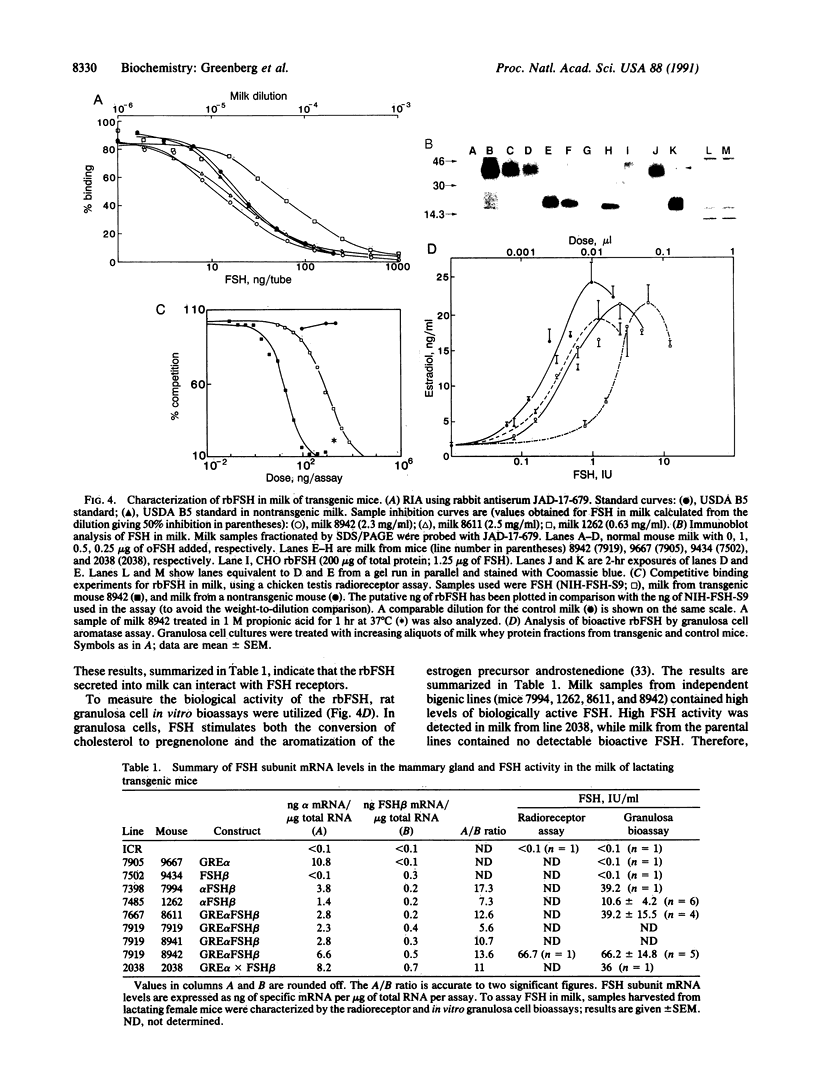
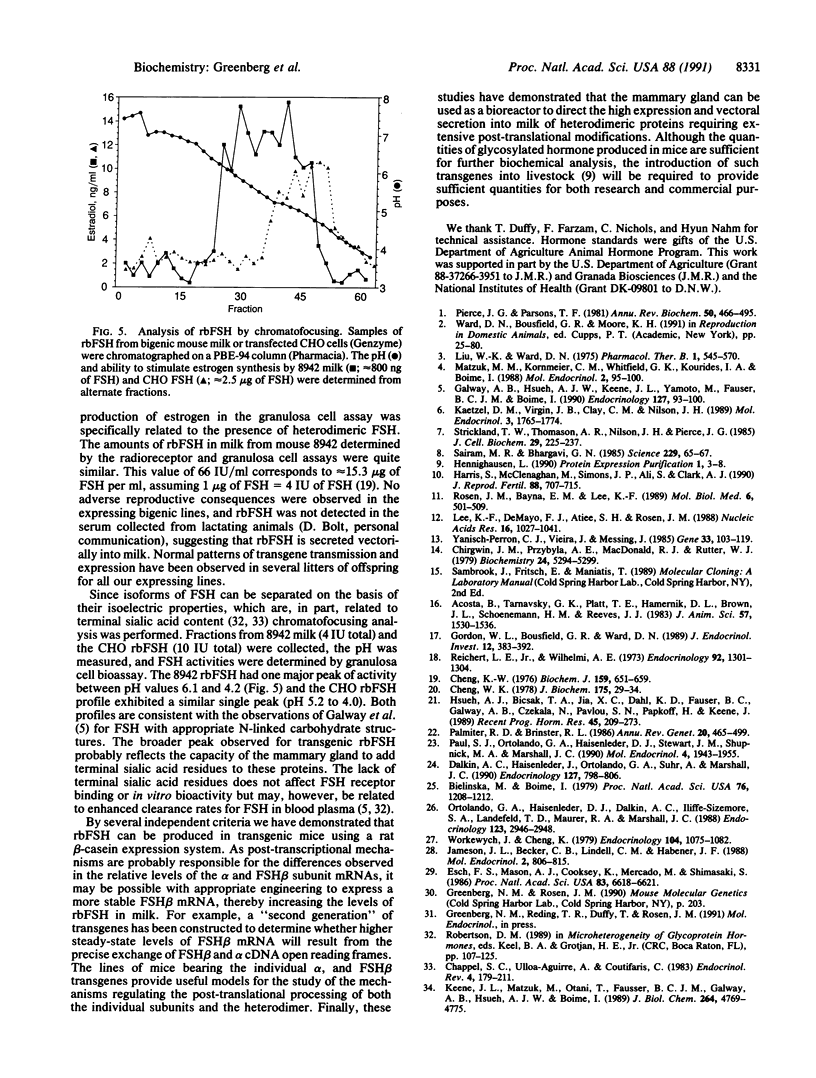
Abstract
Follicle-stimulating hormone (FSH; follitropin) is a pituitary glycoprotein composed of two post-translationally modified subunits, which must properly assemble to be biologically active. FSH has been difficult to purify and to obtain in quantities sufficient for detailed biochemical studies. We have targeted FSH expression to the mammary gland of transgenic mice by using cDNAs encoding the bovine alpha and FSH beta subunits and a modified rat beta-casein gene-based expression system. Lines of bigenic mice expressing both subunits have been generated either by coinjection of the subunit transgenes or by mating mice that acquired and expressed transgenes encoding an individual subunit. Up to 60 international units (15 micrograms) of biologically active FSH per ml was detected in the milk of the bigenic mice. These lines provide a model system for studying the post-transcriptional mechanisms that effect the expression and secretion of this heterodimeric hormone.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acosta B., Tarnavsky G. K., Platt T. E., Hamernik D. L., Brown J. L., Schoenemann H. M., Reeves J. J. Nursing enhances the negative effect of estrogen on LH release in the cow. J Anim Sci. 1983 Dec;57(6):1530–1536. doi: 10.2527/jas1983.5761530x. [DOI] [PubMed] [Google Scholar]
- Bielinska M., Boime I. Glycosylation of human chorionic gonadotropin in mRNA-dependent cell-free extracts: post-translational processing of an asparagine-linked mannose-rich oligosaccharide. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1208–1212. doi: 10.1073/pnas.76.3.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappel S. C., Ulloa-Aguirre A., Coutifaris C. Biosynthesis and secretion of follicle-stimulating hormone. Endocr Rev. 1983 Spring;4(2):179–211. doi: 10.1210/edrv-4-2-179. [DOI] [PubMed] [Google Scholar]
- Cheng K. W. Isolation and characterization of the subunits of bovine follitropin. Biochem J. 1978 Oct 1;175(1):29–34. doi: 10.1042/bj1750029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. W. Purification and properties of bovine pituitary follitropin. Biochem J. 1976 Dec 1;159(3):651–659. doi: 10.1042/bj1590651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dalkin A. C., Haisenleder D. J., Ortolano G. A., Suhr A., Marshall J. C. Gonadal regulation of gonadotropin subunit gene expression: evidence for regulation of follicle-stimulating hormone-beta messenger ribonucleic acid by nonsteroidal hormones in female rats. Endocrinology. 1990 Aug;127(2):798–806. doi: 10.1210/endo-127-2-798. [DOI] [PubMed] [Google Scholar]
- Esch F. S., Mason A. J., Cooksey K., Mercado M., Shimasaki S. Cloning and DNA sequence analysis of the cDNA for the precursor of the beta chain of bovine follicle stimulating hormone. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6618–6621. doi: 10.1073/pnas.83.17.6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galway A. B., Hsueh A. J., Keene J. L., Yamoto M., Fauser B. C., Boime I. In vitro and in vivo bioactivity of recombinant human follicle-stimulating hormone and partially deglycosylated variants secreted by transfected eukaryotic cell lines. Endocrinology. 1990 Jul;127(1):93–100. doi: 10.1210/endo-127-1-93. [DOI] [PubMed] [Google Scholar]
- Gordon W. L., Bousfield G. R., Ward D. N. Comparative binding of FSH to chicken and rat testis. J Endocrinol Invest. 1989 Jun;12(6):383–392. doi: 10.1007/BF03350707. [DOI] [PubMed] [Google Scholar]
- Harris S., McClenaghan M., Simons J. P., Ali S., Clark A. J. Gene expression in the mammary gland. J Reprod Fertil. 1990 Mar;88(2):707–715. doi: 10.1530/jrf.0.0880707. [DOI] [PubMed] [Google Scholar]
- Hennighausen L. The mammary gland as a bioreactor: production of foreign proteins in milk. Protein Expr Purif. 1990 Sep;1(1):3–8. doi: 10.1016/1046-5928(90)90037-y. [DOI] [PubMed] [Google Scholar]
- Hsueh A. J., Bicsak T. A., Jia X. C., Dahl K. D., Fauser B. C., Galway A. B., Czekala N., Pavlou S. N., Papkoff H., Keene J. Granulosa cells as hormone targets: the role of biologically active follicle-stimulating hormone in reproduction. Recent Prog Horm Res. 1989;45:209–277. doi: 10.1016/b978-0-12-571145-6.50009-1. [DOI] [PubMed] [Google Scholar]
- Jameson J. L., Becker C. B., Lindell C. M., Habener J. F. Human follicle-stimulating hormone beta-subunit gene encodes multiple messenger ribonucleic acids. Mol Endocrinol. 1988 Sep;2(9):806–815. doi: 10.1210/mend-2-9-806. [DOI] [PubMed] [Google Scholar]
- Kaetzel D. M., Virgin J. B., Clay C. M., Nilson J. H. Disruption of N-linked glycosylation of bovine luteinizing hormone beta-subunit by site-directed mutagenesis dramatically increases its intracellular stability but does not affect biological activity of the secreted heterodimer. Mol Endocrinol. 1989 Nov;3(11):1765–1774. doi: 10.1210/mend-3-11-1765. [DOI] [PubMed] [Google Scholar]
- Keene J. L., Matzuk M. M., Otani T., Fauser B. C., Galway A. B., Hsueh A. J., Boime I. Expression of biologically active human follitropin in Chinese hamster ovary cells. J Biol Chem. 1989 Mar 25;264(9):4769–4775. [PubMed] [Google Scholar]
- Lee K. F., DeMayo F. J., Atiee S. H., Rosen J. M. Tissue-specific expression of the rat beta-casein gene in transgenic mice. Nucleic Acids Res. 1988 Feb 11;16(3):1027–1041. doi: 10.1093/nar/16.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W. K., Ward D. N. The purification and chemistry of pituitary glycoprotein hormones. Pharmacol Ther B. 1975;1(3):545–570. doi: 10.1016/0306-039x(75)90053-7. [DOI] [PubMed] [Google Scholar]
- Matzuk M. M., Kornmeier C. M., Whitfield G. K., Kourides I. A., Boime I. The glycoprotein alpha-subunit is critical for secretion and stability of the human thyrotropin beta-subunit. Mol Endocrinol. 1988 Feb;2(2):95–100. doi: 10.1210/mend-2-2-95. [DOI] [PubMed] [Google Scholar]
- Ortolano G. A., Haisenleder D. J., Dalkin A. C., Iliff-Sizemore S. A., Landefeld T. D., Maurer R. A., Marshall J. C. Follicle-stimulating hormone beta subunit messenger ribonucleic acid concentrations during the rat estrous cycle. Endocrinology. 1988 Dec;123(6):2946–2948. doi: 10.1210/endo-123-6-2946. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S. J., Ortolano G. A., Haisenleder D. J., Stewart J. M., Shupnik M. A., Marshall J. C. Gonadotropin subunit messenger RNA concentrations after blockade of gonadotropin-releasing hormone action: testosterone selectively increases follicle-stimulating hormone beta-subunit messenger RNA by posttranscriptional mechanisms. Mol Endocrinol. 1990 Dec;4(12):1943–1955. doi: 10.1210/mend-4-12-1943. [DOI] [PubMed] [Google Scholar]
- Pierce J. G., Parsons T. F. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- Reichert L. E., Jr, Wilhelmi A. E. A summary of the biological activities of the various lots of pituitary hormones produced under the programs of the National Institute of Arthritis, Metabolism and Digestive Diseases. Endocrinology. 1973 May;92(5):1301–1304. doi: 10.1210/endo-92-5-1301. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Bayna E., Lee K. F. Analysis of milk protein gene expression in transgenic mice. Mol Biol Med. 1989 Dec;6(6):501–509. [PubMed] [Google Scholar]
- Sairam M. R., Bhargavi G. N. A role for glycosylation of the alpha subunit in transduction of biological signal in glycoprotein hormones. Science. 1985 Jul 5;229(4708):65–67. doi: 10.1126/science.2990039. [DOI] [PubMed] [Google Scholar]
- Strickland T. W., Thomason A. R., Nilson J. H., Pierce J. G. The common alpha subunit of bovine glycoprotein hormones: limited formation of native structure by the totally nonglycosylated polypeptide chain. J Cell Biochem. 1985;29(3):225–237. doi: 10.1002/jcb.240290307. [DOI] [PubMed] [Google Scholar]
- Workewych J., Cheng K. W. Development of glycoprotein hormones and their alpha- and beta-subunits in bovine fetal pituitary glands. II. Quantitation of free alpha- and beta-subunits by radioimmunoassays and correlation of free alpha-subunit with thyrotropin, follicle-stimulating hormone, and luteinizing hormone. Endocrinology. 1979 Apr;104(4):1075–1082. doi: 10.1210/endo-104-4-1075. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]