Abstract
Prokaryotic insertion sequence (IS) elements behave like parasites in terms of their ability to invade and proliferate in microbial gene pools and like symbionts when they coevolve with their bacterial hosts. Here we investigated the evolutionary history of IS605 and IS607 of Helicobacter pylori, a genetically diverse gastric pathogen. These elements contain unrelated transposase genes (orfA) and also a homolog of the Salmonella virulence gene gipA (orfB). A total of 488 East Asian, Indian, Peruvian, and Spanish isolates were screened, and 18 and 14% of them harbored IS605 and IS607, respectively. IS605 nucleotide sequence analysis (n = 42) revealed geographic subdivisions similar to those of H. pylori; the geographic subdivision was blurred, however, due in part to homologous recombination, as indicated by split decomposition and homoplasy tests (homoplasy ratio, 0.56). In contrast, the IS607 populations (n = 44) showed strong geographic subdivisions with less homologous recombination (homoplasy ratio, 0.2). Diversifying selection (ratio of nonsynonymous change to synonymous change, ≫1) was evident in ∼15% of the IS605 orfA codons analyzed but not in the IS607 orfA codons. Diversifying selection was also evident in ∼2% of the IS605 orfB and ∼10% of the IS607 orfB codons analyzed. We suggest that the evolution of these elements reflects selection for optimal transposition activity in the case of IS605 orfA and for interactions between the OrfB proteins and other cellular constituents that potentially contribute to bacterial fitness. Taken together, similarities in IS elements and H. pylori population genetic structures and evidence of adaptive evolution in IS elements suggest that there is coevolution between these elements and their bacterial hosts.
The insertion sequences (IS) of bacteria are discrete DNA segments that are distinguished by their ability to move within genomes without a need for extensive DNA homology. These elements move within and between species by DNA transfer and transposition, and they proliferate within bacterial genomes by transposition per se (for general reviews see references 3, 15, and 16). Most elements seem to be innocuous or parasitic (19). However, coadaptation between elements and their hosts during long-term association may also make carriage beneficial in certain cases (38, 43).
Chance and natural selection may each contribute to the maintenance of IS elements in bacterial populations. Once a given element has entered a gene pool, its abundance should reflect its rates of interstrain transfer and transposition, coupled with any benefit or cost resulting from its carriage in terms of bacterial survival, growth, or adaptability in variable and often hostile environments. Elements that confer resistance to antibiotics or metals provide dramatic examples of contributions to host fitness. Certain mutations caused by IS element movement may also be adaptive (11, 53, 56), and the product of the transposase gene of at least one element (IS50) contributes to host fitness, independent of movement (25). The known deleterious effects of transposable elements are related to their movement, and they include inactivation of genes by insertion, adjacent deletion, and other chromosomal rearrangements. The position of a mobile element in the spectrum from parasitic to beneficial in its interactions with its host may depend, at least in part, on the selection imposed by the host's background genotype and environment.
Much of our understanding of mechanisms that regulate IS element maintenance and evolution in bacterial populations has come from studies of the elements that are common in natural isolates of Escherichia coli and Salmonella (5, 26, 41, 45, 48, 53). These enteric organisms have strongly clonal population structures and often carry multiple unrelated elements in their chromosomes and/or plasmids. Interstrain transfer is relatively frequent, and transfer between species has also been observed. Consequently, IS phylogenies are often incongruent with the phylogenies of the chromosomal genes of the bacterial hosts; nearly identical elements are often found in divergent bacterial lineages, and conversely, divergent elements are sometimes found in closely related lineages (5, 24, 41, 54). In E. coli nucleotide sequences within each class of IS elements are highly conserved (>99% similar to a consensus sequence), and comparisons with sequences from chromosomal genes suggest that these elements move often among host lineages (41). Nucleotide sequence analysis of IS1 and IS3 homologs from diverse enteric species also indicated that there is significant intragenic recombination, which might contribute to IS element evolution (41).
To gain insight into the role of natural selection in the maintenance and evolution of IS elements in bacterial populations, we investigated the evolutionary histories of IS605 and IS607 of Helicobacter pylori, a gram-negative bacterium that is implicated in peptic ulcer disease and gastric cancer (6, 14). H. pylori is of particular interest for such studies because it is extremely diverse genetically and has robust geographic subdivisions that are far more robust than those of any other bacterial pathogen (1, 22, 23, 36, 46, 52). Its great genetic diversity can be ascribed to multiple factors, including (i) mutation, (ii) recombination, (iii) highly localized (preferentially intrafamilial) transmission and the resulting lack of species-wide selective sweeps, and (iv) selection for different bacterial traits in different human hosts (20, 21, 47, 61). Barriers to gene flow resulting from geographic isolation of ancestral H. pylori populations may also have caused considerable genetic drift and thus differentiation of various H. pylori subpopulations (22). Each of these factors may also have influenced the evolutionary dynamics of IS elements in the H. pylori gene pool.
IS605 and IS607 belong to a novel family of transposable elements that are chimeric; each element contains two genes, orfA and orfB, which apparently have different phylogenetic origins (9, 10, 34, 35). The orfA genes of IS605 and IS607 encode transposases belonging to two different families. IS607 orfA is predicted to encode a serine recombinase, most homologs of which catalyze only site-specific recombination (59), although one case in which this enzyme mediates both site-specific recombination and transposition has also been reported (39). In contrast, the IS605 orfA product is not considered to be a serine recombinase based on amino acid homologies. Consistent with transposase differences, the elements differ in their insertion specificities. IS605 inserts preferentially downstream of a pentanucleotide motif, 5′-TTTAA, whereas IS607 inserts preferentially between G residues in a GG target sequence. The second gene of these elements, orfB, which is related to the genes encoding several putative bacterial transposases (10), is not required for transposition in the E. coli model, but it is related to the gene encoding the Salmonella prophage-encoded GipA protein, which promotes Salmonella's growth and survival in Peyer's patches of the intestine (60).
Nucleotide sequence analysis of IS elements in bacterial populations can provide insights into the evolutionary histories of the elements and sometimes also into the histories of their microbial hosts (5, 41). In this study, we elucidated the evolutionary histories of IS605 and IS607 in the context of H. pylori's extensive genetic diversity and geographic population subdivision.
MATERIALS AND METHODS
Bacterial isolates and geographical origins.
H. pylori isolates were cultured by using standard conditions and were maintained as frozen stocks at −70°C in sterile brain heart infusion broth containing 15% glycerol. A total of 488 H. pylori isolates from different parts of the world were included in this study. The individual population profiles are described below.
Sixty-eight Spanish isolates obtained from Teresa Alarcon and Manuel Lopez Brea have been described previously (37), as have 76 isolates cultured from biopsies provided by Abhijit Choudhury from ethnic Bengali patients in Calcutta, India (47). Fifty-one strains isolated from Amerindian (native Peruvian) patients from Lima, Peru, were provided by Robert H. Gilman. For the East Asian strains, 140 isolates were obtained from ethnic Japanese patients in Fukui, Honshu, Japan, 103 isolates were obtained from ethnic Japanese or Okinawan patients in Okinawa, and 50 isolates were obtained from ethnic Chinese patients in Hong Kong. All isolates were obtained from patients with gastric complaints that had undergone diagnostic endoscopy with informed consent.
PCR amplification, dot blot hybridization, and nucleotide sequencing.
Chromosomal DNA was prepared from confluent cultures on brain heart infusion agar plates either by the hexadecyltrimethylammonium bromide extraction method as described previously (39) or with a QIAamp DNA minikit (QIAGEN Inc., Chatsworth, Calif.). Primers used for generating probes for hybridization, PCR, and nucleotide sequencing are listed in Table 1. Dot blot hybridization was performed by using Hybond-N1 nylon membranes and an ECL kit (Amersham Pharmacia Biotech, Piscataway, N.J.) according to the manufacturer's instructions. Probes for the IS605 orfA and orfB genes were generated by PCR by using H. pylori strain 26695 genomic DNA as the template with primer pairs ORF18F-ORF18R and ORF19F-ORF19R, respectively. A probe for IS607 was generated by using H. pylori strain CPY41 genomic DNA as the template with primers FlkF and F10. Specific PCR were carried out in 20-μl mixtures containing 10 ng of DNA, 1 U of Taq polymerase (Promega, Madison, Wis.), 10 pmol of each primer, each deoxynucleoside triphosphate at a concentration of 0.25 mM, and 2 to 3 mM MgCl2 in standard PCR buffer for 30 cycles, generally under the following conditions: 94°C for 40 s, 55°C for 40 s, and 72°C for a time chosen based on the size of the expected fragment (1 min/kb).
TABLE 1.
Primers used in the present study
| Gene | Primer | Nucleotide sequence | Location |
|---|---|---|---|
| IS605 orfA | ORF18F | 5′-CGCCTTGATCGTTTCAGGATTAGC | 112 bp from left end of IS605 |
| ORF18R | 5′-CAACCAACCGAAGCAAGCATAATC | 482 bp from left end of IS605 | |
| IS605 orfB | ORF19F | 5′-GGCTGTTCTAGGGTCGTGTATAAC | 658 bp from left end of IS605 |
| ORF19R | 5′-CAAGCTAGATGCAATCTAGCTACC | 1319 bp from left end of IS605 | |
| IS607 orfA | FlkF | 5′-GGCTACAAACAGAAACTAAAAT | 237 bp from left end of IS607 |
| F3 | 5′-ATGCGTGATTGAGATAGCTG | 740 bp from left end of IS607 | |
| IS607 orfB | R6 | 5′-GCGCTAGATTGTATGGCTCT | 1386 bp from right end of IS607 |
| F2 | 5′-GCATAGATATTTAAGCCATTAGA | 1351 bp from left end of IS607 | |
| F10 | 5′-CTCTTAATTTAGGATTTTTGTTG | 1984 bp from left end of IS607 |
To assess the extent of geographic subdivision in IS element populations, PCR products obtained from IS605 (n = 42) and IS607 (n = 44) were sequenced. Isolates were selected primarily to sample the geographic extremes of East Asia (Japan, Hong Kong) and Europe (Spain). Peruvian and Indian H. pylori isolates in our collection exhibited substantial European influence (36, 46). Therefore, for the purposes of this study, Indian, Spanish, and Peruvian strains were placed in the Indo-European group, and strains from Japan (including Honshu and Okinawa) and Hong Kong were placed in the East Asian group. Sampling within each group was randomized to test the hypothesis of geographic isolation between the two groups.
The IS605 orfA and orfB genes were amplified in a single PCR with primers ORF18F and ORF19R. To ensure twofold redundancy, the PCR product was sequenced by using primers ORF18F and ORF19R and also primers ORF18R and ORF19F (internal primers) (Table 1). Similarly, the IS607 FlkF-F10 PCR product was sequenced by using primers FlkF and F10 and also primers F3, R6, and F2 (internal primers) (Table 1). PCR products were sequenced directly after purification with a QIAquick gel extraction kit (QIAGEN Inc.) by using a Big-Dye terminator cycle sequencing kit (Perkin-Elmer Applied Biosystems, Foster City, Calif.) and an ABI 377 automated sequencer. Of the 86 IS elements for which nucleotide sequences were determined, 38 were resequenced to check for possible sequencing or PCR errors. The IS elements selected for verification included those which had identical sequences or those which had stop codons in the coding region. The resequencing data were 100% concordant with the first data set.
Computations.
Preliminary sequence data management, including editing and alignment, was done by using the VectorNTI suite of programs (Informax Inc., Carlsbad, Calif.).
(i) Polymorphism and divergence.
Tests to obtain estimates of the average nucleotide diversity within populations (average nucleotide divergence per site [π]), the average nucleotide diversity at synonymous sites (πS) and nonsynonymous sites (πNS) corrected for multiple hits, the average nucleotide divergence between populations (Dxy), and FST and permutation tests for geographic isolation and genetic differentiation were done with DNASP, version 4 (58). A permutation (randomization) approach was used to detect geographic subdivision and genetic differentiation (30, 31). The significance of the observed values for KS*, KST, ZS, ZS*, and SNN test statistics was obtained by 10,000 random permutations as implemented in DNASP, version 4.
(ii) Phylogenetic analysis.
Phylogenetic reconstruction of IS605 and IS607 nucleotide sequences was performed by using the maximum-likelihood (ML) approach implemented in PAUP*4b10 (63). All available sequences were used. An optimal model of DNA substitution that best described the pattern of nucleotide variation and sequence evolution of IS elements was determined by using MODELTEST, version 3.06 (55). For this purpose, an initial neighbor-joining tree was computed with the uncorrected p-distance measure and used as the input to estimate the likelihood parameters for 56 nested models of evolution. Models that were nested (i.e., where the simpler model [null hypothesis, H0] was a special case of the more general model [alternate hypothesis, H1]) were compared statistically by using the hierarchical likelihood ratio test (LRT) (32). The LRT includes generating a likelihood ratio statistic (LRS), which is computed as follows: LRS = 2 × [(−InLH1) − (−InLH0)], where InL is the log-likelihood score of a given model. The product 2LRS follows a χ2 distribution with the degrees of freedom equal to the difference in the number of parameters between the two models. An ML phylogeny was reconstructed under the best-fit model by using a combination of heuristic searches and branch swapping (with the tree bisection reconnection algorithm) to further optimize the likelihood score and substitution parameters, including among- and within-site rate variation. The significance of observed groupings in the phylogeny was assessed by a bootstrap analysis performed with 1,000 replicates under the distance optimality criterion, while the ML-optimized model and parameters were incorporated by using a combination of heuristic searches and branch swapping. Phylogenetic trees were visualized with TreeView, version 1.6.6 (49), and were edited further in Microsoft PowerPoint.
Homologous recombination in IS605 and IS607 was assessed by split decomposition (SD) analysis (2) and the homoplasy test (44). SD analysis uses a relaxed condition for estimating splitting patterns among taxa, and both the signal (suggesting the tree structure) and the noise (suggesting patterns inconsistent with the tree structure, such as those arising from recombination and/or convergent evolution) can be presented simultaneously. Unlike standard tree-building methods, SD analysis can usually produce a network that helps delineate anomalies in the history of gene trees (3, 50, 62). In particular, recombination generates sequences linked by multiple ancestors, which with this method are depicted as an interconnected network of phylogenetic relationships (50). For the analysis reported below, nucleotide data were transformed into a matrix of pairwise distances incorporating ML-optimized substitution parameters. In some cases, SD analysis was also done by using the uncorrected p-distance measure. The analysis was done with SplitsTree, version 3.2 (33). The homoplasy test was done by using the program HOMOPLASY (kindly provided by Mark Achtman). Estimates of Se (effective site number) and the homoplasy ratio (h) were obtained by assuming all three levels of expression (low, medium, and high) in three independent runs. Results were reported by averaging over all runs.
(iii) Analysis of selection pressures.
The selection pressure on a protein-encoding gene can be measured by comparing the nonsynonymous substitution rate (dN) (amino acid altering) to the synonymous substitution rate (dS) (silent, no amino acid change) to obtain the dN/dS ratio (ω). An ω of 1 indicates neutral evolution (relaxed selective constraint; nonsynonymous changes have no associated fitness advantage and are fixed at the same rate as synonymous changes); an ω of <1 indicates purifying selection (strong functional constraint; nonsynonymous changes are deleterious for protein function and are fixed at a lower rate than synonymous changes); and an ω of >1 indicates positive selection (adaptive evolution; nonsynonymous changes are favored because they confer a fitness advantage and are fixed at a higher rate than synonymous changes).
Here we used an ML-based approach for measuring selective pressures acting on H. pylori's IS elements. ML analysis with site-specific codon substitution models that allow ω to vary among sites but not among lineages (68) detects positive selection at individual sites only if the average dN over all lineages is higher than the average dS. Thus, the test is still relatively conservative in terms of detecting positive selection. ω for codons of IS607 and IS605 orfA and orfB were computed by using ML-optimized phylogenies of the orf genes (available from us upon request). Sequences with stop codons were excluded from this analysis.
The following six site-specific models of codon substitution were used in this study. (i) M0 assumes that all codons (sites) are subject to the same selection pressure, so a single ω value is estimated. (ii) M1 divides codons into two categories, representing the codons that are invariant (p0), with ω0 fixed at 0, and codons that are neutral (p1), where ω1 is 1. (iii) M2 accounts for positive selection by adding a third category of codons (p2) with ω2, which is estimated from the data and can be >1. (iv) M3 also invokes three codon site classes and provides a more sensitive test for positive selection by estimating all ω values from the data, and all ω values may be >1. (v) M7 uses a discrete β distribution (with 10 rate categories), whose shape varies depending on the parameters p and q, to model ω among codons; with M7, no class of codons can have an ω of >1. (vi) M8 also uses a β distribution, but an extra class of codons is incorporated, in which ω can be >1.
Positive selection was inferred when codons with an ω of >1 were identified and the likelihood of the codon substitution model in question was significantly higher than the likelihood of a nested model that did not take positive selection into account. When the more general models indicated the presence of sites with ω of >1, the comparison constituted an LRT (see above) of positive selection (68). Finally, by using Bayesian methods we calculated the probability that a specific codon belonged to the neutral, negative, or positively selected class. This analysis was done with the CODEML program in the PAML suite, version 3.13d (67).
Nucleotide sequence accession numbers.
Sequence data determined in this study have been deposited in the GenBank/EMBL/DDBJ databases under accession numbers AY687641 to AY687726, AY687742 to AY687785, and AY687787 to AY687828.
RESULTS
Population structure of H. pylori IS elements: geographic distribution of IS605 and IS607 in H. pylori populations.
The distribution of IS605 and IS607 in H. pylori isolates from several parts of the world was analyzed by dot blot hybridization, followed by confirmatory PCR. IS605 was found in 18% of the isolates worldwide, but the frequency varied geographically, ranging from only 5% in Japanese (Honshu) isolates to 41% in Spanish isolates (Table 2). These outcomes agree with previous reports of IS605 carriage in 28 to 50% of mainly Western H. pylori collections (9, 29, 34), but they indicate that this element is significantly less common in East Asian isolates (P < 0.05 for all pairwise comparisons except Hong Kong versus India).
TABLE 2.
Geographic prevalence of IS elements
| Geographic origin | No. of strains | IS element prevalence
|
||
|---|---|---|---|---|
| IS605 | IS607 | Both IS605 and IS607 | ||
| HongKong | 50 | 7 (14)a | 9 (18) | 4 (8) |
| Japan (Honshu) | 140 | 7 (5) | 7 (5) | 0 (0) |
| Japan (Okinawa) | 103 | 8 (7.5) | 18 (17.5) | 1 (0.97) |
| India | 76 | 19 (25) | 10 (13) | 1 (1.3) |
| Peru | 51 | 18 (35) | 14 (27) | 1 (1.96) |
| Spain | 68 | 28 (41) | 12 (18) | 2 (2.94) |
The numbers in parentheses are percentages.
IS607 was found in 14% of the isolates tested, but the frequency also varied geographically, ranging from 5% in Japanese (Honshu) H. pylori isolates to 27% in Peruvian isolates (Table 2). As observed with IS605, significantly fewer Honshu isolates than isolates from elsewhere carried IS607 (P < 0.05). However, the IS607 prevalence in Okinawan and Hong Kong isolates did not differ significantly from that in Spanish, Indian, or Peruvian isolates (P > 0.1). IS607 was less abundant than IS605, especially among Spanish isolates (for IS607 versus IS605, P < 0.01); this general trend was also detected among Peruvian and Indian isolates, although the differences were not statistically significant.
Of the 157 isolates that were positive for IS605 and IS607, only 9 (6%) carried both elements (Table 2). The co-occurrence of these elements in most geographic regions was extremely low and ranged from 0% in Japan (Honshu) to 8% in Hong Kong.
Nucleotide sequence diversity.
We determined the nucleotide sequences for 378 bp of orfA and 549 bp of orfB from IS605 in 42 strains and for 537 bp of orfA and 563 bp of orfB from IS607 in 44 strains; in each case the strain was chosen as described in Materials and Methods. The average nucleotide diversity (i.e., nucleotide divergence per site) in these elements ranged from 0.021 (IS607 orfA) to 0.051 (IS605 orfA). The average nucleotide diversity at synonymous sites, where the accumulated changes reflect the ages of alleles (4), ranged from 0.078 (IS607 orfA) to 0.153 (IS605 orfA). The IS π and πS values were fairly similar to those of five other H. pylori normal chromosomal genes (housekeeping genes) (Table 3). The average nucleotide diversity values for nonsynonymous sites ranged from 0.007 in IS607 orfA to 0.029 in IS605 orfA. Stop codons due to nucleotide substitutions were identified in 1 of 44 IS607 orfA genes, in 5 of 42 IS605 orfA genes, and in one IS607 orfB gene. In all other cases the orfA and orfB sequences were not interrupted.
TABLE 3.
DNA divergence in H. pylori IS elements and chromosomal genes
| Gene | No. of sequences | Sequence length (bp) | Mean nucleotide diversity [π(JC corrected)]b | Mean nucleotide diversity at synonymous sites [πS(JC corrected)] | Mean nucleotide diversity at nonsynonymous sites [πNS(JC corrected)] |
|---|---|---|---|---|---|
| IS605 orfA | 42 | 378 | 0.051 | 0.153 | 0.029 |
| IS605 orfB | 42 | 549 | 0.035 | 0.128 | 0.013 |
| IS607 orfA | 44 | 537 | 0.021 | 0.078 | 0.007 |
| IS607 orfB | 44 | 563 | 0.033 | 0.113 | 0.013 |
| atpAa | 310 | 627 | 0.032 | 0.136 | 0.002 |
| efpa | 303 | 410 | 0.035 | 0.175 | 0.001 |
| ppaa | 317 | 398 | 0.025 | 0.107 | 0.005 |
| yphCa | 332 | 510 | 0.068 | 0.164 | 0.014 |
| ureIa | 335 | 585 | 0.031 | 0.123 | 0.006 |
Sequences for the housekeeping genes listed were downloaded from http://helicobacter.mlst.net and have been described previously (22).
Jukes-Cantor correction was applied for multiple hits.
Phylogenetic relationships inferred from IS605 sequences.
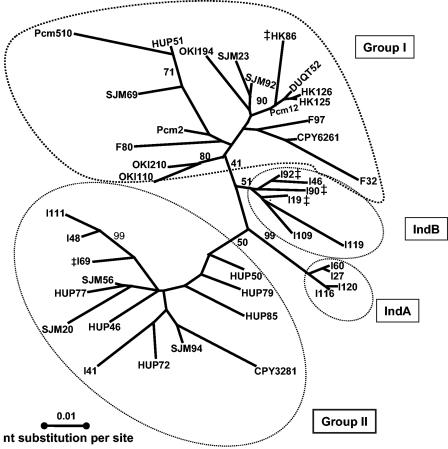
A 927-bp sequence obtained from concatenating the IS605 orfA and orfB sequences was used for phylogenetic reconstruction. Figure 1 shows an unrooted ML tree of IS605 nucleotide sequences obtained from 42 H. pylori isolates. The East Asian isolates formed one weakly supported group (group I; bootstrap support, 41%) and sequences from Hong Kong, Honshu, and Okinawa were intermingled with sequences from Spain and Peru. A second weakly supported group (group II; bootstrap support, 50%) consisted mostly of Spanish isolates that were intermingled with Peruvian and Indian isolates; one Honshu isolate (CPY3281) also clustered with group II. Three distinct groups were seen among 14 Indian H. pylori isolates. IS605 sequences from four Indian H. pylori isolates (I-41, I-69, I-111, and I-48) clustered with group II Spanish and Peruvian H. pylori isolates, and IS605 sequences of the other 10 isolates formed two additional clusters, group IndA (four isolates; bootstrap support, 99%) and group IndB (six isolates; bootstrap support, 51%). Taken together, the IS605 phylogeny exhibited blurred population subdivisions, in which three of the four major groups were only weakly supported.
FIG. 1.
Unrooted, radial gene tree generated by the ML method by using a 927-bp concatenated sequence from IS605 orfA (378 bp) and orfB (549 bp). All available sequences were used in the phylogenetic reconstruction. orfA sequences with stop codons are indicated by double daggers. The most appropriate model for IS605 sequence evolution (TIM + I + Γ) was used for phylogenetic reconstruction with a discrete gamma distribution (Γ) shape parameter (α = 0.803) and an assumed proportion of invariate sites (I = 0.69). The TIM model (a constrained submodel of the general time reversible model) specifies a rate substitution matrix in which transitions (A↔G and C↔T) have only one rate category and the four possible transversions (A↔C, G↔T, A↔T, and C↔G) have four distinct rate categories. Bootstrap values of ≥50 are indicated at the nodes. H. pylori strain designations indicate the geographic origins, as follows: HUP, Spain; SJM, Peru; I, India; OKI, Okinawa, Japan; F or CPY, Honshu, Japan; and HK, DUQT, or Pcm, Hong Kong. Major sequence similarity clusters are circled. nt, nucleotide.
The nucleotide sequence diversity within subpopulations ranged from 1% in group IndA to 3.4% in group II (Table 4). In all pairwise comparisons of IS605 subpopulations, the mean divergence between groups (Dxy) was higher than the mean divergence within groups, which supported the phylogenetic distinctness (51). Statistical tests that measured the extent and significance of genetic differentiation (30, 31) suggested that IS605 sequences from different populations had evolved with significant geographic isolation (P < 0.01 for KS*, KST, ZS, ZS*, and SNN test statistics) and had undergone very great genetic differentiation (FST = 0.28l) (Table 4) (27). Individual pairwise comparisons also revealed significant geographic isolation and genetic differentiation among IS605 subpopulations.
TABLE 4.
Sequence divergence within and between phylogenetically distinct IS605 taxa
| Comparison | Genes | Mean nucleotide diversity within groupa | Mean nucleotide diversity between groupsa | No. of fixed polymorphismsb | No. of shared polymorphisms | FST | PM test P valuec |
|---|---|---|---|---|---|---|---|
| Group I vs Group II | orfA + orfB | πI = 0.033; πII = 0.034 | 0.05 | 0 | 52 | 0.303 | <0.001 |
| Group I vs IndA | orfA + orfB | πI = 0.033; πIndA = 0.01 | 0.047 | 9 (6S + 3N) | 5 | 0.341 | <0.001 |
| Group I vs IndB | orfA + orfB | πI = 0.033; πIndB = 0.025 | 0.037 | 0 | 29 | 0.225 | <0.001 |
| Group II vs IndA | orfA + orfB | πII = 0.034; πIndA = 0.01 | 0.041 | 1N | 5 | 0.163 | <0.05 |
| Group II vs IndB | orfA + orfB | πII = 0.034; πIndB = 0.025 | 0.048 | 0 | 25 | 0.364 | <0.001 |
| IndA vs IndB | orfA + orfB | πIndA = 0.01; πIndB = 0.025 | 0.035 | 14 (10S + 4N) | 9 | 0.252 | <0.01 |
| All groups | orfA + orfB | NAd | NA | NA | NA | 0.281 | <0.01 |
Values were calculated with the Jukes-Cantor correction (correction for multiple hits).
S, synonymous polymorphisms; N, nonsynonymous polymorphisms.
A permutation test was done with 10,000 replications; P values were calculated for the Ks*, KST, Zs, Zs* (30), and Snn (31) test statistics. All test statistics demonstrated identical significance levels.
NA, not applicable.
Subpopulations that diverge from a common ancestor and subsequently evolve in isolation are expected to accumulate fixed polymorphisms (sites at which all sequences in one population differ from all sequences in a second population), shared polymorphisms, and unique polymorphisms (i.e., sites polymorphic in one population but monomorphic in a second population) in their DNA sequences (28). The distributions of these polymorphisms are useful in inferring the times of population divergence; recently split populations show only shared polymorphisms, and populations that split a long time ago show an inverse relationship between fixed and shared polymorphisms or only fixed polymorphisms (64). Of six possible pairwise comparisons of IS605 subpopulations, only three provided evidence for the presence of fixed polymorphisms, and all three of these involved the well-supported IndA group (Table 4). For all other comparisons, only polymorphisms shared by subpopulations were observed, suggesting that either the subpopulations diverged very recently or that recombination between subpopulations had obscured population differentiation (see below).
Phylogenetic relationships inferred from IS607 sequences.
An 1,100-bp sequence obtained from concatenating IS607 orfA and orfB was used for phylogenetic reconstruction. Figure 2A shows an unrooted ML tree for IS607 nucleotide sequences obtained from the 44 H. pylori isolates. Two distinct sequence similarity clusters were evident that broadly distinguished group I (n = 20), consisting of East Asian H. pylori isolates, and group II (n = 22), consisting of Spanish, Peruvian, and Indian H. pylori isolates. Only 3 of the 23 East Asian H. pylori isolates, F-37 from Japan and pcm2 and DUQT-52 from Hong Kong, were not assigned to group I. The phylogeny also provided evidence for population subdivision within each group. Specifically, IS607 elements from Japanese (Honshu) isolates and from Okinawan-Hong Kong H. pylori isolates formed two well-supported groups, designated group Ia (bootstrap support, 99%) and group Ib (bootstrap support, 85%), although two Okinanwan IS607 sequences clustered with group Ia. IS607 sequences from three Indian isolates formed a strongly supported group, designated group IIa (bootstrap support, 99), which was distinct from all other group II IS607 sequences. Taken together, the IS607 phylogeny exhibited well-supported geographic clustering that was markedly stronger than that in the IS605 phylogeny.
FIG. 2.
(A) Unrooted, radial gene tree generated by the ML method by using an 1,100-bp concatenated sequence from IS607 orfA (537 bp) and orfB (563 bp). Sequences with stop codons in orfA and orfB are indicated by double daggers and solid diamonds, respectively. The TVM + I + Γ model was used in phylogenetic reconstruction with α = 0.304 and I = 0.46. The TVM rate substitution matrix specifies that transitions have two distinct rate categories and transversions have only two specified rate categories (A↔C and G↔T have one rate and A↔T and C↔G have one rate). Bootstrap values of ≥50 are indicated at the nodes; distinct IS607 sequence similarity clusters are circled. H. pylori strain GS1 is from Honshu, Japan; for an explanation of all other strain designations see the legend to Fig. 1. nt, nucleotide. (B) Summary of polymorphisms in IS607 subpopulations: Venn diagram summarizing the unique, shared, and fixed polymorphisms observed in IS607group Ia (n = 9), group Ib (n = 11), and group II (n = 19). Fixed differences between populations are indicated below the arrows outside the conjoined circles.
The nucleotide diversity within IS607 subpopulations ranged from 0.4% in group Ia to 1.8% in group II (Table 5). As seen with IS605 populations, Dxy, the mean divergence between groups, was higher than π, the mean divergence within groups, suggesting the phylogenetic distinctness of IS607 subpopulations. The probabilities for the KS*, KST*, ZS, ZS*, and SNN test statistics obtained by the permutation test with 10,000 replicates were highly significant (P < 0.001) (Table 5). This suggested that (i) the data deviated significantly from the null hypothesis of no genetic differentiation or geographic isolation and (ii) the observed IS607 population structure was highly improbable by chance alone under a neutral model. The FST value was 0.65, which suggested very great genetic differentiation (27) between IS607 subpopulations.
TABLE 5.
Sequence divergence within and between phylogenetically distinct IS607 taxaa
| Comparison | Genes | Mean nucleotide diversity within group | Mean nucleotide diversity between groups | No. of fixed polymorphisms | No. of shared polymorphisms | FST | PM test P value |
|---|---|---|---|---|---|---|---|
| Group Ia vs Group II | orfA + orfB | πIa = 0.004; πII = 0.018 | 0.035 | 11 (8S + 3N) | 6 | 0.691 | <0.001 |
| Group Ib vs Group II | orfA + orfB | πIb = 0.015; πII = 0.018 | 0.034 | 1S | 18 | 0.499 | <0.001 |
| Group Ia vs Group Ib | orfA + orfB | πIa = 0.004; πIb = 0.015 | 0.026 | 8 (5S + 3N) | 6 | 0.634 | <0.001 |
| Group Ia vs Group IIa | orfA + orfB | πIa = 0.004; πIIa = 0.005 | 0.028 | 24 (19S + 5N) | 0 | 0.839 | <0.001 |
| Group Ib vs Group IIa | orfA + orfB | πIb = 0.015; πIIa = 0.005 | 0.031 | 16 (12S + 4N) | 1 | 0.659 | <0.001 |
| Group II vs Group IIa | orfA + orfB | πII = 0.018; πIIa = 0.005 | 0.029 | 4 (2S + 2N) | 3 | 0.593 | <0.001 |
| All groups | orfA + orfB | NA | NA | NA | NA | 0.648 | <0.001 |
See footnotes to Table 4.
Pairwise comparisons of IS607 groups Ia, Ib, II, and IIa revealed a negative correlation between fixed and shared polymorphisms (Table 5), which indicated that these subpopulations had diverged from a common ancestor. Since group IIa had only three sequences, a detailed analysis of polymorphisms is presented here for only groups Ia, Ib, and II. Group Ia IS607 shared six polymorphisms with group Ib and six polymorphisms with group II, and groups Ib and II shared 18 polymorphisms (Fig. 2B). Furthermore, group Ia IS607 showed only 11 unique polymorphisms, in contrast to the 58 and 89 unique polymorphisms in groups Ib and group II, respectively. These patterns suggest that there was strong genetic drift from few founders (founder effect) or a population bottleneck in group Ia IS607 rather than a long time of separation from other IS607 populations. A long time of separation would have allowed steady accumulation of unique polymorphisms and greater nucleotide diversity in group Ia, similar to the diversity seen in group Ib and group II (Table 5). Only one fixed difference between group Ib and group II with correspondingly large numbers of shared polymorphisms was identified. This suggests either that group Ib experienced a recent increase in population size or that the two groups diverged recently from their common ancestor.
Homologous recombination among H. pylori IS elements.
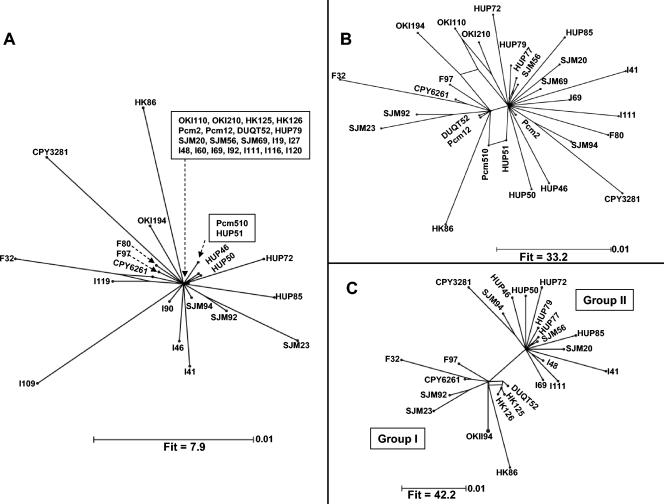
The impact of recombination on IS605 and IS607 population structure was analyzed by using split decomposition. The splits graph of the complete IS605 data set showed that the majority of sequences were linked to a single multifurcating node (star phylogeny) (Fig. 3A), which reaffirmed the lack of the sharp population subdivision seen in the IS605 ML tree. Distant outgroups often show large random fluctuations and also different systematic biases (i.e., deviations between a parameter and parameter estimate due incorrect assumptions in the estimation method), with a tendency to hide information on in-group systematic bias (62). In such situations the splits graph loses resolution, as indicated by a low fit parameter (a fit parameter of 100 indicates that all conflicts in the data set are fully resolved). With the IS605 splits graph a low fit parameter was obtained (fit parameter, 7.9), indicating that very few conflicts (multiple pathways linking sequences) in the data set could be resolved. Recalculation of the splits graph after removal of cluster IndA and IndB IS605 sequences resulted in an improved fit parameter and revealed networks among IS605 sequences (Fig. 3B). The multiple pathways linking East Asian and Spanish-Peruvian-Indian IS605 sequences suggest that recombination had blurred the phylogenetic distinction between lineages. This suggestion was strengthened by removing sequences forming the network linking East Asian and Indo-European sequences in the splits graph. This revealed distinct East Asian and Indo-European IS605 subpopulations (Fig. 3C).
FIG. 3.
Split decomposition analysis of IS605. (A) Annotated splits graph of the concatenated 927-bp IS605 orfA and orfB sequence generated with a pairwise TIM + I + Γ distance matrix. A fit parameter of 7.9 indicated the virtual absence of a tree-like structure. (B) Recalculation of the splits graph after removal of IS605 sequences from clusters IndA and IndB improved the fit parameter and resolved networks linking the East Asian and Indo-European elements. (C) Splits graph of IS605 showing population subdivision within IS605, which was masked by a history of recombinational exchanges between the two lineages. All branch lengths are drawn to scale.
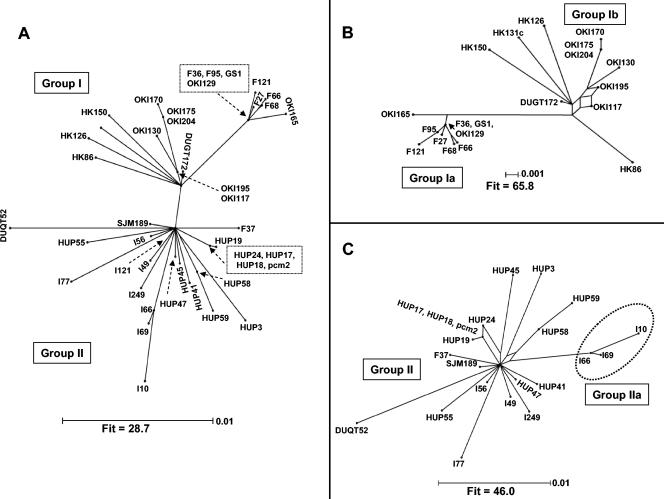
In the case of IS607, the splits graph showed the clear phylogenetic distinctness of the East Asian elements from the elements in other regions but provided no evidence for networked evolution (Fig. 4A). Because the great length of the branch linking the two groups could have obscured networked evolution within groups, we also analyzed separately sequences from the two major lineages corresponding to groups I and II. In both groups few sequences formed interconnected networks, suggesting that there was a history of limited recombination (Fig. 4B and C).
FIG. 4.
Split decomposition analysis of IS607. (A) Annotated splits graph of the 1,100-bp concatenated sequence from IS607 orfA and orfB generated with a pairwise TVM + I + Γ distance matrix. A fit parameter of 28.7 indicated that all conflicts in the data set could not be resolved. Removal of distant outgroups in the data set improved the fit parameter (indicating better resolution of conflicts), as shown in the annotated splits graph for group I IS607 (B) and the annotated splits graph for group II IS607 (C). All branch lengths are drawn to scale.
Homoplasy ratio calculations complemented the outcomes from the SD analysis (Table 6). The complete concatenated IS607 data set yielded an extremely low h value (0.2), indicating a lack of gene flow between IS607 subpopulations. Even within IS607 subpopulations, only low to moderate levels of recombination were detected. In contrast, the h values for IS605 were in greater accord with values observed for other H. pylori chromosomal genes, which typically range from 0.6 to 1 (1, 61). Furthermore, for the complete data set, intragenic recombination seemed to be relatively more frequent within IS605 orfA (h = 0.53) than within orfB (h = 0.32). Consistently low levels of recombination were detected in both IS607 orfA (h = 0.21) and orfB (h = 0.201). These outcomes suggest that recombination between divergent lineages has been more common in the evolution of IS605 than in the evolution of IS607.
TABLE 6.
Homologous recombination within IS elements
| IS element | No. of sequences | No. of variable sites | No. of informative sites | Mean shuffled h | h ratioa |
|---|---|---|---|---|---|
| IS607AB | 44 | 61 | 40 | 89.2 | 0.217 |
| Group Ia | 7 | NDb | ND | ND | ND |
| Group Ib | 11 | 26 | 14 | 17.7 | 0.348 |
| Group II | 19 | 59 | 39 | 119.2 | 0.525 |
| IS605ABc | 42 | 67 | 53 | 261.9 | 0.558 |
| GroupI | 10 | 41 | 18 | 19.2 | 0.654 |
| GroupII | 14 | 54 | 39 | 90 | 0.701 |
The h ratio is the average of h ratio assuming high, low, and medium expression.
ND, not determined due to insufficient number of synonymous variable sites.
IS605 group designations are based on the split decomposition analysis shown in Fig. 3C.
Natural selection and evolution of H. pylori IS elements.
We assessed the relative contributions of selective forces on the evolution of H. pylori IS elements by measuring site-specific dN/dS ratios using the ML approach implemented in the CODEML program of PAML, version 3.13d (68).
(i) IS605.
The IS605 orfA gene encodes a 142-amino-acid transposase that is not related to the IS607 transposase. In the 126 codons analyzed here, the average ω ranged from 0.31 to 0.47, implying that purifying selection dominated the evolution of this transposase (Table 7). Models that allowed for positive selection (M2, M3, and M8) all suggested the presence of positively selected sites. M3 suggested more sites than M2 or M8 because the parameter estimates with this model identified an additional 12.4% of the sites that were under relatively weak positive (diversifying) selection (ω1 = 1.12). Since the ML approach explicitly assumes a phylogenetic tree, to test whether high levels of recombination in IS605 orfA (h = 0.56 to 0.7) could have produced a false-positive signal for positive selection, we repeated the analysis assuming a star phylogeny. In a star phylogeny lineages diverge simultaneously from a single root node, thereby removing the effect of phylogenetic history. This analysis again produced significant evidence for positive selection (P < 0.0001 for M3 versus M0, M1, and M2 and for M8 versus M7), and in general the same sites fell into the positively selected class that they fell into in the original analysis (data not shown). This indicated that recombination did not adversely affect the ability of the ML method to detect positive selection in the IS605 phylogeny. Taken together, these data provide evidence for adaptive evolution in IS605 transposase.
TABLE 7.
Selection pressures acting on IS605 orfA: ML parameter estimates
| Model | lnLa | dN/dSb | Estimates of parameters | LRT (df)c | χ2 | P | Positively selected sites (ω > 1)d |
|---|---|---|---|---|---|---|---|
| M0 (one ratio) | −1,716.813 | 0.263 | ω = 0.263 | None | |||
| M1 (neutral) | −1,679.783 | 0.336 | p0 = 0.663, ω0 = 0; p1 = 0.336, ω1 = 1 | Not allowed | |||
| M2 (selection) | −1,663.452 | 0.475 | p0 = 0.656; p1 = 0.321; (p2 = 0.021), ω2 = 7.066 | M0 vs M2 (2) | 74.06 | <0.000 | 6E, 19P, 104R |
| M1 vs M2 (2) | 32.66 | <0.000 | |||||
| M3 (discrete) | −1,649.582 | 0.318 | p0 = 0.856, ω0 = 0.073; p1 = 0.124, ω1 = 1.125; p2 = 0.019, ω2 = 5.842 | M0 vs M3 (4) | 134.46 | <0.000 | 6E, 10N, 18I, 19P, 48H, 50I, 62N, 63F, 92S, 104R, 105D, 59T, 3K, 21N |
| M1 vs M3 (4) | 60.40 | <0.000 | |||||
| M2 vs M3 (2) | 27.74 | <0.000 | |||||
| M7 (β) | −1,667.806 | 0.212 | p = 0.134, q = 0.497 | Not allowed | |||
| M8 (β and ω) | −1,654.705 | 0.302 | p0 = 0.972; p = 0.210, q = 0.940; p2 = 0.027, ω2 = 4.767 | M7 vs M8 (2) | 26.20 | <0.000 | 6E, 19P, 104R |
InL, log-likelihood score. Tree lengths and kappa estimates were homogeneous for all models.
dN/dS averaged over all lineages and all sites.
Likelihood ratio test (degrees of freedom).
Site probability in boldface type, >95%; site probability underlined, >75% and <95%; site probability not in boldface type and not underlined, >50% and <75%.
In 183 of the 427 IS605 orfB codons analyzed, the average ω for all sites ranged from 0.12 to 0.15 for all models except M1, which, although better than M0, was rejected in favor of all other models that allowed for sites under positive selection (Table 8). M3 fit the data better than M0, M1, or M2, and parameter estimates from M3 suggested that ∼90% of the sites were under functional constraints (purifying selection) with an ω0 of 0.071, ∼8% of the sites were moderately conserved with an ω1 of 0.47 (which also implies purifying selection), and ∼1.5% of the sites were under positive selection with an ω2 of 2.613. Both M3 and M8 identified sites 100 and 183 with a P value of >99%.
TABLE 8.
Selection pressures acting on IS605 orfB: ML parameter estimatesa
| Model | lnL | dN/dS | Estimates of parameters | LRT (df) | χ2 | P | Positively selected sites (ω > 1) |
|---|---|---|---|---|---|---|---|
| M0 (one ratio) | −1,947.005 | 0.141 | ω = 0.141 | None | |||
| M1 (neutral) | −1,942.164 | 0.27 | p0 = 0.729, ω0 = 0; p1 = 0.270, ω1 = 1 | Not allowed | |||
| M2 (selection) | −1,923.208 | 0.125 | p0 = 0.0, p1 = 0.054; (p2 = 0.945), ω2 = 0.075 | M0 vs M2 (2) | 47.59 | <0.000 | None |
| M1 vs M2 (2) | 37.91 | <0.000 | |||||
| M3 (discrete) | −1,920.305 | 0.145 | p0 = 0.90, ω0 = 0.071; p1 = 0.084, ω1 = 0.474; p2 = 0.015, ω2 = 2.613 | M0 vs M3 (4) | 53.40 | <0.000 | 100D, 183T |
| M1 vs M3 (4) | 43.71 | <0.000 | |||||
| M2 vs M3 (2) | 5.80 | <0.05 | |||||
| M7 (β) | −1,927.993 | 0.1531 | p = 0.202, q = 1.096 | Not allowed | |||
| M8 (β and ω) | −1,920.445 | 0.145 | p0 = 0.982; p = 0.869, q = 7.280; p2 = 0.017, ω2 = 2.511 | M7 vs M8 (2) | 15.09 | <0.000 | 100D, 183T |
See Table 7 footnotes.
(ii) IS607.
In 179 of the 217 orfA codons analyzed, the average ω ranged from 0.2 to 0.25, indicating that the transposase was under purifying selection (Table 9). The more complex models (M1, M2, and M3) were significantly better than M0, thereby suggesting that selective pressures (indicated by ω) varied among sites. The best-supported M1 (neutral) model estimated that ∼75% of the codons analyzed were subject to strong purifying selection (with ω0 fixed at 0), while the rest of the codons underwent neutral evolution (ω1 = 1). These data suggest that the IS607 transposase evolution was dominated by purifying and neutral selection, in contrast to the positive selection evident in the unrelated IS605 transposase.
TABLE 9.
Selection pressures acting on IS607 orfA: ML parameter estimatesa
| Model | lnL | dN/dS | Estimates of parameters | LRT (df) | χ2 | P | Positively selected sites (ω > 1) |
|---|---|---|---|---|---|---|---|
| M0 (one ratio) | −1,393.247 | 0.207 | ω = 0.207 | None | |||
| M1 (neutral) | −1,385.371 | 0.246 | p0 = 0.753, ω0 = 0; p1 = 0.246, ω1 = 1 | Not allowed | |||
| M2 (selection) | −1,384.631 | 0.206 | p0 = 0.673, p1 = 0.119; (p2 = 0.207), ω2 = 0.41 | M0 vs M2 (2) | 17.232 | <0.000 | None |
| M1 vs M2 (2) | 1.48 | NSb | |||||
| M3 (discrete) | −1,384.77 | 0.208 | p0 = 0.115, ω0 = 0.0001; p1 = 0.62, ω1 = 0.01; p2 = 0.26, ω2 = 0.76 | M0 vs M3 (4) | 16.954 | <0.001 | None |
| M1 vs M3 (4) | 1.202 | NS | |||||
| M2 vs M3 (2) | −0.278 | NS | |||||
| M7 (β) | −1,384.701 | 0.208 | p = 0.086, q = 0.329 | Not allowed | |||
| M8 (β and ω) | −1,384.483 | 0.217 | p0 = 0.98; p = 0.144, q = 0.638; p2 = 0.011, ω2 = 3.059 | M7 vs M8 (2) | 0.138 | NS | None |
See Table 7 footnotes.
NS, not significant.
In 189 of the 419 IS607 orfB codons analyzed, the average ω ranged from 0.21 to 0.27 among the best-fitting models (Table 10). Parameter estimates and LRTs suggested the presence of sites under positive selection. For example, the discrete model (M3), which fit the data significantly better than M0, M1, or M2, estimated that ∼10% of the codons analyzed were under positive selection with an ω2 of 1.665. Similarly, parameter estimates from M8, which fit the data better than M7, also suggested that ∼10% of the orfB codons analyzed were under positive selection with an ω2 of 1.65. The failure of M2 to detect positive selection in biological data sets has been noted previously and is apparently due to the fact that the M1 model, on which M2 is based, is unrealistic and does not optimally account both for sites for which ω is >0 but <1 and for sites for which ω is >1 (68).
TABLE 10.
Selection pressures acting on IS607 orfB: ML parameter estimatesa
| Model | lnL | dN/dS | Estimates of parameters | LRT (df) | χ2 | P | Positively selected sites (ω > 1)a |
|---|---|---|---|---|---|---|---|
| M0 (one ratio) | −1,903.608 | 0.224 | ω = 0.224 | None | |||
| M1 (neutral) | −1,866.1 | 0.239 | p0 = 0.760, ω0 = 0; p1 = 0.239, ω1 = 1 | Not allowed | |||
| M2 (selection) | −1,864.976 | 0.203 | p0 = 0.0, p1 = 0.190; (p2 = 0.809), ω2 = 0.023 | M0 vs M2 (2) | 77.264 | <0.000 | 173G |
| M1 vs M2 (2) | 2.248 | NSb | |||||
| M3 (discrete) | −1,860.354 | 0.245 | p0 = 0.74, ω0 = 0.01; p1 = 0.146, ω1 = 0.4; p2 = 0.104, ω2 = 1.665 | M0 vs M3 (4) | 86.508 | <0.000 | 155N, 168V, 173G, 174V, 180K, 186Q, 50T, 51N, 53L, 94R, 148L, 179Y, 29A, 178E |
| M1 vs M3 (4) | 11.492 | <0.02 | |||||
| M2 vs M3 (2) | 10.694 | <0.01 | |||||
| M7 (β) | −1,865.261 | 0.217 | p = 0.041, q = 0.158 | Not allowed | |||
| M8 (β and ω) | −1,862.362 | 0.245 | p0 = 0.893; p = 0.158, q = 1.755; p2 = 0.106, ω2 = 1.65 | M7 vs M8 (2) | 5.798 | <0.05 | 155N, 168V, 173G, 174V, 180K, 186Q, 50T, 51N, 53L, 94R, 148L, 179Y, 29A, 178E |
See Table 7 footnotes.
NS, not significant.
DISCUSSION
Population structures of IS605 and IS607.
Analysis of IS605 and IS607 sequences indicated that most East Asian elements were phylogenetically distinct from Indo-European elements. Statistical tests for population subdivision suggest that the differences resulted from evolution in geographic isolation. The nucleotide sequences of normal chromosomal (housekeeping) genes of East Asian and Indo-European H. pylori strains also form distinct phylogenetic clusters (1, 22, 23, 36, 46, 52). Thus, the IS605 and IS607 population structures seemed to be similar to those of their H. pylori hosts. This was most striking in the case of IS607, in which there was little recombination between subpopulations (Fig. 2 and 4A and Table 6). In the case of IS605, geographic isolation between East Asian and Indo-European elements (Fig. 3C and Table 4) was blurred by recombination between subpopulations (Fig. 3B and Table 6). Similarities in the IS and H. pylori population structures suggest parallel evolutionary histories and ancient host-element associations. Differences in the detailed population genetic structure of IS605 and IS607 are discussed below.
Population differences in IS element alleles might be evolutionarily stable, reflecting long-term IS residence in the H. pylori gene pool, or might reflect transient polymorphisms that arose recently as elements spread among lineages and that remain to be eliminated. The levels of nucleotide divergence at synonymous sites, which are generally more uniform than those at nonsynonymous sites (27), can be used to test these alternatives because changes accumulated at synonymous sites reflect the ages of alleles (4, 66). The cumulative (all populations) mean divergence values at synonymous sites (corrected for multiple hits) for the IS607 orfA and orfB and IS605 orfA and orfB sequences were 7.8, 11.3, 15.3, and 12.8%, respectively (Table 3). These values resembled the mean divergence values at synonymous sites seen in several of H. pylori's normal chromosomal genes, such as ppa (10.7%), ureI (12.3%), atpA (13.6%), yphC (16.4%), and efp (17.5%) (22). The levels of nonsynonymous site divergence, which is subject to greater selective constraints, were generally less than the synonymous site divergence levels for both IS and other chromosomal genes (Table 3). These data suggest that IS elements are ancient components of the H. pylori gene pool and evolved at approximately the same rate as normal chromosomal genes. Long-term IS residence in the H. pylori gene pool would provide ample opportunities for element adaptation to the host or coevolution.
Polymorphism levels within geographic subdivisions depend on population history, including bottlenecks that directly decrease effective population sizes (8, 27). Assuming that the IS elements each invaded the H. pylori gene pool prior to separation of populations and that only a fraction of the ancestral H. pylori strains also harbored each IS element, the effect of random genetic drift, which reduces the net nucleotide diversity, would have been greater on IS elements than on normal H. pylori genes, because of smaller IS population sizes. Additionally, constraints on lateral movement of these elements (e.g., due to H. pylori's preferential intrafamilial transmission) or possible selection against IS element carriage in certain lineages would increase drift by keeping the fraction of IS element-containing strains small (8). Analysis of IS607 subpopulations suggested that there was particularly strong drift among group Ia elements (Fig. 2a); the nucleotide diversity in this group (π = 0.4%) was ∼5- to 10-fold less than that in normal chromosomal genes from Japanese strains (π ranged from 1.5 to 2.8% for six chromosomal genes [Dailide and Berg, unpublished data] but see GenBank accession numbers AY152917 to AY152929, AY152970 to AY152986, AY153290 to AY153309, AY153217 to AY153238, AY153368 to AY153388, and AY153164 to AY153177). In contrast, the nucleotide diversity in IS607 group II (π = 1.8%) was ∼1.3- to 3-fold less than that in Indo-European strains (π ranged from 2.4 to 4.6% for six chromosomal genes [Dailide and Berg, unpublished] but see GenBank accession numbers AY152881 to AY152893, AY152930 to AY152941, AY153261 to AY153289, AY153204 to AY153216, AY153338 to AY1533367, and AY153151 to AY153263). The Indo-European H. pylori population is far more complex than the Japanese population; further sampling of IS607 and H. pylori populations would help us judge better how reduced the nucleotide diversity in group II is and how much of the diversity stems from genetic drift.
In contrast to IS607, the nucleotide diversity within East Asian (group I π = 3.3%) and Indo-European (group II π = 3.4%) IS605 elements (Table 4) was similar to that in normal chromosomal genes from corresponding populations. However, the data also suggested greater recombination among IS605 subpopulations than the recombination observed with IS607 (Fig. 3 and Table 6). Recombination between different subpopulations can increase the net nucleotide divergence within a given subpopulation (13) and can also reduce genetic differentiation between populations (12). This is seen in the FST values for IS605 populations (FST = 0.281; PM test P values, <0.01) relative to those of IS607 (FST = 0.648; PM test P values, <0.001) (Tables 4 and 5).
In most studies of IS element population genetics the workers have focused on elements from model enteric bacterial species (E. coli, Salmonella) and have used strains from North American or European collections. We anticipate, however, that additional sampling of Asian enteric strains would reveal little, if any, geographic clustering. The strong geographic clustering of IS element sequences seen in H. pylori is ascribed to the different population genetic structures and biology of the bacterial host species (H. pylori versus E. coli and Salmonella).
Strains of E. coli containing any one IS element were far more likely to contain additional, unrelated elements than would be expected by chance (26). This suggested that enteric IS elements were often transferred on high-capacity plasmid vectors. In contrast, only 9 (6%) of the 157 H. pylori isolates of the 488 isolates that we screened that carried either IS607 or IS605 carried both elements. This predominance of strains carrying only single elements would be explained if such high-capacity mobilizable plasmid vectors are uncommon in H. pylori populations.
Natural selection and IS element evolution.
We sought evidence for positive selection in IS genes based on the premise that IS element adaptation to its host should accelerate the accumulation of new divergent alleles (4, 66). ML analysis of ratios of nonsynonymous to synonymous nucleotide changes provided a measure of selective pressures acting along a protein-encoding sequence, and the results suggested that the IS605 and IS607 genes had been subjected to different selective pressures at different sites (Tables 7 to 10). Purifying selection dominated at most codon sites of IS elements, but positive (diversifying) selection was evident at specific codons in IS605 orfA (12.9% of the codons with an ω1 of 1.125 and 1.9% of the codons with an ω2 of 5.842), in IS605 orfB (1.5% of the codons with an ω2 of 2.613), and in IS607 orfB (10% of the codons with an ω2 of 1.665). In contrast, only purifying selection and neutral selection were detected in IS607 orfA (ω0 was 0 in 75% of the codons, and ω1 was 1 in ∼25% of the codons); no positive selection was detected.
The finding that there was positive selection in ∼15% of IS605 orfA (transposase) codons but not in IS607 orfA suggests that the unrelated transposases evolved under different selective regimens. We also detected positive selection (ω2 = 1.63) at ∼10% of the codons in the transposase of ISHp608 (data not shown), which is related to the transposase of IS605 but not to the transposase of IS607 (37). Several bacterial IS incorporate host proteins in their transposition complexes (10, 15); given the genetic variability in natural H. pylori populations, we suggest that IS605 and ISHp608 transposases adapted to interact with more than one accessory factor (or common factor with polymorphic features), whose abundance varies geographically. This proposed difference in selective forces acting on the unrelated transposases of IS605 and IS607 is consistent with the distinct insertion specificities (34, 35) and the possibility of fundamentally different mechanisms of transposition. Differences in selective pressures, recombination frequency, and random genetic drift (see above) together suggest that IS605 and IS607 have distinct evolutionary dynamics.
The dominance of purifying selection seen here, which is also evident in eukaryotic mobile elements (18, 40, 65), is consistent with a model in which transposition causes IS element proliferation in the host gene pool (38). Bacterial IS elements tend to be more active when they are introduced into a naïve cell (free of the homologous IS element) than after they are established (3, 10, 57). Counteracting the short-term selective advantage of any element that transposes frequently would be host defenses that minimize potentially deleterious consequences of transposition (usually gene inactivation or genome rearrangements) plus negative regulating mechanisms of the element itself. Several different mechanisms for regulating transposition have been identified in various elements (3, 10, 15).
Mutational studies have shown that wild-type transposases themselves are less than maximally active and that certain amino acid substitutions can increase their transposition activity (10, 17, 57). Accordingly, an alternate hypothesis for the observed positive selection involves selection for optimal (but not necessarily maximal) IS605 transposition activity in different host backgrounds. Changes that reduce transposition of established elements would often be selected because they decrease deleterious effects of transposition on host fitness (see above). Compensatory mutations that increase transposition might be selected at other times (e.g., under conditions that favor spread of the element to other genetic lineages or, in cases of transposition, that contribute to host fitness by increasing genome plasticity) (45, 48, 53). Much as in other cases of evolution in rugged landscapes (7), independent histories of mutations that cause decreased and then compensatory increased transposition activities would result in different evolutionary trajectories and manifest as positive selection (i.e., for amino acid change) at specific sites, as observed here.
Because IS orfB genes are not needed for transposition, at least in an E. coli model, selection (purifying and positive) of IS orfB genes suggests that the proteins that are encoded have other roles. The amino acid sequences of IS605 and IS607 OrfB proteins are ∼25 to 30% identical to those of the Salmonella phage-encoded GipA protein, which promotes Salmonella's growth and survival in the Peyer's patches of the intestine (60). A single C-terminal Zn(II) binding tetracysteine motif, CX2CX15-16CX2C (C4-type zinc finger), is well conserved among GipA and H. pylori IS OrfB proteins (60) and potentially would facilitate DNA or RNA binding or protein-protein interactions (42). It is thus appealing to consider the possibility that IS OrfB proteins may regulate chromosomal gene expression and/or perhaps IS element transposition and thereby contribute to H. pylori host adaptation, growth, and/or survival.
Concluding remarks.
The present study of sequences from two representative IS elements of H. pylori provided a new perspective on the evolutionary dynamics of IS elements in bacterial populations, which was not evident from studies of the unrelated IS elements of enteric bacterial models (5, 26, 41). The distinctive geographic clustering of H. pylori IS element sequences likely reflects the extreme genetic diversity and the nonclonal, geographically partitioned population structure of H. pylori hosts and the probable paucity of readily transferable, high-capacity plasmid vectors in this gastric pathogen. Some of the patterns observed here may also result from features of these IS elements themselves (such as orfB) that distinguish them from the previously studied enteric elements. Long-term association of transposable elements with their hosts can result in coevolution (38, 43). Apparently parallel evolutionary histories of IS605, IS607, and H. pylori populations and adaptive evolution in IS genes suggest such coevolutionary dynamics. Understanding when and how IS605 and IS607 affect bacterial fitness and any reciprocal effects of host factors on the fitness of IS elements themselves (the give and take of any evolutionary process) may in turn provide new insights into H. pylori's transmission, colonization, and virulence mechanisms and the evolution of its genome.
Acknowledgments
We thank many colleagues for the H. pylori strains used in this study.
This study was supported by research grants RO1 AI38166, RO3 AI49161, RO1 AI63041, RO1 DK53727, RO1 DK057826, and P30 DK52574 from the National Institutes of Health.
REFERENCES
- 1.Achtman, M., T. Azuma, D. E. Berg, Y. Ito, G. Morelli, Z. J. Pan, S. Suerbaum, S. A. Thompson, A. van der Ende, and L. J. van Doorn. 1999. Recombination and clonal groupings within Helicobacter pylori from different geographical regions. Mol. Microbiol. 32:459-470. [DOI] [PubMed] [Google Scholar]
- 2.Bandelt, H. J., and A. W. Dress. 1992. Split decomposition: a new and useful approach to phylogenetic analysis of distance data. Mol. Phylogenet. Evol. 1:242-252. [DOI] [PubMed] [Google Scholar]
- 3.Berg, D. E., and M. M. Howe. 1989. Mobile DNA. ASM Press, Washington, D.C.
- 4.Bergelson, J., M. Kreitman, E. A. Stahl, and D. Tian. 2001. Evolutionary dynamics of plant R-genes. Science 292:2281-2285. [DOI] [PubMed] [Google Scholar]
- 5.Bisercic, M., and H. Ochman. 1993. The ancestry of insertion sequences common to Escherichia coli and Salmonella typhimurium. J. Bacteriol. 175:7863-7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaser, M. J., and D. E. Berg. 2001. Helicobacter pylori genetic diversity and risk of human disease. J. Clin. Investig. 107:767-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burch, C. L., and L. Chao. 1999. Evolution by small steps and rugged landscapes in the RNA virus phi6. Genetics 151:921-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavalli-Sfroza, L. L., and W. F. Bodmer. 1999. The genetics of human populations. Dover Publications Inc., New York, N.Y.
- 9.Censini, S., C. Lange, Z. Xiang, J. E. Crabtree, P. Ghiara, M. Borodovsky, R. Rappuoli, and A. Covacci. 1996. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 93:14648-14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandler, M., and J. Mahillon. 2002. Insertion sequences revisited, p. 305-367. In N. C. Craig, R. Craigie, M. Gellert, and A. M. Lambowitz (ed.), Mobile DNA II. ASM Press, Washington, D.C.
- 11.Chao, L., C. Vargas, B. B. Spear, and E. C. Cox. 1983. Transposable elements as mutator genes in evolution. Nature 303:633-635. [DOI] [PubMed] [Google Scholar]
- 12.Cherry, J. L. 2004. Selection, subdivision, and extinction and recolonization. Genetics 166:1105-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohan, F. M. 2002. Sexual isolation and speciation in bacteria. Genetica 116:359-370. [PubMed] [Google Scholar]
- 14.Cover, T. L., D. E. Berg, M. J. Blaser, and H. L. T. Mobely. 2001. Helicobacter pylori pathogenesis, p. 509-558. In E. A. Groisman (ed.), Principles of bacterial pathogenesis. Academic Press, New York, N.Y.
- 15.Craig, N. C., R. Craigie, M. Gellert, and A. M. Lambowitz (ed.). 2002. Mobile DNA II. ASM Press, Washington, D.C.
- 16.Curcio, M. J., and K. M. Derbyshire. 2003. The outs and ins of transposition: from mu to kangaroo. Nat. Rev. Mol. Cell Biol. 4:865-877. [DOI] [PubMed] [Google Scholar]
- 17.Derbyshire, K. M., and N. D. Grindley. 1996. cis preference of the IS903 transposase is mediated by a combination of transposase instability and inefficient translation. Mol. Microbiol. 21:1261-1272. [DOI] [PubMed] [Google Scholar]
- 18.Doak, T. G., D. J. Witherspoon, C. L. Jahn, and G. Herrick. 2003. Selection on the genes of Euplotes crassus Tec1 and Tec2 transposons: evolutionary appearance of a programmed frameshift in a Tec2 gene encoding a tyrosine family site-specific recombinase. Eukaryot. Cell 2:95-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doolittle, W. F., and C. Sapienza. 1980. Selfish genes, the phenotype paradigm and genome evolution. Nature 284:601-603. [DOI] [PubMed] [Google Scholar]
- 20.Dubois, A., D. E. Berg, E. T. Incecik, N. Fiala, L. M. Heman-Ackah, J. Del Valle, M. Yang, H. P. Wirth, G. I. Perez-Perez, and M. J. Blaser. 1999. Host specificity of Helicobacter pylori strains and host responses in experimentally challenged nonhuman primates. Gastroenterology 116:90-96. [DOI] [PubMed] [Google Scholar]
- 21.Falush, D., C. Kraft, N. S. Taylor, P. Correa, J. G. Fox, M. Achtman, and S. Suerbaum. 2002. Recombination and mutation during long-term gastric colonization by Helicobacter pylori: estimates of clock rates, recombination size, and minimal age. Proc. Natl. Acad. Sci. USA 98:15056-15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falush, D., T. Wirth, B. Linz, J. K. Pritchard, M. Stephens, M. Kidd, M. J. Blaser, D. Y. Graham, S. Vacher, G. I. Perez-Perez, Y. Yamaoka, F. Megraud, K. Otto, U. Reichard, E. Katzowitsch, X. Wang, M. Achtman, and S. Suerbaum. 2003. Traces of human migrations in Helicobacter pylori populations. Science 299:1582-1585. [DOI] [PubMed] [Google Scholar]
- 23.Ghose, C., G. I. Perez-Perez, M. G. Dominguez-Bello, D. T. Pride, C. M. Bravi, and M. J. Blaser. 2002. East Asian genotypes of Helicobacter pylori strains in Amerindians provide evidence for its ancient human carriage. Proc. Natl. Acad. Sci. USA 99:15107-15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guedon, G., F. Bourgoin, M. Pebay, Y. Roussel, C. Colmin, J. M. Simonet, and B. Decaris. 1995. Characterization and distribution of two insertion sequences, IS1191 and iso-IS981, in Streptococcus thermophilus: does intergeneric transfer of insertion sequences occur in lactic acid bacteria co-cultures? Mol. Microbiol. 16:69-78. [DOI] [PubMed] [Google Scholar]
- 25.Hartl, D. L., D. E. Dykhuizen, R. D. Miller, L. Green, and J. de Framond. 1983. Transposable element IS50 improves growth rate of E. coli cells without transposition. Cell 35:503-510. [DOI] [PubMed] [Google Scholar]
- 26.Hartl, D. L., and S. A. Sawyer. 1988. Why do unrelated insertion sequences occur together in the genome of Escherichia coli. Genetics 118:537-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartl, D. L., and A. G. Clark. 1997. Principles of population genetics. Sinauer Associates Inc., Sunderland, Mass.
- 28.Hey, J. 1991. The structure of genealogies and the distribution of fixed differences between DNA sequence samples from natural populations. Genetics 128:831-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hook-Nikanne, J., D. E. Berg, R. M. Peek, Jr., D. Kersulyte, M. K. Tummuru, and M. J. Blaser. 1998. DNA sequence conservation and diversity in transposable element IS605 of Helicobacter pylori. Helicobacter 3:79-85. [DOI] [PubMed] [Google Scholar]
- 30.Hudson, R. R., D. D. Boos, and N. L. Kaplan. 1992. A statistical test for detecting geographic subdivision. Mol. Biol. Evol. 9:138-151. [DOI] [PubMed] [Google Scholar]
- 31.Hudson, R. R. 2000. A new statistic for detecting genetic differentiation. Genetics 155:2011-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huelsenbeck, J. P., and B. Rannala. 1997. Phylogenetic methods come of age: testing hypotheses in an evolutionary context. Science 276:227-232. [DOI] [PubMed] [Google Scholar]
- 33.Huson, D. H. 1998. SplitsTree: analyzing and visualizing evolutionary data. Bioinformatics 14:68-73. [DOI] [PubMed] [Google Scholar]
- 34.Kersulyte, D., N. S. Akopyants, S. W. Clifton, B. A. Roe, and D. E. Berg. 1998. Novel sequence organization and insertion specificity of IS605 and IS606: chimaeric transposable elements of Helicobacter pylori. Gene 223:175-186. [DOI] [PubMed] [Google Scholar]
- 35.Kersulyte, D., A. K. Mukhopadhyay, M. Shirai, T. Nakazawa, and D. E. Berg. 2000. Functional organization and insertion specificity of IS607, a chimeric element of Helicobacter pylori. J. Bacteriol. 182:5300-5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kersulyte, D., A. K. Mukhopadhyay, B. Velapatino, W. Su, Z. Pan, C. Garcia, V. Hernandez, Y. Valdez, R. S. Mistry, R. H. Gilman, Y. Yuan, H. Gao, T. Alarcon, M. Lopez-Brea, G. Balakrish Nair, A. Chowdhury, S. Datta, M. Shirai, T. Nakazawa, R. Ally, I. Segal, B. C. Wong, S. K. Lam, F. O. Olfat, T. Boren, L. Engstrand, O. Torres, R. Schneider, J. E. Thomas, S. Czinn, and D. E. Berg. 2000. Differences in genotypes of Helicobacter pylori from different human populations. J. Bacteriol. 182:3210-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kersulyte, D., B. Velapatino, G. Dailide, A. K. Mukhopadhyay, Y. Ito, L. Cahuayme, A. J. Parkinson, R. H. Gilman, and D. E. Berg. 2002. Transposable element IShp608 of Helicobacter pylori: nonrandom geographic distribution, functional organization, and insertion specificity. J. Bacteriol. 184:992-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kidwell, M. G., and D. R. Lisch. 2002. Perspective: transposable elements, parasitic DNA, and genome evolution. Evol. Int. J. Org. Evol. 55:1-24. [DOI] [PubMed] [Google Scholar]
- 39.Kiss, J., M. Szabo, and F. Olasz. 2003. Site-specific recombination by the DDE family member mobile element IS30 transposase. Proc. Natl. Acad. Sci. USA 100:15000-15005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lampe, D. J., D. J. Witherspoon, F. N. Soto-Adames, and H. M. Robertson. 2003. Recent horizontal transfer of mellifera subfamily mariner transposons into insect lineages representing four different orders shows that selection acts only during horizontal transfer. Mol. Biol. Evol. 20:554-562. [DOI] [PubMed] [Google Scholar]
- 41.Lawrence, J. G., H. Ochman, and D. L. Hartl. 1992. The evolution of insertion sequences within enteric bacteria. Genetics 131:9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leon, O., and M. Roth. 2000. Zinc fingers: DNA binding and protein-protein interactions. Biol. Res. 33:21-30. [DOI] [PubMed] [Google Scholar]
- 43.Levin, B. R. 1993. The accessory genetic elements of bacteria: existence conditions and (co)evolution. Curr. Opin. Genet. Dev. 3:849-854. [DOI] [PubMed] [Google Scholar]
- 44.Maynard Smith, J., and N. H. Smith. 1998. Detecting recombination from gene trees. Mol. Biol. Evol. 15:590-599. [DOI] [PubMed] [Google Scholar]
- 45.Modi, R. I., L. H. Castilla, S. Puskas-Rozsa, R. B. Helling, and J. Adams. 1992. Genetic changes accompanying increased fitness in evolving populations of Escherichia coli. Genetics 130:241-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mukhopadhyay, A. K., D. Kersulyte, J. Y. Jeong, S. Datta, Y. Ito, A. Chowdhury, S. Chowdhury, A. Santra, S. K. Bhattacharya, T. Azuma, G. B. Nair, and D. E. Berg. 2000. Distinctiveness of genotypes of Helicobacter pylori in Calcutta, India. J. Bacteriol. 182:3219-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mukhopadhyay, A. K., J. Y. Jeong, D. Dailidiene, P. S. Hoffman, and D. E. Berg. 2003. The fdxA ferredoxin gene can down-regulate frxA nitroreductase gene expression and is essential in many strains of Helicobacter pylori. J. Bacteriol. 185:2927-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naas, T., M. Blot, W. M. Fitch, and W. Arber. 1995. Dynamics of IS-related genetic rearrangements in resting Escherichia coli K-12. Mol. Biol. Evol. 12:198-207. [DOI] [PubMed] [Google Scholar]
- 49.Page, R. D. M. 1996. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 50.Page, R. D. M., and E. C. Holmes. 1998. Molecular evolution: a phylogenetic approach. Blackwell Science Inc., Malden, Mass.
- 51.Palys, T., L. K. Nakamura, and F. M. Cohan. 1997. Discovery and classification of ecological diversity in the bacterial world: the role of DNA sequence data. Int. J. Syst. Bacteriol. 47:1145-1156. [DOI] [PubMed] [Google Scholar]
- 52.Pan, Z. J., D. E. Berg, R. W. van der Hulst, W. W. Su, A. Raudonikiene, S. D. Xiao, J. Dankert, G. N. Tytgat, and A. van der Ende. 1998. Prevalence of vacuolating cytotoxin production and distribution of distinct vacA alleles in Helicobacter pylori from China. J. Infect. Dis. 178:220-226. [DOI] [PubMed] [Google Scholar]
- 53.Papadopoulos, D., D. Schneider, J. Meier-Eiss, W. Arber, R. E. Lenski, and M. Blot. 1999. Genomic evolution during a 10,000-generation experiment with bacteria. Proc. Natl. Acad. Sci. USA 96:3807-3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Picardeau, M., T. J. Bull, and V. Vincent. 1997. Identification and characterization of IS-like elements in Mycobacterium gordonae. FEMS Microbiol. Lett. 154:95-102. [DOI] [PubMed] [Google Scholar]
- 55.Posada, D., and K. A. Crandall. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817-818. [DOI] [PubMed] [Google Scholar]
- 56.Reynolds, A. E., J. Felton, and A. Wright. 1981. Insertion of DNA activates the cryptic bgl operon in E. coli K12. Nature 293:625-629. [DOI] [PubMed] [Google Scholar]
- 57.Reznikoff, W. S. 2003. Tn5 as a model for understanding DNA transposition. Mol. Microbiol. 47:1199-1206. [DOI] [PubMed] [Google Scholar]
- 58.Rozas, J., and R. Rozas. 1999. DnaSP version 3: an integrated program for molecular population genetics and molecular evolution analysis. Bioinformatics 15:174-175. [DOI] [PubMed] [Google Scholar]
- 59.Smith, M. C., and H. M. Thorpe. 2002. Diversity in the serine recombinases. Mol. Microbiol. 44:299-307. [DOI] [PubMed] [Google Scholar]
- 60.Stanley, T. L., C. D. Ellermeier, and J. M. Slauch. 2000. Tissue-specific gene expression identifies a gene in the lysogenic phage Gifsy-1 that affects Salmonella enterica serovar Typhimurium survival in Peyer's patches. J. Bacteriol. 182:4406-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suerbaum, S., J. M. Smith, K. Bapumia, G. Morelli, N. H. Smith, E. Kunstmann, I. Dyrek, and M. Achtman. 1998. Free recombination in Helicobacter pylori. Proc. Natl. Acad. Sci. USA 95:12619-12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swofford, D. L., G. J. Olsen, P. J. Wadell, and D. M. Hillis. 1996. Phylogenetic inference, p. 407-514. In D. M. Hillis, C. Moritc, and B. K. Mable (ed.), Molecular systematics. Sinauer Associates Inc., Sunderland, Mass.
- 63.Swofford, D. L. 2003. PAUP. Phylogenetic analysis using parsimony (and other methods), version 4. Sinauer Associates, Sunderland, Mass.
- 64.Wakeley, J., and J. Hey. 1997. Estimating ancestral population parameters. Genetics 145:847-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Witherspoon, D. J. 1999. Selective constraints on P element evolution. Mol. Biol. Evol. 16:472-478. [DOI] [PubMed] [Google Scholar]
- 66.Woolhouse, M. E., J. P. Webster, E. Domingo, B. Charlesworth, and B. R. Levin. 2003. Biological and biomedical implications of the co-evolution of pathogens and their hosts. Nat. Genet. 32:569-577. [DOI] [PubMed] [Google Scholar]
- 67.Yang, Z. 1997. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput. Appl. Biosci. 13:555-556. [DOI] [PubMed] [Google Scholar]
- 68.Yang, Z., R. Nielsen, N. Goldman, and A. M. Pedersen. 2000. Codon-substitution models for heterogeneous selection pressure at amino acid sites. Genetics 155:431-449. [DOI] [PMC free article] [PubMed] [Google Scholar]