by recombination between the two flanking IDS DNA repeats, resulting in resistance (R) to kanamycin and susceptibility (S) to chloramphenicol. B. Deletion frequencies. The values are the means ± standard deviations (error bars) from four to six replicate experiments. As expected, H. pylori strains with the cassette in vacA (control) showed progressively higher deletion frequencies with increasing size of the IDS. Strains with the deletion cassette in recG show significantly (25- and 7-fold) higher intragenomic recombination frequencies between flanking DNA repeats of 50 and 100 bp, respectively (P values of <0.05) compared to the control mutants with comparable cassettes in vacA.
Recovery from DNA damage.
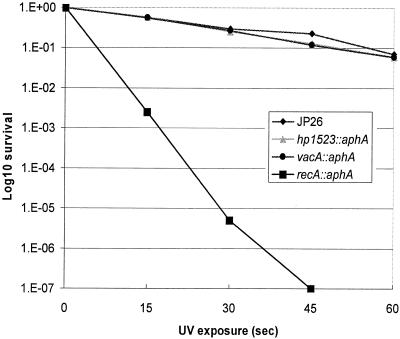
E. coli recG mutants are sensitive to DNA-damaging agents, such as UV radiation (34), due to their decreased ability to overcome replication fork-halting lesions. Hypothesizing that the H. pylori hp1523 mutant would show a similar survival defect, we exposed mutant and wild-type H. pylori strains to UV irradiation, which primarily damages the DNA template by creating pyrimidine dimers (57). However, in contrast to our expectations, the hp1523 mutant did not display a substantial increase in susceptibility to UV damage compared to the wild-type or control strain (Fig. 4).
FIG. 4.
Susceptibility of H. pylori wild-type and mutant strains to UV exposure. H. pylori wild-type strain JP26 and recA, recG, and vacA mutant strains were assayed for survival after exposure to UV (wavelength of 312 nm) for 0 to 60 s. As expected, the recA mutant showed significantly greater susceptibility to UV compared to that of wild-type JP26. The susceptibilities of recG and vacA (control) mutants were not significantly different from that of the wild-type strain. The results from one representative experiment are shown (four trials were performed). 1.E-07, 10−7.
To examine the role of hp1523 in recovery from a different type of DNA damage, we measured the susceptibility of the hp1523 mutant and wild-type H. pylori strains to the fluoroquinolone ciprofloxacin. Fluoroquinolones are bactericidal agents that primarily target DNA gyrase or topoisomerase IV (16, 30), creating a complex composed of protein, fluoroquinolone, and DNA that both halts replication forks and generates loose DNA ends (13, 40). Since Staphylococcus aureus recG mutants have increased susceptibility to quinolones, it is believed that RecG helps overcome the damage induced by these ternary complexes by acting at damaged replication forks (48).
To determine whether E. coli RecG and HP1523 play similar roles in recovery from quinolone-induced damage, we examined the susceptibilities of E. coli recG mutant and wild-type strains and H. pylori hp1523 mutant and wild-type strains to ciprofloxacin. Our results indicate that the E. coli recG mutant is more susceptible than the wild-type strain to ciprofloxacin, similar to the findings for S. aureus (48). In contrast, the hp1523 mutant was slightly less susceptible to ciprofloxacin than wild-type H. pylori cells, and complementation of HP1523 in trans restores ciprofloxacin resistance to wild-type levels, confirming that the effect is specific to HP1523 (Table 3).
TABLE 3.
Susceptibilities of wild-type, recG mutant, and complemented E. coli and H. pylori strains to ciprofloxacina
| Strain | Ciprofloxacin MIC (μg/ml) |
|---|---|
| E. coli | |
| AB1157 | 0.018 ± 0.003 |
| N4452 | 0.008 ± 0.001b |
| N4452-HPrG | 0.017 ± 0.002 |
| N4452-ECrG | 0.017 ± 0.002 |
| N4452-AD1 | 0.009 ± 0.001b |
| H. pylori | |
| JP26 | 0.103 ± 0.013 |
| JP26/HP1523::aphA | 0.272 ± 0.040c |
| JP26 HPrG comp | 0.106 ± 0.013 |
| JP26 ECrG comp | 0.176 ± 0.052c |
The E. coli and H. pylori strains were assayed by inhibition within lawns on plates containing a ciprofloxacin E-test strip (AB Biodisk) in concentrations from 0.002 to 32 μg/ml. Results shown are the means (± standard deviations) of at least four replicate determinations.
These values are significantly different (P < 0.05) from the value for AB1157 (wild type).
These values are significantly different (P < 0.05) from the value for JP26 (wild type).
Influence of HP1523 on cell morphology.
We then explored bacterial filamentation, another manifestation of DNA damage (25, 31, 42), that has been documented in most bacterial species, including H. pylori (15, 28, 36, 60). In E. coli, defects in recombination proteins, such as PriA, RecA, and RecG, lead to filamented cells (25, 42, 49). Therefore, using light microscopy, we examined the HP1523 mutant and wild-type H. pylori strains and control E. coli strains to determine whether filamentation could be observed. As expected, filamentation was apparent (5%) for the E. coli recG mutant after 24 h of growth to stationary phase (25), but not in the wild-type strain (<0.1%). For the wild-type H. pylori strain, JP26, no (<0.1%) filamentation was observed after 48 h of growth (reflecting stationary phase); no filamentation was observed in the HP1523 mutant, in contrast to the E. coli findings. Thus, in a second phenotype related to DNA damage, hp1523 appears to differ in function from its E. coli homolog.
Cross-species complementation of RecG.
Despite the strong similarity of HP1523 to other RecG homologs (Fig. 1), HP1523 does not play a role in survival after DNA damage or filamentation, as is expected for a RecG helicase. These findings suggest either that HP1523 may not be a true member of the RecG family or that the milieu in which recombination and DNA repair proteins operate differs in E. coli and H. pylori, resulting in different RecG phenotypes. To distinguish between these possibilities, as well as to determine the extent of functional similarity between HP1523 and E. coli RecG, cross-species complementation studies were performed between H. pylori and E. coli.
To determine whether the E. coli RecG protein could complement H. pylori HP1523 mutants, pAD1-ECrG was used to transform HP1523::aphA, creating strain JP26 ECrG comp. Intergenomic recombination frequencies (Fig. 2) and susceptibilities to ciprofloxacin (Table 3) were used to assess phenotype. Our results show that E. coli RecG can partially complement H. pylori HP1523 mutants, but not to the same extent as native HP1523.
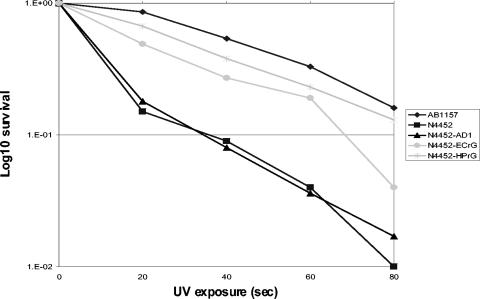
To determine whether the H. pylori HP1523 protein could complement in E. coli, the E. coli recG mutant strain, N4452 (27), was transformed with HP1523 in a shuttle plasmid to create N4452-HPrG, with E. coli RecG in the same vector to create N4452-ECrG (positive control), or with the vector alone to create N4452-AD1 (negative control). Susceptibilities to UV exposure and ciprofloxacin were used to assess phenotype. As expected, N4452-ECrG, but not N4452-AD1, displayed an increase in survival after exposure to either UV (Fig. 5) or ciprofloxacin (Table 3), demonstrating that complementation with the E. coli gene in trans was achieved. After exposure to UV or ciprofloxacin, strain N4452-HPrG also showed survival to nearly wild-type levels (Fig. 5 and Table 3); the findings were nearly identical in magnitude to those achieved in trans with E. coli RecG. These observations indicate that HP1523 can perform known RecG functions involved in DNA repair when expressed in E. coli, but not in H. pylori.
FIG. 5.
Susceptibility of cross-complemented E. coli strains to UV exposure. E. coli strains transformed with a plasmid containing E. coli RecG (pAD1-ECrG), H. pylori HP1523(pAD1-HPrG), or no insert (pAD1) were examined for susceptibility to UV for 0 to 80 s. The results from one representative experiment are shown (three trials were performed). 1.E-02, 10−2.
DISCUSSION
Recombination is essential for chromosomal repair after DNA damage and also permits generation of variation through intrachromosomal rearrangements and assimilation of foreign DNA (11, 52). Such generation of diversity is important in prokaryotes, facilitating adaptation in response to environmental stresses (1, 54). E. coli is widely accepted as a model organism for understanding enzymes involved in recombination, leading to paradigms that have been generalized to other prokaryotes. However, we now show that even among related gram-negative organisms, such as E. coli and H. pylori, intracellular host milieu is sufficiently different to affect RecG phenotypes. HP1523 (and JHP1412) have been annotated as being RecG homologs (3, 64); our alignment and phylogenetic analyses are consistent with this, and the presence of conserved RecG motifs (35) provides further strong support. Although we conclude that HP1523 is indeed a member of the RecG family, our experimental studies indicate several phenotypic differences.
In previous studies of S. aureus, Streptococcus pneumoniae, and E. coli, RecG has been important in recovery after exposure to DNA-damaging agents (34, 41, 48), whereas our studies indicate that RecG is not involved in DNA repair in H. pylori after damage due to exposure to UV (Fig. 4) or methyl methanesulfonate (data not shown). However, the cross-species complementation studies indicate that H. pylori RecG has the ability to repair DNA lesions in E. coli and complements E. coli recG mutants to the same extent as the native E. coli RecG. Why, then, is it not involved in H. pylori DNA repair?
One possibility is that H. pylori has evolved alternative, possibly more efficient mechanisms to repair DNA, obviating the need for RecG in recovery from DNA damage. Genome analysis and prior studies indicate that H. pylori has the capacity to repair base and nucleotide excision (63). Having highly competent DNA repair may be especially important for H. pylori, which induces host inflammatory responses involving neutrophils, lymphocytes, and macrophages, releasing DNA-damaging free radicals (26).
The repertoire of putative recombination proteins in H. pylori differs from that in E. coli. RecFO, RecCD, DnaC, and other important recombination pathway components in E. coli are not present in H. pylori on the basis of in silico analyses in sequenced strains (3, 64), as well as an ortholog search using the COG database (data not shown). Although it is possible that H. pylori has alternative recombination pathways that have not been identified yet, the absence of such regulatory enzymes may contribute to its extraordinarily high rate of recombination (59). The only partial complementation by E. coli RecG suggests that H. pylori RecG has additional domains required for full function in H. pylori.
E. coli recG mutants display increased susceptibility to quinolones (Table 3), clearly showing a role for recG in repair. The unexpected finding that the H. pylori recG mutants are less susceptible to ciprofloxacin than the wild type may result from enhanced recombination, which aids recovery from quinolone-induced DNA damage (16). Thus, the extent of the recG mutation on recombination may supersede a more limited effect on repair. The fact that H. pylori recG apparently is not involved in repair of UV-induced damage is consistent with this hypothesis, since both UV and quinolones induce replication fork blockage.
Another possibility is that although RecG is not essential for recombinational repair of DNA lesions, it is involved in a redundant repair pathway. The ternary complexes formed by quinolones are not the cause of cell death per se (12, 20, 30, 33, 61), but an additional step, most likely an aborted repair attempt, is required for the generation of a lethal DNA double-strand break (12, 33). Therefore, the repair pathway through which a ternary complex is processed ultimately determines the organism's level of susceptibility. If repair of ternary complexes represents a balance between repair and recombination phenomena, the differing recG phenotypes in these respects may explain the observed results. Since E. coli and H. pylori possess different repair pathway proteins, the presence or absence of RecG may alter quinolone susceptibility in different ways. The cross-species complementation results (Table 3 and Fig. 5) support this hypothesis.
H. pylori colonizes an environmental niche that is essentially isolated from other organisms, unlike E. coli, which must compete in its niche with other prokaryotes and is exposed to diverse genetic material. High rates of intragenomic recombination may be a way H. pylori can maximize the probability of survival in dynamic host environments (10), allowing self-generation of a genetically diverse population from which the variants that are most fit can be selected (13). In every species, there is an intrinsic tension between fidelity, implied by DNA repair mechanisms, and diversification at particular loci, represented by recombination. In H. pylori, as in RNA viruses, the requirement for diversification may be so great that fidelity is a lower priority.
Therefore, the existence of a DNA helicase, RecG, that preserves genomic integrity in this highly diverse organism by limiting both intra- and intergenomic recombination is of interest. Helicases that maintain genome stability by limiting recombination have been found in other bacteria and eukaryotes, including humans (17, 21). The conservation of RecG in H. pylori, despite the absence of involvement in DNA repair, suggests that its major role could involve maximizing genomic integrity, especially in H. pylori, whose genome contains substantial repetitive DNA that can foster illegitimate exchanges (6).
The increased intergenomic recombination displayed by the H. pylori recG mutant is consistent with the prior understanding of RecG function, involving recognition and unwinding of branched DNA structures (43, 66). The fact that the H. pylori recG mutants also display increased intragenomic recombination frequencies between direct DNA repeats suggests that the mechanisms involved in deletion include the formation of branched DNA structures. On the basis of studies in E. coli (45, 66), we hypothesize that in wild-type H. pylori cells, RecG recognizes and unwinds such intermediate structures, preventing many deletion events from reaching completion. Since these studies are the first, to our knowledge, to examine recombination between chromosomal DNA repeats, such a RecG phenotype may be present in other species.
Acknowledgments
This work was supported in part by grants RO1GM62370 and 5T32 GM07308 from the National Institutes of Health and the Medical Research Service of the Department of Veterans Affairs.
REFERENCES
- 1.Achaz, G., E. P. Rocha, P. Netter, and E. Coissac. 2002. Origin and fate of repeats in bacteria. Nucleic Acids Res. 30:2987-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Achtman, M., T. Azuma, D. E. Berg, Y. Ito, G. Morelli, Z. J. Pan, S. Suerbaum, S. A. Thompson, A. van der Ende, and L. J. van Doorn. 1999. Recombination and clonal groupings within Helicobacter pylori from different geographical regions. Mol. Microbiol. 32:459-470. [DOI] [PubMed] [Google Scholar]
- 3.Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 4.Ando, T., D. A. Israel, K. Kusugami, and M. J. Blaser. 1999. HP0333, a member of the dprA family, is involved in natural transformation in Helicobacter pylori. J. Bacteriol. 181:5572-5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aras, R. A., W. Fischer, G. I. Perez-Perez, M. Crosatti, T. Ando, R. Haas, and M. J. Blaser. 2003. Plasticity of repetitive DNA sequences within a bacterial (type IV) secretion system component. J. Exp. Med. 198:1349-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aras, R. A., J. Kang, A. I. Tschumi, Y. Harasaki, and M. J. Blaser. 2003. Extensive repetitive DNA facilitates prokaryotic genome plasticity. Proc. Natl. Acad. Sci. USA 100:13579-13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aras, R. A., Y. Lee, S. K. Kim, D. Israel, R. M. Peek, Jr., and M. J. Blaser. 2003. Natural variation in populations of persistently colonizing bacteria affect human host cell phenotype. J. Infect. Dis. 188:486-496. [DOI] [PubMed] [Google Scholar]
- 8.Aras, R. A., T. Takata, T. Ando, A. van der Ende, and M. J. Blaser. 2001. Regulation of the HpyII restriction-modification system of Helicobacter pylori by gene deletion and horizontal reconstitution. Mol. Microbiol. 42:369-382. [DOI] [PubMed] [Google Scholar]
- 9.Bachmann, B. J. 1996. Derivations and genotypes of some mutant derivatives of Escherichia coli K-12, p. 2460-2488. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 2. ASM Press, Washington, D.C. [Google Scholar]
- 10.Blaser, M. J., and J. C. Atherton. 2004. Helicobacter pylori persistence: biology and disease. J. Clin. Investig. 113:321-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown, E. W., J. E. LeClerc, M. L. Kotewicz, and T. A. Cebula. 2001. Three R's of bacterial evolution: how replication, repair, and recombination frame the origin of species. Environ. Mol. Mutagen. 38:248-260. [DOI] [PubMed] [Google Scholar]
- 12.Chen, A. Y., and L. F. Liu. 1994. DNA topoisomerases: essential enzymes and lethal targets. Annu. Rev. Pharmacol. Toxicol. 34:191-218. [DOI] [PubMed] [Google Scholar]
- 13.Chen, C. R., M. Malik, M. Snyder, and K. Drlica. 1996. DNA gyrase and topoisomerase IV on the bacterial chromosome: quinolone-induced DNA cleavage. J. Mol. Biol. 258:627-637. [DOI] [PubMed] [Google Scholar]
- 14.Cromie, G. A., J. C. Connelly, and D. R. Leach. 2001. Recombination at double-strand breaks and DNA ends: conserved mechanisms from phage to humans. Mol. Cell 8:1163-1174. [DOI] [PubMed] [Google Scholar]
- 15.DeLoney, C. R., and N. L. Schiller. 1999. Competition of various beta-lactam antibiotics for the major penicillin-binding proteins of Helicobacter pylori: antibacterial activity and effects on bacterial morphology. Antimicrob. Agents Chemother. 43:2702-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drlica, K., and X. Zhao. 1997. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol. Mol. Biol. Rev. 61:377-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellis, N. A., J. Groden, T. Z. Ye, J. Straughen, D. J. Lennon, S. Ciocci, M. Proytcheva, and J. German. 1995. The Bloom's syndrome gene product is homologous to RecQ helicases. Cell 83:655-666. [DOI] [PubMed] [Google Scholar]
- 18.Falush, D., C. Kraft, N. S. Taylor, P. Correa, J. G. Fox, M. Achtman, and S. Suerbaum. 2001. Recombination and mutation during long-term gastric colonization by Helicobacter pylori: estimates of clock rates, recombination size, and minimal age. Proc. Natl. Acad. Sci. USA 98:15056-15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feinstein, S. I., and K. B. Low. 1986. Hyper-recombining recipient strains in bacterial conjugation. Genetics 113:13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galperin, M. Y., and E. V. Koonin. 1999. Functional genomics and enzyme evolution. Homologous and analogous enzymes encoded in microbial genomes. Genetica 106:159-170. [DOI] [PubMed] [Google Scholar]
- 21.Gray, M. D., J. C. Shen, A. S. Kamath-Loeb, A. Blank, B. L. Sopher, G. M. Martin, J. Oshima, and L. A. Loeb. 1997. The Werner syndrome protein is a DNA helicase. Nat. Genet. 17:100-103. [DOI] [PubMed] [Google Scholar]
- 22.Hacker, J., U. Hentschel, and U. Dobrindt. 2003. Prokaryotic chromosomes and disease. Science 301:790-793. [DOI] [PubMed] [Google Scholar]
- 23.Hanada, K., T. Ukita, Y. Kohno, K. Saito, J. Kato, and H. Ikeda. 1997. RecQ DNA helicase is a suppressor of illegitimate recombination in Escherichia coli. Proc. Natl. Acad. Sci. USA 94:3860-3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris, R. S., K. J. Ross, and S. M. Rosenberg. 1996. Opposing roles of the Holliday junction processing systems of Escherichia coli in recombination-dependent adaptive mutation. Genetics 142:681-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishioka, K., H. Iwasaki, and H. Shinagawa. 1997. Roles of the recG gene product of Escherichia coli in recombination repair: effects of the ΔrecG mutation on cell division and chromosome partition. Genes Genet. Syst. 72:91-99. [DOI] [PubMed] [Google Scholar]
- 26.Israel, D. A., and R. M. Peek. 2001. Pathogenesis of Helicobacter pylori-induced gastric inflammation. Aliment. Pharmacol. Ther. 15:1271-1290. [DOI] [PubMed] [Google Scholar]
- 27.Jaktaji, R. P., and R. G. Lloyd. 2003. PriA supports two distinct pathways for replication restart in UV-irradiated Escherichia coli cells. Mol. Microbiol. 47:1091-1100. [DOI] [PubMed] [Google Scholar]
- 28.Karoui, M. H. 1988. DNA repair and its relation to cell division in E. coli. Arch. Inst. Pasteur Tunis 65:59-68. (In French.) [PubMed] [Google Scholar]
- 29.Kersulyte, D., H. Chalkauskas, and D. E. Berg. 1999. Emergence of recombinant strains of Helicobacter pylori during human infection. Mol. Microbiol. 31:31-43. [DOI] [PubMed] [Google Scholar]
- 30.Khodursky, A. B., and N. R. Cozzarelli. 1998. The mechanism of inhibition of topoisomerase IV by quinolone antibacterials. J. Biol. Chem. 273:27668-27677. [DOI] [PubMed] [Google Scholar]
- 31.Knezevic-Vukcevic, J., B. Vukovic, and D. Simic. 1987. Role of rec genes in SOS-induced inhibition of cell division in Escherichia coli. Mutat. Res. 192:247-252. [DOI] [PubMed] [Google Scholar]
- 32.Krasin, F., and F. Hutchinson. 1977. Repair of DNA double-strand breaks in Escherichia coli, which requires recA function and the presence of a duplicate genome. J. Mol. Biol. 116:81-98. [DOI] [PubMed] [Google Scholar]
- 33.Kreuzer, K. N., and N. R. Cozzarelli. 1979. Escherichia coli mutants thermosensitive for deoxyribonucleic acid gyrase subunit A: effects on deoxyribonucleic acid replication, transcription, and bacteriophage growth. J. Bacteriol. 140:424-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lloyd, R. G., and C. Buckman. 1991. Genetic analysis of the recG locus of Escherichia coli K-12 and of its role in recombination and DNA repair. J. Bacteriol. 173:1004-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lloyd, R. G., and G. J. Sharples. 1991. Molecular organization and nucleotide sequence of the recG locus of Escherichia coli K-12. J. Bacteriol. 173:6837-6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Love, P. E., and R. E. Yasbin. 1984. Genetic characterization of the inducible SOS-like system of Bacillus subtilis. J. Bacteriol. 160:910-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahdi, A. A., G. S. Briggs, G. J. Sharples, Q. Wen, and R. G. Lloyd. 2003. A model for dsDNA translocation revealed by a structural motif common to RecG and Mfd proteins. EMBO J. 22:724-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahdi, A. A., P. McGlynn, S. D. Levett, and R. G. Lloyd. 1997. DNA binding and helicase domains of the Escherichia coli recombination protein RecG. Nucleic Acids Res. 25:3875-3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maiden, M. C. 1998. Horizontal genetic exchange, evolution, and spread of antibiotic resistance in bacteria. Clin. Infect. Dis. 27(Suppl. 1):S12-S20. [DOI] [PubMed] [Google Scholar]
- 40.Mamber, S. W., B. Kolek, K. W. Brookshire, D. P. Bonner, and J. Fung-Tomc. 1993. Activity of quinolones in the Ames Salmonella TA102 mutagenicity test and other bacterial genotoxicity assays. Antimicrob. Agents Chemother. 37:213-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin, B., G. J. Sharples, O. Humbert, R. G. Lloyd, and J. P. Claverys. 1996. The mmsA locus of Streptococcus pneumoniae encodes a RecG-like protein involved in DNA repair and in three-strand recombination. Mol. Microbiol. 19:1035-1045. [DOI] [PubMed] [Google Scholar]
- 42.McCool, J. D., and S. J. Sandler. 2001. Effects of mutations involving cell division, recombination, and chromosome dimer resolution on a priA2::kan mutant. Proc. Natl. Acad. Sci. USA 98:8203-8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGlynn, P., A. A. Al-Deib, J. Liu, K. J. Marians, and R. G. Lloyd. 1997. The DNA replication protein PriA and the recombination protein RecG bind D-loops. J. Mol. Biol. 270:212-221. [DOI] [PubMed] [Google Scholar]
- 44.McGlynn, P., and R. G. Lloyd. 2002. Genome stability and the processing of damaged replication forks by RecG. Trends Genet. 18:413-419. [DOI] [PubMed] [Google Scholar]
- 45.McGlynn, P., and R. G. Lloyd. 1999. RecG helicase activity at three- and four-strand DNA structures. Nucleic Acids Res. 27:3049-3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Michel, B., S. D. Ehrlich, and M. Uzest. 1997. DNA double-strand breaks caused by replication arrest. EMBO J. 16:430-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muller, H. J. 1964. The relation of recombination to mutational advance. Mutat. Res. 106:2-9. [DOI] [PubMed] [Google Scholar]
- 48.Niga, T., H. Yoshida, H. Hattori, S. Nakamura, and H. Ito. 1997. Cloning and sequencing of a novel gene (recG) that affects the quinolone susceptibility of Staphylococcus aureus. Antimicrob. Agents Chemother. 41:1770-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nurse, P., K. H. Zavitz, and K. J. Marians. 1991. Inactivation of the Escherichia coli priA DNA replication protein induces the SOS response. J. Bacteriol. 173:6686-6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peek, R. M., Jr., and M. J. Blaser. 2002. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer 2:28-37. [DOI] [PubMed] [Google Scholar]
- 51.Pride, D. T., and M. J. Blaser. 2002. Concerted evolution between duplicated genetic elements in Helicobacter pylori. J. Mol. Biol. 316:629-642. [DOI] [PubMed] [Google Scholar]
- 52.Radman, M., F. Taddei, and I. Matic. 2000. DNA repair systems and bacterial evolution. Cold Spring Harbor Symp. Quant. Biol. 65:11-19. [DOI] [PubMed] [Google Scholar]
- 53.Rayssiguier, C., D. S. Thaler, and M. Radman. 1989. The barrier to recombination between Escherichia coli and Salmonella typhimurium is disrupted in mismatch-repair mutants. Nature 342:396-401. [DOI] [PubMed] [Google Scholar]
- 54.Rocha, E. P., A. Danchin, and A. Viari. 1999. Analysis of long repeats in bacterial genomes reveals alternative evolutionary mechanisms in Bacillus subtilis and other competent prokaryotes. Mol. Biol. Evol. 16:1219-1230. [DOI] [PubMed] [Google Scholar]
- 55.Salama, N., K. Guillemin, T. K. McDaniel, G. Sherlock, L. Tompkins, and S. Falkow. 2000. A whole-genome microarray reveals genetic diversity among Helicobacter pylori strains. Proc. Natl. Acad. Sci. USA 97:14668-14673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharples, G. J., S. M. Ingleston, and R. G. Lloyd. 1999. Holliday junction processing in bacteria: insights from the evolutionary conservation of RuvABC, RecG, and RusA. J. Bacteriol. 181:5543-5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sinha, R. P., and D. P. Hader. 2002. UV-induced DNA damage and repair: a review. Photochem. Photobiol. Sci. 1:225-236. [DOI] [PubMed] [Google Scholar]
- 58.Smeets, L. C., N. L. Arents, A. A. van Zwet, C. M. Vandenbroucke-Grauls, T. Verboom, W. Bitter, and J. G. Kusters. 2003. Molecular patchwork: chromosomal recombination between two Helicobacter pylori strains during natural colonization. Infect. Immun. 71:2907-2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suerbaum, S., J. M. Smith, K. Bapumia, G. Morelli, N. H. Smith, E. Kunstmann, I. Dyrek, and M. Achtman. 1998. Free recombination within Helicobacter pylori. Proc. Natl. Acad. Sci. USA 95:12619-12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takeuchi, H., M. Shirai, J. K. Akada, M. Tsuda, and T. Nakazawa. 1998. Nucleotide sequence and characterization of cdrA, a cell division-related gene of Helicobacter pylori. J. Bacteriol. 180:5263-5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tatusov, R. L., M. Y. Galperin, D. A. Natale, and E. V. Koonin. 2000. The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 28:33-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson, S. A., and M. J. Blaser. 1995. Isolation of the Helicobacter pylori recA gene and involvement of the recA region in resistance to low pH. Infect. Immun. 63:2185-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thompson, S. A., R. L. Latch, and J. M. Blaser. 1998. Molecular characterization of the Helicobacter pylori uvrB gene. Gene 209:113-122. [DOI] [PubMed] [Google Scholar]
- 64.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, J. C. Venter, et al. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 65.Vilenchik, M. M., and A. G. Knudson. 2003. Endogenous DNA double-strand breaks: production, fidelity of repair, and induction of cancer. Proc. Natl. Acad. Sci. USA 100:12871-12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vincent, S. D., A. A. Mahdi, and R. G. Lloyd. 1996. The RecG branch migration protein of Escherichia coli dissociates R-loops. J. Mol. Biol. 264:713-721. [DOI] [PubMed] [Google Scholar]
- 67.Wang, Y., K. P. Roos, and D. E. Taylor. 1993. Transformation of Helicobacter pylori by chromosomal metronidazole resistance and by a plasmid with a selectable chloramphenicol resistance marker. J. Gen. Microbiol. 139:2485-2493. [DOI] [PubMed] [Google Scholar]