Abstract
We generated mice expressing cervid prion protein to produce a transgenic system simulating chronic wasting disease (CWD) in deer and elk. While normal mice were resistant to CWD, these transgenic mice uniformly developed signs of neurological dysfunction ∼230 days following intracerebral inoculation with four CWD isolates. Inoculated transgenic mice homozygous for the transgene array developed disease after ∼160 days. The brains of sick transgenic mice exhibited widespread spongiform degeneration and contained abnormal prion protein and abundant amyloid plaques, many of which were florid plaques. Transmission studies indicated that the same prion strain caused CWD in the analyzed mule deer and elk. These mice provide a new and reliable tool for detecting CWD prions.
The transmissible spongiform encephalopathies (TSEs), are fatal neurodegenerative conditions which include human Creutzfeldt-Jakob disease (CJD), scrapie of sheep and goats, and bovine spongiform encephalopathy (BSE). Because of their extraordinary biology and the unique properties of the infectious agent, these diseases attracted interest well before the advent and epizootic spread of BSE (40) and the subsequent appearance of a variant of CJD (vCJD) (41). The time between infection and disease is extremely long, and for this reason, these diseases were originally thought to be caused by “slow viruses.” However, while the molecular structure of the agent still eludes definitive identification, it is widely accepted that these diseases are caused by prions, which are defined as proteinaceous infectious particles that lack informational nucleic acid (25). Considerable evidence suggests that prions consist largely, if not exclusively, of a disease-associated version of the prion protein (PrP). This isoform, referred to as PrPSc, is an abnormally folded, protease-resistant, β-sheet-rich version of a normally benign cellular protein referred to as PrPC, which is protease sensitive and rich in α-helix. According to the prion hypothesis, the central event in the propagation of prion infectivity is the coercion of cellular prion protein by PrPSc to adopt the disease-associated conformation.
The various prion diseases share a number of characteristic features, the most consistent being the neuropathologic changes that accompany disease in the central nervous system (CNS). These include neuronal vacuolation and degeneration, which confer a spongiform appearance upon the cerebral grey matter, and a reactive proliferation of astroglial cells. The lack of an inflammatory response is also an important trait. While by no means a constant feature, some examples of prion disease are characterized by the deposition of amyloid plaques composed of insoluble aggregates of PrP.
Another important aspect of prion diseases is their transmissibility. Inoculation of diseased brain material into individuals of the same species will typically reproduce the disease. In contrast, the passage of prions from one species to another is generally inefficient and is referred to as the species barrier. Expression of foreign and chimeric prion protein genes in transgenic (Tg) mice has been an effective way to probe the molecular basis of the species barrier (30, 31, 33, 37, 38). Experiments in Tg mice demonstrated that the degree of homology between PrP molecules in the host and inoculum was an important determinant of the species barrier (26).
An equally important component affecting prion transmission barriers is the strain of prion. Mammalian prion strains are classically defined in terms of their incubation times in susceptible animals and the profile of lesions they produce in the CNS. Differences in the neuroanatomic distribution of PrPSc are a parameter that has also been used to define prion strains (11, 15, 18, 19). More recently attempts have been made to use biochemical and/or immunological properties of PrPSc as markers of prion strain differences (6, 24, 28, 36). Seminal studies suggesting that PrPSc conformation was the basis of prion strain diversity arose from investigations of transmissible mink encephalopathy, which, upon transmission, produced different clinical symptoms and produced PrPSc with different resistances to proteinase K digestion and altered amino-terminal proteinase K cleavage sites (3). Evidence supporting the concept that strain diversity is encoded in the tertiary structure of PrPSc emerged from transmission studies of inherited and sporadic human prion diseases in Tg mice (15, 17, 36). Banding patterns of PrPSc forms with different glycosylation patterns and sizes of PrPSc fragments following proteinase K treatment have also been used to classify CJD strains (6, 12, 23).
Of all the prion diseases, chronic wasting disease (CWD) is perhaps the least understood. CWD was first recognized as a spongiform encephalopathy in captive mule deer (Odocoileus hemionus hemionus) in north central Colorado in 1978 (42) and subsequently was diagnosed in free-ranging deer and Rocky Mountain elk (Cervus elaphus nelsoni) in southeastern Wyoming and northeastern Colorado. The origins of the disease are obscure, and, like scrapie in sheep, the natural route of CWD transmission remains unknown. CWD was recently detected in free-ranging white-tailed deer (Odocoileus virginianus) in Wisconsin (14) and Illinois. Whether CWD in mule deer, white-tailed deer, and elk is caused by the same or different prion strains is unknown; whether different CWD prion strains cause disease in captive and free-ranging mule deer and elk in the original areas of endemicity in northern Colorado as well as other areas of North America is also unknown.
While CWD is transmissible after intracerebral inoculation of mule deer with incubation periods of up to 2 years (44), experimental transmission of CWD to other species has had mixed results. The inefficient primary transmission of CWD prions to mice (M. Bruce, Neuropathogenesis Unit, Edinburgh, personal communication) and to ferrets (2) is an example of the species barrier. Based on previous studies demonstrating that expression of foreign PrP in Tg mice is an extremely efficient means of abrogating prion species barriers (4, 5, 7, 30, 31, 37-39), we hypothesized that expression of cervid PrP (CerPrP) in Tg mice would eliminate the barrier to CWD prion transmission, resulting in CWD susceptibility simulating that in cervids. To produce Tg(CerPrP) mice, the open reading frame (ORF) cassette of the CerPrP S2 allele (GenBank accession no.AF009180) was released from plasmid sequences following digestion with SalI and XhoI and purified ORF fragments were ligated to the SalI-cut cosSHa.Tet cosmid expression vector. The cosSHa.Tet cosmid expression vector contains a 49-kb DNA fragment encompassing the Syrian hamster PrP gene (32) and has been used to produce numerous Tg models of prion diseases (35), including mice in which the species barriers to Syrian hamster, human, and bovine prions are eliminated (1, 26, 30, 33, 37, 38). To increase CerPrP expression in Tg mice, we modified the CerPrP S2 allele plasmid nucleotide sequence by site-directed mutagenesis immediately upstream of the initiating ATG to produce a consensus Kozak translation initiation sequence. The isolation of recombinant cosmid clones and production of Tg mice were achieved by previously described methods (32). Two founders were generated by microinjection of fertilized embryos from Prnp0/0 knockout mice on an FVB/N background (FVB/Prnp0/0). Brain PrP expression was estimated by comparing serially diluted brain extracts of F1 Tg mice and wild-type mice followed by immuno-dot blotting or Western blotting with the monoclonal antibody 6H4 (Prionics AG, Schlieren). By this approach, the levels of CerPrP expression in brain extracts of Tg(CerPrP)1536+/− and Tg(CerPrP)1534+/− mice, both hemizygous for the transgene array, were estimated to be five- and threefold higher, respectively, than the level of wild-type PrP expression in FVB mice. Analysis of PrP expression in Tg mice by Western blotting of extracts from brain, lung, spleen, muscle, liver, kidney, and heart using monoclonal antibody 6H4 showed that the cosSHa.Tet cosmid expression vector directed expression exclusively to the CNS (data not shown).
Groups of Tg(CerPrP)1536+/− mice were intracerebrally inoculated with 30 μl of 1% homogenate prepared in phosphate-buffered saline (PBS) of a pooled collection of infected brains from CWD-affected mule deer held captive at the Colorado Division of Wildlife, Wildlife Research Center. We also compared the transmission of CWD isolates from individual captive mule deer and elk in Tg(CerPrP)1536+/− mice. Samples D10 and Db99 refer to captive mule deer does that developed CWD at the Colorado Division of Wildlife, Wildlife Research Center, and sample 7378 refers to an adult female captive elk with natural clinical CWD from the Wyoming Game and Fish Department's Sybille Wildlife Research Unit, Wheatland, Wyo. Inoculated Tg(CerPrP)1536+/− mice developed signs of prion disease between 220 and 270 days after inoculation, and the average incubation periods produced by all three CWD isolates and the CWD pool were similar (Table 1). By mating Tg(CerPrP)1536+/− mice to each other, we produced offspring, designated Tg(CerPrP)1536+/+, that were homozygous for the CerPrP transgene array, which resulted in a doubling of the level of expression of CerPrPC. Tg(CerPrP)1536+/+ mice developed signs of prion disease between 153 and 169 days after inoculation with CWD isolate D10 (Table 1). The pooled CWD inoculum produced disease in the inoculated Tg(CerPrP)1534+/− mice between 261 and 273 days (Table 1). The neurologic signs that accompanied prion disease in sick Tg mice included truncal ataxia and slowed movement, increased tone of the tail, dorsal kyphosis, head bobbing or tilting and roughened coat. At the time of writing, Tg(CerPrP)1536+/− mice inoculated with PBS or the Rocky Mountain Laboratory (RML) strain of mouse-adapted scrapie prions have not shown signs of neurological dysfunction ∼360 and 380 days postinoculation, respectively. Wild-type mice inoculated with the CWD pool also failed to develop signs of neurological dysfunction ∼600 days postinoculation.
TABLE 1.
Transmission of CWD prions to Tg(CerPrP) mice
| Inoculum | Species | Recipient | Mean ± SE incubation time in daysa |
|---|---|---|---|
| CWD pool | Mule deer | Tg(CerPrP)1536+/− | 264 ± 3 (7/7) |
| Db99 | Mule deer | Tg(CerPrP)1536+/− | 259 ± 4 (7/7) |
| D10 | Mule deer | Tg(CerPrP)1536+/− | 225 ± 1 (8/8) |
| 7378 | Elk | Tg(CerPrP)1536+/− | 235 ± 2 (8/8) |
| RML | Mouse-adapted scrapie | Tg(CerPrP)1536+/− | >385 (0/8) |
| PBS | Tg(CerPrP)1536+/− | >360 (0/8) | |
| D10 | Mule deer | Tg(CerPrP)1536+/+ | 160 ± 3 (7/7) |
| CWD pool | Mule deer | Tg(CerPrP)1534+/− | 268 ± 2 (10/10) |
| PBS | Tg(CerPrP)1534+/− | >300 (0/6) | |
| CWD pool | Mule deer | Wild-type mice | >596 (0/7) |
The number of mice developing clinical signs of prion disease divided by the original number of inoculated mice is shown in parentheses.
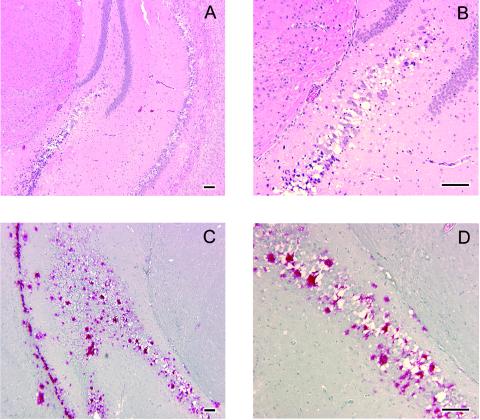
Histopathologic findings were similar for all four inocula and included multiple to coalescing foci of spongiform degeneration of the perikaryon and neuropil. Foci of degeneration were often severe, with a central focus of pale eosinophilic reticulated material surrounded by vacuoles. Neurons adjacent to foci of spongiform change often had shrunken scalloped hyperchromatic nuclei. While spongiform change was widespread in the brain, there was striking and severe vacuolation of the hippocampus (Fig. 1A and B), piriform cortex, and parenchyma adjacent to the ventricular and aqueduct system throughout the brain. In all brains, spongiform degeneration was present in many nuclei in the subcerebellar white matter and brain stem. Patchy foci of degeneration were often present in the middle lamina of the neocortex, within the granular layer of the cerebellar cortex and within the olfactory bulb. Amyloid plaque pathology, long recognized as a pathognomonic feature in cervids with CWD (9, 10, 43), was dramatically reproduced in Tg mice (Fig. 1C and D). All foci of spongiform change had strong positive immunostaining (Fig. 1C and D), often with large central stained plaques partly bordered or surrounded by nonstaining vacuoles. Such florid PrP plaque pathology has also been recognized as a neuropathologic feature of CWD in mule deer (16). Sham-inoculated mice analyzed in parallel had no histologic lesions or positive immunostaining; neither was immunostaining identified in CWD-positive deer brain or CWD-inoculated Tg mice when an irrelevant primary antibody was used and when no primary antibody was applied (data not shown). Brain tissue from a CWD-positive deer had excellent positive immunostaining with the protocol used (data not shown).
FIG. 1.
Neuropathology of Tg(CerPrP)1536+/− mice inoculated with CWD prions. Brains of sick animals from each study group were dissected rapidly after sacrifice and immersion fixed in 10% buffered paraformaldehyde. Tissue was embedded in paraffin, and sections were prepared and stained with hematoxylin and eosin for evaluation of spongiform degeneration. (A and B) Hematoxylin-and-eosin staining of sections through the hippocampus of Tg(CerPrP)1536+/− mice inoculated with brain tissue from CWD-affected mule deer D10 showing spongiform degeneration. Panel B is a higher magnification of an area in panel A. Note shrunken, scalloped neuronal nuclei adjacent to foci of spongiform change. (C and D) Immunohistochemistry of an adjacent section from the same inoculated Tg(CerPrP)1536+/− mouse showing amyloid plaque deposits. Panel D is a magnification of the area indicated in panel C. Note large immunoreactive plaques bordered by vacuoles. Slides were deparaffinized and hydrated followed by immersion in 88% formic acid solution, treatment with 25-mg/ml proteinase-K solution at 26°C for 10 min, followed by autoclaving for 20 min at 121°C in Tris-buffered solution. Tissue preparations were stained with anti-PrP polyclonal antibody R505 (8), followed by anti-rabbit immunoglobulin G-biotinylated secondary antibody streptavidin conjugated to alkaline phosphatase, and then developed with Fast Red A, naphthol, and Fast Red B chromogen. Hematoxylin was used as counterstain. Bar = 100 μm in all cases.
Biochemical analysis of prion proteins in brain extracts from clinically sick Tg mice showed that protease-resistant PrPSc was present in all inoculated groups. The diglycosylated form of PrPSc predominated in the brains of sick Tg(CerPrP)1536+/− mice (Fig. 2A). A similar PrPSc glycosylation pattern has been observed in previous analyses of CWD-affected deer and elk (27). Comparison of PrPSc profiles in brain extracts of sick Tg(CerPrP)1536+/− mice showed that the molecular weight and glycosylation pattern of PrPSc were consistent among all inoculated groups. However, while the amounts of diglycosylated and unglycosylated PrPSc in CWD-affected cervids and CWD-affected Tg(CerPrP)1536+/− mice remained constant, the amount of monoglycosylated PrPSc was consistently lower following transmission of Db99, D10, and 7378 brain extracts to Tg(CerPrP)1536+/− mice (Fig. 2B). Similar differences in glycoform ratios of the same prion strain propagated in mice and human brain have been observed previously (12).
FIG. 2.
Western blots of PrP in brains from Tg(CerPrP)1536+/− mice inoculated with prions from mule deer and elk with CWD. (A) The brains of Tg(CerPrP)1536+/− mice inoculated with D10, 7378, Db99, and the CWD pool were analyzed for the presence of protease-resistant PrPSc. Brain extracts of three individual brains from each inoculated group were treated (+) or not treated (−) with 40-μg/ml proteinase K (PK) in the presence of 2% Sarkosyl for 1 h at 37°C. Uninoc., uninoculated. In panel B, PrPSc in brain homogenates of Tg(CerPrP)1536+/− mice was directly compared with the corresponding CWD inocula from deer and elk. Immunoblots were probed with recombinant Fab Hum-P, which recognizes an epitope on PrP between amino acid residues 96 and 105 (29). The positions of protein molecular mass markers at 28.7 and 21.3 kDa (from top to bottom) are shown to the left of the immunoblots.
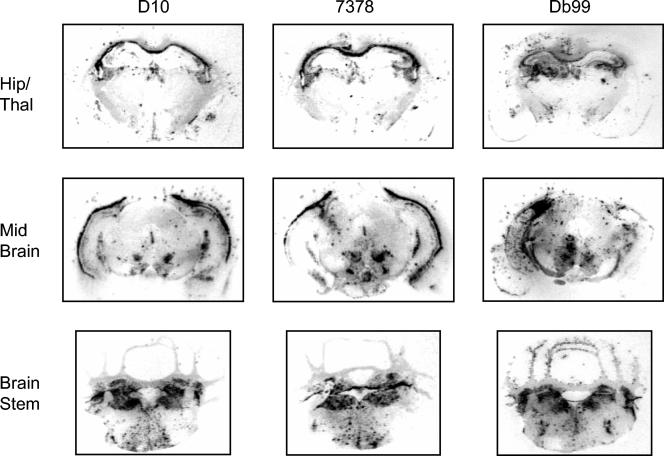
The neuroanatomic distribution of PrPSc was assessed by histoblotting as described previously (34). The most notable feature of histoblotted Tg(CerPrP)1536+/− mouse brains inoculated with CWD prions from D10, Db99 mule deer, and 7378 elk was the widespread punctate deposition of PrPSc (Fig. 3), which likely corresponds to the PrPSc-containing plaques detected by immunohistochemistry (Fig. 1). The concordant patterns of PrPSc deposition in coronal sections of Tg(CerPrP)1536+/− mice inoculated with prions from the D10 CWD-positive mule deer and the 7478 CWD-positive elk, along with the similar incubation times, histopathologic findings, and biochemical properties of PrPSc, indicate that the same CWD prion strain caused disease in these analyzed mule deer and elk. Although the incubation time in Tg(CerPrP)1536+/− mice of the Db99 CWD mule deer isolate was similar to that of the D10/7378 strain, the difference in the neuroanatomic distribution of PrPSc in Db99-inoculated Tg(CerPrP)1536+/− mice (Fig. 3) suggests that a different prion strain caused CWD in the Db99-infected mule deer. Additional passaging studies are required to further characterize the strain properties of these CWD isolates.
FIG. 3.
Regional distribution of PrPSc in the brains of Tg(CerPrP) 1536+/− mice inoculated with CWD prions. Histoblots of 10-μm-thick cryostat sections were generated as previously described (34).To eliminate PrPC from the section, the membranes were air dried, rehydrated for 30 min, and exposed for 1 h at 37°C to 400-mg/ml proteinase K. To enhance immunostaining of PrPSc, the histoblots were exposed to 3 M guanidinium isothiocyanate before immunostaining with PrP recombinant Fab Hum-P followed by alkaline phosphatase-conjugated goat anti-Hu secondary antibody. Histoblotted coronal sections through the hippocampus and thalamus (Hip/Thal), midbrain, and brain stem of Tg(CerPrP)1536+/− mice inoculated with CWD mule deer isolates D10 and Db99 and CWD elk isolate 7378 are shown.
The simulation of CWD in deer and elk following transmission to Tg(CerPrP) mice represents a breakthrough in CWD research. Tg(CerPrP) mice should find broad use in the future to study the biology of CWD prions and CWD pathogenesis. There is currently no quantitative information available regarding the infectivity of any CWD prion preparations, and Tg(CerPrP) mice promise to be a reliable experimental host in which to bioassay CWD prions. Using Tg(CerPrP) mice, it will be possible to expand these preliminary investigations of CWD prion strain prevalence in captive and wild populations of mule deer, white-tailed deer, and Rocky Mountain elk and to assess the effect of cervid PrP polymorphisms on CWD susceptibility (13, 22). In the long term, it should also be possible to gain insights into the origins and mode of transmission of CWD using Tg(CerPrP) mice. Efficient horizontal rather than maternal transmission has been shown to be important in sustaining CWD epidemics (21). The most plausible natural routes of CWD transmission are via ingestion of forage or water contaminated by secretions, excretions, or other sources of agent—for example, carcasses (20). Using CWD-susceptible Tg(CerPrP) mice, it will be possible to bioassay CWD prions in blood and other tissues, body fluids, and secretions of deer and elk that may provide insights into the mode of transmission of CWD and ultimately lead to better disease control in wild cervids.
Acknowledgments
This work was supported in part by grants from the U.S. Public Health Service RO1 NS/AI40334 from the National Institute of Neurological Disorders and Stroke and N01-AI-25491 from the National Institute of Allergy and Infectious Diseases.
We thank Katherine O' Rourke, U.S. Department of Agriculture, Agricultural Research Service, Animal Disease Research Unit, 3003 ADBF, Pullman, Wash., for supplying the S2 mule deer allele, and Stanley Prusiner, Institute for Neurodegenerative Diseases, Department of Neurology, University of California, San Francisco, for supplying Prnp0/0 knockout mice and the cosSHa.Tet vector.
REFERENCES
- 1.Asante, E. A., J. M. Linehan, M. Desbruslais, S. Joiner, I. Gowland, A. L. Wood, J. Welch, A. F. Hill, S. E. Lloyd, J. D. Wadsworth, and J. Collinge. 2002. BSE prions propagate as either variant CJD-like or sporadic CJD-like prion strains in transgenic mice expressing human prion protein. EMBO J. 21:6358-6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartz, J. C., R. F. Marsh, D. I. McKenzie, and J. M. Aiken. 1998. The host range of chronic wasting disease is altered on passage in ferrets. Virology 251:297-301. [DOI] [PubMed] [Google Scholar]
- 3.Bessen, R. A., and R. F. Marsh. 1994. Distinct PrP properties suggest the molecular basis of strain variation in transmissible mink encephalopathy. J. Virol. 68:7859-7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buschmann, A., E. Pfaff, K. Reifenberg, H. M. Muller, and M. H. Groschup. 2000. Detection of cattle-derived BSE prions using transgenic mice overexpressing bovine PrP(C). Arch. Virol. Suppl. 2000:75-86. [DOI] [PubMed] [Google Scholar]
- 5.Castilla, J., A. Gutierrez Adan, A. Brun, B. Pintado, M. A. Ramirez, B. Parra, D. Doyle, M. Rogers, F. J. Salguero, C. Sanchez, J. M. Sanchez-Vizcaino, and J. M. Torres. 2003. Early detection of PrPres in BSE-infected bovine PrP transgenic mice. Arch. Virol. 148:677-691. [DOI] [PubMed] [Google Scholar]
- 6.Collinge, J., K. C. L. Sidle, J. Meads, J. Ironside, and A. F. Hill. 1996. Molecular analysis of prion strain variation and the aetiology of “new variant” CJD. Nature 383:685-690. [DOI] [PubMed] [Google Scholar]
- 7.Crozet, C., F. Flamant, A. Bencsik, D. Aubert, J. Samarut, and T. Baron. 2001. Efficient transmission of two different sheep scrapie isolates in transgenic mice expressing the ovine PrP gene. J. Virol. 75:5328-5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garssen, G. J., L. J. Van Keulen, C. F. Farquhar, M. A. Smits, J. G. Jacobs, A. Bossers, R. H. Meloen, and J. P. Langeveld. 2000. Applicability of three anti-PrP peptide sera including staining of tonsils and brainstem of sheep with scrapie. Microsc. Res. Tech. 50:32-39. [DOI] [PubMed] [Google Scholar]
- 9.Guiroy, D. C., E. S. Williams, R. Yanagihara, and D. C. Gajdusek. 1991. Immunolocalization of scrapie amyloid (PrP27-30) in chronic wasting disease of Rocky Mountain elk and hybrids of captive mule deer and white-tailed deer. Neurosci. Lett. 126:195-198. [DOI] [PubMed] [Google Scholar]
- 10.Guiroy, D. C., E. S. Williams, R. Yanagihara, and D. C. Gajdusek. 1991. Topographic distribution of scrapie amyloid-immunoreactive plaques in chronic wasting disease in captive mule deer (Odocoileus hemionus hemionus). Acta Neuropathol. 81:475-478. [DOI] [PubMed] [Google Scholar]
- 11.Hecker, R., A. Taraboulos, M. Scott, K.-M. Pan, M. Torchia, K. Jendroska, S. J. DeArmond, and S. B. Prusiner. 1992. Replication of distinct prion isolates is region specific in brains of transgenic mice and hamsters. Genes Dev. 6:1213-1228. [DOI] [PubMed] [Google Scholar]
- 12.Hill, A. F., M. Desbruslais, S. Joiner, K. C. L. Sidle, I. Gowland, J. Collinge, L. J. Doey, and P. Lantos. 1997. The same prion strain causes vCJD and BSE. Nature 389:448-450. [DOI] [PubMed] [Google Scholar]
- 13.Johnson, C., J. Johnson, M. Clayton, D. McKenzie, and J. Aiken. 2003. Prion protein gene heterogeneity in free-ranging white-tailed deer within the chronic wasting disease affected region of Wisconsin. J. Wildl. Dis. 39:576-581. [DOI] [PubMed] [Google Scholar]
- 14.Joly, D. O., C. A. Ribic, J. A. Langenberg, K. Beheler, C. A. Batha, B. J. Dhuey, R. E. Rolley, G. Bartelt, T. R. Van Deelen, and M. D. Samuel. 2003. Chronic wasting disease in free-ranging Wisconsin white-tailed deer. Emerg. Infect. Dis. 9:599-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korth, C., K. Kaneko, D. Groth, N. Heye, G. Telling, J. Mastrianni, P. Parchi, P. Gambetti, R. Will, J. Ironside, C. Heinrich, P. Tremblay, S. J. DeArmond, and S. B. Prusiner. 2003. Abbreviated incubation times for human prions in mice expressing a chimeric mouse-human prion protein transgene. Proc. Natl. Acad. Sci. USA 100:4784-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberski, P. P., D. C. Guiroy, E. S. Williams, A. Walis, and H. Budka. 2001. Deposition patterns of disease-associated prion protein in captive mule deer brains with chronic wasting disease. Acta Neuropathol. 102:496-500. [DOI] [PubMed] [Google Scholar]
- 17.Mastrianni, J., F. Nixon, R. Layzer, S. J. DeArmond, and S. B. Prusiner. 1997. Fatal sporadic insomnia: fatal familial insomnia phenotype without a mutation of the prion protein gene. Neurology 48(Suppl.):A296. [Google Scholar]
- 18.Mastrianni, J. A., S. Capellari, G. C. Telling, D. Han, P. Bosque, S. B. Prusiner, and S. J. DeArmond. 2001. Inherited prion disease caused by the V210I mutation: transmission to transgenic mice. Neurology 57:2198-2205. [DOI] [PubMed] [Google Scholar]
- 19.Mastrianni, J. A., R. Nixon, R. Layzer, G. C. Telling, D. Han, S. J. DeArmond, and S. B. Prusiner. 1999. Prion protein conformation in a patient with sporadic fatal insomnia. N. Engl. J. Med. 340:1630-1638. [DOI] [PubMed] [Google Scholar]
- 20.Miller, M., E. Williams, N. Hobbs, and L. Wolfe. 2004. Environmental sources of prion transmission in mule deer. Emerg. Infect. Dis. 10:1003-1006. [Online.] www.cdc.gov/eid. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller, M. W., and E. S. Williams. 2003. Prion disease: horizontal prion transmission in mule deer. Nature 425:35-36. [DOI] [PubMed] [Google Scholar]
- 22.O'Rourke, K. I., T. E. Besser, M. W. Miller, T. F. Cline, T. R. Spraker, A. L. Jenny, M. A. Wild, G. L. Zebarth, and E. S. Williams. 1999. PrP genotypes of captive and free-ranging Rocky Mountain elk (Cervus elaphus nelsoni) with chronic wasting disease. J. Gen. Virol. 80:2765-2769. [DOI] [PubMed] [Google Scholar]
- 23.Parchi, P., R. Castellani, S. Capellari, B. Ghetti, K. Young, S. G. Chen, M. Farlow, D. W. Dickson, A. A. F. Sima, J. Q. Trojanowski, R. B. Petersen, and P. Gambetti. 1996. Molecular basis of phenotypic variability in sporadic Creutzfeldt-Jakob disease. Ann. Neurol. 39:767-778. [DOI] [PubMed] [Google Scholar]
- 24.Peretz, D., R. A. Williamson, G. Legname, Y. Matsunaga, J. Vergara, D. R. Burton, S. J. DeArmond, S. B. Prusiner, and M. R. Scott. 2002. A change in the conformation of prions accompanies the emergence of a new prion strain. Neuron 34:921-932. [DOI] [PubMed] [Google Scholar]
- 25.Prusiner, S. B. 1982. Novel proteinaceous infectious particles cause scrapie. Science 216:136-144. [DOI] [PubMed] [Google Scholar]
- 26.Prusiner, S. B., M. Scott, D. Foster, K.-M. Pan, D. Groth, C. Mirenda, M. Torchia, S.-L. Yang, D. Serban, G. A. Carlson, P. C. Hoppe, D. Westaway, and S. J. DeArmond. 1990. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell 63:673-686. [DOI] [PubMed] [Google Scholar]
- 27.Race, R. E., A. Raines, T. G. M. Baron, M. W. Miller, A. Jenny, and E. S. Williams. 2002. Comparison of abnormal prion protein glycoform patterns from transmissible spongiform encephalopathy agent-infected deer, elk, sheep, and cattle. J. Virol. 76:12365-12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Safar, J., H. Wille, V. Itri, D. Groth, H. Serban, M. Torchia, F. E. Cohen, and S. B. Prusiner. 1998. Eight prion strains have PrPSc molecules with different conformations. Nat. Med. 4:1157-1165. [DOI] [PubMed] [Google Scholar]
- 29.Safar, J. G., M. Scott, J. Monaghan, C. Deering, S. Didorenko, J. Vergara, H. Ball, G. Legname, E. Leclerc, L. Solforosi, H. Serban, D. Groth, D. R. Burton, S. B. Prusiner, and R. A. Williamson. 2002. Measuring prions causing bovine spongiform encephalopathy or chronic wasting disease by immunoassays and transgenic mice. Nat. Biotechnol. 20:1147-1150. [DOI] [PubMed] [Google Scholar]
- 30.Scott, M., D. Foster, C. Mirenda, D. Serban, F. Coufal, M. Wälchli, M. Torchia, D. Groth, G. Carlson, S. J. DeArmond, D. Westaway, and S. B. Prusiner. 1989. Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell 59:847-857. [DOI] [PubMed] [Google Scholar]
- 31.Scott, M., D. Groth, D. Foster, M. Torchia, S.-L. Yang, S. J. DeArmond, and S. B. Prusiner. 1993. Propagation of prions with artificial properties in transgenic mice expressing chimeric PrP genes. Cell 73:979-988. [DOI] [PubMed] [Google Scholar]
- 32.Scott, M. R., R. Köhler, D. Foster, and S. B. Prusiner. 1992. Chimeric prion protein expression in cultured cells and transgenic mice. Protein Sci. 1:986-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott, M. R., J. Safar, G. Telling, O. Nguyen, D. Groth, M. Torchia, R. Koehler, P. Tremblay, D. Walther, F. E. Cohen, S. J. DeArmond, and S. B. Prusiner. 1997. Identification of a prion protein epitope modulating transmission of bovine spongiform encephalopathy prions to transgenic mice. Proc. Natl. Acad. Sci. USA 94:14279-14284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taraboulos, A., K. Jendroska, D. Serban, S.-L. Yang, S. J. DeArmond, and S. B. Prusiner. 1992. Regional mapping of prion proteins in brains. Proc. Natl. Acad. Sci. USA 89:7620-7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Telling, G. C. 2000. Prion protein genes and prion diseases: studies in transgenic mice. Neuropathol. Appl. Neurobiol. 26:209-220. [DOI] [PubMed] [Google Scholar]
- 36.Telling, G. C., P. Parchi, S. J. DeArmond, P. Cortelli, P. Montagna, R. Gabizon, J. Mastrianni, E. Lugaresi, P. Gambetti, and S. B. Prusiner. 1996. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science 274:2079-2082. [DOI] [PubMed] [Google Scholar]
- 37.Telling, G. C., M. Scott, K. K. Hsiao, D. Foster, S. L. Yang, M. Torchia, K. C. Sidle, J. Collinge, S. J. DeArmond, and S. B. Prusiner. 1994. Transmission of Creutzfeldt-Jakob disease from humans to transgenic mice expressing chimeric human-mouse prion protein. Proc. Natl. Acad. Sci. USA 91:9936-9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Telling, G. C., M. Scott, J. Mastrianni, R. Gabizon, M. Torchia, F. E. Cohen, S. J. DeArmond, and S. B. Prusiner. 1995. Prion propagation in mice expressing human and chimeric PrP transgenes implicates the interaction of cellular PrP with another protein. Cell 83:79-90. [DOI] [PubMed] [Google Scholar]
- 39.Vilotte, J.-L., S. Soulier, R. Essalmani, M.-G. Stinnakre, D. Vaiman, L. Lepourry, J. C. Da Silva, N. Besnard, M. Dawson, A. Buschmann, M. Groschup, S. Petit, M.-F. Madelaine, S. Rakatobe, A. Le Dur, D. Vilette, and H. Laude. 2001. Markedly increased susceptibility to natural sheep scrapie of transgenic mice expressing ovine PrP. J. Virol. 75:5977-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wells, G. A. H., A. C. Scott, C. T. Johnson, R. F. Gunning, R. D. Hancock, M. Jeffrey, M. Dawson, and R. Bradley. 1987. A novel progressive spongiform encephalopathy in cattle. Vet. Rec. 121:419-420. [DOI] [PubMed] [Google Scholar]
- 41.Will, R. G., J. W. Ironside, M. Zeidler, S. N. Cousens, K. Estibeiro, A. Alperovitch, S. Poser, M. Pocchiari, A. Hofman, and P. G. Smith. 1996. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 347:921-925. [DOI] [PubMed] [Google Scholar]
- 42.Williams, E. S., and S. Young. 1980. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J. Wildl. Dis. 16:89-98. [DOI] [PubMed] [Google Scholar]
- 43.Williams, E. S., and S. Young. 1993. Neuropathology of chronic wasting disease of mule deer (Odocoileus hemionus) and Elk (Cervus elaphus nelsoni). Vet. Pathol. 30:36-45. [DOI] [PubMed] [Google Scholar]
- 44.Williams, E. S., and S. Young. 1992. Spongiform encephalopathies in Cervidae. Rev. Sci. Tech. Off. Int. Epiz. 11:551-567. [DOI] [PubMed] [Google Scholar]