Abstract
The innate immune response, through the induction of proinflammatory cytokines and antiviral factors, plays an important role in protecting the host from pathogens. Several components of the innate response, including tumor necrosis factor alpha (TNF-α), monocyte chemoattractant protein 1, interferon-inducible protein 10, and RANTES, are upregulated in the brain following neurovirulent retrovirus infection in humans and in animal models. However, it remains unclear whether this immune response is protective, pathogenic, or both. In the present study, by using TNF-α−/− mice we analyzed the contribution of TNF-α to neurological disease induced by four neurovirulent murine retroviruses, with three of these viruses encoding portions of the same neurovirulent envelope protein. Surprisingly, only one retrovirus (EC) required TNF-α for disease induction, and this virus induced less TNF-α expression in the brain than did the other retroviruses. Analysis of glial fibrillary acidic protein and F4/80 in EC-infected TNF-α−/− mice showed normal activation of astrocytes but not of microglia. Thus, TNF-α-mediated microglial activation may be important in the pathogenic process initiated by EC infection. In contrast, TNF-α was not required for pathogenesis of the closely related BE virus and the BE virus induced disease in TNF-α−/− mice by a different mechanism that did not require microglial activation. These results provide new insights into the multifactorial mechanisms involved in retrovirus-induced neurodegeneration and may also have analogies to other types of neurodegeneration.
The innate immune response, which is associated with upregulation of proinflammatory cytokines and chemokines, has a critical role as a first line of defense against many viral infections. However, in certain organs such as the brain, the activation of cytokines and chemokines may actually contribute to pathogenesis rather than protect the host. In human, primate, feline, and murine retrovirus infections of the brain, increased expression of several cytokines and chemokines, including monocyte chemoattractant protein 1 (MCP-1, CCL2), macrophage inflammatory protein 1α (CCL3), RANTES (CCL5), interferon-inducible protein 10 (CXCL10), and tumor necrosis factor alpha (TNF-α), is often associated with neurological disorders and may contribute to disease development (12, 21, 25, 26, 35, 37, 41, 47).
We have previously analyzed the potential contributions of proinflammatory responses to neurological disease development, using the neurovirulent murine retrovirus Fr98 and related recombinant viruses (25). The envelope gene of Fr98 contains two distinct regions involved in neurovirulence. These regions appear to act by different but complementary mechanisms that give rise to a more rapid disease tempo when combined in the Fr98 virus (7). These viruses infect microglia and endothelial cells in the brain, resulting in the upregulation of several proinflammatory cytokines and chemokine and the onset of severe neurological disease. Despite the upregulation of proinflammatory factors, there is little evidence of lymphocyte recruitment in the brain (25). Instead, the primary signs of neuroinflammation induced by Fr98 appear to be microglial nodules and astrogliosis (28, 33). In previous studies with knockout mice, we found that the chemokine receptor CCR2, but not CCR5, contributed to Fr98-induced neurological disease (24). However, absence of CCR2 did not prevent the eventual development of clinical disease, suggesting that other proinflammatory cytokines and chemokines may also contribute to pathogenesis.
TNF-α upregulation in the brain is often correlated with retroviral neurovirulence (6, 25, 26, 43), suggesting that TNF-α is one component of the innate immune response that could contribute to neuropathogenesis. This conclusion has been supported by studies with TNF-α-blocking drugs (23, 39) and with TNF-α knockout (TNF-α−/−) mice (8), as well as human gene polymorphism studies (31). However, other studies have suggested that TNF-α may not directly contribute to retrovirus-induced neuropathogenesis (9, 18, 36), and TNF-α has even been suggested to be neuroprotective (34).
To analyze the role of TNF-α in retrovirus-induced neuropathogenesis, we used TNF-α−/− mice and analyzed the effect of TNF-α deficiency on neurological disease induced by the neurovirulent retrovirus Fr98, as well as three other related neurovirulent viruses, FrCasE, BE, and EC. Surprisingly, the requirement for TNF-α in neurological disease induction varied significantly among these viruses and only one virus required TNF-α for pathogenesis. TNF-α appeared to contribute to retrovirus-induced neurological disease by mediating activation of microglia and/or macrophages in the central nervous system (CNS).
MATERIALS AND METHODS
Mice.
129SvEv wild-type and TNF-α−/− mice, derived from littermate controls and maintained as separate colonies, were kindly provided by Frances Balkwill and have been previously described (19, 38). All animal experiments were carried out in accordance with the regulations of the Rocky Mountain Laboratories Animal Care and Use Committee and the guidelines of the National Institutes of Health. Comparison of the 129SvEv mouse strain to the IRW mouse strain used in previous studies (25, 28, 33) indicated that the murine retroviruses used in this study induced the same clinical signs of neurological disease in both mouse strains, although the onset of clinical disease was slower in 129SvEv mice.
Viruses and infection.
The construction of virus clones Fr54, Fr98, FrCasE, and EC was previously described (27, 28). The BE construct was generated by cloning the BbsI-to-EcoRI fragment of the Fr98 envelope gene into a plasmid containing the Fr54 virion. This plasmid was transfected into Mus dunni cells to produce BE virus stocks as previously described (7). Stocks of all of the viruses used were prepared from the supernatants of confluently infected M. dunni fibroblast cells. Virus titers were determined by focal infectivity assay with envelope-specific monoclonal antibody 514 (32) or 667 (27). Wild-type and TNF-α−/− mice were injected intraperitoneally (i.p.) with 104 focus-forming units (FFU) of virus in a 100-μl volume within 24 h of birth. Mock-infected mice were inoculated i.p. with 100 μl of supernatant from uninfected M. dunni cultures. Following infection, mice infected with Fr98, EC, or BE were observed daily for neurological disease, which was characterized by obvious signs of ataxia and/or seizures (28). Mice infected with FrCasE were followed for clinical signs of tremor, hind limb weakness, and paralysis (27).
RNA purification.
Infected mice were exsanguinated by axillary incision under deep isoflurane anesthesia. Brains were removed from infected mice at the indicated times and divided into two sagittal sections for RNA and immunohistochemical analysis. In some instances, whole-brain tissue was incubated in RNAlater (Ambion, Austin, Tex.) overnight at 4°C to harden tissue while preserving mRNA. The brain tissue was then separated into four regions: the anterior, middle, cerebellum, and brain stem with a dissecting microscope. The tissues were immediately frozen in liquid nitrogen and stored at −80°C. Total RNA from the brain tissue was prepared with Trizol reagent (Life Technologies, Rockville, Md.) in accordance with the manufacturer's instructions. RNA was quantified by spectroscopy at 260 nm. The RNA was then treated with DNase (Ambion) for 30 min to remove any contaminating DNA and purified over RNeasy columns (QIAGEN, Valencia, Calif.).
Primers and probes for gene detection.
Virus expression was analyzed by detection of the viral gag gene with the forward primer FB29 GAG-1169F (5′-AAA CCA ATG TGG CCA TGT CAT T-3′), the reverse primer FB29 GAG-1244R (5′-AAA TCT TCT AAC CGC TCT AAC TTT CG-3′), and probe FB29 GAG-1192T (5′-6FAM-ATC TGG CAG TCC GCC CCG G-TAMRA-3′). F4/80 mRNA expression was detected with the forward primer F4/80-1958F (5′-TTA CGA TGG AAT TCT CCT TGT ATA TCA-3′), the reverse primer F4/80-2051R (5′-CAC AGC AGG AAG GTG GCT ATG-3′), and probe F4/80-2001T (6FAM-AGT CAT CTC CCT GGT ATG TCT TGC CTT GG-TAMRA). Glial fibrillary acidic protein (GFAP) mRNA expression was detected with the forward primer GFAP-16F (5′-CGT TTC TCC TTG TCT CGA ATG AC-3′), the reverse primer GFAP-112R (5′-TCG CCC GTG TCT CCT TGA-3′), and probe GFAP-42T (6FAM-TCC ACT CCC TGC CAG GGT GGA CTT-TAMRA). Predeveloped primer and probe sets (PDAR) were used to detect TNF-α expression (Applied Biosystems, Foster City, Calif.). Primer and probes from the rodent GAPDH (glyceraldehyde-3-phosphate dehydrogenase) control reagent kit (Applied Biosystems) were used to detect GAPDH expression.
Real-time PCR analysis of gene expression.
Reactions were run in triplicate with the one-step reverse transcription-PCR master mix (Applied Biosystems) in a 10-μl volume with approximately 10 ng of DNase-treated total RNA, 500 nM forward and reverse primers, and 250 nM probe on an ABI PRISM 7900 sequence detection system (Applied Biosystems). Lack of DNA contamination was confirmed by running reactions without reverse transcriptase. The cycle number at which each sample reached a fixed fluorescence threshold (CT) was used to quantitate gene expression. Data were calculated as the CT value (log2) of the gene for GAPDH minus the CT value of the gene of interest for each sample (ΔCT = CT GAPDH − CT gene of interest) to control for variations in RNA amounts in each sample. The data are presented as the fold expression of the gene of interest relative to that of the gene for GAPDH. For example, a value of 1 indicates the same level of mRNA expression as GAPDH (ΔCT = 0), whereas a value of 2 indicates a level of mRNA expression twofold higher than that of the GAPDH mRNA (ΔCT = 1). For TNF-α expression (see Fig. 2A and 4), values were multiplied by 103 for graphing purposes; therefore, a value of 1 is actually 0.001-fold of the GAPDH expression level. For F4/80 expression (see Fig. 6C and D and 7), values were multiplied by 102.
FIG. 2.
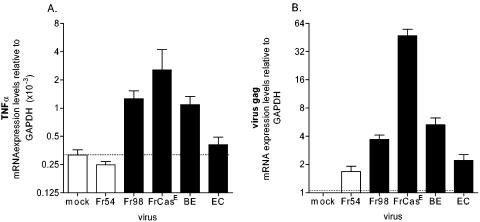
Expression of TNF-α (A) or viral gag (B) mRNA following polytropic retrovirus infection. Brain tissue was removed from Fr54, Fr98, FrCasE, BE, and EC virus-infected and mock-infected mice at 17 to 21 days postinfection and processed for mRNA as described in Materials and Methods. mRNA expression was determined by real-time PCR with triplicate wells for each sample. Data are shown as fold expression of the gene of interest relative to that of the gene for GAPDH as described in Materials and Methods. Data are the means and standard errors for three to eight mice per group. Statistical analysis was done by a one-way analysis of variance with the Newman-Keuls posttest. The P values for TNF-α (A) compared to Fr54 virus-infected controls were <0.01 for the FrCasE virus, <0.01 for the Fr98 virus, <0.05 for the BE virus, and >0.05 for the EC virus. For viral gag expression (B), EC virus levels were significantly lower compared to BE (P < 0.01) and Fr98 (P < 0.05) virus levels.
FIG. 4.
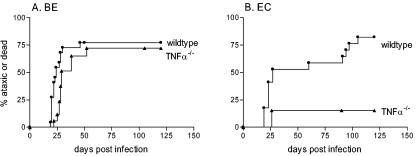
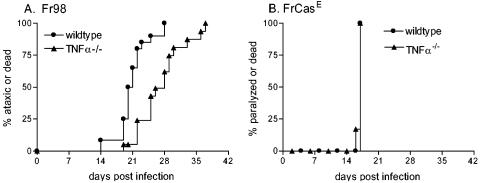
Development of neurological disease in wild-type and TNF-α−/− mice following infection with the BE (A) or EC (B) chimeric virus. Neonatal wild-type and TNF-α−/− mice were inoculated with 104 FFU of BE or EC virus stocks, by i.p. injection, less than 24 h after birth. Mice were then followed for the development of clinical signs of ataxia and seizures until the conclusion of the experiment at 120 days postinfection. Data are presented as the percentage of mice with severe neurological disease for 22 wild-type and 15 TNF-α−/− mice in panel A and 14 wild-type and 13 TNF-α−/− mice in panel B. Statistical analysis was done as described for Fig. 1. The P value for the two-tailed log rank test for trend between wild-type and TNF-α−/− mice was 0.095 for panel A and 0.002 for panel B.
FIG. 6.
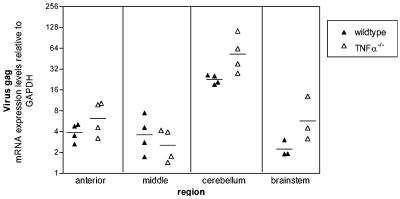
Viral gag mRNA expression in EC virus-infected wild-type and TNF-α−/− mice. Brains were dissected into four regions as shown in Fig. 5A. Viral gag mRNA expression was determined by real-time PCR with triplicate wells for each sample. Data are shown as the fold expression of the gene of interest relative to that of the gene for GAPDH as described in Materials and Methods. Each symbol represents a single animal; filled triangles represent EC virus-infected wild-type mice, while open triangles represent EC virus-infected TNF-α−/− mice. Brains were removed at 21 days postinfection. Statistical analysis was performed with a two-tailed Mann-Whitney test for each brain region. The P value between EC virus-infected wild-type versus EC virus-infected TNF-α−/− mice was P = 0.4 for the anterior, 0.5 for the middle, 0.029 for the cerebellum, and 0.1 for the brain stem region.
FIG. 7.
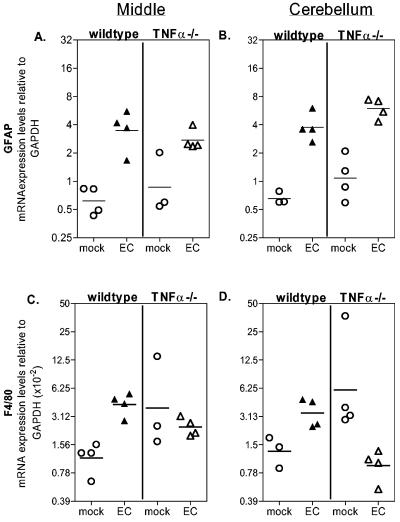
GFAP (A and B) and F4/80 (C and D) mRNA expression in the middle (A and C) and cerebellum (B and D) regions of wild-type and TNF-α−/− mice. Brains were dissected into regions as shown in Fig. 5A. GFAP and F4/80 mRNA expression was determined by real-time PCR with triplicate wells for each sample. Data are shown as the fold expression of the gene of interest relative to that of GAPDH as described in Materials and Methods. The data shown are from one of two representative experiments. Each circle represents a single animal. All mice were 21 days postinfection at the time of tissue removal. Statistical analysis was performed with a one-tailed Mann-Whitney test for each brain region. (A and B) GFAP levels were significantly higher (P < 0.03) in both EC virus-infected wild-type and TNF-α−/− mice compared to uninfected controls in both the middle (A) and cerebellum (B) regions. (C and D) F4/80 expression was significantly upregulated (P < 0.001) by EC virus infection in the middle (C) and cerebellum (D) regions of wild-type mice. No significant increase in F4/80 expression was observed in any region by EC virus infection of TNF-α−/− mice.
RESULTS
Delayed onset of neurological disease in Fr98-infected TNF-α−/− mice.
In previous studies, the neurovirulent virus Fr98 induced the upregulation of TNF-α in the brain prior to the onset of clinical disease (25). To directly analyze the role of TNF-α in Fr98-induced neurological disease, wild-type and TNF-α−/− 129Sv/Ev mice were infected with Fr98 and followed for development of the usual clinical signs of ataxia and seizures. A small but significant delay in the onset of clinical signs was observed in TNF-α−/− mice compared to wild-type controls (Fig. 1A). This result indicated that TNF-α was an important contributing factor in the kinetics of Fr98-induced neurological disease.
FIG. 1.
Development of neurological disease in wild-type and TNF-α−/− mice following infection with Fr98 (A) or FrCasE (B). Neonatal wild-type and TNF-α−/− mice were inoculated with 104 FFU of virus by i.p. injection, prior to 24 h postbirth. Mice were then followed for the development of clinical signs of severe ataxia and seizures (A) or paralysis (B). Data are presented as the percentage of mice with severe neurological disease for 22 wild-type and 16 TNF-α−/− mice for panel A and 7 wild-type and 6 TNF-α−/− mice for panel B. Statistical analysis was done by Kaplan-Meier survival curve analysis with GraphPad Prism (GraphPad, San Diego, Calif.). The P value for the two-tailed log rank test for trend between Fr98 virus-infected wild-type and TNF-α−/− mice was <0.0001.
As a comparison to Fr98-induced disease, we analyzed the contribution of TNF-α to neurological disease induced by another neurovirulent murine retrovirus, FrCasE. Although FrCasE encodes the same long terminal repeat and gag and pol genes as Fr98, it encodes a different envelope protein and uses a different cellular receptor to infect microglia. Additionally, FrCasE differs from Fr98 in the type of clinical signs (tremor, hind limb weakness, and paralysis) and pathology (spongiform degeneration) that it induces (28). Interestingly, no difference in disease onset (Fig. 1B) or pathology (data not shown) was observed between wild-type and TNF-α−/− mice infected with FrCasE. Thus, TNF-α was not required for FrCasE-induced neurological disease.
Induction of TNF-α by neurovirulent retroviruses.
Previously, both Fr98 and FrCasE were shown to induce TNF-α expression in IRW mice (1, 25). To confirm that both Fr98 and FrCasE induced TNF-α expression in the 129 mouse strain used in this study, we analyzed TNF-α mRNA expression by real-time PCR. Both Fr98 and FrCasE infections resulted in significant upregulation of TNF-α mRNA in the brain compared to mock infection or infection with an avirulent retrovirus, Fr54 (Fig. 2A). Interestingly, the level of TNF-α expression was slightly higher in FrCasE-infected mice than in Fr98-infected mice. This correlated with a higher level of virus in the brains of FrCasE-infected mice as detected by viral gag mRNA expression (Fig. 2B) and immunohistochemical analysis (data not shown). However, on the basis of the lack of alteration of FrCasE disease kinetics in TNF-α−/− mice, the high TNF-α upregulation in FrCasE infection did not have a determining role in the disease process.
Envelope determinants influence TNF-α expression.
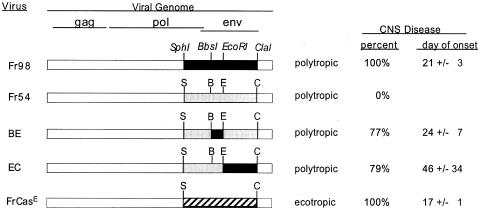
Both FrCasE- mediated pathogenesis and Fr98-mediated pathogenesis are dependent on the virus envelope protein (2, 28). The Fr98 envelope protein encodes two separate neurovirulence determinants that induce neurological disease (Fig. 3) (7). Chimeric viruses encoding either the N-terminal BbsI-to-EcoRI (BE) region or the middle EcoRI-to-ClaI (EC) region of the Fr98 envelope-encoding gene are known to induce the same clinical neurological disease as Fr98, but with decreased incidence and slower kinetics compared to those of Fr98 itself (Fig. 3). In the present study, the chimeric BE virus induced high levels of TNF-α in the brain in the 129 mouse strain (Fig. 2A). In contrast, the chimeric EC virus did not induce a significant increase in TNF-α expression in this same mouse strain (Fig. 2A). This was similar to results previously obtained with IRW mice, where no increase in TNF-α mRNA expression was observed in EC virus-infected mice prior to or during the onset of clinical signs (25). Interestingly, the level of TNF-α expression correlated strongly with the level of viral gag mRNA expression in the brain (Fig. 2B). Thus, a high level of virus infection might be one factor required for inducing TNF-α upregulation.
FIG. 3.
Disease induction by different murine retroviruses. Fr98 virus envelope sequences are shown as black bars, while Fr54 virus envelope sequences are shown as grey bars. The FrCasE virus envelope sequence is shown as a striped bar. The region between the 5′ end of the virus genome and the SphI site is the same in all of the virus clones. Virus stocks with the same titer (105 FFU/ml) were used for inoculation of mice. CNS disease was defined as signs of severe ataxia and/or seizures following infection. For FrCasE virus-infected mice, CNS disease was defined as paralysis. The day of onset is the average day at which severe clinical signs were first noted in the mice that developed clinical sign ± standard deviation. Survival curve analysis of 129 wild-type mice infected with the Fr98 (Fig. 1), BE, or EC (see Fig. 4) virus demonstrated a significant difference in disease development induced by Fr98 virus infection compared to either BE (P < 0.001) or EC (P < 0.0001) virus infection. However, no significant difference was observed between EC virus-induced disease and BE virus-induced disease (P = 0.57). Survival curve analysis was done by the Kaplan-Meier method and the two-tailed log rank test for trend with GraphPad Prism.
Effect of TNF-α deficiency on BE- and EC-induced neurological disease.
To determine if the BE and EC viruses also vary in the requirement for TNF-α during pathogenesis, wild-type and TNF-α−/− mice were infected with either BE or EC virus and followed for clinical disease. BE virus infection induced an incidence and kinetics of neurological disease in TNF-α−/− mice similar to those of wild-type controls (Fig. 4A). In contrast, after EC virus infection only 15% of TNF-α−/− mice developed clinical disease, compared to 79% of wild-type mice (Fig. 4B), demonstrating that TNF-α is required for EC-mediated neurovirulence in the majority of mice. Thus, EC, the neurovirulent retrovirus that induced minimal TNF-α upregulation (Fig. 2A), was highly dependent on TNF-α for pathogenesis (Fig. 4B), whereas BE, which induced extensive TNF-α upregulation (Fig. 2A), was not dependent on TNF-α for its pathogenic effects.
TNF-α expression in EC-infected mice.
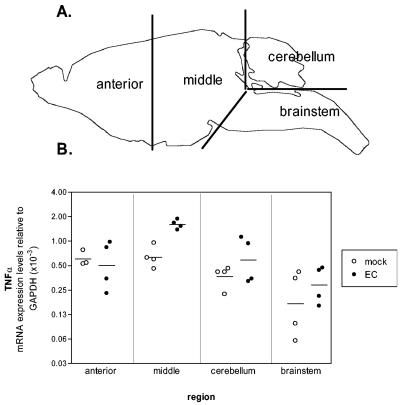
It was unexpected that TNF-α was important in EC pathogenesis because brains of EC-infected mice had previously shown little upregulation of TNF-α (Fig. 2A) (3). One possible explanation for this paradox could be that TNF-α upregulation might only occur in small areas of the brain and might have been overlooked by analyzing the entire brain in our initial studies (Fig. 2A). To study this possibility, brains of EC-infected mice were divided into four regions (anterior, middle, cerebellum, and brain stem) prior to analysis for mRNA expression (Fig. 5A). A significant (2- to 2.5-fold) increase in TNF-α mRNA expression was observed in the middle region of all EC-infected mice compared to that of mock-infected controls (Fig. 5B). Increased expression of TNF-α was also found in the cerebellum but was not consistent in all of the mice (Fig. 5B). No significant difference in TNF-α expression was detected in the brain stem or anterior brain region of EC-infected mice. Thus, both the regional restriction and the relatively low magnitude of TNF-α expression induced by EC infection appeared to account for the previous failure to detect TNF-α upregulation when whole-brain preparations were analyzed (Fig. 2A).
FIG. 5.
TNF-α mRNA expression in mock-infected and EC virus-infected wild-type mice at 21 days postinfection. (A) Brains were dissected into four regions, anterior, middle, cerebellum, and brain stem, as shown. (B) TNF-α mRNA expression was determined by real-time PCR with triplicate wells for each sample. Each circle represents a single animal. Open circles represent mock-infected wild-type controls at 21 days postinfection, while filled circles represent EC virus-infected wild-type mice at 21 days postinfection with clinical signs of ataxia. Data are shown as the fold expression of the gene of interest relative to the gene for GAPDH as described in Materials and Methods. Data are from one of two representative experiments. Statistical analysis was done with a one-tailed Mann-Whitney test for each brain region. A significant difference (P = 0.014) was observed in the middle region between mock-infected and EC virus-infected wild-type mice.
Viral loads in TNF-α−/− and wild-type mice.
To study the possibility that TNF-α might be important for infection or spread of the EC virus, brain tissue from EC virus-infected mice was quantitatively analyzed by real-time reverse transcription-PCR for viral gag mRNA expression. In these experiments, no significant decrease in viral gag mRNA was detected in TNF-α−/− mice compared to wild-type mice in any region of the brain (Fig. 6). Instead, the viral gag mRNA level was actually higher in the cerebellums of TNF-α−/− mice than in those of wild-type mice (Fig. 6). The high levels of virus replication in the cerebellum compared to those in other regions of the brain were in agreement with previous enzyme-linked immunosorbent assay and immunohistochemistry studies (30). Immunohistochemical analysis for virus envelope protein revealed no difference in the location or number of EC virus-infected cells in TNF-α−/− mice compared to those in wild-type controls (data not shown). Thus, TNF-α did not appear to decrease EC virus infection in the brain at the time of disease onset. To rule out the possibility that TNF-α affected virus replication at an earlier stage in the periphery or brain, we also analyzed viral gag mRNA expression in the spleen and in the middle and cerebellum regions of the brain at 10 days postinfection. Similar levels of viral mRNA (P > 0.8) were present in EC virus-infected TNF-α−/− mice compared to those in wild-type mice in all three tissue sections (data not shown). Therefore, the effect of TNF-α on EC virus-induced neuropathogenesis was not via suppression of viral infection.
EC virus-induced astrocyte activation in TNF-α−/− mice.
The primary pathological alterations associated with EC virus infection are astrogliosis and microglial nodules (28). Both in vitro and in vivo astrocyte activation can be induced by TNF-α (3, 46). To determine if astrocyte activation was inhibited in TNF-α−/− mice, we analyzed RNA expression levels of the astrocyte activation marker GFAP. In wild-type mice, EC infection significantly upregulated GFAP expression by two- to sixfold in the middle (Fig. 7A), cerebellum (Fig. 7B), and anterior (data not shown) regions of the brain. Interestingly, EC infection of TNF-α−/− mice induced a similar level of GFAP upregulation (Fig. 7A and B). Thus, TNF-α was not necessary for EC-induced astrocyte activation, and this activation did not appear to be the mechanism by which TNF-α contributes to EC virus-induced neurological disease.
TNF-α deficiency influences the activation and/or recruitment of microglia and/or macrophages.
The primary brain cell types infected by the EC virus are microglia, macrophages, and endothelia (30). In this system, infection and activation of microglia are strongly correlated with pathogenesis (28, 29, 33). To analyze microglial activation and/or possible macrophage recruitment to the brain, we analyzed mRNA expression of the microglia and macrophage activation marker F4/80. EC virus infection of wild-type mice resulted in a two- to fourfold increase in F4/80 mRNA expression in the middle(Fig. 7C), cerebellum (Fig. 7D), and anterior (data not shown) regions compared to those of uninfected controls, demonstrating that the EC virus induced microglia and/or macrophage activation. Uninfected TNF-α−/− mice had basal levels of F4/80 expression higher than those of uninfected wild-type mice (Fig. 7C and D), suggesting either a higher number of microglia in the brains of TNF-α−/− mice or an increased state of microglial activation. However, EC infection of TNF-α−/− mice did not induce microglia and/or macrophage activation, as F4/80 expression was lower in EC virus-infected mice than in the uninfected controls (Fig. 7 C and D). This lack of F4/80 upregulation in EC virus-infected TNF-α−/− mice suggested that TNF-α may be involved in EC virus-induced activation of microglia and/or recruitment of macrophages to the brain. Immunohistochemical analysis of microglia and macrophages in the brain with an antibody against Iba1 revealed no discernible difference in microglial or macrophage numbers between EC virus-infected wild-type and TNF-α−/− mice (data not shown). Possibly, the difference in F4/80 mRNA expression is indicative of the activation state of microglia or macrophages rather than of the proliferation or recruitment of these cell types.
Effect of TNF-α on F4/80 expression in BE virus-infected mice.
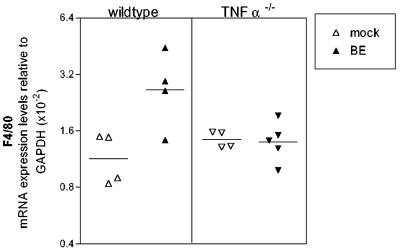
Microglial activation is also observed in BE virus-infected mice (data not shown). The lack of an effect of TNF-α deficiency on BE virus-induced neurological disease suggests either that TNF-α is not necessary for activation of microglia following BE virus infection or that activation of microglia is not required for BE virus-induced disease. To determine if F4/80 expression is upregulated by BE virus infection in the absence of TNF-α, we analyzed whole-brain tissue from BE virus-infected wild-type and TNF-α−/− mice. As expected wild-type mice infected with BE virus had increased expression of F4/80 compared to that of uninfected controls (Fig. 8). However, in TNF-α−/− mice BE virus infection did not induce F4/80 upregulation (Fig. 8) in spite of the presence of clinical disease. Thus, the activation of microglia and/or the recruitment of macrophages, as measured by F4/80 mRNA expression, does not appear to be necessary for BE virus-induced neurological disease.
FIG. 8.
Expression of F4/80 mRNA following BE virus infection. Brain tissue was removed from BE virus-infected wild-type and TNF-α−/− mice at 21 days postinfection and analyzed for F4/80 mRNA as described for Fig. 7. Each symbol represents a single animal. Statistical analysis was performed by one-way analysis of variance with the Newman-Keuls posttest. The P value between mock-infected and BE virus-infected wild-type mice was <0.01, while the P value between mock-infected and BE virus-infected TNF-α−/− mice was >0.05.
DISCUSSION
In previous studies, two separate neurovirulence determinants were detected in the envelope protein of the murine retrovirus Fr98 (7). In the present study, we demonstrated that viruses encoding these separate determinants have different requirements for TNF-α. Deficiency in TNF-α significantly inhibited EC virus-mediated disease (Fig. 4B), indicating that TNF-α was involved in an important mechanism by which the EC virus induced neurovirulence (Fig. 9). In contrast, TNF-α was not involved in BE virus-mediated disease (Fig. 4A), suggesting that other factors, possibly MCP-1 (24), are responsible for BE virus-induced disease (Fig. 9). Fr98, which encodes the neurovirulence determinants of both the EC and BE viruses, may use both mechanisms to mediate pathogenesis (Fig. 9). This would explain the increased incidence and tempo of neurological disease observed in Fr98 virus infection compared to those seen in either EC or BE virus infection (Fig. 3). In the absence of TNF-α, the Fr98 virus may mediate disease solely through the BE virus pathway (Fig. 9), resulting in the delayed onset of kinetics observed in Fr98 virus-infected TNF-α−/− mice (Fig. 1A). These results indicate that the mechanisms involved in retrovirus-induced neurodegeneration are complex and multifactorial. Similarly, the mechanisms behind other, nonretroviral neurodegenerative disorders may also be dependent on the interactions of multiple components, including cytokine and chemokine expression.
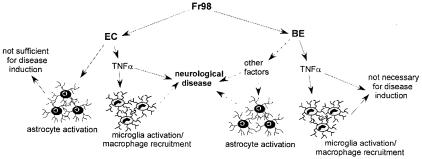
FIG. 9.
Pathways of Fr98, EC, and BE virus-mediated neurovirulence. Solid arrows indicate results from the present study. Broken arrows are hypothesized pathways that have not been demonstrated. Fr98 encodes both the EC and BE virus neurovirulence determinants and may use both pathways to mediate neurovirulence. EC virus-mediated disease requires TNF-α (Fig. 4B), which is also necessary for EC virus-induced microglia activation (Fig. 7). EC virus-induced astrocyte activation was not dependent on TNF-α (Fig. 7), and the activation of astrocytes in nonsymptomatic EC virus-infected TNF-α−/− mice indicates that astrocyte activation alone is not sufficient for disease induction. BE virus-induced neurovirulence did not require either TNF-α (Fig. 4A) or microglia and/or macrophage activation (Fig. 8). Thus, BE virus appears to mediate disease through other pathways, possibly through astrocyte production of MCP-1, as suggested in a previous report (24).
The differences in the pathology and clinical symptoms induced by FrCasE infection and those induced by Fr98, EC, or BE virus infection indicate that the mechanism by which FrCasE mediates disease is also different from that of these other viruses (28). The present results indicate that TNF-α does not contribute to FrCasE-induced spongiform neurodegeneration in the brain (Fig. 1B). Similarly, TNF-α does not appear to play a role in spongiform degeneration induced by another murine retrovirus, Moloney murine leukemia virus ts1 (9). Instead, recent evidence has suggested that both FrCasE and Moloney murine leukemia virus ts1 may mediate spongiform neurodegeneration through induction of endoplasmic reticulum stress or mitochondrial stress (5, 11, 15), rather than TNF-α.
TNF-α has been suggested to play both toxic and protective roles in neurological diseases such as human immunodeficiency virus (HIV)-associate dementia (HAD) and multiple sclerosis (14, 22, 34, 43). In the present experiments, elimination of TNF-α expression did not influence either BE or FrCasE virus-mediated disease (Fig. 2 and 4). However, both of these viruses induced significant levels of TNF-α upregulation in wild-type mice (Fig. 2). These results suggest that TNF-α does not have either direct neuroprotective or neurotoxic activity in these diseases. This conclusion is in agreement with previous studies of transgenic mice expressing TNF-α under control of the GFAP promoter, which indicated that overexpression of TNF-α did not induce direct neuronal damage in the brain but induced disease by recruiting inflammatory cells (40). Possibly, TNF-α requires another component of the host response for induction of neuronal pathogenesis. Additionally, the cellular source of TNF-α expression and/or kinetics of TNF-α expression may influence the contribution of TNF-α to disease induction.
TNF-α may contribute to EC virus-induced neurological disease through activation of microglia. Microglial activation is found in many instances of neurological disease, including multiple sclerosis, Alzheimer's disease, HAD, and other retrovirus-induced neurological diseases (17, 20, 45), and may play an important role in pathogenesis. In the present study, EC virus infection upregulated the expression of F4/80 in wild-type mice but not in TNF-α−/− mice (Fig. 7). This suggested that TNF-α may regulate retrovirus-induced activation of microglia cells. Alternatively, increased F4/80 expression levels may indicate the recruitment of a small number of peripheral macrophages into the brain not discernible by immunohistochemical analysis.
Activated astrocytes are often associated with neurological disorders and may also contribute to neuronal pathogenesis. The presence of activated astrocytes in EC virus-infected TNF-α−/− mice demonstrated that astrocyte activation alone was not sufficient for the induction of neurological disease (Fig. 7 and 9). Furthermore, it indicated that astrocyte activation was not dependent on TNF-α. Several studies have demonstrated that TNF-α can induce astrocyte activation (3, 46). However, other proinflammatory cytokines and chemokines, such as interleukin-1 and -6, SDF-α, and RANTES, can also induce astrocyte activation (3, 4, 16). Possibly, expression of one of these other proteins may induce astrocyte activation in the absence of TNF-α.
Unlike EC virus-induced neurological disease, BE virus-induced neurological disease does not appear to require either TNF-α (Fig. 4A) or microglial activation (Fig. 8). However, the BE virus infects the brain at a two- to threefold higher level than the EC virus (Fig. 2B) and induces the expression of other proinflammatory cytokines and chemokines not readily detected in EC virus-infected mice (data not shown) (25). At least one of the proinflammatory chemokines, MCP-1, has been shown to contribute to retrovirus-induced neurological disease (24). Therefore, BE may mediate disease through the upregulation of chemokines, rather than TNF-α. Additionally, the high level of virus in the brain and strong innate immune response during BE virus infection may limit the requirement for any one protein, such as TNF-α, in disease induction.
TNF-α has been implicated as a possible mechanism of HAD. Analysis of genetic polymorphisms in humans revealed a correlation with a high-expressor TNF-α allele and an increased risk of HAD (31). Furthermore, increased TNF-α mRNA expression has been found in brain samples from HAD patients compared to that of HIV-infected patients without dementia (44). Thus, TNF-α may contribute to the development of HAD. However, similar to the Fr98 virus-related polytropic murine retroviruses, HIV envelope variants can differ in the ability to induce TNF-α expression (10, 13), and TNF-α mRNA expression levels varied in HAD patients (44). Possibly, HIV strains that vary in the envelope protein may have different dependencies on TNF-α for the induction of neurological disease. Thus, treatment of HAD patients with TNF-α inhibitors may have disparate results depending on the viral envelope variants in the brain. Additionally, the level of TNF-α expression in cerebrospinal fluid may not correlate with the dependency of TNF-α in disease development. This would make it difficult to analyze the potential effect of TNF-α inhibitors on disease prevention or progression.
Acknowledgments
We thank Sonja Best, John Portis, and Sue Priola for critical reviews of the manuscript and Cynthia Favara for technical assistance with histology.
REFERENCES
- 1.Askovic, S., C. Favara, F. J. McAtee, and J. L. Portis. 2001. Increased expression of MIP-1α and MIP-1β mRNAs in the brain correlates spatially and temporally with the spongiform neurodegeneration induced by a murine oncornavirus. J. Virol. 75:2665-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Askovic, S., F. J. McAtee, C. Favara, and J. L. Portis. 2000. Brain infection by neuroinvasive but avirulent murine oncornaviruses. J. Virol. 74:465-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balasingam, V., T. Tejada-Berges, E. Wright, R. Bouckova, and V. W. Yong. 1994. Reactive astrogliosis in the neonatal mouse brain and its modulation by cytokines. J. Neurosci. 14:846-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonavia, R., A. Bajetto, S. Barbero, P. Pirani, T. Florio, and G. Schettini. 2003. Chemokines and their receptors in the CNS: expression of CXCL12/SDF-1 and CXCR4 and their role in astrocyte proliferation. Toxicol. Lett. 139:181-189. [DOI] [PubMed] [Google Scholar]
- 5.Dimcheff, D. E., S. Askovic, A. H. Baker, C. Johnson-Fowler, and J. L. Portis. 2003. Endoplasmic reticulum stress is a determinant of retrovirus-induced spongiform neurodegeneration. J. Virol. 77:12617-12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glass, J. D., S. L. Wesselingh, O. A. Selnes, and J. C. McArthur. 1993. Clinical-neuropathologic correlation in HIV-associated dementia. Neurology 43:2230-2237. [DOI] [PubMed] [Google Scholar]
- 7.Hasenkrug, K. J., S. J. Robertson, J. Porti, F. McAtee, J. Nishio, and B. Chesebro. 1996. Two separate envelope regions influence induction of brain disease by a polytropic murine retrovirus (FMCF98). J. Virol. 70:4825-4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iida, R., K. Saito, K. Yamada, A. S. Basile, K. Sekikawa, M. Takemura, H. Fujii, H. Wada, M. Seishima, and T. Nabeshima. 2000. Suppression of neurocognitive damage in LP-BM5-infected mice with a targeted deletion of the TNF-α gene. FASEB J. 14:1023-1031. [DOI] [PubMed] [Google Scholar]
- 9.Jolicoeur, P., C. Hu, T. W. Mak, J. C. Martinou, and D. G. Kay. 2003. Protection against murine leukemia virus-induced spongiform myeloencephalopathy in mice overexpressing Bcl-2 but not in mice deficient for interleukin-6, inducible nitric oxide synthetase, ICE, Fas, Fas ligand, or TNF-R1 genes. J. Virol. 77:13161-13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khanna, K. V., X. F. Yu, D. H. Ford, L. Ratner, J. K. Hildreth, and R. B. Markham. 2000. Differences among HIV-1 variants in their ability to elicit secretion of TNF-α. J. Immunol. 164:1408-1415. [DOI] [PubMed] [Google Scholar]
- 11.Kim, H. T., K. Waters, G. Stoica, W. Qiang, N. Liu, V. L. Scofield, and P. K. Wong. 2004. Activation of endoplasmic reticulum stress signaling pathway is associated with neuronal degeneration in MoMuLV-ts1-induced spongiform encephalomyelopathy. Lab. Investig. 84:816-827. [DOI] [PubMed] [Google Scholar]
- 12.Kolb, S. A., B. Sporer, F. Lahrtz, U. Koedel, H. W. Pfister, and A. Fontana. 1999. Identification of a T cell chemotactic factor in the cerebrospinal fluid of HIV-1-infected individuals as interferon-gamma inducible protein 10. J. Neuroimmunol. 93:172-181. [DOI] [PubMed] [Google Scholar]
- 13.Kong, L. Y., B. C. Wilson, M. K. McMillian, G. Bing, P. M. Hudson, and J. S. Hong. 1996. The effects of the HIV-1 envelope protein gp120 on the production of nitric oxide and proinflammatory cytokines in mixed glial cell cultures. Cell. Immunol. 172:77-83. [DOI] [PubMed] [Google Scholar]
- 14.The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. 1999. TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. Neurology 53:457-465. [PubMed] [Google Scholar]
- 15.Liu, N., X. Kuang, H. T. Kim, G. Stoica, W. Qiang, V. L. Scofield, and P. K. Wong. 2004. Possible involvement of both endoplasmic reticulum- and mitochondria-dependent pathways in MoMuLV-ts1-induced apoptosis in astrocytes. J. Neurovirol. 10:189-198. [DOI] [PubMed] [Google Scholar]
- 16.Luo, Y., M. A. Berman, Q. Zhai, F. R. Fischer, S. R. Abromson-Leeman, Y. Zhang, W. A. Kuziel, C. Gerard, and M. E. Dorf. 2002. RANTES stimulates inflammatory cascades and receptor modulation in murine astrocytes. Glia 39:19-30. [DOI] [PubMed] [Google Scholar]
- 17.Minagar, A., P. Shapshak, R. Fujimura, R. Ownby, M. Heyes, and C. Eisdorfer. 2002. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J. Neurol. Sci. 202:13-23. [DOI] [PubMed] [Google Scholar]
- 18.Miura, Y., N. Misawa, Y. Kawano, H. Okada, Y. Inagaki, N. Yamamoto, M. Ito, H. Yagita, K. Okumura, H. Mizusawa, and Y. Koyanagi. 2003. Tumor necrosis factor-related apoptosis-inducing ligand induces neuronal death in a murine model of HIV central nervous system infection. Proc. Natl. Acad. Sci. USA 100:2777-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore, R. J., D. M. Owens, G. Stamp, C. Arnott, F. Burke, N. East, H. Holdsworth, L. Turner, B. Rollins, M. Pasparakis, G. Kollias, and F. Balkwill. 1999. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat. Med. 5:828-831. [DOI] [PubMed] [Google Scholar]
- 20.Nelson, P. T., L. A. Soma, and E. Lavi. 2002. Microglia in diseases of the central nervous system. Ann. Med. 34:491-500. [DOI] [PubMed] [Google Scholar]
- 21.Orandle, M. S., A. G. MacLean, V. G. Sasseville, X. Alvarez, and A. A. Lackner. 2002. Enhanced expression of proinflammatory cytokines in the central nervous system is associated with neuroinvasion by simian immunodeficiency virus and the development of encephalitis. J. Virol. 76:5797-5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perry, S. W., S. Dewhurst, M. J. Bellizzi, and H. A. Gelbard. 2002. Tumor necrosis factor-alpha in normal and diseased brain: conflicting effects via intraneuronal receptor crosstalk? J. Neurovirol. 8:611-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persidsky, Y., J. Limoges, J. Rasmussen, J. Zheng, A. Gearing, and H. E. Gendelman. 2001. Reduction in glial immunity and neuropathology by a PAF antagonist and an MMP and TNF-α inhibitor in SCID mice with HIV-1 encephalitis. J. Neuroimmunol. 114:57-68. [DOI] [PubMed] [Google Scholar]
- 24.Peterson, K. E., J. S. Errett, T. Wei, D. E. Dimcheff, R. Ransohoff, W. A. Kuziel, L. Evans, and B. Chesebro. 2004. MCP-1 and CCR2 contribute to non-lymphocyte-mediated brain disease induced by Fr98 polytropic retrovirus infection in mice: role for astrocytes in retroviral neuropathogenesis. J. Virol. 78:6449-6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson, K. E., S. J. Robertson, J. L. Portis, and B. Chesebro. 2001. Differences in cytokine and chemokine responses during neurological disease induced by polytropic murine retroviruses map to separate regions of the viral envelope gene. J. Virol. 75:2848-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poli, A., M. Pistello, M. A. Carli, F. Abramo, G. Mancuso, E. Nicoletti, and M. Bendinelli. 1999. Tumor necrosis factor-alpha and virus expression in the central nervous system of cats infected with feline immunodeficiency virus. J. Neurovirol. 5:465-473. [DOI] [PubMed] [Google Scholar]
- 27.Portis, J. L., S. Czub, C. F. Garon, and F. J. McAtee. 1990. Neurodegenerative disease induced by the wild mouse ecotropic retrovirus is markedly accelerated by long terminal repeat and gag-pol sequences from nondefective Friend murine leukemia virus. J. Virol. 64:1648-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Portis, J. L., S. Czub, S. Robertson, F. McAtee, and B. Chesebro. 1995. Characterization of a neurologic disease induced by a polytropic murine retrovirus: evidence for differential targeting of ecotropic and polytropic viruses in the brain. J. Virol. 69:8070-8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poulsen, D. J., C. Favara, E. Y. Snyder, J. Portis, and B. Chesebro. 1999. Increased neurovirulence of polytropic mouse retroviruses delivered by inoculation of brain with infected neural stem cells. Virology 263:23-29. [DOI] [PubMed] [Google Scholar]
- 30.Poulsen, D. J., S. J. Robertson, C. A. Favara, J. L. Portis, and B. W. Chesebro. 1998. Mapping of a neurovirulence determinant within the envelope protein of a polytropic murine retrovirus: induction of central nervous system disease by low levels of virus. Virology 248:199-207. [DOI] [PubMed] [Google Scholar]
- 31.Quasney, M. W., Q. Zhang, S. Sargent, M. Mynatt, J. Glass, and J. McArthur. 2001. Increased frequency of the tumor necrosis factor-alpha-308 A allele in adults with human immunodeficiency virus dementia. Ann. Neurol. 50:157-162. [PubMed] [Google Scholar]
- 32.Robertson, M. N., M. Miyazawa, S. Mori, B. Caughey, L. H. Evans, S. F. Hayes, and B. Chesebro. 1991. Production of monoclonal antibodies reactive with a denatured form of the Friend murine leukemia virus gp70 envelope protein: use in a focal infectivity assay, immunohistochemical studies, electron microscopy and Western blotting. J. Virol. Methods 34:255-271. [DOI] [PubMed] [Google Scholar]
- 33.Robertson, S. J., K. J. Hasenkrug, B. Chesebro, and J. L. Portis. 1997. Neurologic disease induced by polytropic murine retroviruses: neurovirulence determined by efficiency of spread to microglial cells. J. Virol. 71:5287-5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saha, R. N., and K. Pahan. 2003. Tumor necrosis factor-alpha at the crossroads of neuronal life and death during HIV-associated dementia. J. Neurochem. 86:1057-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sasseville, V. G., M. M. Smith, C. R. Mackay, D. R. Pauley, K. G. Mansfield, D. J. Ringler, and A. A. Lackner. 1996. Chemokine expression in simian immunodeficiency virus-induced AIDS encephalitis. Am. J. Pathol. 149:1459-1467. [PMC free article] [PubMed] [Google Scholar]
- 36.Sato-Matsumura, K. C., J. Berger, J. A. Hainfellner, P. Mazal, and H. Budka. 1998. Development of HIV encephalitis in AIDS and TNF-α regulatory elements. J. Neuroimmunol. 91:89-92. [DOI] [PubMed] [Google Scholar]
- 37.Schmidtmayerova, H., H. S. Nottet, G. Nuovo, T. Raabe, C. R. Flanagan, L. Dubrovsky, H. E. Gendelman, A. Cerami, M. Bukrinsky, and B. Sherry. 1996. Human immunodeficiency virus type 1 infection alters chemokine beta peptide expression in human monocytes: implications for recruitment of leukocytes into brain and lymph nodes. Proc. Natl. Acad. Sci. USA 93:700-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott, K. A., R. J. Moore, C. H. Arnott, N. East, R. G. Thompson, B. J. Scallon, D. J. Shealy, and F. R. Balkwill. 2003. An anti-tumor necrosis factor-alpha antibody inhibits the development of experimental skin tumors. Mol. Cancer Ther. 2:445-451. [PubMed] [Google Scholar]
- 39.Sei, Y., K. Nishida, Y. Kustova, S. P. Markey, H. C. Morse III, and A. S. Basile. 1997. Pentoxifylline decreases brain levels of platelet activating factor in murine AIDS. Eur. J. Pharmacol. 325:81-84. [DOI] [PubMed] [Google Scholar]
- 40.Stalder, A. K., M. J. Carson, A. Pagenstecher, V. C. Asensio, C. Kincaid, M. Benedict, H. C. Powell, E. Masliah, and I. L. Campbell. 1998. Late-onset chronic inflammatory encephalopathy in immune-competent and severe combined immune-deficient (SCID) mice with astrocyte-targeted expression of tumor necrosis factor. Am. J. Pathol. 153:767-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sui, Y., R. Potula, D. Pinson, I. Adany, Z. Li, J. Day, E. Buch, J. Segebrecht, F. Villinger, Z. Liu, M. Huang, O. Narayan, and S. Buch. 2003. Microarray analysis of cytokine and chemokine genes in the brains of macaques with SHIV-encephalitis. J. Med. Primatol. 32:229-239. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki, T., I. Hide, K. Ido, S. Kohsaka, K. Inoue, and Y. Nakata. 2004. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J. Neurosci. 24:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tyor, W. R., J. D. Glass, J. W. Griffin, P. S. Becker, J. C. McArthur, L. Bezman, and D. E. Griffin. 1992. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann. Neurol. 31:349-360. [DOI] [PubMed] [Google Scholar]
- 44.Wesselingh, S. L., C. Power, J. D. Glass, W. R. Tyor, J. C. McArthur, J. M. Farber, J. W. Griffin, and D. E. Griffin. 1993. Intracerebral cytokine messenger RNA expression in acquired immunodeficiency syndrome dementia. Ann. Neurol. 33:576-582. [DOI] [PubMed] [Google Scholar]
- 45.Williams, K. C., and W. F. Hickey. 2002. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu. Rev. Neurosci. 25:537-562. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, L., W. Zhao, B. Li, D. L. Alkon, J. L. Barker, Y. H. Chang, M. Wu, and D. R. Rubinow. 2000. TNF-α induced over-expression of GFAP is associated with MAPKs. Neuroreport 11:409-412. [DOI] [PubMed] [Google Scholar]
- 47.Zink, M. C., G. D. Coleman, J. L. Mankowski, R. J. Adams, P. M. Tarwater, K. Fox, and J. E. Clements. 2001. Increased macrophage chemoattractant protein-1 in cerebrospinal fluid precedes and predicts simian immunodeficiency virus encephalitis. J. Infect. Dis. 184:1015-1021. [DOI] [PubMed] [Google Scholar]