Abstract
Interferon regulatory factor 7 (IRF-7) is implicated in the regulation of Epstein-Barr virus (EBV) latency. EBV transforms primary B cells, and the major EBV oncoprotein, latent membrane protein 1 (LMP-1), is required for the process. LMP-1 both induces the expression of IRF-7 and activates the IRF-7 protein by phosphorylation and nuclear translocation. Here we report that the expression of IRF-7 is increased in EBV-immortalized B lymphocytes compared with that in primary B cells. IRF-7 was phosphorylated and predominantly localized in the nucleus in the immortalized cells. The expression of IRF-7 was detected in 19 of 27 specimens of primary lymphomas of the human central nervous system by immunohistochemical analysis. The association between LMP-1 and IRF-7 was statistically highly significant for these specimens. An appreciable amount of the IRF-7 expressed in lymphoma cells was localized in the nucleus. Furthermore, IRF-7 promoted the anchorage-independent growth of NIH 3T3 cells. LMP-1 and IRF-7 showed additive effects on the growth transformation of NIH 3T3 cells. IRF-7-expressing NIH 3T3 cells formed tumors in athymic mice. Thus, IRF-7 has oncogenic properties and, along with LMP-1, may mediate or potentiate the EBV transformation process in the pathogenesis of EBV-associated lymphomas.
Interferon regulatory factors (IRFs) are a small family of transcription factors with multiple functions. The hallmark of these factors is a conserved N-terminal DNA binding domain which mediates binding to the core region of the interferon (IFN)-stimulated response element and the regulation of IFN-responsive promoters. The C-terminal portion is variable and promotes a variety of biologic functions (26, 46, 59). Some IRFs are involved in oncogenesis. IRF-2 and IRF-4 are proven oncogenes, and the overexpression of IRF-4 has been implicated in human malignancy (12, 14, 27, 44, 46). Human herpesvirus 8 (HHV-8) encodes several viral IRF homologs (vIRFs), and vIRF1 has been demonstrated to posses oncogenic potential both in vitro and in vivo (9). HHV-8 is associated with Kaposi's sarcoma and has substantial sequence homology to Epstein-Barr virus (EBV).
IRF-7 was initially identified within the biological context of EBV latency (60). EBV is a human herpesvirus of increasing clinical importance and almost certainly triggers two fatal malignancies in immunosuppressed patients without the necessity for cofactors; these malignancies are AIDS-associated central nervous system (CNS) lymphoma and posttransplantation lymphoproliferative disorders (reviewed in reference 31). In addition, EBV infection is intimately associated with the development of nasopharyngeal carcinoma (16, 28, 31, 33, 34, 37). EBV has been implicated in other malignancies, including Burkitt's lymphoma, Hodgkin's disease, and gastric carcinoma (16, 31, 37).
IRF-7 has multiple functions. IRF-7 may be involved in switching EBV latency types and in the maintenance of latency through its regulation of the viral BamHI Q promoter (Qp) for Epstein-Barr nuclear antigen 1 (EBNA-1), the usage of which is indicative of viral latency types I and II (16, 37, 56, 57, 60). In EBV-infected cells, the expression of IRF-7 is linked with that of latent membrane protein 1 (LMP-1), the principal EBV oncogene. LMP-1 both induces the expression of IRF-7 and activates the IRF-7 protein by phosphorylation and nuclear translocation (57, 58, 62). In addition, IRF-7 mediates the activation of the cellular transporter associated with antigen processing 2 (Tap-2) by LMP-1, suggesting that IRF-7 plays an important role in host immune system regulation (58). Moreover, it participates in a regulatory circuit with LMP-1 in that IRF-7 up-regulates LMP-1 promoter activity (29). Also, the IRF-7 promoter itself is activated by IRF-7-containing VAF complexes induced directly by virus infection as well as by IFN (30). Apart from its functions in EBV latent infection, IRF-7 has been shown by several laboratories to be induced and to be a primary activator of IFN genes upon infection with other viruses, pointing to the importance of IRF-7 in innate cellular defense mechanisms (2, 22, 32, 52, 61). Finally, IRF-7 is involved in monocyte differentiation processes (20). Thus, it is apparent that IRF-7 plays important roles in a variety of biological processes.
Several lines of evidence suggest that IRF-7 may have oncogenic properties. EBV transforms primary B cells into immortalized cell lines and establishes type III latency (16, 37). The expression and activation of IRF-7 are consistently associated with type III latency, which is characterized by an EBV transformation state in which LMP-1 is expressed (60). LMP-1, which is required for transformation by EBV in vitro, is consistently related to but not required for the expression of IRF-7. Based on these associations and the properties of other IRFs, we reasoned that IRF-7 may have oncogenic properties and may play a role in viral transformation. In this report, we provide evidence by the use of both cell culture systems and human lymphoma tissues that the expression and activation of IRF-7 are associated with EBV transformation processes and that IRF-7 by itself appears to have oncogenic potential.
MATERIALS AND METHODS
Cells, plasmids, and antibodies.
NIH 3T3 cells were maintained in Dulbecco's minimum essential medium supplemented with 10% calf serum. Jijoye is an EBV-positive Burkitt's lymphoma cell line (35); four newly transformed lymphoblastoid cell lines (LCL-1 to -4) were gifts from Kenneth Izumi. These cells were maintained in RPMI 1640 plus 10% fetal bovine serum. Fresh blood was purchased from a local Red Cross Station. Primary B cells were isolated by the use of CD19 antibody-conjugated magnetic beads according to the manufacturer's recommendations (Dynal Inc.). The isolated B cells were immediately lysed in 2× sodium dodecyl sulfate (SDS) protein loading buffer for Western blot analysis or were released from the beads for immunostaining experiments (1, 8, 41, 42). An LMP-1 monoclonal antibody (CS1-4) was purchased from Dako. IRF-1, IRF-2, and IRF-7 antibodies were obtained from Santa Cruz. Purified antibodies against full-length IRF-7 and the IRF-7A expression plasmid are described elsewhere (60). An LMP-1-expressing plasmid was made by the introduction of cDNA sequences into the pCDNA3.1(+)-hyg mammalian expression vector (Invitrogen).
NIH 3T3 cell transformation assays.
Subconfluent cultures of NIH 3T3 cells seeded in 60-mm-diameter tissue culture dishes were transfected with 500-ng aliquots of desired expression plasmids by the calcium phosphate method. Two days after transfection, the cultures were subcultured into growth medium supplemented with G418 and/or hygromycin to select for drug-resistant stably transfected cell populations. NIH 3T3 cells stably expressing target proteins were pooled and analyzed for anchorage-independent growth and for tumor formation in athymic (nu/nu) nude mice.
For anchorage-independent growth assays, single-cell suspensions (5 × 104 cells per 60-mm-diameter dish) of each cell line were suspended in growth medium supplemented with 0.3% agar. The appearance of proliferating colonies of cells was monitored and quantitated for up to 4 weeks.
For tumor formation in nude mice, 106 cells were trypsinized, suspended in phosphate-buffered saline solution, and then injected subcutaneously into the flanks of genetically athymic (nu/nu) mice, and the appearance of tumors was monitored biweekly.
Western blot analysis with ECL.
The separation of proteins by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) was done according to standard methods. After the proteins were transferred to a nitrocellulose or Immobilon membrane, the membrane was blocked with 5% nonfat dry milk in TBST (50 mM Tris [pH 7.5], 200 mM NaCl, 0.05% Tween 20) at room temperature for 10 min. It was then washed briefly with water and incubated with a primary antibody in 5% milk in TBST for 1 to 2 h at room temperature or overnight at 4°C. After three rinses with TBST for 10 min each time, the membrane was incubated with the secondary antibody at room temperature for 1 h. It was then washed three times with TBST as before, treated with ECL (Amersham) or SuperSignal (Pierce) detection reagents, and exposed to Kodak XAR-5 film.
Clinical samples.
A total of 27 primary CNS lymphomas was collected from the archives of the Institute of Pathology, Lausanne University, Lausanne, Switzerland. The tumors were histologically and immunohistochemically characterized according to the latest World Health Organization Classification of Brain Tumors (17). The ages of the patients ranged from 27 to 82 years (Table 1). Two patients suffered from other human immunodeficiency virus type 1 (HIV-1)-associated opportunistic infections in addition to the CNS lymphoma. Finally, in two cases, the CNS lymphoma extended through the subarachnoid space.
TABLE 1.
Immunohistochemical detection of cellular IRF-7 and viral proteins in CNS lymphomasa
| Case no. | Age (yrs) | Gender | Immunohistochemistryb
|
|||||
|---|---|---|---|---|---|---|---|---|
| CD79a | CD20 | CD3 | HIV p24 | EBV LMP-1 | IRF-7 | |||
| 1 | 47 | Female | − | ++ | − | ++ | +++ | ++ |
| 2 | 38 | Male | − | ++ | − | ++ | ++ | ++ |
| 3 | 58 | Female | + | + | − | ++ | + | ++ |
| 4 | 62 | Female | − | ++ | − | + | + | ++ |
| 5 | 35 | Male | − | + | − | +++ | ++ | + |
| 6 | 62 | Female | − | +++ | − | ++ | ++ | + |
| 7 | 38 | Male | + | + | − | + | + | − |
| 8 | 69 | Female | ++ | ++ | − | + | +++ | ++ |
| 9 | 27 | Male | + | ++ | − | +++ | ++ | − |
| 10 | 42 | Male | + | ++ | − | ++ | +++ | ++ |
| 11 | 39 | Male | + | +++ | − | ++ | + | + |
| 12 | 49 | Male | − | ++ | − | ++ | + | + |
| 13 | 77 | Female | +++ | ++ | − | + | + | − |
| 14 | 80 | Male | + | + | − | − | − | − |
| 15 | 63 | Female | ++ | ++ | − | − | − | − |
| 16 | 60 | Male | ++ | + | − | + | +++ | + |
| 17 | 82 | Male | − | +++ | − | − | − | − |
| 18 | 69 | Male | ++ | +++ | − | ++ | +++ | ++ |
| 19 | 67 | Male | ++ | ++ | − | + | ++ | + |
| 20 | 79 | Male | +++ | +++ | − | ++ | + | + |
| 21 | 76 | Male | +++ | ++ | − | + | ++ | + |
| 22 | 72 | Female | + | ++ | − | + | + | + |
| 23 | 78 | Female | − | +++ | − | ++ | + | − |
| 24 | 65 | Female | ++ | +++ | − | − | + | ++ |
| 25 | 86 | Male | +++ | +++ | − | ++ | + | − |
| 26 | 67 | Female | + | ++ | − | + | + | + |
| 27 | 69 | Female | + | +++ | − | + | ++ | + |
Diagnoses of primary CNS lymphomas were based on the WHO classification of Brain Tumors.
−, negative reactivity; +, 1 to 30% of cells were positive; ++, 31 to 60% of cells were positive; +++, >60% of cells were positive. The association between LMP-1 and IRF-7 is highly significant (R = 0.5449; P = 0.0033). The association between HIV p24 and EBV LMP-1 is also highly significant (R = 0.8478; P < 0.0001). Multiple regression analysis was done with Statistica 6.1 (StatSoft, Inc.).
Histological and immunohistochemical analysis.
Formalin-fixed, paraffin-embedded tissues were sectioned into 4-μm-thick sections and stained with hematoxylin and eosin for routine histological characterization. Immunohistochemistry was performed by use of an avidin-biotin-peroxidase complex system according to the manufacturer's instructions (Vectastain Elite ABC peroxidase kit; Vector Laboratories, Inc.). The samples were deparaffinized in xylene and rehydrated with alcohols, and nonenzymatic antigen retrieval was performed with 0.01 M sodium citrate buffer (pH 6.0) for 40 min at 95°C. After a cooling step, peroxidase quenching was performed with methanol-3% H2O2 for 20 min. The sections were then blocked in 5% normal horse or goat serum for 2 h at room temperature. The primary antibodies were incubated overnight at room temperature in a humidified chamber. The primary antibodies used for this study included two mouse monoclonal EBV LMP-1 antibodies (a cocktail of monoclonal antibodies, including clones CS1 to -4 from DAKO, used at a 1:100 dilution, and clone CS1 from Novocastra Laboratories, used at a 1:100 dilution), a mouse monoclonal antibody for the detection of CD20 (clone L26; DAKO) (1:200 dilution), a mouse monoclonal antibody for the detection of HIV p24 (clone Kal-1; DAKO) (1:20 dilution), a rabbit polyclonal IRF-7 antibody from Santa Cruz (1:50 dilution), and a purified rabbit polyclonal antibody against the full-length IRF-7 protein (1:500 dilution) (60). Biotinylated secondary mouse or rabbit antibodies were incubated for 1 h at room temperature followed by incubation with the avidin-biotin complex; finally, the sections were developed with diaminobenzidine. The slides were counterstained with hematoxylin, dehydrated, cleared in xylene, and mounted with Permount (Sigma Laboratories).
For confocal microscopy analysis, CNS specimens were deparaffinized and fixed in 4% paraformaldehyde. The sections were then blocked in phosphate-buffered saline solution plus Tween 20 plus 1% bovine serum albumin for 30 min at room temperature. The primary antibodies were incubated for 2 h at room temperature in a humidified chamber. The secondary antibodies were incubated for 1 h at room temperature followed by examination by confocal microscopy. The primary antibodies used for this study included a monoclonal antibody to LMP-1 (clones CS1 to -4; DAKO), a rabbit polyclonal antibody to IRF-7 from Santa Cruz, and a purified rabbit polyclonal antibody against the full-length IRF-7 protein (60). Cy-2-, Cy3-, and Cy5-conjugated secondary antibodies against mouse or rabbit immunoglobulin G were purchased from The Jackson Laboratory.
Phosphorylation analysis and immunoprecipitation.
CD19-positive primary B cells and freshly immortalized LCLs were labeled with [32P]orthophosphate for 4 h, washed once with 1× phosphate-buffered saline solution, and lysed in a buffer containing 0.5% NP-40, 50 mM Tris-Cl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 100 mM NaF, 1 mM sodium orthovanadate, and 1 mM phenylmethylsulfonyl fluoride. The cell lysates were precleared with a preimmune serum and protein G-agarose beads under gentle agitation at 4°C for 30 min. An IRF-7 antiserum and protein G-agarose were then added, and the lysates were incubated overnight at 4°C. The beads were washed three times with 1 ml of immunoprecipitation washing buffer, resuspended in 50 μl of SDS-PAGE sample loading buffer, and boiled for 5 min. The samples were resolved by electrophoresis in an SDS-polyacrylamide gel and then transferred onto an Immobilon membrane. The membrane was dried and autoradiographed. After autoradiography, the membrane was rehydrated with 100% methanol for 30 s and used for Western blotting to visualize the total amount of IRF-7.
RESULTS
Expression and activation of IRF-7 are associated with EBV immortalization of lymphocytes.
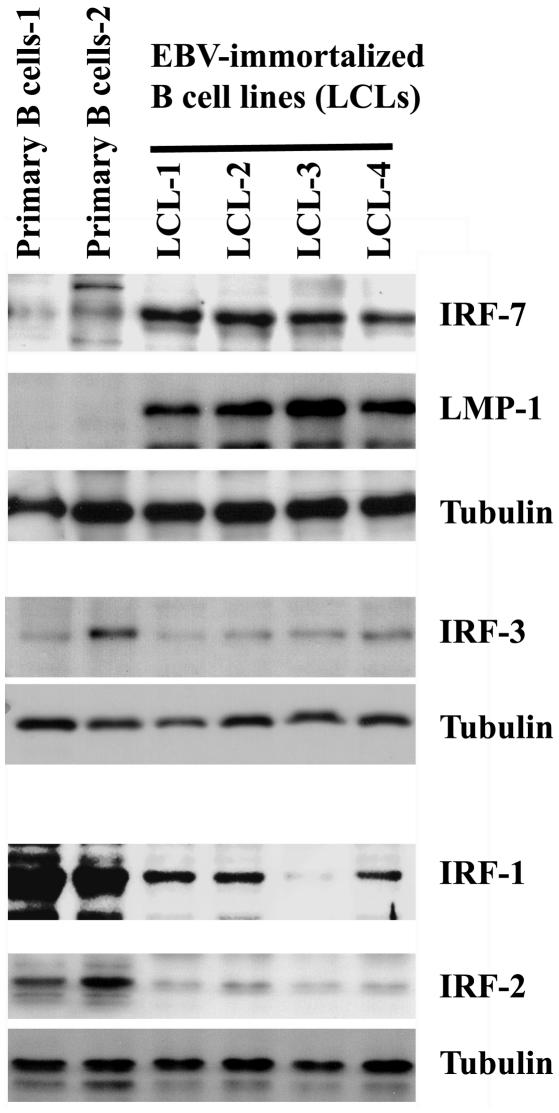
EBV efficiently immortalizes and transforms primary B lymphocytes into continually growing LCLs in vitro. LMP-1 is required for this process. We tested whether the expression of IRF-7 is induced during cell immortalization. Primary B cells from two individuals were isolated by the use of CD19 antibody-conjugated magnetic beads according to an established protocol (1, 8, 41, 42). Western blot analysis was done to compare the expression of IRF-7 in primary and recently EBV-immortalized B cells. As shown in Fig. 1, a low level of IRF-7 was detected in primary cells, but highly elevated levels were expressed in all four of the immortalized cell lines. In contrast, the expression of IRF-3, the closest IRF family member to IRF-7 (46), was not associated with cell immortalization by EBV in vitro. Neither the expression of IRF-1 nor that of IRF-2 was increased during EBV transformation (Fig. 1). Although IRF-2 is associated with EBV type III latency (56), it should be noted that cellular genes associated with type III latency may or may not be expressed in EBV-transformed cells. Bcl-2 is induced by LMP-1 and associated with type III latency (13). However, Bcl-2 is not associated with EBV transformation (24), and we confirmed this finding in our system (55).
FIG. 1.
Expression of IRF-7 is associated with EBV immortalization of B lymphocytes in vitro. Primary B cells were isolated from fresh human blood by the use of CD19 antibody-conjugated magnetic beads. CD19 is a pan-B cell marker. Lysates from primary B cells and EBV-immortalized B cells were separated by SDS-PAGE. The expression levels of IRF-7 and other proteins as indicated were determined by Western blotting.
The activation status of IRF-7 in primary B cells and that in the newly immortalized LCLs were compared. In primary B cells, IRF-7 was predominantly expressed in the cytoplasmic compartment; however, in LCLs, IRF-7 was mainly detected in the nucleus (Fig. 2A). In addition, IRF-7 was phosphorylated in the transformed cells, but phosphorylated IRF-7 could not be detected in primary uninfected B cells under our experimental conditions (Fig. 2B). The phosphorylation and translocation of IRF-7 from the cytoplasm into the nucleus may be due to the expression of LMP-1 (58), and these results suggest that the expression and activation of IRF-7 are associated with EBV transformation.
FIG. 2.
Activation of IRF-7 is detected in EBV-immortalized B cells. (A) IRF-7 is predominantly localized in the nuclei of EBV-immortalized B cells. Primary B cells were isolated from fresh human blood by the use of CD19 antibody-conjugated magnetic beads and were released from the beads before fixation with paraformaldehyde. The subcellular localization of IRF-7 in newly transformed LCLs and primary B cells was detected by staining with an IRF-7 antibody and a Cy-2-labeled donkey anti-rabbit secondary antibody, followed by examination by confocal microscopy. Propidium iodide was used to stain the nuclei. The colors were artificially mounted to facilitate viewing. Blue, nuclei; green, IRF-7. (B) IRF-7 is phosphorylated in EBV-immortalized B cells. Primary B cells isolated from fresh human blood and EBV-immortalized LCLs were labeled with [32P]orthophosphate for 4 h. The cell lysates were used for immunoprecipitation with an IRF-7 antibody. The immunoprecipitates were separated by SDS-PAGE and transferred to a membrane for Western blotting with the IRF-7 antibody, which was done after autoradiography for the detection of phospho-IRF-7.
IRF-7 is expressed in EBV-positive CNS lymphoma cells.
Primary CNS lymphomas occur with a high frequency in patients with AIDS and in recipients of organ transplants. CNS lymphomas are currently considered an HIV-defining disease. EBV has been detected in virtually all AIDS-associated CNS lymphoma specimens, and the virus is believed to be the primary causative infectious agent of the disease (10, 21, 38, 39, 43). EBV establishes type III latency in neoplastic lymphocytes, in which expression of LMP-1 is frequently detected. Because these lymphomas resemble in vitro-transformed B cells, we examined 27 human CNS lymphoma specimens by histological and immunohistochemical analyses (Table 1). The tumors were characterized according to the latest World Health Organization Classification of Brain Tumors (17). The patients had been diagnosed with CNS lymphoma and presented without the involvement of any other organ or tissue. Histologically, the tumors were characterized by abundant homogeneous neoplastic lymphocytes, located predominantly within the Virchow-Robin space in a concentric pattern. In the majority of the cases, the neoplastic cells had infiltrated the brain parenchyma (Fig. 3A). At a higher magnification, the neoplastic cells showed pleomorphic, hyperchromatic nuclei and a scant cytoplasm; mitotic figures and areas of necrosis were frequently observed (Fig. 3B). In order to characterize the variety of neoplastic cells, we performed immunohistochemistry with pan-B (CD20) and pan-T (CD3) markers. Initially, detection of the very specific but less sensitive CD79a marker was also performed. All of the tumors studied demonstrated cytoplasmic expression of CD20 and had the concentric perivascular pattern and brain parenchyma infiltration by neoplastic cells (Fig. 3C and D, respectively). The results show that only B-cell, and not T-cell, lymphomas were analyzed.
FIG. 3.
Histological and immunohistochemical characterization and expression of LMP and IRF-7 in CNS lymphomas. Hematoxylin and eosin (H.E.) staining of a CNS lymphoma demonstrated the perivascular concentric accumulation of neoplastic cells (A). At a higher magnification, the tumors were seen to be composed of numerous homogeneous round cells with atypical nuclei and a scant cytoplasm (B). Immunohistochemistry for the pan-B-cell marker CD20 highlighted the neoplastic lymphocytes in the Virchow-Robin space (C) as well as neoplastic lymphocytes infiltrating the brain parenchyma (D). The latent membrane protein (LMP-1) of EBV was robustly expressed in the cytoplasm of neoplastic lymphocytes in both the perivascular space and the brain parenchyma (E and F, respectively). Finally, the expression of IRF-7 was detected in numerous neoplastic lymphocytes (G and H, respectively). The original magnifications of the images and the identities of the proteins are indicated.
We further examined the EBV status and IRF-7 expression of the CNS lymphomas. LMP-1 was detected by immunohistochemistry in numerous neoplastic lymphocytes in both the Virchow-Robin space and individual lymphocytes infiltrating the brain parenchyma (Fig. 3E and F, respectively). Immunohistochemistry with IRF-7 antibodies showed a robust immunolabeling of neoplastic lymphocytes (Fig. 3E and F). With this assay, IRF-7 was detected in 19 of 27 tumor specimens examined (70.4%). In addition, LMP-1 was detected in 24 of the 27 specimens, and the association between LMP-1 and IRF-7 was statistically highly significant (R = 0.5449; P = 0.0033) (Table 1). Due to the fact that high levels of LMP-1 and IRF-7 are required for detection by immunochemistry, these data strongly suggest that high levels of LMP-1 are associated with high levels of IRF-7 in CNS lymphomas. In addition, EBV LMP-1 was associated with HIV infection, as shown by the detection of the p24 antigen in the tumor specimens in all but four cases (Table 1).
IRF-7 is associated with LMP-1-expressing CNS lymphoma cells.
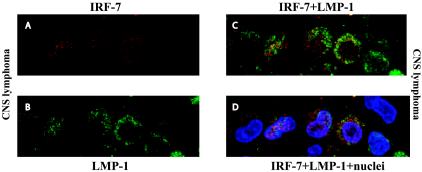
Finally, we used another assay to detect the relationship between LMP-1 and IRF-7 in CNS lymphomas, namely, double immunolabeling followed by confocal microscopy. With confocal microscopy, IRF-7 was detected in virtually all of the specimens, as the fluorescence signals could be amplified with a higher sensitivity. Since primary B cells expressed IRF-7 (Fig. 1 and 2), the detection of IRF-7 in these B-cell lymphomas was anticipated. As shown in Fig. 4, however, LMP-1-positive lymphoma cells expressed higher levels of IRF-7 than did LMP-1-negative cells (panel D). In addition, some IRF-7 localized in the nucleus, sometimes predominantly, and was presumably activated in LMP-1-expressing cells. Because LMP-1 is a marker for CNS lymphoma, these results provide further evidence that IRF-7 is highly expressed and can move into the nucleus or be activated in CNS lymphoma cells in vivo. In EBV-negative cells, IRF-7 expression was lower than in LMP-1-positive cells (Fig. 4). Also, due to paraffin embedding, the background was higher than in isolated primary B cells (compare Fig. 2 and 4). Thus, the subcellular localization of IRF-7 in LMP-1-negative cells is hardly conclusive. In addition, due to the fact that LMP-1 is a marker for CNS lymphoma, we cannot be certain that the LMP-1-negative cells were actually CNS lymphomas without another credible tumor marker. Based on the subcellular localization of IRF-7 in primary B cells (Fig. 2A) and confocal imaging of the CNS lymphoma specimens, IRF-7 is likely localized in the cytoplasm in LMP-1-negative cells. Thus, by using immunohistological staining as well as confocal imaging, we concluded that IRF-7 is highly expressed in EBV-positive CNS lymphoma cells and, furthermore, that some IRF-7 is activated, i.e., localized in the nucleus, in LMP-1-positive lymphoma cells.
FIG. 4.
IRF-7 is expressed and localized in the nuclei of LMP-1-expressing CNS lymphoma cells. A CNS lymphoma specimen was stained with IRF-7 (rabbit) and LMP-1 (mouse) antibodies. Cy5- and Cy2-labeled secondary antibodies were used to distinguish the signals from IRF-7 and LMP-1, respectively. Propidium iodide was used to stain the nuclei. The colors were artificially mounted to facilitate viewing. Blue, nuclei; green, LMP-1; red, IRF-7. (A) IRF-7 signal only; (B) LMP-1 signal only; (C) LMP-1 plus IRF-7 signals; (D) LMP-1, IRF-7, and nucleus signals are mixed.
IRF-7 causes anchorage-independent growth in NIH 3T3 cells.
The foregoing data suggest that IRF-7 may be a mediator for EBV, or LMP-1 in particular, during the transformation process. Because some IRFs are known to have oncogenic potential, whether IRF-7 has similar properties was evaluated by use of the in vitro transformation assay system used to evaluate other IRF members. We first examined whether IRF-7 is capable of transforming NIH 3T3 mouse fibroblasts. An IRF-7 plasmid was transfected into NIH 3T3 cells, and the IRF-7-expressing cells were selected in G418-containing medium. Stable cell lines were pooled, and the expression of IRF-7 was confirmed by immunoprecipitation and Western blot analyses (Fig. 5B). IRF-7 has several splicing forms. For this work, we used an expression plasmid for the full-length IRF-7A because it is the major form, at least in lymphocytes (60). As shown in Fig. 5, IRF-7 could induce the growth of NIH 3T3 cells in soft agar. These data indicate that IRF-7 has transforming activity in cell culture.
FIG. 5.
IRF-7 causes anchorage-independent growth of NIH 3T3 cells. (A) A soft-agar assay was used for an IRF-7 stably expressing cell line established in NIH 3T3 cells. The numbers are averages from two experiments. (B) Expression of IRF-7 in NIH 3T3 cells, as determined by immunoprecipitation followed by Western blotting with an IRF-7 antibody.
IRF-7-expressing NIH 3T3 cells cause tumor formation in nude mice.
The induction of anchorage-independent growth by IRF-7 in NIH 3T3 cells raised the possibility that IRF-7 might have oncogenic potential. We further tested whether IRF-7-expressing NIH 3T3 cells are capable of inducing tumor formation in vivo. Cells from the parental NIH 3T3 cell lines, the empty pcDNA3 (neo) vector control, and pcDNA-IRF-7A(neo)-transfected cells (106 cells) were injected subcutaneously into the flanks of athymic (nu/nu) mice, and the appearance of tumor formation was monitored every 2 weeks. As shown in Table 2, the IRF-7-expressing cells were capable of inducing tumor formation. In addition, IRF-7 continued to be expressed in the tumors growing in the mice (Fig. 6B). It should be noted that human IRF-7 (hIRF-7) and mouse IRF-7 (mIRF-7) have different molecular masses (69 versus 46 kDa) and can thus be distinguished. Also, the IRF-7 expressed in these tumor-derived cells was predominantly localized in the cytoplasm, as determined by immunostaining (data not shown); however, we could not readily distinguish the signal derived from the endogenous mIRF-7 from that derived from the ectopically expressed hIRF-7 with the available antibodies.
TABLE 2.
IRF-7 causes tumor formation in nude micea
| Expt or cell type | Tumor formation (no. of mice with tumors/total no. of mice)
|
||
|---|---|---|---|
| 1 month | 2 months | 3 months | |
| 1 | |||
| Parental (NIH 3T3) | 0/3 | 0/3 | 0/3 |
| Vector (pcDNA3) | 0/3 | 0/3 | 1/3 |
| IRF-7A | 0/3 | 2/3 | 3/3 |
| 2 | |||
| Parental (NIH 3T3) | 0/8 | 0/8 | |
| Vector (pcDNA3) | 0/8 | 1/8 | |
| IRF-7A | 0/8 | 8/8 | |
Nude mice were injected with parental cells (NIH 3T3) or IRF-7-expressing cells (IRF-7A) or a vector control cell line (pcDNA3). The numbers of mice with tumors were counted at the indicated times.
FIG. 6.
IRF-7 causes tumor formation in nude mice. (A) IRF-7-expressing cells induce tumors. A tumor in the flank of a nude mouse injected with IRF-7-expressing cells is shown. (B) Tumors express IRF-7. Cell lysates from the IRF-7-derived tumors were analyzed by Western blotting with an IRF-7 antibody. The positive control was a cell lysate from Jijoye cells. (C and D) Paraffin sections from IRF-7-containing tumors were stained with hematoxylin and eosin and viewed at magnifications of ×40 and ×100, respectively.
IRF-7 and LMP-1 have an additive effect on anchorage-independent growth of NIH 3T3 cells.
Because the expression of LMP-1 and IRF-7 seemed to be closely associated in human CNS lymphomas in vivo (Fig. 4), we examined whether LMP-1 and IRF-7 might have synergistic effects. IRF-7, with or without the LMP-1 plasmid, was transfected, and stably expressing clones were pooled and assayed for anchorage-independent growth. As shown in Fig. 7, LMP-1 and IRF-7 had an additive and possibly cooperative, but not synergistic, effect on the anchorage-independent growth of NIH 3T3 cells. LMP-1 itself also caused cellular transformation, as reported previously (11, 49, 50). It should be mentioned that the additive or cooperative effect of LMP-1 and IRF-7 was apparent only in the in vitro situation, since a corresponding effect has not been observed for the tumor formation assay with nude mice in vivo (data not shown). Possible explanations include the fact that LMP-1 itself is a strong inducer of tumors in mice and needs only 3 weeks for tumor formation; however, IRF-7 may need up to 2 months for tumor formation. In addition, NIH 3T3 cells express endogenous murine IRF-7 that may be activated by LMP-1.
FIG. 7.
IRF-7 and LMP-1 have an additive effect on anchorage-independent growth of NIH 3T3 cells. Soft-agar assays were used for a stably expressing cell line established in NIH 3T3 cells. The colony numbers are averages from two experiments.
DISCUSSION
The unique characteristic of EBV as a human virus is its ability to immortalize and transform primary human B cells in vitro. LMP-1 is the principal oncoprotein required for immortalization and transformation. In addition, the expression of LMP-1 in nasopharyngeal carcinomas, AIDS-associated CNS lymphomas, and posttransplantation lymphoproliferative disorders is consistent. Because in lymphomas immortalization takes place in cells lacking genetic mutations acquired during cell culture, at least initially, LMP-1 must contribute to cellular transformation by altering the expression and activity of cellular genes that are involved in oncogenesis.
One of the genes regulated by LMP-1 is IRF-7. Some IRFs are already known to have oncogenic properties. IRF-2 and IRF-4 are considered oncogenes, and IRF-4 has been implicated in multiple myelomas because a chromosomal translocation of the IRF-4 gene was found in some of these neoplasms (14, 27, 44, 46). HHV-8 vIRF1 is apparently also oncogenic, as shown by the transformation of NIH 3T3 cells and tumor formation in nude mice (9).
IRF-7 may contribute to oncogenesis by LMP-1. First, IRF-7 itself has oncogenic potential, as shown by its ability to cause anchorage-independent growth in NIH 3T3 cells as well as to induce tumors in nude mice (Fig. 5 and 6). These data suggest that the expression of IRF-7 per se is able to transform 3T3 cells. It is interesting that IRF-7 exists in at least two forms, an activated and an inactivated form. LMP-1-activated IRF-7 is a phosphoprotein and is localized to the nucleus (58). However, IRF-7 itself is also a phosphoprotein, and trace amounts are localized to the nucleus even in the absence of LMP-1. Trace amounts of activated IRF-7 can be detected when IRF-7 is overexpressed (19, 23, 58). Although the exact nature of activated IRF-7 is unknown, our data do not eliminate the possibility that these trace amounts contribute to or are even responsible for the oncogenic properties of IRF-7. Our data show that (i) LMP-1 and IRF-7 have a cooperative or at least an additive effect on transformation (Fig. 7) which may be related to the activation processes of IRF-7 (58) and (ii) IRF-7 can activate the EBV LMP-1 promoter and increase the expression of LMP-1 (29). Because IRF-7 is expressed in human B cells (Fig. 1 and 2), these data provide evidence that IRF-7 may contribute to the transformation capability of LMP-1 directly by a cooperative (or additive) functional interaction with LMP-1 or indirectly by the induction of LMP-1.
One possible mechanism whereby IRF-7 transforms 3T3 cells is by acting like IRF-2, which blocks the functions of IRF-1, a putative tumor suppressor gene (46). The mechanism used by IRF-2 to transform cells is to compete with IRF-1 for DNA binding, thus disrupting the tumor suppressor function of IRF-1. The ratio of IRF-1 to IRF-2 is thought to be important for tumor development (27, 45, 53). IRF-7 also competes with IRF-1 for DNA binding, at least in vitro (60). The same competition between the two IRFs may also occur in vivo, especially when IRF-7 is activated. A similar mechanism might also apply to the transformation of rodent cells by IRF-7. It is noteworthy that the ratios of IRF-1 to IRF-2 are similar for primary B cells and EBV-transformed B cells; however, the ratios of IRF-1 to IRF-7 are drastically changed during the transformation process (Fig. 1). Thus, one possible mechanism for EBV oncogenesis is that IRF-7, which is induced by LMP-1, modifies the genes that are regulated by the putative tumor suppressor gene IRF-1. It should be stressed that IRF-7 may use additional mechanisms for transformation.
IRF-7 may play a role in EBV-associated tumors. One malignancy that is believed to be caused by EBV is CNS lymphoma in immunocompromised hosts. EBV establishes type III latency with LMP-1 expression in these B-lymphoma cells. The cells are phenotypically similar to in vitro-transformed LCLs. We have shown that the expression and activation of IRF-7 are associated with the EBV immortalization and transformation process in vitro (Fig. 1 and 2). Furthermore, for CNS lymphoma cells, the association between high levels of LMP-1 and IRF-7 is highly significant (Table 1). IRF-7 is expressed and activated in LMP-1-expressing CNS lymphoma cells in vivo (Table 1; Fig. 3 and 4). The cooperative activity by LMP-1 and IRF-7 may contribute to the oncogenesis of at least some CNS lymphomas (Table 1; Fig. 3, 4, and 7). Moreover, IRF-7 can induce the LMP-1 promoter, and the activation of IRF-7 may enhance this induction (29). This circuit may potentiate LMP-1's activity in vivo by enhancing its expression (29).
The data strongly support the contention that IRF-7 expression in human cancer is meaningful and suggest that nuclear IRF-7 is relevant to viral transformation. However, whether activated IRF-7 is an effector in cellular transformation, especially if it is triggered by LMP-1, remains to be determined. It should be noted that posttranslationally activated proteins can have oncogenic roles. A well-characterized example is the activated STAT-3 protein, which plays essential roles in cellular transformation and the growth of cancer cells (3).
Generally, the transformation of a cell requires multiple molecular events (6, 15, 18, 36, 40). LMP-1 is an oncogenic protein that is required for the transformation of primary B cells in vitro and is believed to drive the EBV transformation process in vivo. LMP-1, through its signaling from the cell membrane, activates a variety of cellular genes involved in cell proliferation, apoptosis, angiogenesis, immune regulation, and signal transduction, to which may now be added IRF-7, that collectively transform and enhance the malignant potential of cells (4, 5, 7, 13, 25, 47-49, 51, 54).
Acknowledgments
We thank Charles Wood and Clinton Jones for the use of equipment, Guoqing Lu for statistical analyses, and Ke Hong and Sarah Johnson for technical help.
This work was supported in part by a grant from the National Institute of Allergy and Infectious Diseases (AI 42371) to J.S.P. and by grants from the Tobacco Settlement Biomedical Research Enhancement Fund, the Layman Foundation, and the Nebraska Center for Virology, funded by the National Institutes of Health (RR15635) from the COBRE Program of the National Center for Research Resources, to L.Z.
REFERENCES
- 1.Allday, M. J., A. Sinclair, G. Parker, D. H. Crawford, and P. J. Farrell. 1995. Epstein-Barr virus efficiently immortalizes human B cells without neutralizing the function of p53. EMBO J. 14:1382-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Au, W. C., P. A. Moore, D. W. LaFleur, B. Tombal, and P. M. Pitha. 1998. Characterization of the interferon regulatory factor-7 and its potential role in the transcription activation of interferon A genes. J. Biol. Chem. 273:29210-29217. [DOI] [PubMed] [Google Scholar]
- 3.Bromberg, J. F., M. H. Wrzeszczynska, G. Devgan, Y. Zhao, R. G. Pestell, C. Albanese, and J. E. J. Darnell. 1999. Stat3 as an oncogene. Cell 98:295-303. [DOI] [PubMed] [Google Scholar]
- 4.Cahir-McFarland, E. D., D. M. Davidson, S. L. Schauer, J. Duong, and E. Kieff. 2000. NF-kappa B inhibition causes spontaneous apoptosis in Epstein-Barr virus-transformed lymphoblastoid cells. Proc. Natl. Acad. Sci. USA 97:6055-6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, H., L. Hutt-Fletcher, L. Cao, and S. D. Hayward. 2003. A positive autoregulatory loop of LMP1 expression and STAT activation in epithelial cells latently infected with Epstein-Barr virus. J. Virol. 77:4139-4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole, M. D., and S. B. McMahon. 1999. The Myc oncoprotein: a critical evaluation of transactivation and target gene regulation. Oncogene 18:2916-2924. [DOI] [PubMed] [Google Scholar]
- 7.Fries, K. L., W. E. Miller, and N. Raab-Traub. 1996. Epstein-Barr virus latent membrane protein 1 blocks p53-mediated apoptosis through the induction of the A20 gene. J. Virol. 70:8653-8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frost, V., S. Delikat, S. Al-Mehairi, and A. J. Sinclair. 2001. Regulation of p27KIP1 in Epstein-Barr virus-immortalized lymphoblastoid cell lines involves non-apoptotic caspase cleavage. J. Gen. Virol. 82:3057-3066. [DOI] [PubMed] [Google Scholar]
- 9.Gao, S. J., C. Boshoff, S. Jayachandra, R. A. Weiss, Y. Chang, and P. S. Moore. 1997. KSHV ORF K9 (vIRF) is an oncogene which inhibits the interferon signaling pathway. Oncogene 15:1979-1985. [DOI] [PubMed] [Google Scholar]
- 10.Guterman, K. S., L. S. Hair, and S. Morgello. 1996. Epstein-Barr virus and AIDS-related primary central nervous system lymphoma. Viral detection by immunohistochemistry, RNA in situ hybridization, and polymerase chain reaction. Clin. Neuropathol. 15:79-86. [PubMed] [Google Scholar]
- 11.Hammerschmidt, W., B. Sugden, and V. R. Baichwal. 1989. The transforming domain alone of the latent membrane protein of Epstein-Barr virus is toxic to cells when expressed at high levels. J. Virol. 63:2469-2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harada, H., M. Kitagawa, N. Tanaka, H. Yamamoto, K. Harada, M. Ishihara, and T. Taniguchi. 1993. Anti-oncogenic and oncogenic potentials of interferon regulatory factors-1 and -2. Science 259:971-974. [DOI] [PubMed] [Google Scholar]
- 13.Henderson, S., M. Rowe, C. Gregory, D. Croom-Carter, F. Wang, R. Longnecker, E. Kieff, and A. Rickinson. 1991. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell 65:1107-1115. [DOI] [PubMed] [Google Scholar]
- 14.Iida, S., P. H. Rao, M. Butler, P. Corradini, M. Boccadoro, B. Klein, R. S. Chaganti, and R. Dalla-Favera. 1997. Deregulation of MUM1/IRF4 by chromosomal translocation in multiple myeloma. Nat. Genet. 17:226-230. [DOI] [PubMed] [Google Scholar]
- 15.Kelekar, A., and M. D. Cole. 1986. Tumorigenicity of fibroblast lines expressing the adenovirus E1a, cellular p53, or normal c-Myc genes. Mol. Cell. Biol. 6:7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kieff, E. 1996. Epstein-Barr virus and its replication, p. 2343-2396. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 17.Kleihues, P., D. N. Louis, B. W. Scheithauer, L. B. Rorke, G. Reifenberger, P. C. Burger, and W. K. Cavenee. 2002. The W.H.O. classification of tumors of the nervous system. J. Neuropathol. Exp. Neurol. 61:215-225. [DOI] [PubMed] [Google Scholar]
- 18.Kohl, N. E., and H. E. Ruley. 1987. Role of c-myc in the transformation of REF52 cells by viral and cellular oncogenes. Oncogene 2:41-48. [PubMed] [Google Scholar]
- 19.Lin, R., P. Genin, Y. Mamane, and J. Hiscott. 2000. Selective DNA binding and association with the CREB binding protein coactivator contribute to differential activation of alpha/beta interferon genes by interferon regulatory factors 3 and 7. Mol. Cell. Biol. 20:6342-6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu, R., and P. M. Pitha. 2001. Monocyte differentiation to macrophage requires interferon regulatory factor 7. J. Biol. Chem. 276:45491-45496. [DOI] [PubMed] [Google Scholar]
- 21.MacMahon, E. M., J. D. Glass, S. D. Hayward, R. B. Mann, P. S. Becker, P. Charache, J. C. McArthur, and R. F. Ambinder. 1991. Epstein-Barr virus in AIDS-related primary central nervous system lymphoma. Lancet 338:969-973. [DOI] [PubMed] [Google Scholar]
- 22.Marie, I., J. E. Durbin, and D. E. Levy. 1998. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 17:6660-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marie, I., E. Smith, A. Prakash, and D. E. Levy. 2000. Phosphorylation-induced dimerization of interferon regulatory factor 7 unmasks DNA binding and a bipartite transactivation domain. Mol. Cell. Biol. 20:8803-8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin, J. M., D. Veis, S. J. Korsmeyer, and B. Sugden. 1993. Latent membrane protein of Epstein-Barr virus induces cellular phenotypes independently of expression of Bcl-2. J. Virol. 67:5269-5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller, W. E., H. S. Earp, and N. Raab-Traub. 1995. The Epstein-Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor. J. Virol. 69:4390-4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen, H., J. Hiscott, and P. M. Pitha. 1997. The growing family of interferon regulatory factors. Cytokine Growth Factor Rev. 8:293-312. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen, H., A. Mustafa, J. Hiscott, and R. Lin. 1995. Transcription factor IRF-2 exerts its oncogenic phenotype through the DNA binding/transcription repression domain. Oncogene 11:537-544. [PubMed] [Google Scholar]
- 28.Niedobitek, G. 2000. Epstein-Barr virus infection in the pathogenesis of nasopharyngeal carcinoma. Mol. Pathol. 53:248-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ning, S., A. Hahn, L. Huye, and J. S. Pagano. 2003. Interferon regulatory factor 7 regulates expression of Epstein-Barr virus latent membrane protein 1: a regulatory circuit. J. Virol. 77:9359-9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ning, S., L. Huye, and J. S. Pagano. Submitted for publication.
- 31.Pagano, J. S. 1999. Epstein-Barr virus: the first human tumor virus and its role in cancer. Proc. Assoc. Am. Physicians 111:573-580. [DOI] [PubMed] [Google Scholar]
- 32.Qing, J., C. Liu, L. Choy, R. Y. Wu, J. S. Pagano, and R. Derynck. 2004. Transforming growth factor beta/Smad3 signaling regulates IRF-7 function and transcriptional activation of the beta interferon promoter. Mol. Cell. Biol. 24:1411-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raab-Traub, N. 1992. Epstein-Barr virus and nasopharyngeal carcinoma. Cancer Biol. 3:297-307. [PubMed] [Google Scholar]
- 34.Raab-Traub, N. 1996. Pathogenesis of Epstein-Barr virus and its associated malignancies. Semin. Virol. 7:315-323. [Google Scholar]
- 35.Ragona, G., I. Ernberg, and G. Klein. 1980. Induction and biological characterization of the Epstein-Barr virus carried by the Jijoye lymphoma cell line. Virology 101:553-557. [DOI] [PubMed] [Google Scholar]
- 36.Ralston, R. 1991. Complementation of transforming domains in E1a/myc chimaeras. Nature 353:866-868. [DOI] [PubMed] [Google Scholar]
- 37.Rickinson, A. B., and E. Kieff. 1996. Epstein-Barr Virus, p. 2397-2446. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 38.Rodriguez, M. M., P. I. Delgado, and C. K. Petito. 1997. Epstein-Barr virus-associated primary central nervous system lymphoma in a child with the acquired immunodeficiency syndrome. A case report and review of the literature. Arch. Pathol. Lab. Med. 121:1287-1291. [PubMed] [Google Scholar]
- 39.Scadden, D. T. 2000. Epstein-Barr virus, the CNS, and AIDS-related lymphomas: as close as flame to smoke. J. Clin. Oncol. 18:3323-3324. [DOI] [PubMed] [Google Scholar]
- 40.Shalloway, D., P. J. Johnson, E. O. Freed, D. Coulter, and W. A. Flood, Jr. 1987. Transformation of NIH 3T3 cells by cotransfection with c-Src and nuclear oncogenes. Mol. Cell. Biol. 7:3582-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinclair, A. J., I. Palmero, A. Holder, G. Peters, and P. J. Farrell. 1995. Expression of cyclin D2 in Epstein-Barr virus-positive Burkitt's lymphoma cell lines is related to methylation status of the gene. J. Virol. 69:1292-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinclair, A. J., I. Palmero, G. Peters, and P. J. Farrell. 1994. EBNA-2 and EBNA-LP cooperate to cause G0 to G1 transition during immortalization of resting human B lymphocytes by Epstein-Barr virus. EMBO J. 13:3321-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taiwo, B. O. 2000. AIDS-related primary CNS lymphoma: a brief review. AIDS Read. 10:486-491. [PubMed] [Google Scholar]
- 44.Tanaka, N., and T. Taniguchi. 2000. The interferon regulatory factors and oncogenesis. Semin. Cancer Biol. 10:73-81. [DOI] [PubMed] [Google Scholar]
- 45.Taniguchi, T., H. Harada, and M. Lamphier. 1995. Regulation of the interferon system and cell growth by the IRF transcription factors. J. Cancer Res. Clin. Oncol. 121:516-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taniguchi, T., K. Ogasawara, A. Takaoka, and N. Tanaka. 2001. IRF family of transcription factors as regulators of host defense. Annu. Rev. Immunol. 19:623-655. [DOI] [PubMed] [Google Scholar]
- 47.Wakisaka, N., S. Kondo, T. Yoshizaki, S. Murono, M. Furukawa, and J. S. Pagano. 2004. Epstein-Barr virus latent membrane protein 1 induces synthesis of hypoxia-inducible factor 1 alpha. Mol. Cell. Biol. 24:5223-5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wakisaka, N., S. Murono, T. Yoshizaki, M. Furukawa, and J. S. Pagano. 2002. Epstein-Barr virus latent membrane protein 1 induces and causes release of fibroblast growth factor-2. Cancer Res. 62:6337-6344. [PubMed] [Google Scholar]
- 49.Wang, D., D. Leibowitz, and E. Kieff. 1985. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell 43:831-840. [DOI] [PubMed] [Google Scholar]
- 50.Wang, D., D. Liebowitz, and E. Kieff. 1988. The truncated form of the Epstein-Barr virus latent-infection membrane protein expressed in virus replication does not transform rodent fibroblasts. J. Virol. 62:2337-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, F., C. Gregory, C. Sample, M. Rowe, D. Liebowitz, R. Murray, A. Rickinson, and E. Kieff. 1990. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J. Virol. 64:2309-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wathelet, M. G., C. H. Lin, B. S. Parekh, L. V. Ronco, P. M. Howley, and T. Maniatis. 1998. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-beta enhancer in vivo. Mol. Cell 1:507-518. [DOI] [PubMed] [Google Scholar]
- 53.Willman, C. L., C. E. Sever, M. G. Pallavicini, H. Harada, N. Tanaka, M. L. Slovak, H. Yamamoto, K. Harada, T. C. Meeker, A. F. List, et al. 1993. Deletion of IRF-1, mapping to chromosome 5q31.1, in human leukemia and preleukemic myelodysplasia. Science 259:968-971. [DOI] [PubMed] [Google Scholar]
- 54.Yoshizaki, T., H. Sato, M. Furukawa, and J. S. Pagano. 1998. The expression of matrix metalloproteinase 9 is enhanced by Epstein-Barr virus latent membrane protein 1. Proc. Natl. Acad. Sci. USA 95:3621-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang, L., K. Hong, J. Zhang, and J. Pagano. 2004. Multiple signal transducers and activators of transcription (STATs) are induced by EBV LMP-1. Virology 323:141-152. [DOI] [PubMed] [Google Scholar]
- 56.Zhang, L., and J. S. Pagano. 1999. Interferon regulatory factor 2 represses the Epstein-Barr virus BamHI Q latency promoter in type III latency. Mol. Cell. Biol. 19:3216-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang, L., and J. S. Pagano. 2000. Interferon regulatory factor 7 is induced by Epstein-Barr virus latent membrane protein 1. J. Virol. 74:5748-5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang, L., and J. S. Pagano. 2001. Interferon regulatory factor 7 mediates the activation of Tap-2 by Epstein-Barr virus latent membrane protein 1. J. Virol. 75:341-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang, L., and J. S. Pagano. 2001. Interferon regulatory factor 7: a key cellular mediator of LMP-1 in EBV latency and transformation. Semin. Cancer Biol. 11:445-453. [DOI] [PubMed] [Google Scholar]
- 60.Zhang, L., and J. S. Pagano. 1997. IRF-7, a new interferon regulatory factor associated with Epstein-Barr virus latency. Mol. Cell. Biol. 17:5748-5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, L., and J. S. Pagano. 2002. Review: structure and function of IRF-7. J. Interferon Cytokine Res. 22:95-101. [DOI] [PubMed] [Google Scholar]
- 62.Zhang, L., L. H. Wu, K. Hong, and J. S. Pagano. 2001. Intracellular signaling molecules activated by Epstein-Barr virus for induction of interferon regulatory factor 7. J. Virol. 75:12393-12401. [DOI] [PMC free article] [PubMed] [Google Scholar]